Abstract
Prostate cancer is the second leading cause of cancer deaths among men. For patients with hormone-refractory disease, few treatments are available once the tumor has metastasized beyond the prostate. In the present study, two conjugated lytic peptide sequences (named JCHLHRH and JC21LHRH) were designed to target luteinizing hormone-releasing hormone receptors (LHRH-R). Our results indicate that human prostate cancer cell lines were sensitive to both LHRH-conjugated and non-conjugated lytic peptides, with IC50 concentrations for LNCaP cells, 4.4 and 9.1 µM; for DU-145 cells, 4.8 and 5.7 µM; and for PC-3 cells, 4.4 and 8.2 µM, respectively. JCHLHRH and JC21LHRH were nontoxic to normal primary human prostate epithelial cells or to bone marrow stromal cells in co-culture. There were morphological changes in PC-3 cells after 3 hr of exposure to either peptide; after 6 hr, there were significant reductions in cell numbers. Exposure of PC-3 cells for 24 hr to either JCHLHRH or JC21LHRH blocked their growth over 3 days. Since JCHLHRH and JC21LHRH have specificity for and anti-proliferative activity against tumor cells, and low toxicity for normal prostate cells, these peptides could serve as a new type of therapy for prostate cancer.
Keywords: Lytic Peptides, Prostate Cancer, Luteinizing Hormone-Releasing Hormone, Luteinizing Hormone-Releasing Hormone Receptors, Chemotherapy
1. Introduction
In the United States, prostate cancer is currently the most commonly diagnosed cancer and the second-leading cause of cancer death in men [1–3]. Following trends of increasing and decreasing rates, prostate cancer rates have been, since 2001, decreasing by 4.4%/year. Likewise, since the early 1990s, there has been a more substantial decrease in prostate cancer deaths among African American men than their white counterparts. Yet prostate cancer incidence and death rates among African Americans remain more than twice as high as those for whites. Furthermore, African American men and Jamaican men of African descent have the highest incidences of prostate cancer in the world [2, 3]. Many factors have been suggested to contribute to the higher prostate cancer incidence and mortality in African American men. For these men, over-expression of the androgen receptor (AR) [4, 5] and elevated luteinizing hormone (LH) levels have been associated with advanced disease progression. Although substantial effort has been applied to identify agents with efficacy against prostate cancer, few treatment options, including classical chemotherapeutic agents, have not proven to be effective against this disease.
Recently, the design and use of lytic peptides for numerous cancers has been reported [6, 7]. Lytic peptides are a ubiquitous feature of nearly all multi-cellular and some single-cellular life forms. They generally consist of between 10–40 amino acid sequences, which have potential for forming discrete secondary structures. Often, they exhibit the property of amphipathy., which may be depicted as a cylinder with one curved face composed primarily of nonpolar amino acids, while the other face is composed of polar amino acids. Most lytic peptides that have been previously described [6, 8, 9], appear to fall into one of three different classes based on the arrangement of amphipathy and high positive charge density within the molecule: 1) Cecropins (35 amino acids in length and derived from the Giant Silk Moth), 2) Magainins (23 amino acids in length and derived from the African Clawed Frog), 3) Melittin (26 amino acids in length and derived from the Honeybee),
The conservation of these physical properties is a requisite for activity, but the requirements seem to be somewhat nonspecific in terms of amino acid sequence. In a survey of the physical properties of natural osmotically-active–proteins, we discovered similarities between natural and synthetically prepared lytic peptides [10–12]. Further, synthetic peptides were more active and less toxic than their naturally occurring counterparts [6, 13]. For example, we have synthesized highly sequence-divergent analogs for each of the peptide classes and have found some of them to be more active and less toxic than their natural counterparts (17–20).
Therapies directed at preventing or limiting the tumor’s transition to the more aggressive invasive and metastatic stages offer benefits different from those designed to kill prostate cancer cells. To enhance the anti-tumor activity of these synthetic peptides, two 23-amino acid sequences were manipulated, resulting in the generation of two sequences, JCH and JC21. Both peptides have centers of hydrophobicity that have been altered from previously designed peptides and their positive charge density increased in order to maximize physical properties known to enhance cancer cell killing activity. Since prostate cancer cells express high levels of luteinizing hormone releasing hormone receptors (LHRH-R), which have been a target for hormonal therapy [14–16], JCH and JC21 were linked with a modified LHRH binding sequence for enhanced tumor targeting. The resulting products were designated JCHLHRH and JC21LHRH.
To evaluate the efficacy of these peptides in inhibiting prostate cancer, a prostate cancer progression model was utilized. Through the utilization of androgen-responsive LNCaP cells, and androgen unresponsive DU-145 and PC-3 prostate cancer cells, this approach represents the stages of prostate cancer progression. Herein, we provide evidence that JCH and JC21 inhibit cell proliferation at low concentrations, and, when conjugated with a modified LHRH sequence, both peptides have enhanced anti-proliferative activity. Furthermore, JCHLHRH and JC21LHRH demonstrate minimal anti-proliferative effects on normal prostate cells. Real-time imaging revealed that these compounds exert toxicity within 6 hr of exposure to PC-3 cells, apparently through different modes of action. Pretreatment of PC-3 cells with these peptides blocks their growth over a 3-day period. Thus, JCHLHRH and JC21LHRH are possible new therapies for prostate cancer.
