Abstract
BACKGROUND
Stress myocyte biomarkers are used prognostically in patients with cardiovascular disease. We examined associations between amino-terminal pro–B-type natriuretic peptide (NT-proBNP), midregional pro–A-type natriuretic peptide (MR-proANP), and midregional proadrenomedullin (MR-proADM) concentrations and cardiac chamber volumes in chest pain patients without heart failure by use of computed tomography (CT).
METHODS
At the time of 64-slice CT scan, we acquired plasma and serum samples for these biomarkers from 346 patients [mean (SD) age 53 (12) years, 65% men]. Left atrial volume (LAV) and left ventricular volumes at end-diastole (LVEDV) and end-systole (LVESV) were measured and indexed to body surface area (LAVI, LVEDI, LVESI).
RESULTS
Concentrations of both natriuretic peptides were correlated with LAV and LAVI (r=0.19–0.32, all P ≤ 0.0005) and MR-proADM with LV volumes and indices (r=−0.14 to −0.21, all P ≤ 0.01). NT-proBNP and MR-proANP concentrations were higher in the top quartiles of patients than the lowest quartiles using LAV and LAVI, whereas MR-proADM concentrations were lower in the top quartiles of LV measures. In adjusted analyses, patients had 2- to 4-fold increased risk of LA enlargement for every incremental increase in log10NT-proBNP [LAV odds ratio (OR) 2.4, P = 0.03; LAVI OR 4.0, P = 0.003] and 10- to 13-fold increased risk of LA enlargement for every incremental increase in log10MR-proANP (LAV OR 10.7, P = 0.009; LAVI OR 13.1, P = 0.004).
CONCLUSIONS
In patients without heart failure, both NT-proBNP and MR-proANP concentrations are independently associated with LA enlargement, whereas MR-proADM concentrations are correlated with LV volumes. This may partially explain the well-recognized value of natriuretic peptides for use in risk stratification.
Circulating natriuretic peptides have been shown to have prognostic value in a wide spectrum of cardiovascular diseases. Amino-terminal pro–B-type natriuretic peptide (NT-proBNP)6 is a well-established biomarker used for diagnostic and prognostic purposes in the evaluation of patients with cardiovascular disease, including heart failure (HF) (1–2) and myocardial infarction (MI) (3). Newer myocyte stress biomarkers, such as midregional pro–A-type natriuretic peptide (MR-proANP) and the midregional fragment of proadrenomedullin (MR-proADM), have since been discovered that have potential clinical importance.
NT-proBNP is produced primarily in the ventricular myocytes (4, 5). Concentrations of NT-proBNP increase with age and in women (6), are higher in patients with decreased left ventricular ejection fraction (LVEF) (7), and are increased in those with HF (8). The development of a midregional assay for the propeptide of ANP has allowed for a better understanding of the peptide. MR-proANP is secreted from both the left atrium and the left ventricle (5) and is stimulated to enter circulation by atrial stretch (9). Like NT-proBNP, MR-proANP is increased in HF patients, aids with diagnosis of acute HF in patients with dyspnea (10), and predicts survival in post-MI and chronic HF patients (2, 11–14). Adrenomedullin (ADM) is produced in many tissues and cell types including heart, brain, lung, kidney, and gastrointestinal organs (15, 16), and the mRNA is expressed in endothelial cells (17). Much like ANP, ADM is rapidly cleared from the circulatory system (18), making it difficult to analyze and thus less well studied. Nevertheless, ADM concentrations have been shown to be higher in HF patients (19). MR-proADM, which is the more stable form of ADM (20), has recently been shown to be increased in patients with acute MI and acute HF (2, 21).
Little is known about the relation of NT-proBNP, MR-proANP, and MR-proADM to left atrial (LA) and left ventricular (LV) volumes in patients without HF. The use of cardiac computed tomography (CT) has increased dramatically in the past few years. With contrast-enhanced CT, LA and LV volumes can easily be measured with excellent reproducibility (22, 23). Thus, we aimed to describe the relationship of the stress myocyte biomarkers, NT-proBNP, MR-proANP, and MR-proADM, with left atrial and ventricular volumes in a large cohort of patients without HF. We also sought to evaluate these biomarkers for their association with LA and LV enlargement.
Materials and Methods
STUDY POPULATION
The Rule Out Myocardial Infarction Using Computer Assisted Tomography (ROMICAT) trial was a prospective observational cohort study of consecutive adult patients at low-to-intermediate likelihood of acute coronary syndrome who presented to the emergency department (ED) of a tertiary hospital with acute chest pain and in whom the initial electrocardiogram (ECG) was normal or inconclusive and serum troponin was negative. The details of the study design have been reported (24). Briefly, eligible patients who consented underwent ECG-gated contrast-enhanced 64-slice CT and had serum and plasma biomarkers drawn at time of CT acquisition. Enrollment occurred over a cumulative period of 18 months ending May 2007. No patients had incident or prevalent HF. In this substudy, we included 346 patients in whom the concentrations of serum NT-proBNP, plasma MR-proANP, and plasma MR-proADM were measured at time of CT and in whom there was full visualization of the LA and LV on the multiphase reformatted dataset of the CT. Our institutional review board approved the study protocol, and all patients provided written informed consent.
CT DATA ACQUISITION AND MEASUREMENT
CT imaging was performed using a standard 64-slice multidetector CT coronary angiography (Sensation 64, Siemens Medical Solutions) protocol that was acquired at end inspiration and included the administration of sublingual nitroglycerin (0.6 mg) and intravenous β-blocker (metoprolol 5–20 mg) for those with baseline heart rate >60 beats per minute and no other contraindications. A test bolus protocol was used and contrast agent based on scan range at 5 mL/s. CT images were acquired in spiral mode, gantry rotation time of 330 ms, 64 × 0.6 mm slice collimation, tube voltage 120 kV, effective tube current 850 –950 mAs, with ECG-correlated tube current modulation when appropriate. For volumetric and functional analysis, a multiphase reformatted dataset was reconstructed at 10% RR interval (10 phases) with 1.5-mm slice thickness and 1.5-mm increments of the transaxial images.
