Abstract
Chronic inflammation may play an etiologic role in endometrial cancer. Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce inflammatory activity by inhibiting the pro-inflammatory cyclooxygenase enzymes and therefore may decrease cancer risk. However, few studies have examined the association between NSAID use and endometrial cancer. We conducted a prospective study among 72,524 women in the NIH-AARP Diet and Health Study. Women completed a questionnaire in 1996–97 on lifestyle and health-related factors, including type and frequency of NSAID use within the past year, and were followed through 2003 by linkages to cancer registries and vital status databases. During 488,261 person-years of follow-up, there were 732 incident endometrial cancers. NSAID use, compared with non-use of NSAIDs, was not significantly associated with endometrial cancer risk (relative risk (RR) = 0.90, 95% CI: 0.74–1.09). Null associations also were observed by type of NSAID use (aspirin only, RR = 0.88, 95% CI: 0.70–1.11; non-aspirin NSAID [NA-NSAID] only, RR= 1.01, 95% CI: 0.79–1.29; both aspirin and NA-NSAIDs, RR=0.85, 95% CI: 0.68–1.06). Generally, results were not statistically significant by frequency of use for aspirin or non-aspirin NSAIDs. Results did not change when women with a history of heart disease, hypertension, or diabetes were excluded to minimize the potential for confounding by indication. Overall, our data do not support an association between aspirin or NA-NSAID use and endometrial cancer risk.
Keywords: nonsteroidal anti-inflammatory drug, aspirin, analgesic, endometrial cancer, epidemiology
INTRODUCTION
Recent interest has focused on the role of chronic inflammation in the etiology of several cancers (1). Although many established risk factors for endometrial cancer, such as obesity, parity, oral contraceptive use, and unopposed estrogen use, traditionally have been viewed as acting via estrogen and progesterone pathways, Modugno et. al. (2005) hypothesized that they also may influence endometrial cancer through their effects on inflammation (2). Cyclooxygenase (COX) is an enzyme for the rate-limiting step in the synthesis of prostaglandins, key mediators of the inflammatory process, and is inhibited by nonsteroidal anti-inflammatory drugs (NSAIDs) (reviewed in (3, 4)). Increased COX expression has been observed in endometrial tumors (5, 6). Thus, if chronic inflammation plays an etiologic role, NSAID use might be hypothesized to decrease endometrial cancer risk (2).
Few studies to date have described associations between NSAID use and endometrial cancer. Aspirin use was examined in one case-control (7) and one prospective study (8), which also examined associations with non-aspirin NSAIDs (NA-NSAIDs). Neither study observed a significant relationship between NSAID use and endometrial cancer risk overall. However, in each, aspirin use was associated with a reduction in endometrial cancer risk among obese women, and the authors proposed two explanations for the association (7, 8). First, obesity is associated with increased levels of inflammatory cytokines (9), and thus the anti-inflammatory effects of NSAIDs may have a greater health benefit among obese women. Second, COX-2 and aromatase levels are positively correlated (10), and therefore inhibition of COX-2 by NSAID use may reduce estrogen production through decreased aromatization of androgens in adipose tissue (11, 12). However, because obesity is a strong risk factor for endometrial cancer, it is possible that effect modification observed on a relative risk scale does not reflect meaningful differences on an absolute scale. Thus, it is important for studies to consider both relative and absolute measures of risk. Further, although all NSAIDs inhibit COX activity (4), there are important mechanistic differences by NSAID type. Aspirin is distinct from other NSAIDs because it permanently inactivates COX, whereas other NSAIDs reversibly inactivate COX (reviewed in (13, 14)).
Therefore, we sought to determine associations, on both the relative and absolute scale, between endometrial cancer risk and aspirin and non-aspirin NSAID use in the prospective NIH-AARP Diet and Health Study.
MATERIALS AND METHODS
The NIH-AARP Diet and Health Study
The National Institutes of Health (NIH)-AARP Diet and Health Study was established in 1995–96 when 3.5 million AARP members ages 50 to 71 years were sent a questionnaire to collect demographic, anthropometric, lifestyle, and health information (15). Questionnaires were mailed to AARP residents in six states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia and Detroit, Michigan) that had high-quality cancer registries and substantial AARP membership. The baseline questionnaire was satisfactorily completed and returned by 566,402 members.