2. Materials and Methods
2.1. Cell Culture
DU-145, a cell line originally derived from a brain metastasis of a human prostate adenocarcinoma [17], retains the androgen independence of the original tumor and does not express a functional AR [18]. This cell line has both LHRH-R and epidermal growth factor receptors (EGFR) and produces the EGFR ligands, transforming growth factor α (TGF-α) and EGF [19, 20]. PC-3 cells were derived from a bone metastasis of a grade IV prostatic adenocarcinoma [21]; LNCaP cells were derived from a lymph node metastasis [22]. Normal primary human prostate epithelial cells (hPrEC) were obtained from Clonetics (Lonza Inc., Walkersville, MD). All cells lines were purchased from ATCC and maintained in T-media (Gibco Invitrogen, CA) supplemented with 10% fetal bovine serum (FBS) (Gibco Invitrogen, CA) and 1% penicillin/streptomycin (Cellgro Mediatech, Inc., VA).
2.2 Preparation of Lytic Peptides
Two synthetic peptides targeting the LHRH sequence, designated JCHLHRH and JC21LHRH, were prepared by Chem Prep Inc (Miami, FL) and were determined to be 95% pure by HPLC. They contained 20 and 33 amino acids, and their molecular weights were 3742.73 and 3150.36, respectively. For experimental use, the peptides were dissolved in sterile water.
2.3. Cell Proliferation Assays
Cytotoxicity was evaluated using the methylthiazol tetrazolium (MTT) assay (Sigma Aldrich, MO), which determines the number of viable cells from the formazan crystals produced by metabolic activity. Cells were plated in 96-well plates at 2.5 × 103 cells/well. They were allowed to reattach overnight and were then exposed to peptides (1–20 µM) for 72 hr. The MTT reagent [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, 50 µL) in T-media was added to each well, and the plates were incubated for 2 hr. The supernatant media was aspirated, and formazan crystals were dissolved with 100 µL dimethylsulfoxide per well at 37°C for 10 min. The quantity of solublized formazan per well was determined by a Spectromax plate reader at 540 nm (Molecular Devices, CA). Each assay was performed at least three times with six replicates per data point.
2.3. Real-Time Imaging
Imaging was performed utilizing an Olympus DSU (Disk Scanning Units) Confocal microscope (Olympus America Inc., PA). Cells were maintained in 5% C02 at 37°C in an incubation chamber (Pathology Devices, Inc., MD). Images were taken every 15 min and processed utilizing MetaMorph Imaging Software (Molecular Devices, CA).
2.4 Co-cultures
Co-cultures were performed as previously described [23, 24], with modifications. Co-cultures consisted of 50,000 cells/cm2 of GFP-HS-27a bone marrow stromal cells and 2,000 cells/cm2 of RFP- PC-3 prostate cancer cells. Co-cultures were maintained in Dulbecco's Modified Eagle Medium, and cell death was monitored in real-time (as described above) by differential fluorescence. To determine the relative intensity, we measured the total intensity area of each image as well as the threshold intensity for each channel utilizing Metamorph Imaging Software, CA. The significance between the two intensities was then analyzed using excel. Bar graph represents n=4 images sectioned and individually analyzed for total area. All quantitative data was normalized to each control image.
2.6 siRNA for LHRH-R
A total of 2 × 105 cells, equaling approximately 60–70% confluency, were plated in six-well plates. The LHRH-R siRNA (Santa Cruz, CA) was diluted in 200 ml of Opti-MEM (Invitrogen, CA). A 4 ml aliquot of Lipofectamine 2000 (Invitrogen, CA) was diluted in 200 ml of Opti-MEM and incubated for 5 min at room temperature. The diluted siRNA and Lipofectamine 2000 were mixed according to manufacturer’s directions and incubated for 20 min at room temperature. The resulting complexes were added to each well and incubated for 24 h. Media were changed after 24 h, and replaced with fresh media and incubated for an additional 24 h. LHRH-R expression was determined by immunofluorescence utilizing anti-LHRH-R antibody (Lab Vision, CA) and Alexa 488 secondary antibody (Invitrogen, CA).
2.7 Statistics
For all experiments, statistics were performed with Microsoft Excel. Independent Student’s t-test was utilized to determine statistical differences between experimentals and controls. The IC50 values were derived through regression analysis of the continuous data by cubic spline interpolation utilizing Aabel™ (Gigawiz), a software application that integrates statistics (inferential and multivariate) with graphical representation.
3. Results
3.1 Lytic Peptides Inhibit Growth of Prostate Cancer Cells
Although the cytotoxic activity of lytic peptides on prostate cancer cells has been previously described, these peptides are required in high concentrations to achieve substantial anti-cancer activity [7, 25]. Therefore, we sought to design new peptides based on maintaining a hydrophobic center from previously designed peptides. Furthermore, physical properties affecting charge, density, length, amphipathy, and spatial orientation that would maximize lytic activity on cancer cells. To achieve this, two 33-amino acid peptide sequences, designated JCH and JC21, were synthesized (Figure 1A). Since LHRH-R over-expression is associated with aggressive prostate cancers [7, 26–28], a modified LHRH 10-amino acid sequence (QHWSYGLRP), was utilized for selective targeting, with a single amino acid modification (QHWSWGLRP) to increase hydrophobicity and enhance anti-tumor activity while limiting this activity on non-cancerous cells (Figure 1B).
Figure 1. Three-dimensional molecular modeling of peptides.