Two experienced readers, blinded to the clinical outcome, performed the quantitative CT measurements. We used a highly reproducible threshold-based method for quantifying LA volume (LAV) 3-dimensionally without geometric shape assumptions, as described and validated (22). Briefly, we calculated LAV by pure volumetric summation of manually traced regions of interests on sequential axial 1.5-mm thick slices with a threshold window width set at 100–1000 Hounsfield units using a dedicated semiautomated volumetric software program (Volume Viewer, Leonardo, Siemens Medical Solutions). We measured the LAV as the maximum volume measured from the end-systolic phase just before the mitral valve opening with the largest LA cavity and smallest LV cavity, as determined qualitatively from multiplanar LV short-axis, 2-chamber, and 4-chamber views. LAV included the left atrial appendage but excluded the pulmonary veins. LAV was indexed to body surface area (BSA) for the LAVI measurement. We obtained quantitative LV measurements using Vitrea software (Vital Images). We derived LV volumes and function by manually corrected semiautomated delineation of the endocardial borders. We measured the LV volumes at end-diastolic (LVEDV) and end-systolic (LVESV) phases and indexed the volumes to BSA (LVEDI and LVESI, respectively). LVEF was calculated as (LVEDV − LVESV)/LVEDV×100%.
BIOMARKER TESTING
Blood samples for biomarker testing were taken at the time of CT angiography. The samples were obtained from a peripheral vein and collected into EDTA-coated and noncoated tubes. The blood was immediately centrifuged, and the aliquoted plasma and serum were stored in microcentrifuge tubes at −80 °C until assayed. Specimens were tested on the first freeze-thaw cycle. We performed serum NT-proBNP measurements by use of an electrochemiluminescence sandwich immunoassay (Elecsys 2010, Roche Diagnostics). For NT-proBNP, the interassay CV was 1.38% and intraassay CV was 2.58%. To convert NT-proBNP results from ng/L to pmol/L, divide by 8.457. We measured plasma MR-proANP and MR-proADM concentrations by use of immunoluminometric assays (BRAHMS AG), as described elsewhere (25, 26). For MR-proANP, the interassay CV was 2.33% and intraassay CV was 3.65%. The in vitro stability of MR-proANP is 24 h at room temperature. For MR-proADM, the inter- and intraassay CVs were 2.43% and 2.17%, respectively. All analyses were run in a blinded fashion.
COVARIATES OF INTEREST
Cardiovascular risk factors and medical history were assessed at the time of subject enrollment based on self-report or obtained from the medical records during the index hospitalization. Body mass index (BMI) was defined as weight (kilograms) divided by the height squared (meters). BSA was calculated using the Dubois formula (27). Hypertension was defined as systolic blood pressure of at least 140 mmHg or diastolic blood pressure of at least 90 mmHg or current antihypertensive treatment. Diabetes mellitus was defined as a fasting plasma glucose ≥126 mg/dL or treatment with a hypoglycemic agent. Hyperlipidemia was defined as total cholesterol of ≥200 mg/dL or treatment with a lipid-lowering medication. Subjects were classified as smokers if they smoked at least 1 cigarette a day currently or in the past. Documented history of coronary artery disease (CAD) included previous MI or coronary revascularization. Family history of CAD was defined as having a first-degree female (<65 years) or male (<55 years) relative with a documented history of myocardial infarction or sudden cardiac death. Estimated glomerular filtration rate (eGFR) was determined using the Modification of Diet in Renal Disease (MDRD) formula (28).
STATISTICAL ANALYSIS
We expressed descriptive statistics as mean (SD) or median with interquartile range for continuous variables and as frequency and percentages for nominal variables, as appropriate. Because MR-proADM was normally distributed, we performed log10 transformation only on NT-proBNP and MR-proANP to make them normally distributed before incorporating them into our regression models. We applied Pearson correlation when correlating normally distributed continuous variables and Spearman correlation with non–normally distributed data. We used t-test for the sex comparison of normally distributed covariates and Wilcoxon rank sum of non–normally distributed covariates. We used generalized linear model (GLM) regression analyses to test for sex–biomarker and age–biomarker interaction for all the volumetric measures. For analyses of LA and LV enlargement, we dichotomized the LA and LV volumes and their indices into top and lowest quartiles. We used logistic regression to assess for the risk of volumetric enlargement per increment increase in biomarker concentration. Thus, owing to log10 transformation, the odds ratios for NT-proBNP and MR-proANP refer to risks associated with a 10-fold increase in the concentrations of these biomarkers. In multivariable logistic regression analyses, we adjusted for potential confounders and included all the covariates based on a priori knowledge that may be associated with volumetric enlargement (P < 0.15) from the univariate analyses. For the multivariable models of log10NT-proBNP and log10MR-proANP and their association to LA enlargement by LAV and LAVI, we adjusted for age, sex, diabetes, and history of CAD. We also included BMI and hyperlipidemia in the LAV model. For the multivariable models of MR-proADM and its association with LV enlargement, we adjusted for age, sex, eGFR, and hypertension. We also included BSA in the LVESV model, history of CAD in the LVESI model, and BSA and diabetes in the LVEDV model. The interobserver reproducibility for the volumes was determined for 20 randomly selected patients using intraclass correlation coefficient (ICC) (LAV 0.998; LVESV 0.985, LVEDV 0.995; all P < 0.001). A 2-tailed P value of <0.05 was considered to indicate statistical significance. All analyses were performed using SAS (version 9.1.3, SAS Institute) and SPSS (version 16.0).