A second questionnaire was mailed in 1996–97 to obtain additional information, including NSAID use. It was completed by 334,908 men and women, excluding individuals who died or moved out of the study area before their questionnaires were scanned. Our analysis additionally excluded individuals who used a proxy respondent for either the baseline (n=6,959) or second (n= 3,424) questionnaire, were male (n=188,117), or reported a diagnosis of cancer other than non-melanoma skin cancer on either questionnaire (n=9,022, including 617 endometrial cancers), a history of hysterectomy (n= 51,515) or unknown hysterectomy status (n= 1,498) on the baseline questionnaire, or menstrual periods that stopped due to surgery (n=1,063) or radiation or chemotherapy (n=58) on the baseline questionnaire. After further excluding women who developed non-epithelial endometrial cancer (n=51) or had missing or inconsistent NSAID information (n=677), there were 72,524 eligible women who had completed both questionnaires. The Special Studies Institutional Review Board of the National Cancer Institute approved the study, and all participants provided consent.
Nonsteroidal Anti-Inflammatory Drug (NSAID) Use
Women were asked whether they had taken aspirin products during the last 12 months and, if they had, their usual frequency of use (<2 or 2–3 times per month, 1–2, 3–4, or 5–6 times per week, daily, or 2 or more times per day). They also were asked if they had taken other pain relievers, listed by their generic and brand names, during the past 12 months, specifically excluding Tylenol, acetaminophen, and any other pain relievers. A single question asked about frequency of NA-NSAID use, based on the same categories as aspirin NSAID use; the number of non-aspirin NSAIDs used in the last year was not assessed.
Endometrial Cancer
Incident cancers through 2003 were identified by probabilistic linkages with cancer registries in the original recruitment areas and three common states of re-location (Arizona, Nevada, Texas). Fatal cancers were identified by linkages with mortality databases (Social Security vital status database, with cause of death from the National Death Index Plus file). The original cancer registries were certified by the North American Association of Central Cancer Registries (NAACCR) as being 95% complete within 18 months of the end of the diagnosis year, and a validation study found that our follow-up methods identify about 90% of cancers in the cohort (16). During 488,261 person-years of follow-up, there were 732 incident endometrial cancers, most of which were early-stage tumors (81% of tumors with known stage were localized).
Statistical Analysis
Cox proportional hazards models were used to estimate relative risks (RRs) and 95% confidence interval (CIs) with age as the time metric. Baseline age was used as a stratification variable to control for (potential) secular trends in endometrial cancer by birth cohort. Women entered analyses at the age they completed the second questionnaire and contributed person-time until they were diagnosed with endometrial cancer, moved out of the registry catchment area (self-reported or identified by the U.S. Postal Service), died, or reached the end of follow-up on December 31, 2003.
The referent group in all analyses was non-users of NSAIDs, defined as women who had not taken aspirin or NA-NSAIDs in the year before the second study questionnaire. NSAID users were women who reported aspirin or NA-NSAID use (with or without a frequency of use), as well as those who skipped the yes/no use question but reported a frequency of use. The small proportion of women with inconsistent responses (i.e., report of non-use with a frequency of use) was excluded.
NSAID use was examined overall and by type (non-use, aspirin only, NA-NSAID only, both aspirin and NA-NSAIDs). For analyses of any NSAID use, results are presented for women with data on both aspirin and NA-NSAIDs (n=71,165) to be consistent with the NSAID type analyses; including the small number of additional women with information on one NSAID type only (n=1,359) did not alter associations. Associations for aspirin and NA-NSAID use were also examined separately, overall and by frequency of use. Analyses were stratified by obesity (body mass index (BMI) <30, ≥30 kg/m2) to enable comparisons to previous studies. To minimize the potential for confounding by indication, we also assessed associations excluding women with a self-reported history of heart disease, hypertension, or diabetes at baseline.
Multivariable models included the following covariates based on a priori consideration of potential confounders: race, age at menarche, age at menopause, postmenopausal hormone use, parity, oral contraceptive use, smoking, body mass index, physical activity, family history of breast cancer, and a personal history of heart disease, high blood pressure, or diabetes (see Table 2 for categories). For covariates with a small amount of missing data, women who did not report information were coded into the most frequently reported category. Additionally, the following variables were considered but not included in the final models because they did not meaningfully change results: education, age at first birth, waist-to-hip ratio, body shape, alcohol consumption, multi-vitamin intake, total calories consumed, hours spent watching TV, amount of sleep, self-rated health, history of oophorectomy, history of other ovarian surgery, and a history of health conditions.