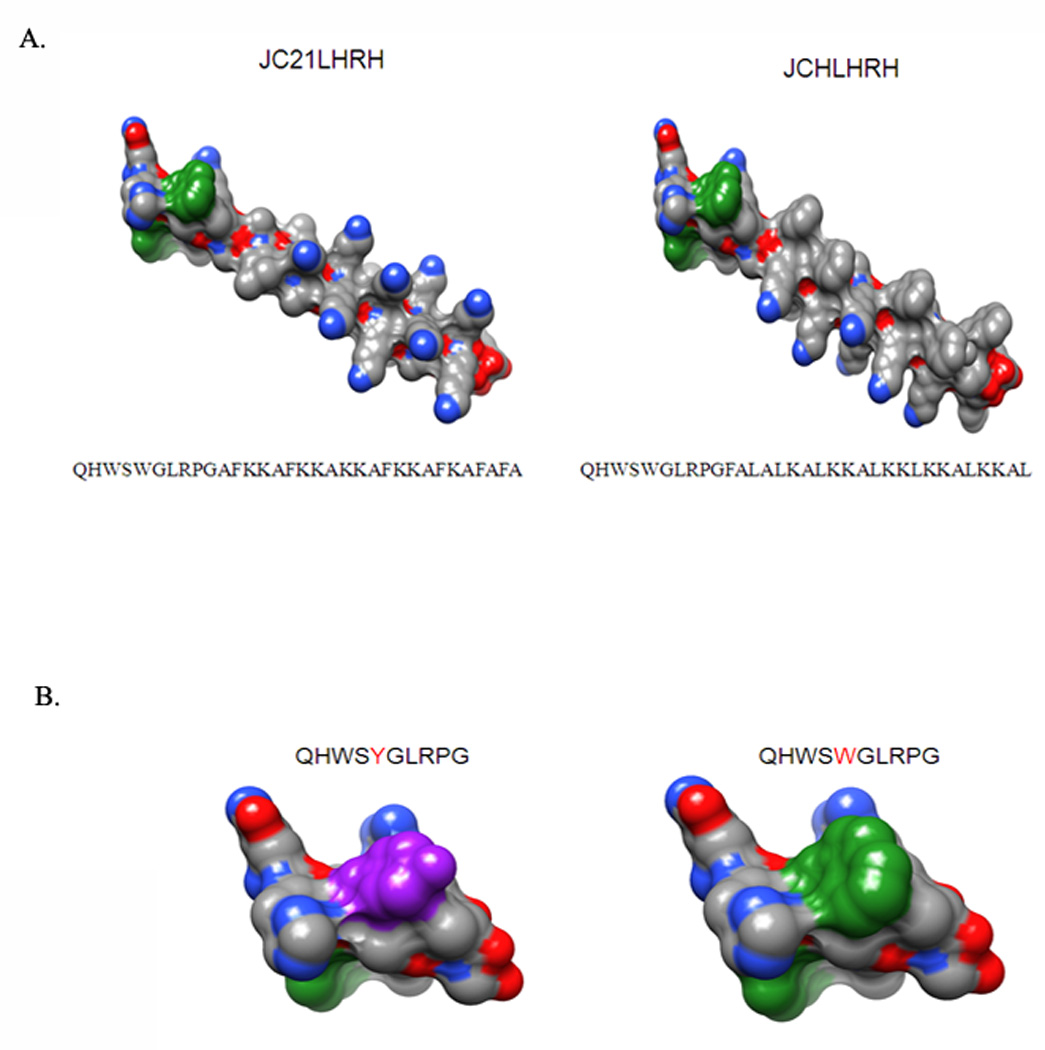
(A) JC21LHRH and JCHLHRH models were derived with the UCSF molecular modeling program, Chiron. The colored atoms are the following: carbon, gray; nitrogen, blue; and oxygen, red. (B) The amino acid residue tyrosine (in the natural LHRH sequence) is red; the modified LHRH sequence possesses an additional tryptophan, both of which are colored green.
To determine the effectiveness of JCH and JC21 with or without LHRH conjugates, androgen-independent, highly metastatic PC-3 cells were exposed to the peptides in increasing concentrations (0–20 µM). Both peptides exhibited a dose-dependent decrease in cell proliferation, with JCH and JC21 having IC50 values of 6.67 and 9.25 µM, respectively (Figure 2A). Addition of LHRH lowered the IC50 concentrations to 4.22 µM for JCHLHRH and 7.24 µM for JC21LHRH. The effectiveness of JCHLHRH and JC21LHRH on two additional human prostate cell lines, the androgen-dependent LNCaP and androgen-independent DU-145 cell lines, was also examined. The IC50 values for LNCaP were 4.36 µM for JCHLHRH and 9.15 µM for JC21LHRH (Figure 2B). For DU-145 cells, the values were 4.81 µM for JCHLHRH and 5.66 µM for JC21LHRH (Figure 2C). All IC50 values are summarized in Table 1.
Figure 2. Cytotoxicity of JC21, JCH, JC21LHRH, and JCHLHRH to prostate cancer cells.