Results
BASELINE CHARACTERISTICS IN THE ROMICAT COHORT
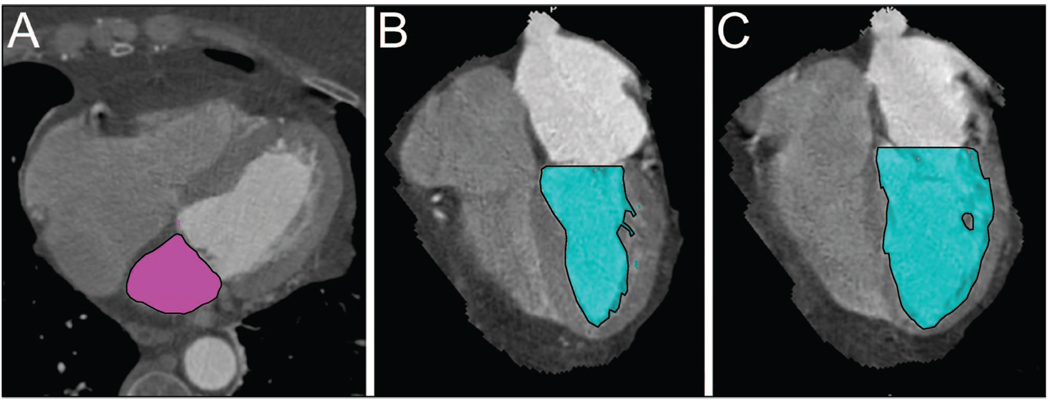
Patient demographics are summarized in Table 1. In this cohort of 346 patients, the average age was 53.5 (11.9) years, and 64.7% (n = 224) were men. The cohort had preserved renal function [mean eGFR 84.2 (18.0) mL/min/1.73 m2], normal LV function [mean LVEF 67.3% (9.8%)], and an average heart rate of 65.3 (11.1) beats per minute during the CT scan. Eleven percent of patients had previously diagnosed CAD, and 25% had a family history of CAD. The means or medians of the volumetric measures and biomarker concentrations are also shown in Table 1. Fig. 1 illustrates examples of measured LAV, LVESV, and LVEDV. For the volumes, LAV ranged from 47.0 to 208.3 mL, LVEDV from 58.0 to 272.0 mL, and LVESV from 10.0 to 219.0 mL. When indexed to BSA, LAVI ranged from 24.0 to 95.3 mL/m2, LVEDI from 29.6 to 139.3 mL/m2, and LVESI from 5.2 to 112.2 mL/m2. For the biomarkers, NT-proBNP concentrations ranged from 5.0 to 1752.0 ng/L, MR-proANP from 14.7 to 321.5 pmol/L, and MR-proADM from 0 to 1.24 nmol/L.
Table 1.
Patient characteristics, CT measures, and biomarker concentrations of the whole cohort and stratified by sex.a
| Total | Men | Women | P | |
|---|---|---|---|---|
| n | 346 | 224 | 122 | |
| Demographics | ||||
| Age, years | 53.5 (11.9) | 51.9 (12.4) | 56.5 (10.6) | 0.0007 |
| BSA, m2 | 2.00 (0.26) | 2.10 (0.22) | 1.82 (0.21) | <0.0001 |
| BMI, kg/m2 | 29.2 (5.8) | 29.5 (5.5) | 28.6 (6.4) | 0.18 |
| Hypertension, n (%) | 146 (42.2) | 92 (41.1) | 54 (44.3) | 0.57 |
| Diabetes mellitus, n (%) | 43 (12.4) | 32 (14.3) | 11 (9.0) | 0.18 |
| Hyperlipidemia, n (%) | 141 (40.8) | 91 (40.6) | 50 (41.0) | 0.95 |
| Smoker, n (%) | 172 (49.7) | 113 (50.5) | 59 (48.4) | 0.74 |
| History of CAD, n (%) | 38 (11.0) | 32 (14.3) | 6 (4.9) | 0.007 |
| Family history of CAD, n (%) | 88 (25.4) | 56 (25.0) | 32 (26.2) | 0.80 |
| eGFR, mL/min/1.73 m2 | 84.2 (18.0) | 85.4 (17.9) | 81.9 (18.2) | 0.08 |
| CT measures | ||||
| LAV, mL | 97.3 (25.9) | 99.9 (27.4) | 92.5 (22.2) | 0.007 |
| LAVI, mL/m2 | 49.0 (12.6) | 47.8 (13.0) | 51.1 (11.6) | 0.02 |
| LVEDV, mL | 118.5 (32.8) | 126.1 (35.2) | 104.6 (21.6) | <0.0001 |
| LVEDI, mL/m2 | 59.3 (14.7) | 60.2 (16.3) | 57.8 (11.0) | 0.10 |
| LVESV, mL | 37.0 (27.0–48.0) | 41.0 (31.0–51.5) | 28.0 (22.0–37.0) | <0.0001 |
| LVESI, mL/m2 | 18.2 (14.1–23.4) | 19.2 (14.9–25.2) | 16.3 (12.7–20.8) | <0.0001 |
| LVEF, % | 67.3 (9.8) | 65.5 (9.6) | 70.6 (9.2) | <0.0001 |
| Biomarkers | ||||
| NT-proBNP, ng/L | 51.3 (24.9–118.0) | 40.9 (20.9–95.0) | 71.2 (37.6–155.0) | <0.0001 |
| MR-proANP, pmol/L | 59.5 (42.1–87.1) | 55.2 (41.1–84.8) | 65.7 (45.6–101.7) | 0.02 |
| MR-proADM, nmol/L | 0.51 (0.17) | 0.49 (0.16) | 0.55 (0.18) | 0.0007 |
Data are mean (SD) or median (IQR) unless noted otherwise.
Fig. 1.
Threshold-based method of (A), LAV with summation of region of interests (outlined in black) from transaxial images during left ventricular end-systole; (B), LVESV (outlined in black); (C), LVEDV (outlined in black).