Table 2.
Use of nonsteroidal anti-inflammatory drugs within the last year, overall and by type, and risk of endometrial cancer in the NIH-AARP Diet and Health Study.
| Cases | Person- Years | Age-adjusted | Multivariable* | |||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |||
| NSAID use in the last year | ||||||
| No | 123 | 71,793 | 1.00 | Referent | 1.00 | Referent |
| Yes | 597 | 407,400 | 0.89 | 0.73 – 1.08 | 0.90 | 0.74 – 1.09 |
| NSAID type | ||||||
| Non-users | 123 | 71,793 | 1.00 | Referent | 1.00 | Referent |
| Aspirin only | 184 | 126,233 | 0.85 | 0.68 – 1.07 | 0.88 | 0.70 – 1.11 |
| NA-NSAID only | 149 | 88,816 | 1.05 | 0.82 – 1.33 | 1.01 | 0.79 – 1.29 |
| Aspirin + NA-NSAID | 264 | 192,351 | 0.85 | 0.68 – 1.05 | 0.85 | 0.68 – 1.06 |
NSAID = nonsteroidal anti-inflammatory drug; NA-NSAID = non-aspirin NSAID
Adjusted for race (white, non-white), age at menarche (≤12, 13–14, ≥15 years), age at menopause (premenopausal, age at menopause: <45, 45–49, 50–54, ≥ 55 years, missing age at menopause/menopausal status), postmenopausal hormone use (non-use, unopposed estrogen use: <10, ≥10 yrs, estrogen plus progestin use: <10, ≥10 years, other/missing hormone use or duration), parity (nulliparous, 1, 2, 3 or more births), oral contraceptive use (non-use, < 1 year, 1 to <10, ≥10 years), smoking (never, former, current, missing), body mass index (<25, 25 to <30, ≥30 kg/m2, missing), physical activity (5 categories of exercise frequency over the past 12 months), family history of breast cancer (yes, no, missing), personal history of diabetes (yes, no), heart disease (yes, no), and high blood pressure (yes, no, missing).
Proportional hazards were assessed using interaction terms with log exit age. The model assumptions appeared reasonable for all models (exposure interaction terms only, all p-values ≥ 0.42; exposure and covariate interaction terms, all p-values ≥ 0.12).
We calculated incidence rates, both crude and standardized to the age distribution of the entire study population, overall, by NSAID use, and by NSAID type. Rates were also stratified by obesity.
RESULTS
Study Population Description
The mean age at baseline was 62.4 years among this primarily Caucasian (>90%) population. Almost half the women reported use of aspirin (26%) or NA-NSAIDs (19%) only, while 40% reported using both and 15% reported using neither in the last year. Most women reported using aspirin or NA-NSAIDs less than weekly (51% and 56%, respectively), with about a quarter reporting weekly and daily use of aspirin (24% and 25%, respectively) or NA-NSAIDs (24% and 20%, respectively). The frequencies of aspirin and NA-NSAID use were positively correlated (ρ = 0.72) and were similar for exclusive and non-exclusive users (e.g., the proportion using aspirin two or more times a day was 4.3% among aspirin-only users and 4.1% among users of both aspirin and NA-NSAIDs).
The average 6.7 years of follow-up per woman was similar across NSAID use category (non-use, aspirin only, NA-NSAID only, both aspirin and NA-NSAID). The majority of women were parous, and among parous women, most had 3 or more children (Table 1). Non-users of NSAIDs within the last year were slightly less likely than NSAID users to be white or have education beyond the high school level. Non-users and users of NSAIDs had similar mean ages at baseline and menarche, smoking status, BMI, and history of hypertension. Despite a similar mean age at baseline, non-users of NSAIDs were less likely than NSAID users to use postmenopausal hormones or oral contraceptives. There was heterogeneity among NSAID users, with aspirin-only users less likely than users of NA-NSAIDs only or both aspirin and NA-NSAIDs to use postmenopausal hormones and oral contraceptives, and more likely to report a history of heart disease.