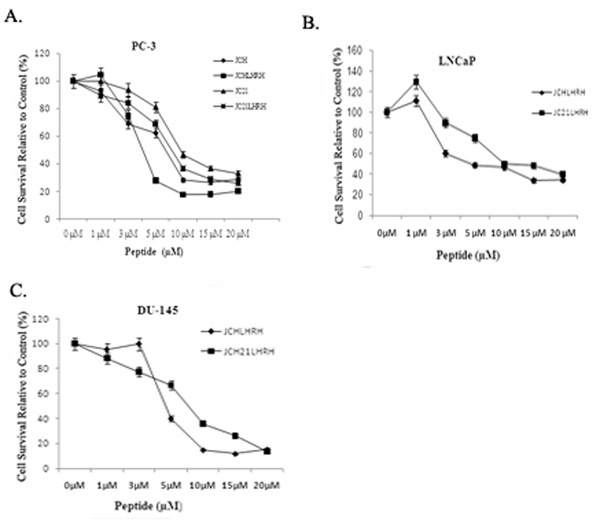
(A) PC-3 cells were exposed to JC21, JC21LHRH, or JCHLHRH (0–20 µM). (B) LnCaP cells were exposed to JC21LHRH and JCHLHRH. C) DU-145 cells were exposed to JC21LHRH or JCHLHRH. Cell viability was determined by MTT. Mean ± s.d. (n=4)
Table 1.
Interpolated Mean IC50 values obtained from prostate cancer cell lines
| Cell line | Peptides | |||
|---|---|---|---|---|
| JC21 | JCH | JC21LHRH | JCHLHRH | |
| LNCaP | n.d. | n.d. | 9.149µM | 4.362µM |
| DU-145 | n.d. | n.d. | 5.66µM | 4.81µM |
| PC-3 | 9.25µM | 6.67µM | 7.42µM | 4.22µM |
LNCaP; Du-145 and PC-3 cells. IC50 was determined by interpolation of 3 individual experiments performed in quadruplicate at 50% cell death. All values were determined to be significant compared to control p<.0001. n.d.= not determined.
Since similar IC50 values were obtained across various prostate cancer cells lines, the highly metastatic PC-3 cell line was utilized for the remainder of the studies. To determine if the addition of the LHRH sequence was directly responsible for the increased activity of both JCHLHRH and JC21LHRH, we assayed the anti-proliferative (anti-growth) effectiveness of the modified LHRH sequence alone. This sequence caused a decrease in cell proliferation at concentrations as low as 1 µM, but higher concentrations failed to cause 50% cell death (Figure 3). Although LHRH activity was not as potent as JC21LHRH and JCHLHRH, the sensitivity to PC-3 cells was sustained, providing evidence that addition of the LHRH sequence adds to lytic activity. To more firmly establish the significance of the LHRH sequence on peptide activity, we treated PC-3 cells with siRNA-LHRH at 50nM and 100nM concentrations and assayed LHRH levels by immunofluorescence. 100nM siRNA-LHRH exhibited the most significant decreases in LHRH levels as determined by lack of fluorescent staining (Figure 3B). Next. PC-3 cells were exposed with both JC21LHRH and JCHLHRH in the presence or absences of previously siRN-ALHRH or siRNA Scrabmled treated cells. Our results, show that deleption of LHRH levels decreased the sensitivity of both peptides sequences by 50%. (Figure 3C). Finally, to determine the selectivity of JC21LHRH and JCHLHRH, we performed a similar experiment with normal hPrEC cells. Neither JC21LHRH nor JCHLHRH caused appreciable decreases in hPrEC cell proliferation (Figure 4).
Figure 3. Cytotoxicity of an LHRH sequence to PC-3 cells.
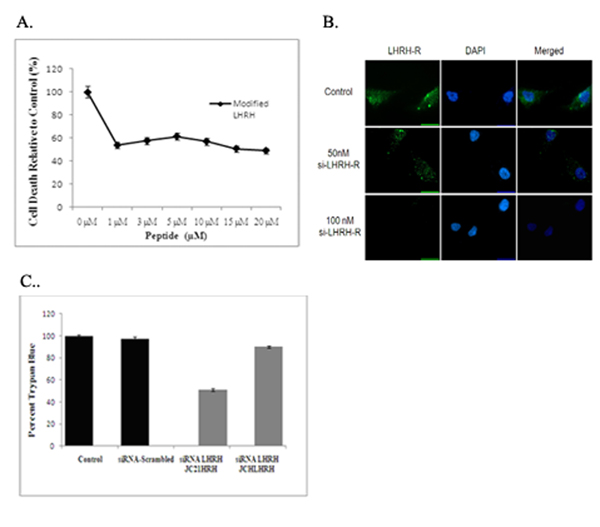
(A) PC-3 cells were exposed to a modified LHRH sequence. The data shown represent the average of three individual experiments performed in quadruplicate. Cell viability was determined by MTT. Mean ± s.d. (n=3). (B). PC-3 cells were treated with siRNA-LHRH at 50nM and 100nM. Immunofluorescence was preformed utilizing anti-LHRH antibody with Alexa 488 secondary antibody. (C). PC-3 cells were treated with JC21LHRH or JCHLHRH in the presence or absence of 100nM siRNA-LHRH or siRNA-scramble control. Cell viability was determined by trypan blue exclusion The values are respresentative of three independent experiments (n=3).
Figure 4. Cytotoxicity of JCHLHRH and JC21LHRH to normal primary hPrEC cells.