Table 1 also shows the difference in distributions of volume measurements and biomarker concentrations as stratified by sex. Men had higher LAV, LVEDV, LVESV, and LVESI than women (all P≤0.007). However, women had slightly higher LAVI (P = 0.02) and better LV function (LVEF% 65.5 vs 70.6, P < 0.0001) than men. There was no detectable difference in LVEDI between the sexes (P=0.10). Interestingly, women had significantly higher concentrations of NT-proBNP, MR-proANP, and MR-proADM (all P ≤ 0.02) than men, though there were no sex– biomarker interactions for any of the volume measures.
ASSOCIATIONS BETWEEN BIOMARKERS AND CT VOLUMES
The correlation among the biomarkers was strongest between the natriuretic peptides (r=0.74, P<0.0001) and modest between MR-proADM and the natriuretic peptides (NT-proBNP r = 0.47, P < 0.0001; MR-proANP r = 0.45, P < 0.0001). Both natriuretic peptides were positively correlated with LAV (NT-proBNP r = 0.21, P < 0.0001; MR-proANP r = 0.19, P = 0.0005) and LAVI (NT-proBNP r = 0.32, P < 0.0001; MR-proANP r = 0.30, P < 0.0001), whereas MR-proADM was not (both P > 0.15). Conversely, although there were no correlations with the natriuretic peptides to LV volumes (all P > 0.18), LV volumes were negatively correlated with MR-proADM (LVEDV r = −0.14, P = 0.01; LVEDI r = −0.19, P = 0.0005; LVESV r=−0.17, P = 0.0013; LVESI r=−0.21, P = 0.0001). None of the biomarkers were found to be correlated with LVEF (all P>0.27). With regard to age, all 3 biomarkers had moderate positive correlation (NT-proBNP r = 0.49, P < 0.0001; MR-proANP r = 0.52, P < 0.0001; MR-proADM r = 0.50, P < 0.0001), although there were no age– biomarker interactions for any of the volume measures.
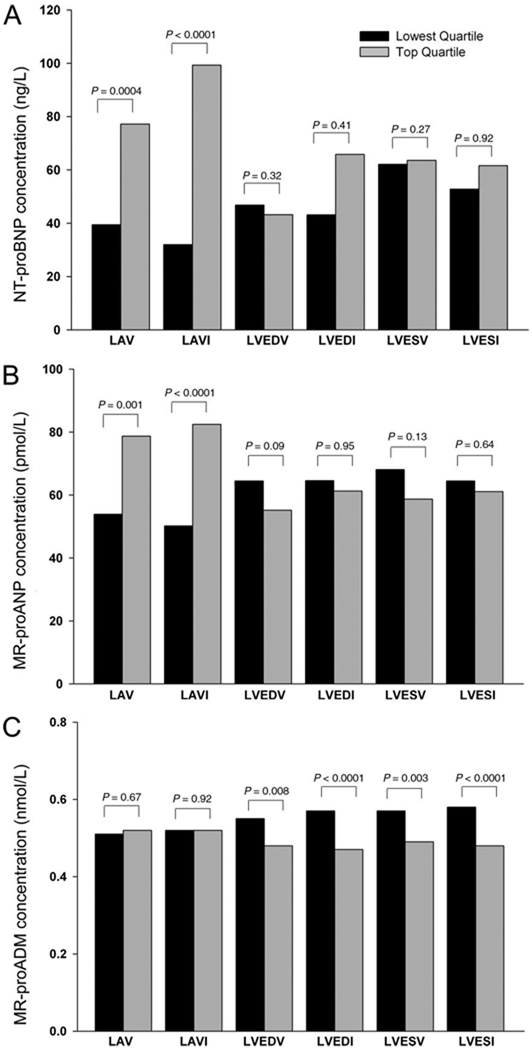
Table 2 and Fig. 2 show the differences in demographics and biomarker concentrations when stratified by highest and lowest quartiles of volume measurements (LAV, 113.2–208.3 vs 47.0 –79.0 mL; LAVI, 56.8 –95.3 vs 24.0 –39.9 mL/m2; LVEDV, 138.0 –272.0 vs 58.0 –95.0 mL; LVESV, 48.0 –219.0 vs 10.0 –27.0 mL; LVEDI, 66.6 –139.3 vs 29.6 –50.0 mL/m2; and LVESI, 23.4 –112.2 vs 5.2–14.1 mL/m2). Median NT-proBNP concentrations were significantly higher in patients in the top quartiles than those in the lowest quartiles for both LAV [median 77 (IQR 40–219) vs 39 (21–103) ng/L; P = 0.0004] and LAVI [99 (48 –219) vs 32 (19– 80) ng/L; P < 0.0001]. Similarly, median MR-proANP concentrations were also increased in the top quartiles compared with the lowest quartiles for LAV [79 (46– 118) vs 54 (39 –77) pmol/L; P = 0.0014] and LAVI [83 (54 –123) vs 50 (35–75) pmol/L; P < 0.0001]. Neither NT-proBNP nor MR-proANP concentrations were significantly different when comparing them in the top vs lowest quartiles of left ventricular measurements (all P values nonsignificant). Mean MR-proADM concentrations were significantly lower in the top quartiles compared with the lowest quartiles of LVEDV [0.48 (0.14) vs 0.55 (0.2) nmol/L, P = 0.008], LVEDI [0.47 (0.13) vs 0.57 (0.19) nmol/L, P<0.0001], LVESV [0.49 (0.15) vs 0.57 (0.19) nmol/L, P = 0.003], and LVESI [0.48 (0.15) vs 0.58 (0.19) nmol/L, P < 0.0001]. MR-proADM concentrations did not differ significantly between patients in the top and lowest quartiles of atrial measurements (all P > 0.67).
Table 2.