Table 1.
Characteristics at study baseline of 72,524 women in the NIH-AARP Diet and Health Study by use of nonsteroidal anti-inflammatory drugs (NSAIDs).
| Any*NSAID Use | NSAID Type* | Aspirin + NA | |||
|---|---|---|---|---|---|
| No | Yes | Aspirin only | NA only | ||
| (n= 10,729) | (n=60,436) | (n= 18,757) | (n= 13,204) | (n= 28,475) | |
| Mean age at baseline (SD) | 63.4 (5.2) | 62.2 (5.5) | 63.3 (5.3) | 61.6 (5.5) | 61.7 (5.5) |
| Percent | Percent | Percent | Percent | Percent | |
| Race/Ethnicity | |||||
| White | 88.8 | 92.8 | 92.8 | 92.0 | 93.3 |
| Non-white | 11.2 | 7.2 | 7.2 | 8.0 | 6.7 |
| Education | |||||
| < High school | 6.2 | 4.1 | 4.6 | 4.0 | 3.7 |
| High school | 26.7 | 23.0 | 25.0 | 23.6 | 21.4 |
| Post-high school | 32.3 | 34.5 | 33.6 | 35.0 | 34.9 |
| College graduate | 32.2 | 36.2 | 34.5 | 35.1 | 37.8 |
| Age at menarche (years) | |||||
| ≤ 12 | 46.6 | 47.6 | 46.2 | 49.7 | 47.5 |
| 13–14 | 43.1 | 43.1 | 44.3 | 41.2 | 43.3 |
| ≥ 15 | 10.0 | 9.0 | 9.3 | 8.8 | 9.0 |
| Menopausal status | |||||
| Premenopausal | 3.5 | 6.6 | 4.0 | 8.1 | 7.5 |
| Postmenopausal | 96.0 | 93.0 | 95.6 | 91.4 | 92.1 |
| Postmenopausal hormone use | |||||
| Non-use | 61.8 | 49.7 | 58.4 | 47.1 | 45.3 |
| E only, <10 years | 5.5 | 6.0 | 5.7 | 6.0 | 6.1 |
| E only, ≥10 years | 0.6 | 0.8 | 0.9 | 0.8 | 0.7 |
| E+P, <10 years | 15.5 | 22.5 | 17.1 | 24.4 | 25.1 |
| E+P, ≥ 10+ years | 4.9 | 7.4 | 5.7 | 7.7 | 8.4 |
| Oral contraceptive use | |||||
| Never or < 1 year | 65.6 | 57.6 | 64.0 | 54.7 | 54.7 |
| 1 to <10 years | 25.0 | 30.9 | 26.3 | 33.0 | 33.0 |
| ≥ 10 years | 8.9 | 10.9 | 9.1 | 11.7 | 11.8 |
| Parity | |||||
| No births | 19.9 | 17.5 | 19.3 | 16.6 | 16.7 |
| 1 | 11.1 | 10.6 | 10.7 | 10.9 | 10.3 |
| 2 | 25.1 | 26.3 | 25.3 | 26.9 | 26.7 |
| ≥ 3 | 43.6 | 45.4 | 44.4 | 45.4 | 46.0 |
| Smoking status | |||||
| Never | 46.9 | 43.7 | 45.1 | 43.2 | 42.9 |
| Former | 37.4 | 40.1 | 37.2 | 41.3 | 41.5 |
| Current | 13.2 | 13.7 | 15.2 | 13.3 | 12.9 |
| Body mass index (kg/m2) | |||||
| <25 | 47.5 | 46.4 | 49.5 | 44.6 | 45.2 |
| 25 to <30 | 28.5 | 30.8 | 30.4 | 30.3 | 31.2 |
| ≥>30 | 20.2 | 20.4 | 17.4 | 22.8 | 21.2 |
| Self-reported: | |||||
| Heart disease | 8.1 | 6.9 | 8.7 | 4.6 | 6.7 |
| Hypertension | 34.4 | 33.2 | 34.0 | 32.4 | 33.2 |
| Diabetes | 7.5 | 5.8 | 6.0 | 6.1 | 5.5 |
Does not include 1,359 women excluded for reasons described in Methods section.
Missing data are not shown and were included in percentage calculations; therefore, percents may not sum to 100.