hPrEC cells were exposed to JCHLHRH or JC21LHRH at 0–20 µM. Cell viability was determined by MTT. Mean ± s.d. (n=4)
3.2 Real-time Monitoring of the effects of JC21LHRH and JCHLHRH
Several assays have been proposed to determine the lytic activity of peptide sequences on the inhibition of cancer cell proliferation [29, 30]. Since the mechanism of death is unclear, real-time imaging was employed for cells in a 5% CO2 and temperature-regulated (37°C) chamber to monitor the effects of JC21LHRH and JCHLHRH on PC-3 cellular responses. There were morphological changes in cells exposed to either JC21LHRH or JCHLHRH for 3 hr (Figure 5A) (supplemental movie). However, as determined by trypan blue exclusion assay, a significant increases in cell death (p<.001) was observed at 3hr and 6hr time periods (Figure 5B). There were different modes of action by which JCHLHRH and JC21LHRH caused cell death. Exposure to JCHLHRH resulted in shrinkage and eventual bursting of the cell membrane. While cells exposed to JC21LHRH showed a condensing of the cellular and nuclear membranes, but did not burst.
Figure 5. Real-time images of cytotoxicity of lytic peptides in hours.
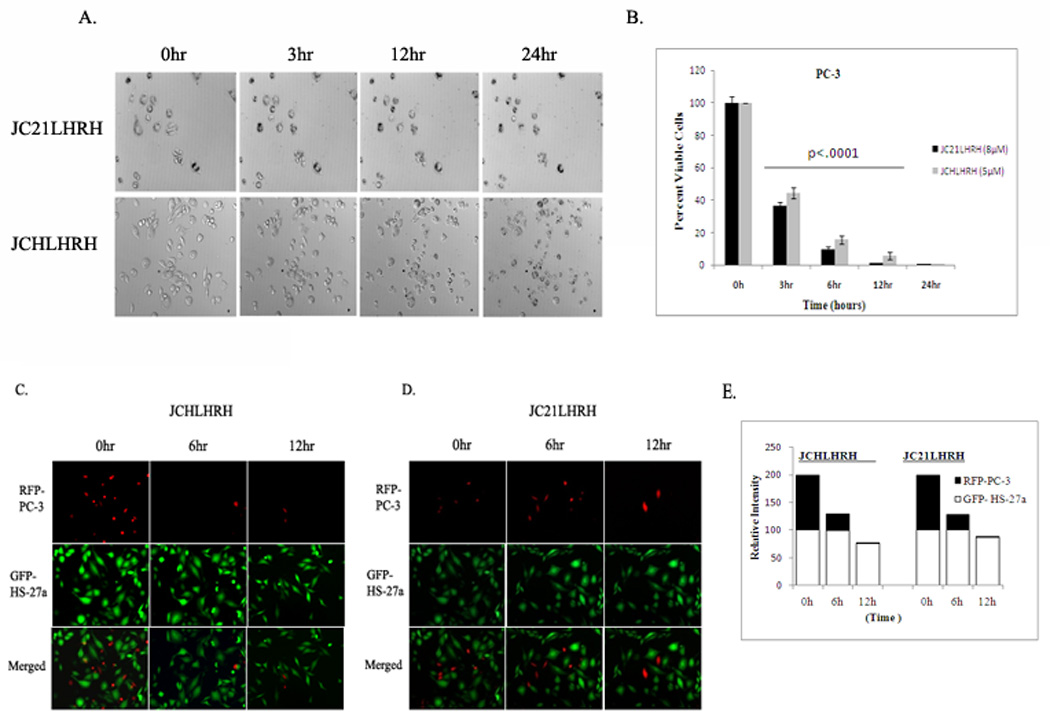
A.) PC-3 cells were exposed to JCHLHRH (5 µM) or JC21LHRH (8 µM) and imaged continuously for 24 hr with time-lapse microscopy. Phase contrast images, selected from time-lapse microscopy at 3, 6, and 12 hr, are representative of two individual experiments with similar findings. The time-lapse movie can be found in Supplemental Data (5E & F). B.) Quantification of total cell number and cell viability was determined by trypan blue exclusion after exposure to JCHLHRH (5 µM) or JC21LHRH (8 µM) over a 24 hr period. Results shown are representative of three individual experiments performed in triplicate (---- (bar) indicates significance at p < 0.001). C). Exposure of JCHLHRH (5 µM) or D.) JC21LHRH (8 µM) to RFP-PC-3/GFP-HS-27a co-cultures was determined by differential immunofluorescence over 12 hr period. Images shown are representative of two individual experiments with similar results. (E.) Relative Intensity of total areas measured differential fluourescence of co-culutres.
Previously, we have described a novel three-dimensional method, utilizing bone marrow stromal cells and prostate cancer cell lines co-cultures to delineate the influence of the host stromal cells on metastatic prostate cancer cells [31]. This method has reproducibly shown to mimic the vivo situation [32]. To demonstrate the tumor targeting specificity of both peptides for metastatic prostate cancer, we utilized a co-culture of GFP-HS-27a bone marrow stromal cells and PC-3 cells. In the presence of either peptide, there was appreciable death of PC-3 cells in 12 hr, but little effect was observed on GFP-HS-27a cells, as determined by differential fluorescence (Figure 5C & D).
Lastly, to determine if cells could recover after JCHLHRH or JC21LHRH exposure, PC-3 cells were exposed to the conjugated peptide sequences for 24 hr, and their growth was measured over a 3-day period. Pretreatment completely inhibited their growth over the 3 days assayed (Figure 6).
Figure 6. Lytic peptide pretreatment blocks PC-3 cell growth.