Demographics as stratified by top and bottom quartiles of cardiac chamber measures.a
| LAV | P | LAVI | P | LVEDV | P | LVEDI | P | LVESV | P | LVESI | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 0.02 | 0.002 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | ||||||
| Top | 56.2 (13.3) | 58.8 (13.0) | 49.1 (11.5) | 49.6 (12.1) | 51.2 (12.7) | 50.9 (13.5) | ||||||
| Bottom | 51.7 (11.4) | 51.7 (11.7) | 57.9 (11.9) | 57.3 (11.9) | 59.0 (11.2) | 58.2 (12.0) | ||||||
| Men, n (%) | 0.07 | 0.003 | <0.0001 | 0.24 | <0.0001 | 0.001 | ||||||
| Top | 65 (74.7) | 50 (57.5) | 76 (86.4) | 65 (75.6) | 78 (83.0) | 68 (78.2) | ||||||
| Bottom | 53 (61.6) | 69 (79.3) | 42 (49.4) | 57 (66.3) | 33 (39.3) | 47 (54.0) | ||||||
| BMI, kg/m2 | 0.0001 | 0.14 | 0.001 | 0.04 | 0.22 | 0.04 | ||||||
| Top | 30.9 (5.5) | 28.5 (5.4) | 31.1 (6.3) | 28.4 (5.8) | 29.8 (5.9) | 28.5 (5.5) | ||||||
| Bottom | 27.6 (5.4) | 29.8 (5.8) | 28.1 (5.6) | 30.3 (5.7) | 28.7 (6.1) | 30.4 (6.1) | ||||||
| Hypertension, n (%) | 0.29 | 0.54 | 0.03 | 0.01 | 0.007 | 0.004 | ||||||
| Top | 43 (49.4) | 43 (49.4) | 30 (34.1) | 29 (33.7) | 35 (37.2) | 32 (36.8) | ||||||
| Bottom | 35 (40.7) | 39 (44.8) | 43 (50.6) | 46 (53.5) | 49 (58.3) | 52 (59.8) | ||||||
| Diabetes mellitus, n (%) | 0.04 | 0.12 | 0.12 | 0.99 | 0.30 | 0.68 | ||||||
| Top | 19 (21.8) | 16 (18.4) | 14 (15.9) | 10 (11.6) | 17 (18.1) | 15 (17.2) | ||||||
| Bottom | 8 (9.3) | 8 (9.2) | 7 (8.2) | 10 (11.6) | 10 (11.9) | 12 (13.8) | ||||||
| Hyperlipidemia, n (%) | 0.12 | 0.44 | 0.76 | 0.34 | 0.37 | 0.99 | ||||||
| Top | 39 (44.8) | 37 (42.5) | 33 (37.5) | 28 (32.6) | 37 (39.4) | 34 (39.1) | ||||||
| Bottom | 28 (32.6) | 31 (35.6) | 34 (40.0) | 35 (40.7) | 39 (46.4) | 34 (39.1) | ||||||
| Smoker, n (%) | 0.65 | 0.65 | 0.93 | 0.99 | 0.93 | 0.99 | ||||||
| Top | 47 (54.0) | 47 (54.0) | 46 (52.3) | 47 (54.6) | 51 (54.3) | 49 (56.3) | ||||||
| Bottom | 43 (50.0) | 51 (58.6) | 45 (52.9) | 47 (54.6) | 45 (53.6) | 49 (56.3) | ||||||
| History of CAD, n (%) | 0.03 | 0.12 | 0.93 | 0.99 | 0.22 | 0.09 | ||||||
| Top | 14 (16.1) | 15 (17.2) | 13 (14.8) | 12 (14.0) | 18 (19.2) | 18 (20.7) | ||||||
| Bottom | 5 (5.8) | 8 (9.2) | 13 (15.3) | 12 (14.0) | 10 (11.9) | 9 (10.3) | ||||||
| Family history of CAD, n (%) | 0.73 | 0.73 | 0.85 | 0.47 | 0.87 | 0.86 | ||||||
| Top | 21 (24.1) | 21 (24.1) | 18 (20.5) | 17 (19.8) | 25 (26.6) | 20 (23.0) | ||||||
| Bottom | 23 (26.7) | 24 (27.6) | 19 (22.4) | 22 (25.6) | 24 (28.6) | 22 (25.3) | ||||||
| eGFR, mL/min/1.73 m2 | 0.98 | 0.67 | 0.12 | 0.01 | 0.02 | 0.004 | ||||||
| Top | 84.2 (18.6) | 83.4 (17.6) | 85.9 (16.8) | 89.3 (18.0) | 86.2 (17.3) | 87.9 (17.7) | ||||||
| Bottom | 84.3 (16.9) | 84.5 (18.4) | 81.5 (19.5) | 82.0 (18.9) | 79.6 (20.4) | 79.6 (19.6) |
Data are mean (SD) unless noted otherwise.
Fig. 2.
Biomarker concentrations of serum NT-proBNP (A), plasma MR-proANP (B), and plasma MR-proADM (C) as stratified by top vs. lowest quartiles of left atrial and ventricular volumes.
Table 3 shows the association between the biomarkers and volumetric enlargement. For every 1-increment increase in log10NT-proBNP, patients had 2- to 6-fold increased crude risk of LA enlargement by LAV [odds ratio (OR) 2.8, P=0.002] and LAVI (OR 6.0, P < 0.0001). Even after multivariable adjustment, there remained a 2- to 4-fold increased risk for LA enlargement by LAV (OR 2.4, P = 0.03) and LAVI (OR 4.0, P = 0.003). For every 1-increment increase in log10MR-proANP, patients had a near 9-fold increased crude risk of LA enlargement and a 10-fold adjusted increased risk of LA enlargement by LAV (OR 8.8, P = 0.0009; adjusted OR 10.7, P = 0.009). By LAVI, there was a 24-fold unadjusted increase in risk for every increment increase of log10MR-proANP, which was attenuated to a 13-fold increased risk after multivariable adjustment (OR 24.3, P < 0.0001; adjusted OR 13.1, P = 0.004). For every 0.1-nmol/L increase of MR-proADM concentration, the risk of LV enlargement decreased by 22%–24% by volume (LVESV OR 0.76, P = 0.004; LVEDV 0.78, P = 0.009) and by 32%–34% by volume index (LVESI OR 0.68, P = 0.0003; LVEDI OR 0.66, P = 0.0002). After multivariable adjustment, although the association between MR-proADM concentrations and LV enlargement by any of the ventricular measurements was no longer significant, there was a possible residual relationship between MR-proADM and LVEDI (adjusted OR 0.79, P = 0.07).