NSAID = nonsteroidal anti-inflammatory drug; NA =non-aspirin; E = estrogen; E+P = estrogen plus progestin.
Aspirin products were defined as: generic aspirin, Bayer, Bufferin, Anacin, Ecotrin, or Excedrin.
Non-aspirin products were defined as: Generic ibuprofen, Advil, Nuprin, Motrin, Aleve, Orudis, Ketoprofen, Naprosyn, Anaprox, Feldene, Piroxicam, Clinoril, Sulindac, Indocin, Indomethacin, Relafen, Nalfon, Nambumetone, or Fenoprofen, excluding Tylenol, acetaminophen, or any other pain relievers.
Multivariable Results
Age- and multivariable-adjusted (Table 2) results were similar. NSAID use was not statistically significantly associated with endometrial cancer risk (RR= 0.90, 95% CI: 0.74–1.09). A null association was observed for use of aspirin only, non-aspirin only, and both aspirin and NA-NSAIDs. Results remained similar in analyses restricted to women with complete covariate information.
Any aspirin use was not significantly associated with endometrial cancer risk (RR=0.86, 95% CI: 0.71–1.06, Table 3). Users of aspirin two or more times per day had a lower risk of endometrial cancer than non-users of NSAIDs (RR=0.55, 95% CI: 0.31–0.95). However, there were only 14 endometrial cancers among these most frequent users, and there was no significant trend by frequency of use (p=0.64). NA-NSAID use was not significantly associated with endometrial cancer risk overall (RR=0.90, 95% CI: 0.74–1.11) or by frequency of use (p-trend=0.45).
Table 3.
Use of aspirin or non-aspirin nonsteroidal anti-inflammatory drugs* within the last year, overall and by frequency of use, and risk of endometrial cancer in the NIH-AARP Diet and Health Study.
| Cases | Person- Years | RR† | 95% CI† | |
|---|---|---|---|---|
| Aspirin use (any) | ||||
| No | 123 | 71,793 | 1.00 | Referent |
| Yes | 453 | 321,814 | 0.86 | 0.71 – 1.06 |
| Frequency of aspirin use | ||||
| Non-use | 123 | 71,793 | 1.00 | Referent |
| < 2 times per month | 135 | 104,342 | 0.83 | 0.65 – 1.07 |
| 2–3 times per month | 71 | 61,329 | 0.75 | 0.56 – 1.00 |
| 1–2 times per week | 53 | 35,534 | 0.94 | 0.68 – 1.30 |
| 3–4 times per week | 48 | 27,955 | 1.04 | 0.74 – 1.46 |
| 5–6 times per week | 24 | 12,886 | 1.07 | 0.69 – 1.65 |
| Daily | 108 | 65,979 | 0.91 | 0.70 – 1.18 |
| 2 or more times per day | 14 | 13,454 | 0.55 | 0.31 – 0.95 |
| p-trend=0.64 | ||||
| NA-NSAID use (any) | ||||
| No | 123 | 71,793 | 1.00 | Referent |
| Yes | 417 | 284,122 | 0.90 | 0.74 – 1.11 |
| Frequency of NA-NSAID use | ||||
| Non-use | 123 | 71,793 | 1.00 | Referent |
| < 2 times per month | 123 | 96,554 | 0.86 | 0.67 – 1.11 |
| 2–3 times per month | 85 | 62,840 | 0.90 | 0.68 – 1.19 |
| 1–2 times per week | 49 | 35,947 | 0.91 | 0.65 – 1.27 |
| 3–4 times per week | 32 | 21,800 | 0.91 | 0.62 – 1.36 |
| 5–6 times per week | 17 | 10,782 | 0.92 | 0.55 – 1.53 |
| Daily | 53 | 24,247 | 1.17 | 0.85 – 1.62 |
| 2 or more times per day | 57 | 31,122 | 0.97 | 0.70 – 1.33 |
| p-trend=0.45 | ||||
NA-NSAID = non-aspirin nonsteroidal anti-inflammatory drug
Among exclusive and non-exclusive users (thus some women are included as both aspirin and non-aspirin users)
Adjusted for race, age at menarche, age at menopause, postmenopausal hormone use, parity, oral contraceptive use, smoking, body mass index, physical activity, family history of breast cancer, personal history of diabetes, heart disease, and high blood pressure.