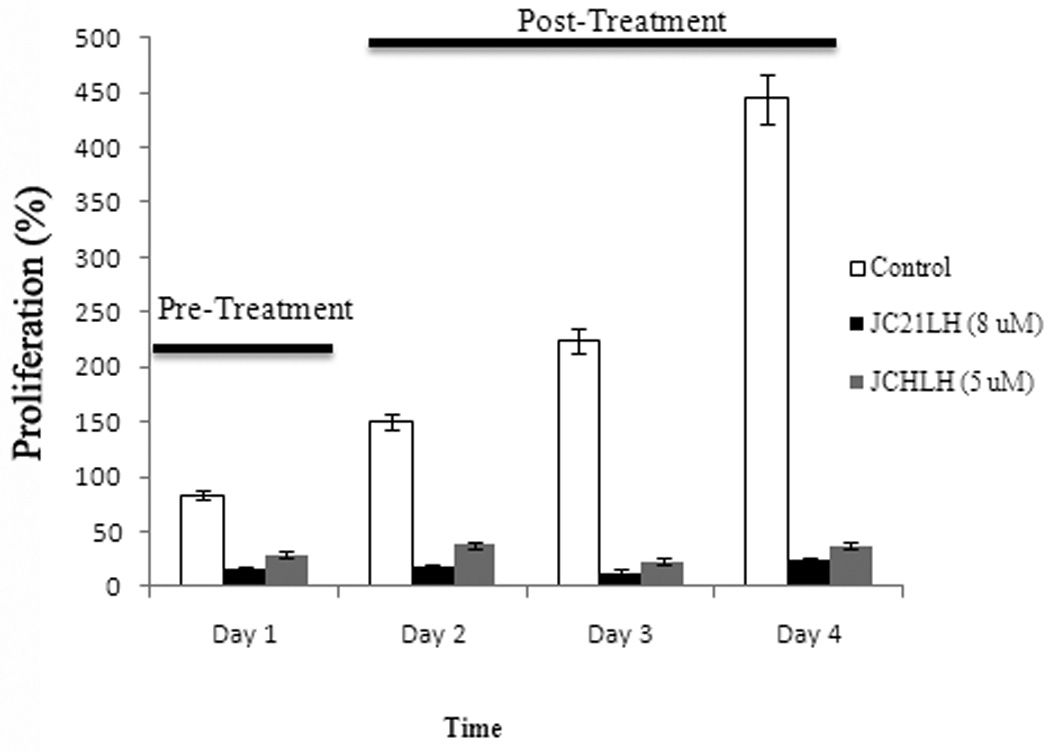
PC-3 cells were exposed to JCHLHRH (5 µM) or JC21LHRH (8 µM) for 24 hr. Growth was measured over a 3-day period. Bars represent the mean number of cells survived as determined by MTT. Mean ± s.d. (n=4)*, p < 0.005, Student’s t-test.
4. Discussion
LHRH-conjugated lytic peptides target the LHRH receptors of prostate cancer cells [6, 7, 26, 28, 33]. In the present study, new lytic peptide sequences and a new LHRH sequence, modified from the full-length LHRH sequence, were utilized to target and destroy androgen-dependent and androgen-independent prostate cancer cells. Both peptides alone showed significant anti-tumor activity; addition of a LHRH conjugate lowered the IC50 concentrations. The results demonstrate that both lytic-LHRH conjugates, JCHLHRH and JC21LHRH, destroy LNCaP, DU-145, and PC-3 prostate cancer cells. Furthermore, both peptides showed minimal effects on normal primary hPrEC cells, even at high concentrations. Interestingly, the LHRH sequence alone was active at 1 µM; however, increasing its concentration failed to demonstrate dose-dependent cell death. Thus, the increase in JCHLHRH and JC21LHRH activity is attributable to the conjugated LHRH sequence.
Our findings parallel those of previously reported, lytic-LHRH conjugated peptides, where the lytic sequence alone was less effective in decreasing proliferation, compared to lytic-LHRH conjugates [6, 7, 28, 33–35]. A major difference, however, between these studies and the present report is the non-requirement of circulating estradiol or follicle-stimulating hormone for our compounds to be active [6]. Previous studies utilizing lytic peptides have highlighted the importance of a steroid presence, since the removal of steroids from the culture media eliminated the sensitivity of prostate cancer cells to the effects of lytic-LHRH conjugates. The present data are not reflective of this, as all culture experiments were performed with serum-free media. Furthermore, the lytic peptides without conjugated LHRH showed substantial activity and selectivity for prostate cancer cells. Thus, it appears that these peptides act directly on the membrane, with the LHRH sequence increasing both activity and enhancing selectivity.