Table 3.
ORs of biomarkers for top vs bottom quartile volumes, where top quartile represents volumetric enlargement.
| Unadjusted OR (95% CI) | P | Adjusted OR (95% CI)a | P | |
|---|---|---|---|---|
| Log10NT-proBNP | ||||
| LAV | 2.8 (1.5–5.2) | 0.002 | 2.4 (1.1–5.4) | 0.03 |
| LAVI | 6.0 (2.9–12.4) | <0.0001 | 4.0 (1.6–9.8) | 0.003 |
| Log10MR-proANP | ||||
| LAV | 8.8 (2.5–31.4) | 0.0009 | 10.7 (1.8–62.5) | 0.009 |
| LAVI | 24.3 (6.2–95.1) | <0.0001 | 13.1 (2.3–74.8) | 0.004 |
| MR-proADM, per 0.1 nmol/L | ||||
| LVEDV | 0.78 (0.64–0.94) | 0.009 | 0.92 (0.71–1.22) | 0.59 |
| LVEDI | 0.66 (0.53–0.83) | 0.0002 | 0.79 (0.62–1.02) | 0.07 |
| LVESV | 0.76 (0.63–0.92) | 0.004 | 1.05 (0.79–1.39) | 0.74 |
| LVESI | 0.68 (0.56–0.84) | 0.0003 | 0.88 (0.68–1.13) | 0.32 |
Multivariable logistic regression models adjusted for all univariate predictors based on a priori knowledge in which the P values are <0.15 in Table 2.
Discussion
We describe the associations between CT cardiac chamber volumes and concentrations of serum NT-proBNP, plasma MR-proANP, and plasma MR-proADM in a large cohort of patients without evidence of heart failure. The strength of our study is the near-simultaneous acquisition of the contrast-enhanced CT scan and the peripheral blood sampling, allowing us to directly compare cardiac chamber volumetric assessments to both established and novel stress myocyte biomarker concentrations. We found that the natriuretic peptides have positive correlation with LAV and LAVI, whereas MR-proADM has weakly negative correlation with LV volumetric measurements. The median NT-proBNP and MR-proANP concentrations were higher in those in the top quartiles of LAV and LAVI but not LV measures, when comparing patients in the top vs lowest quartiles of volumes where the top quartile represents enlargement. Mean MR-proADM concentrations were significantly lower in the top quartiles than the lowest quartiles of LV volumes and indices, but did not differ significantly for the atrial measurements. In crude analysis, MR-proADM concentrations carried a decreased risk of LV volume enlargement, but this association was not significant in adjusted analyses. Most notably, both of the natriuretic peptides remained independently associated with an increased risk of having LA enlargement by CT.
NT-proBNP has been shown to be incrementally additive to general risk factors in predicting first major cardiac events and death across a wide range of patient types (2, 29 –32). In our ED cohort of low- to intermediate-risk patients with suspected acute coronary syndrome, patients presented with acute chest pain instead of dyspnea as their initial presentation and had preserved LV function. Because our patients had both CT and biomarkers for comparison, we were able to assess the association of NT-proBNP to CT-measured cardiac chambers. We found that patients with increased concentrations of NT-proBNP had larger LA volumes and indices, but not LV volumes. Similar to Raymond et al. (6), who reported that concentrations of NT-proBNP had positive correlation increasing with age and stronger correlation in women than men, we also found that increasing age correlated positively with NT-proBNP concentrations and that women had higher NT-proBNP concentrations than men in our cohort. Importantly, after multivariable adjustment including age, sex, and other risk factors, we found that NT-proBNP was independently associated with LA enlargement in patients without HF.
MR-proANP has been shown to be a useful marker in diagnosing patients with acute HF (10), defining prognosis after MI (12, 33), and predicting mortality in HF patients (2, 13, 14). We found a positive correlation between MR-proANP with both LAV and LAVI. As with NT-proBNP, there was positive correlation between age and MR-proANP and sex differences between MR-proANP and LA volumes. In addition, patients without HF who have increased concentrations of MR-proANP are at higher risk for having LA enlargement. Given that LAV has been shown to be a predictor of survival after MI (34), there may be potential use of MR-proANP for risk stratification. One other potential application of this biomarker is in its usage in developing new pharmacotherapy for several cardiovascular disease processes. In animal models, it has been suggested that a histamine-ANP release pathway exists, which if inhibited may stabilize the mast cell degranulation process that is implicated in acute coronary syndrome (35). An α-human atrial natriuretic peptide (carperitide), which has vasodilatory and diuretic effects, is currently being studied for the treatment of acute heart failure patients (36).
Increased ADM concentrations are found in patients with HF (2, 19), with the more stable form, MR-proADM, found to be increased after an acute MI (37). Most recently, in a large population-based study of 5067 asymptomatic patients who were followed for a median of 12.8 years, MR-proADM was found to be an independent predictor of first cardiovascular events (38). MR-proADM has also been shown to be increased patients with HF post-MI (21). In contrast to these studies, we found that MR-proADM concentrations were negatively correlated with LV volumes and had no correlation to LA volumes or LVEF. After multivariable adjustment, however, the association between MR-proADM concentrations and LV volumes was no longer significant. A possible explanation for the contrast with previous results is the difference in our patient population, which is a much lower-risk cohort. Moreover, our cohort does not have HF and has preserved LV function. It is likely that although MR-proADM is increased in HF patients, its concentrations may not be increased and even may be suppressed in patients without HF due to a negative feedback mechanistic loop. Because we performed our analyses on samples acquired nearly simultaneously with the CT scan, we cannot infer the long-term prognostic outcomes of MR-proADM to future cardiovascular events.