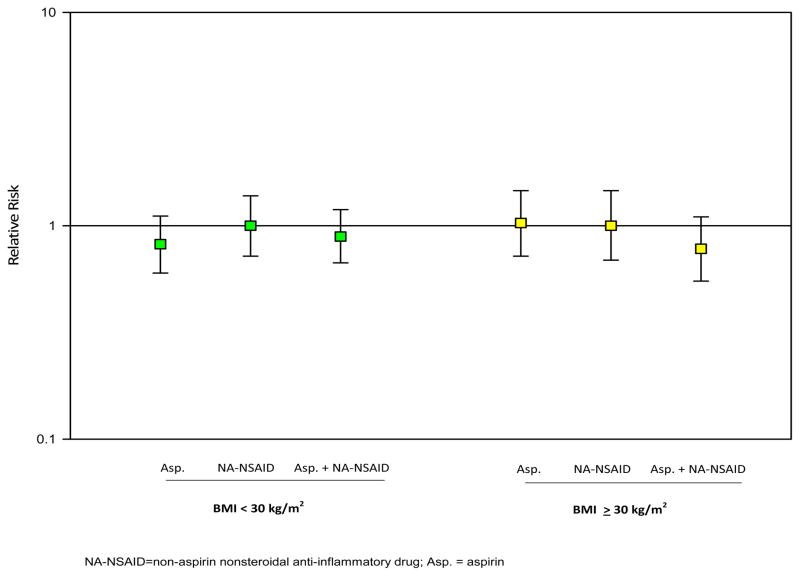
In analyses stratified by obesity, NSAID use (overall, by type, and by frequency) was not associated with endometrial cancer risk among non-obese or obese women (Figure 1, results by NSAID type; other data not shown). Among aspirin users of two or more times per day, point estimates were lower among obese women (RR=0.49, 95% CI: 0.21–1.13) than non-obese women (RR=0.65, 95% CI: 0.31–1.36). However, there were few cases among the aspirin users of two or more times per day (n=6 among obese women and 8 among non-obese women).
Figure 1.
Type of nonsteroidal anti-inflammatory drug (aspirin only, NA-NSAID only, both aspirin and NA-NSAIDs) and endometrial cancer risk by body mass index (BMI).
NA-NSAID=non-aspirin nonsteroidal anti-inflammatory drug; Asp. = aspirin
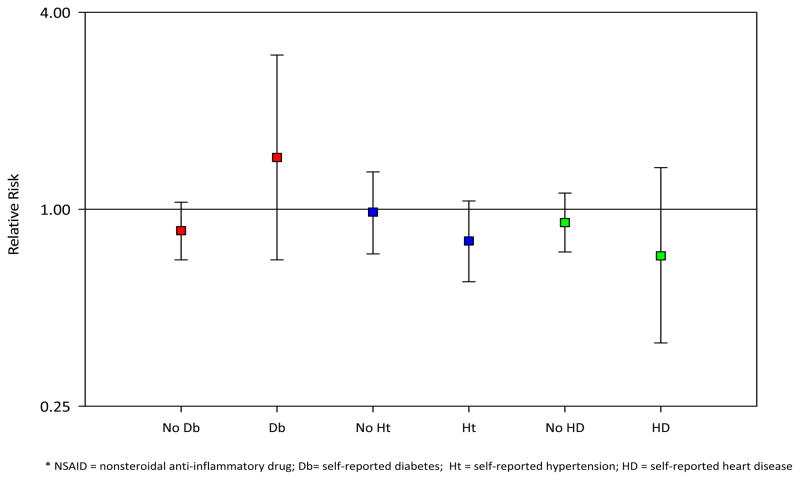
When women with a history of heart disease, hypertension, or diabetes were excluded from the study population, associations for any NSAID use and NSAID type appeared consistent with the primary analyses (Figure 2). In another sensitivity analysis, the referent group was expanded to include infrequent (less than monthly) users; results remained similar to the main findings.
Figure 2.
Ever use of NSAIDs and endometrial cancer risk by self-reported history of diabetes, hypertension, and heart disease.