Although the mechanism associated with the anti-tumor activity of lytic peptides has not been defined, direct interaction with the cell membrane may cause the electrochemical potential to collapse, resulting in cell death [25]. To provide information relating to the mechanism of action, we utilized both a qualitative approach utilizing real-time imaging, and classic makers of apoptosis, Annexin V. JCHLHRH caused membrane rupture and cell bursting; in turn, cells exposed to JC21LHRH showed cell shrinkage without bursting. Additionally, we did not observe expression of apoptotic marker Annexin V (data not shown). This plausible given the rapid mode of action that was observed during the real-time images and trypan blue staining, confirming near 80% cell death after only 6hr of treatment and near 100% cell death after the 24h period. Furthermore this is supported by previous reports utilizing lytic peptides for cancer, that suggest that necrosis is main mode of action compared to apoptosis [26]. Regardless of the mechanism of action, both peptides appeared to cause significant tumor-specific cell death. Tumor cell specificity was evident from the finding that neither peptide had an appreciable anti-proliferative effect on either non-cancerous hPrEC cells or highly proliferative HS-27a bone stromal cells in PC-3/HS-27a co-cultures; thus limiting potential cytotoxic effects from these peptides on the surrounding stroma of cancer cells located at primary and metastatic sites. Further, the effect of exposure to LHRH-conjugated peptides was sufficient to inhibit the growth of PC-3 cells up to 3 days in the absence of the lytic peptide. Whether the lack of PC-3 growth, after pretreatment, was due to a loss of cells or to prevention of cellular proliferation was not determined. The net result, however, is that both peptide sequences were effective in inhibiting cell growth. A possible mechanism for the differential effect of JCHLHRH compared to JC21LHRH may be their direct interaction with the cancer cell membrane. Since lytic peptides differentially target cancer cells [30], it is possible that the peptide sequence determines the type of membrane-mediated cell death.
In conclusion, several labs independently have demonstrated both the in vitro and in vivo efficacy of using LHRH sequences conjugated with lytic peptide sequence compounds in hormonally regulated cancers [7, 28]. In fact, recent reports highlighted that LHRH-lytic conjugated compounds have shown significant anti-cancer effects on prostate and breast cancer metastasis to lymph node and bone microenvironment [6]. That LHRH-Rs are expressed in 86% of human prostate cancers and LHRH-R numbers increase with the increasing metastatic potential of prostate cancer cell lines [14], provides a solid rationale that our in vitro findings will translate in vivo, and ultimately have clinical utility. Additionally, given that the low concentration and unique mode of action of our uniquely designed peptides differ from other classical chemotherapeutic agents, one can extrapolate that the combined use of these conjugated peptides with similar agents or within nanotechnology-directed therapies should enhance their therapeutic values and limit toxicity to non-tumor cells. Although in vivo investigations of their efficacy in animal cancer model systems are warranted and upcoming, these peptides appear to be eventual candidates for use in the treatment of local and metastatic prostate cancer.
Supplementary Material
Acknowledgements
This project was supported by Grants 3P20MD000195 (NIH/NCMHD), U54 CA118623 (NIH/NCI) to T.T. and RR-G12RR03059 and PC07397 (Department of Defense) to C.Y.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Society AC. Cancer Facts & Figures 2009. American Cancer Society. 2009 [Google Scholar]
- 3.Society AC. Cancer Facts & Figures for African Americans 2009–2010. American Cancer Society. 2009 [Google Scholar]
- 4.Bennett CL, Price DK, Kim S, Liu D, Jovanovic BD, Nathan D, et al. Racial variation in CAG repeat lengths within the androgen receptor gene among prostate cancer patients of lower socioeconomic status. J Clin Oncol. 2002;20:3599–3604. doi: 10.1200/JCO.2002.11.085. [DOI] [PubMed] [Google Scholar]
- 5.Irvine RA, Yu MC, Ross RK, Coetzee GA. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer research. 1995;55:1937–1940. [PubMed] [Google Scholar]
- 6.Hansel W, Leuschner C, Enright F. Conjugates of lytic peptides and LHRH or betaCG target and cause necrosis of prostate cancers and metastases. Molecular and cellular endocrinology. 2007;269:26–33. doi: 10.1016/j.mce.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Leuschner C, Enright FM, Gawronska-Kozak B, Hansel W. Human prostate cancer cells and xenografts are targeted and destroyed through luteinizing hormone releasing hormone receptors. Prostate. 2003;56:239–249. doi: 10.1002/pros.10259. [DOI] [PubMed] [Google Scholar]
- 8.Ballweber LM, Jaynes JE, Stamm WE, Lampe MF. In vitro microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1 to 2% D2A21 against Chlamydia trachomatis. Antimicrob Agents Chemother. 2002;46:34–41. doi: 10.1128/AAC.46.1.34-41.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaynes JM, Julian GR, Jeffers GW, White KL, Enright FM. In vitro cytocidal effect of lytic peptides on several transformed mammalian cell lines. Pept Res. 1989;2:157–160. [PubMed] [Google Scholar]
- 10.Jaynes JM, Burton CA, Barr SC, Je€ ers GW, Julian GR, White KL, et al. In vitro cytocidal e€ ffects of novel lytic peptides on Plasmodium falciparum and Trypanosoma cruzi. FASEB J. 1988;2:2878–2883. doi: 10.1096/fasebj.2.13.3049204. [DOI] [PubMed] [Google Scholar]
- 11.Jaynes JM, Julian GR, Jeffers GW, White KL, Enright FM. In vitro cytocidal effect lytic peptides on several transformed mammalian cell lines. Peptide Research. 1989;2:157–160. [PubMed] [Google Scholar]