Several limitations are noteworthy in the interpretation of our study results. The generalizability of our findings may be limited to symptomatic acute chest pain patients presenting to the emergency department. The results obtained in this study cannot be directly applied to all patients with cardiac diseases, especially those with symptomatic heart failure. We arbitrarily used the top vs lowest quartiles in our comparisons of LA and LV enlargement to the biomarker concentrations. Although we were unable to grade the severity of LA and LV enlargement, we believe these comparisons are valid, since the top quartile should represent patients with at least some extent of LA or LV enlargement and those in the lowest quartile group most likely have normal LA and LV size. The radiation exposure inherent in the acquisition of CT images should preclude cardiac CT from being performed solely for the evaluation of volumetric assessments. Dose-saving algorithms, such as ECG tube modulation and use of lower tube current and voltage, could still allow for evaluation of volumetric measures. Last, as it is known that a substantial portion of circulating natriuretic peptide is either uncleaved precursor, variably glycosylated NT-proBNP, or degraded BNP, analysis or expression of these peptides in a molar fashion is not supported (39). Thus, we expressed NT-proBNP in our analysis as ng/L; despite the interesting potential to examine molar associations between NT-proBNP and MR-proANP and cardiac structure, this heterogeneity in circulating forms prevents such an analysis.
Acknowledgments
We gratefully acknowledge the enthusiastic support in patient enrollment of the team of faculty, residents, and nursing and administrative staff of the Emergency Department Services of the Massachusetts General Hospital.
Research Funding: J.L. Januzzi, Roche Diagnostics; Q.A. Truong, NIH grant T32HL076136 and L30HL093896; J.L. Januzzi, Balson Cardiac Scholar Award; A.A. Mahabadi, grant from the German National Academic Foundation. This work was supported by the NIH R01 HL080053, and in part supported by Siemens Medical Solutions and General Electrics Healthcare. Reagents for measurement of the natriuretic peptides were kindly provided for free by the BRAHMS CO.
Expert Testimony: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Nonstandard abbreviations: NT-proBNP, amino-terminal pro–B-type natriuretic peptide; HF, heart failure; MI, myocardial infarction; MR-proANP, midregional pro–A-type natriuretic peptide; MR-proADM, midregional fragment of proad-renomedullin; LVEF, left ventricular ejection fraction; ADM, adrenomedullin; LA, left atrial; CT, computed tomography; ROMICAT, Rule Out Myocardial Infarction Using Computer Assisted Tomography; ED, emergency department; ECG, electrocardiogram; LAV, LA volume; BSA, body surface area; LAVI, index of LAV to BSA; LVEDV, LV volume at end-diastolic phase; LVESV, LV volume at end-systolic phase; LVEDI, index of LVEDV to BSA; LVESI, index of LVESV to BSA; BMI, body mass index; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; GLM, generalized linear model; ICC, intraclass correlation coefficient; OR, odds ratio.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: A. Bergmann, BRAHMS AG; J. Kunde, BRAHMS AG.
Consultant or Advisory Role: None declared.
Stock Ownership: A. Bergmann, BRAHMS AG (immediate family member).
Honoraria: None declared.
References
- 1.Mohammed AA, Januzzi JL., Jr Natriuretic peptides in the diagnosis and management of acute heart failure. Heart Fail Clin. 2009;5:489–500. doi: 10.1016/j.hfc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Gegenhuber A, Struck J, Dieplinger B, Poelz W, Pacher R, Morgenthaler NG, et al. Comparative evaluation of B-type natriuretic peptide, midregional pro-A-type natriuretic peptide, midregional pro-adrenomedullin, and copeptin to predict 1-year mortality in patients with acute destabilized heart failure. J Card Fail. 2007;13:42–49. doi: 10.1016/j.cardfail.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2009;54:357–364. doi: 10.1016/j.jacc.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 4.Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132:1961–1970. doi: 10.1210/endo.132.5.8477647. [DOI] [PubMed] [Google Scholar]
- 5.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 6.Raymond I, Groenning BA, Hildebrandt PR, Nilsson JC, Baumann M, Trawinski J, Pedersen F. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart. 2003;89:745–751. doi: 10.1136/heart.89.7.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bay M, Kirk V, Parner J, Hassager C, Nielsen H, Krogsgaard K, et al. NT-proBNP: a new diagnostic screening tool to differentiate between patients with normal and reduced left ventricular systolic function. Heart. 2003;89:150–154. doi: 10.1136/heart.89.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi H, Yoshida J, Yamamoto K, Sakata Y, Mano T, Akehi N, et al. Elevation of plasma brain natriuretic peptide is a hallmark of diastolic heart failure independent of ventricular hypertrophy. J Am Coll Cardiol. 2004;43:55–60. doi: 10.1016/j.jacc.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Lerman A, Gibbons RJ, Rodeheffer RJ, Bailey KR, McKinley LJ, Heublein DM, Burnett JC., Jr Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet. 1993;341:1105–1109. doi: 10.1016/0140-6736(93)93125-k. [DOI] [PubMed] [Google Scholar]
- 10.Gegenhuber A, Struck J, Poelz W, Pacher R, Morgenthaler NG, Bergmann A, et al. Midregional pro-A-type natriuretic peptide measurements for diagnosis of acute destabilized heart failure in short-of-breath patients: comparison with B-type natriuretic peptide (BNP) and amino-terminal proBNP. Clin Chem. 2006;52:827–831. doi: 10.1373/clinchem.2005.065441. [DOI] [PubMed] [Google Scholar]