* NSAID = nonsteroidal anti-inflammatory drug; Db = self-reported diabetes; Ht = self-reported hypertension; HD = self-reported heart disease
Incidence Rates
The crude incidence rate of endometrial cancer was 17 per 10,000 person-years among non-users of NSAIDs and 15 per 10,000 person-years among NSAID users. There was slight variation by NSAID type, with an endometrial cancer rate per 10,000 person-years of 15 among aspirin-only users, 17 among NA-NSAID-only users, and 14 among both aspirin and NA-NSAID users. The crude and age-standardized rates were similar (data not shown).
Non-obese (BMI <30 kg/m2) women had a much lower rate of endometrial cancer than obese (BMI ≥ 30 kg/m2) women (11 vs. 29 per 10,000 person-years, respectively). Non-users of NSAIDs had rates of 12 and 36 per 10,000 person-years among non-obese and obese women, respectively, while NSAID users had rates of 11 and 28 per 10,000 person-years among non-obese and obese women, respectively. Similarly, among aspirin-only users, non-obese and obese women had crude rates of 10 and 36 per 10,000 person-years, respectively. The crude rate was 2.3 times higher among obese NSAID users, and 3 times higher among obese aspirin users, than the crude rate among non-obese non-users.
DISCUSSION
In this large, prospective study, we did not observe significant associations between ever use of NSAIDs in the past year and endometrial cancer risk overall or by NSAID type. Aspirin use twice or more per day was inversely associated with endometrial cancer, but the trend across frequency categories was not significant and there were few cases among these aspirin users. There was no significant reduction in endometrial cancer risk among daily aspirin users or the most frequent non-aspirin NSAID users. Thus, given the inconsistency of results by NSAID frequency and type, it seems possible that the single significant association among frequent aspirin users may be a spurious finding.
Two other studies did not observe significant overall associations between NSAID use and endometrial cancer. In a hospital-based case-control study (7), duration, frequency, and lifetime or regular use of aspirin were not associated with endometrial cancer risk. In the prospective Nurses’ Health Study (NHS) (8), null associations were observed for recency, duration, dosage, and frequency of aspirin use, as well as frequency of NA-NSAID use. However, aspirin use was inversely associated with endometrial cancer among obese women in both studies (7, 8). In the hospital-based case-control study (7), use of aspirin seven or more times per week (n=23 cases and 16 controls) was associated with a reduced endometrial cancer risk among obese women (RR=0.40, 95% CI: 0.18–0.92), with similar associations for long-duration (over 10 years, RR=0.46) or cumulative (over 10 tablet-years, OR=0.49) aspirin use. In the NHS (8), current use of aspirin was inversely associated with endometrial cancer among obese women (RR=0.66, 95% CI: 0.46–0.95, n=48 and 125 cases among never and current users, respectively) and positively associated among non-obese women (RR=1.41, 95% CI: 1.05–1.89, n=71 and 261 cases among never and current users, respectively).
It is possible that the significant results stratified by obesity were driven by small differences on the absolute scale. Using data presented in the NHS paper (Table 3 in (8)) to calculate crude endometrial cancer rates, never and current aspirin users had similar rates to each other among both obese (12 and 10 per 10,000 person-years, respectively) and non-obese (3 and 4 per 10,000 person-years, respectively) women. Obese aspirin users had a 3.3 times higher rate than non-obese non-aspirin users in the NHS, similar to our findings.
In 2001, NSAIDs accounted for 30 billion over-the-counter and 70 million prescription sales (4). The prevalence of NSAID use in epidemiologic studies reflects the way it was assessed, and the three epidemiologic studies to date have used different methods: use within the last year (our study), regularly updated information (NHS (8)), or retrospective recall of NSAID use prior to illness, with “regular” use defined as use of at least once a week for 6 months (hospital case-control study (7)). Despite different ways of defining NSAID use, all three studies found an overall null association and do not provide evidence of a strong association between NSAID use and endometrial cancer risk.
A limitation of our study is that we did not have information on dose or duration, and our results reflect an unknown average dose and duration. However, the primary results from two previous studies with this information (7, 8) were consistent with our study results. We also did not update exposure information during follow-up and were unable to separately examine non-prescription and prescription NA-NSAIDs.