- 12.Thwaini A, Naase M, Chinegwundoh F, Baithun S, Ghali L, Shergill I, et al. Gonadotropins and prostate cancer: revisited. Urol Int. 2006;77:289–296. doi: 10.1159/000096330. [DOI] [PubMed] [Google Scholar]
- 13.Boman HG. Peptide antibiotics and their role in innate immunity. Annual Review of Immunology. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 14.Qayum A, Gullick W, Clayton RC, Sikora K, Waxman J. The effects of gonadotrophin releasing hormone analogues in prostate cancer are mediated through specific tumour receptors. British journal of cancer. 1990;62:96–99. doi: 10.1038/bjc.1990.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qayum A, Gullick WJ, Waxman J. Gonadotrophin-releasing hormone: physiological significance and relevance to cancer. Progress in growth factor research. 1991;3:115–130. doi: 10.1016/s0955-2235(05)80003-1. [DOI] [PubMed] [Google Scholar]
- 16.Platz EA, Pollak MN, Rimm EB, Majeed N, Tao Y, Willett WC, et al. Racial variation in insulin-like growth factor-1 and binding protein-3 concentrations in middle-aged men. Cancer Epidemiol Biomarkers Prev. 1999;8:1107–1110. [PubMed] [Google Scholar]
- 17.Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145) International journal of cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- 18.Dondi D, Moretti RM, Montagnani Marelli M, Pratesi G, Polizzi D, Milani M, et al. Growth-inhibitory effects of luteinizing hormone-releasing hormone (LHRH) agonists on xenografts of the DU 145 human androgen-independent prostate cancer cell line in nude mice. International journal of cancer. 1998;76:506–511. doi: 10.1002/(sici)1097-0215(19980518)76:4<506::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Jungwirth A, Pinski J, Galvan G, Halmos G, Szepeshazi K, Cai RZ, et al. Inhibition of growth of androgen-independent DU-145 prostate cancer in vivo by luteinising hormone-releasing hormone antagonist Cetrorelix and bombesin antagonists RC-3940-II and RC-3950-II. Eur J Cancer. 1997;33:1141–1148. doi: 10.1016/s0959-8049(97)00072-5. [DOI] [PubMed] [Google Scholar]
- 20.Turner T, Chen P, Goodly LJ, Wells A. EGF receptor signaling enhances in vivo invasiveness of DU-145 human prostate carcinoma cells. Clinical & experimental metastasis. 1996;14:409–418. doi: 10.1007/BF00123400. [DOI] [PubMed] [Google Scholar]
- 21.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Investigative urology. 1979;17:16–23. [PubMed] [Google Scholar]
- 22.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, et al. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 23.Yates C, Shepard CR, Papworth G, Dash A, Beer Stolz D, Tannenbaum S, et al. Novel three-dimensional organotypic liver bioreactor to directly visualize early events in metastatic progression. Advances in cancer research. 2007;97:225–246. doi: 10.1016/S0065-230X(06)97010-9. [DOI] [PubMed] [Google Scholar]
- 24.Yates CC, Shepard CR, Stolz DB, Wells A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. British journal of cancer. 2007;96:1246–1252. doi: 10.1038/sj.bjc.6603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arlotti JA, Cimino TS, Nguyen T, Dhir R, Thomas A, Jaynes JM, et al. Efficacy of a synthetic lytic peptide in the treatment of prostate cancer. Urologic oncology. 2001;6:97–102. doi: 10.1016/s1078-1439(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 26.Bodek G, Kowalczyk A, Waclawik A, Huhtaniemi I, Ziecik AJ. Targeted ablation of prostate carcinoma cells through LH receptor using Hecate-CGbeta conjugate: functional characteristic and molecular mechanism of cell death pathway. Exp Biol Med (Maywood) 2005;230:421–428. doi: 10.1177/15353702-0323006-10. [DOI] [PubMed] [Google Scholar]
- 27.Halmos G, Arencibia JM, Schally AV, Davis R, Bostwick DG. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. The Journal of urology. 2000;163:623–629. [PubMed] [Google Scholar]
- 28.Leuschner C, Hansel W. Targeting breast and prostate cancers through their hormone receptors. Biology of reproduction. 2005;73:860–865. doi: 10.1095/biolreprod.105.043471. [DOI] [PubMed] [Google Scholar]
- 29.Chalekson CP, Neumeister MW, Jaynes J. Treatment of infected wounds with the antimicrobial peptide D2A21. J Trauma. 2003;54:770–774. doi: 10.1097/01.TA.0000047047.79701.6D. [DOI] [PubMed] [Google Scholar]
- 30.Robertson CN, Roberson KM, Pinero A, Jaynes JM, Paulson DF. Peptidyl membrane-interactive molecules are cytotoxic to prostatic cancer cells in vitro. World J Urol. 1998;16:405–409. doi: 10.1007/s003450050091. [DOI] [PubMed] [Google Scholar]
- 31.Josson S, Sharp S, Aneja R, Wang R, Turner T, Chung L, et al. Tumor-Stromal Interactions Influence Radiation Sensitivity in Epithelial- versus Mesenchymal-Like Prostate Cancer Cells. Journal of Onocology. 2010;2010:10. doi: 10.1155/2010/232831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, et al. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer research. 2008;68:9996–10003. doi: 10.1158/0008-5472.CAN-08-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansel W, Leuschner C, Gawronska B, Enright F. Targeted destruction of prostate cancer cells and xenografts by lytic peptide-betaLH conjugates. Reprod Biol. 2001;1:20–32. [PubMed] [Google Scholar]
- 34.Hansel W, Enright F, Leuschner C. Destruction of breast cancers and their metastases by lytic peptide conjugates in vitro and in vivo. Mol Cell Endocrinol. 2007;260–262:183–189. doi: 10.1016/j.mce.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 35.Leuschner C, Enright FM, Gawronska B, Hansel W. Membrane disrupting lytic peptide conjugates destroy hormone dependent and independent breast cancer cells in vitro and in vivo. Breast Cancer Res Treat. 2003;78:17–27. doi: 10.1023/a:1022169525521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