- 11.Potocki M, Breidthardt T, Reichlin T, Hartwiger S, Morgenthaler NG, Bergmann A, et al. Comparison of midregional pro-atrial natriuretic peptide with N-terminal pro-B-type natriuretic peptide in the diagnosis of heart failure. J Intern Med. 2010;267:119–129. doi: 10.1111/j.1365-2796.2009.02135.x. [DOI] [PubMed] [Google Scholar]
- 12.Khan SQ, Dhillon O, Kelly D, Squire IB, Struck J, Quinn P, et al. Plasma N-terminal B-type natriuretic peptide as an indicator of long-term survival after acute myocardial infarction: comparison with plasma midregional pro-atrial natriuretic peptide: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol. 2008;51:1857–1864. doi: 10.1016/j.jacc.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 13.von Haehling S, Jankowska EA, Morgenthaler NG, Vassanelli C, Zanolla L, Rozentryt P, et al. Comparison of midregional pro-atrial natriuretic peptide with N-terminal pro-B-type natriuretic peptide in predicting survival in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1973–1980. doi: 10.1016/j.jacc.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Moertl D, Berger R, Struck J, Gleiss A, Hammer A, Morgenthaler NG, et al. Comparison of midregional pro-atrial and B-type natriuretic peptides in chronic heart failure: influencing factors, detection of left ventricular systolic dysfunction, and prediction of death. J Am Coll Cardiol. 2009;53:1783–1790. doi: 10.1016/j.jacc.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 16.Ichiki Y, Kitamura K, Kangawa K, Kawamoto M, Matsuo H, Eto T. Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett. 1994;338:6–10. doi: 10.1016/0014-5793(94)80106-1. [DOI] [PubMed] [Google Scholar]
- 17.Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, et al. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160–1166. doi: 10.1006/bbrc.1994.1827. [DOI] [PubMed] [Google Scholar]
- 18.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 19.Jougasaki M, Rodeheffer RJ, Redfield MM, Yamamoto K, Wei CM, McKinley LJ, Burnett JC., Jr Cardiac secretion of adrenomedullin in human heart failure. J Clin Invest. 1996;97:2370–2376. doi: 10.1172/JCI118680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Struck J, Tao C, Morgenthaler NG, Bergmann A. Identification of an adrenomedullin precursor fragment in plasma of sepsis patients. Peptides. 2004;25:1369–1372. doi: 10.1016/j.peptides.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Khan SQ, O’Brien RJ, Struck J, Quinn P, Morgenthaler N, Squire I, et al. Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol. 2007;49:1525–1532. doi: 10.1016/j.jacc.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 22.Mahabadi AA, Samy B, Seneviratne SK, Toepker MH, Bamberg F, Hoffmann U, Truong QA. Quantitative assessment of left atrial volume by electrocardiographic-gated contrast-enhanced multidetector computed tomography. J Cardiovasc Comput Tomogr. 2009;3:80–87. doi: 10.1016/j.jcct.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolzmann P, Scheffel H, Leschka S, Schertler T, Frauenfelder T, Kaufmann PA, et al. Reference values for quantitative left ventricular and left atrial measurements in cardiac computed tomography. Eur Radiol. 2008;18:1625–1634. doi: 10.1007/s00330-008-0939-4. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction Using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. doi: 10.1373/clinchem.2003.021204. [DOI] [PubMed] [Google Scholar]
- 26.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823–1829. doi: 10.1373/clinchem.2005.051110. [DOI] [PubMed] [Google Scholar]
- 27.DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:865–871. [Google Scholar]
- 28.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 30.Rehman S, Lloyd-Jones DM, Martinez-Rumayor A, Januzzi JL. Inflammatory markers, amino-terminal pro-brain natriuretic peptide, and mortality risk in dyspneic patients. Am J Clin Pathol. 2008;130:305–311. doi: 10.1309/L7BP57F7UF7YNYKX. [DOI] [PubMed] [Google Scholar]
- 31.Rehman SU, Martinez-Rumayor A, Mueller T, Januzzi JL., Jr Independent and incremental prognostic value of multimarker testing in acute dyspnea: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Clin Chim Acta. 2008;392:41–45. doi: 10.1016/j.cca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 32.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 33.Elmas E, Brueckmann M, Lang S, Kalsch T, Haghi D, Sueselbeck T, et al. Midregional pro-atrial natriuretic peptide is a useful indicator for the detection of impaired left ventricular function in patients with coronary artery disease. Int J Cardiol. 2008;128:244–249. doi: 10.1016/j.ijcard.2007.04.113. [DOI] [PubMed] [Google Scholar]
- 34.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 35.Li D, Wen JF, Jin JY, Quan HX, Cho KW. Cardiac mast cells regulate myocyte ANP release via histamine H2 receptor in beating rabbit atria. Regul Pept. 2009;155:33–38. doi: 10.1016/j.regpep.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Nomura F, Kurobe N, Mori Y, Hikita A, Kawai M, Suwa M, Okutani Y. Multicenter prospective investigation on efficacy and safety of carperitide as a first-line drug for acute heart failure syndrome with preserved blood pressure: COMPASS: Carperitide Effects Observed through Monitoring Dyspnea in Acute Decompensated Heart Failure Study. Circ J. 2008;72:1777–1786. doi: 10.1253/circj.cj-07-0760. [DOI] [PubMed] [Google Scholar]
- 37.Behnes M, Papassotiriou J, Walter T, Fiedler E, Sauer T, Lang S, et al. Long-term prognostic value of mid-regional pro-adrenomedullin and C-terminal pro-endothelin-1 in patients with acute myocardial infarction. Clin Chem Lab Med. 2008;46:204–211. doi: 10.1515/CCLM.2008.040. [DOI] [PubMed] [Google Scholar]
- 38.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apple FS, Wu AH, Jaffe AS, Panteghini M, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage laboratory medicine practice guidelines: analytical issues for biomarkers of heart failure. Clin Biochem. 2008;41:222–226. doi: 10.1016/j.clinbiochem.2007.07.001. [DOI] [PubMed] [Google Scholar]