We did not collect information on the reason for NSAID use. The most common reason for use of non-prescription NSAIDs is relief of pain (4). To reduce the influence of reverse causation, we excluded the first year of follow-up among all participants to ensure the prospective nature of our exposure. As expected given the large number of early-stage tumors in our study, results remained similar to the main analyses. We also were concerned about aspirin use taken for cardiovascular health benefits because inflammation is a key factor in cardiovascular health (17), and thus aspirin use for secondary prevention of cardiovascular disease might obscure a protective association with endometrial cancer risk. In analyses restricted to individuals without a self-reported history of heart disease, hypertension, or diabetes, results generally were similar to the main analyses.
Our study had several strengths. The NIH-AARP Diet and Health Study is a large, prospective study with detailed information on NSAID type and frequency of use. We had data on numerous potential confounders, including other health conditions, and could adjust for and stratify by key variables. We also were able to calculate both absolute and relative risk measures to describe the relationship between NSAID use, BMI, and endometrial cancer.
In conclusion, our results, in conjunction with the existing epidemiologic literature, do not suggest a strong association between aspirin or non-aspirin NSAID use and endometrial cancer risk. Because relatively few studies exist on this topic, future studies will be needed to confirm this finding, ideally with the ability to examine NSAID type, frequency, dose, and duration.
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the National Cancer Institute, NIH. Dr. Danforth was supported in part by the Sallie Rosen Kaplan Fellowship for Women Scientists in Cancer Research, National Cancer Institute, NIH.
References
- 1.Puntoni M, Marra D, Zanardi S, Decensi A. Inflammation and cancer prevention. Ann Oncol. 2008;19(Suppl 7):vii225–9. doi: 10.1093/annonc/mdn442. [DOI] [PubMed] [Google Scholar]
- 2.Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2005;14:2840–7. doi: 10.1158/1055-9965.EPI-05-0493. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekharan NV, Simmons DL. The cyclooxygenases. Genome Biol. 2004;5:241. doi: 10.1186/gb-2004-5-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green GA. Understanding NSAIDs: from aspirin to COX-2. Clin Cornerstone. 2001;3:50–60. doi: 10.1016/s1098-3597(01)90069-9. [DOI] [PubMed] [Google Scholar]
- 5.Tong BJ, Tan J, Tajeda L, et al. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-delta in human endometrial adenocarcinoma. Neoplasia. 2000;2:483–90. doi: 10.1038/sj.neo.7900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrandina G, Legge F, Ranelletti FO, et al. Cyclooxygenase-2 expression in endometrial carcinoma: correlation with clinicopathologic parameters and clinical outcome. Cancer. 2002;95:801–7. doi: 10.1002/cncr.10736. [DOI] [PubMed] [Google Scholar]
- 7.Moysich KB, Baker JA, Rodabaugh KJ, Villella JA. Regular analgesic use and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2923–8. doi: 10.1158/1055-9965.EPI-05-0457. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan AN, Feskanich D, Schernhammer ES, Hankinson SE. Aspirin, NSAID, and acetaminophen use and the risk of endometrial cancer. Cancer Res. 2008;68:2507–13. doi: 10.1158/0008-5472.CAN-07-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancello R, Clement K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG. 2006;113:1141–7. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 10.Fowler JM, Ramirez N, Cohn DE, et al. Correlation of cyclooxygenase-2 (COX-2) and aromatase expression in human endometrial cancer: tissue microarray analysis. Am J Obstet Gynecol. 2005;192:1262–71. doi: 10.1016/j.ajog.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Szymczak J, Milewicz A, Thijssen JH, Blankenstein MA, Daroszewski J. Concentration of sex steroids in adipose tissue after menopause. Steroids. 1998;63:319–21. doi: 10.1016/s0039-128x(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 12.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–43. [PubMed] [Google Scholar]
- 13.Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:234–64S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 14.Cheema AA. Should people on aspirin avoid Ibuprofen? A review of the literature. Cardiol Rev. 2004;12:174–6. doi: 10.1097/01.crd.0000107892.83612.9d. [DOI] [PubMed] [Google Scholar]
- 15.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–25. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 16.Michaud DS, Midthune D, Hermansen S, et al. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J Reg Manage. 2005;32:70–5. [Google Scholar]
- 17.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. 2007;65:S253–9. doi: 10.1111/j.1753-4887.2007.tb00372.x. [DOI] [PubMed] [Google Scholar]