Abstract
Background
We hypothesized that lymph nodes draining sites of cutaneous vaccination could be identified by sentinel node biopsy techniques, and that measuring T-cell response with lymphocytes obtained from these lymph nodes would provide a more sensitive measure of immunogenicity than would the same measurement made with peripheral blood lymphocytes (PBL).
Methods
ELISpot analysis was used to determine the magnitude of vaccine-specific T-cell response in the sentinel immunized nodes (SIN), random lymph nodes, and peripheral blood lymphocytes (PBL) obtained from patients enrolled in clinical trials of experimental melanoma vaccines.
Results
The SIN biopsy was successful in 97%of cases and morbidity was very low. The T-cell response to vaccination was detected with greater sensitivity in the SIN(57%) than in PBL (39%), and evaluation of T-cell responses in the SIN and the PBL together yielded T-cell responses in 63% of patients. When the T-cell responses from a SIN and a random lymph node were compared in four patients, immune responses were detected to one of the vaccine peptides in three of these four patients. In all of those cases, responses were present in the SIN but absent from the random lymph node.
Conclusion
Measurements of T-cell responsiveness to cutaneous immunization are more frequently positive in the SIN than they are in the PBL, however evaluation of both the SIN and PBL permit a more sensitive measure of T-cell immunogenicity than use of either single source.
Unfortunately, cancer vaccines have not yet been as successful as viral vaccines.1 This may be attributed to numerous factors, including immune escape by the tumor cells, T-cell tolerance to cancer antigens, the presence of regulatory T-cells, and dysfunctional effector T-cells.2 To improve cancer vaccine strategies, it is necessary to dissect the response to cancer vaccines so that obstacles to successful immune therapy can be identified and addressed.3 The induction of effective antitumor immunity by cancer vaccines depends on multiple sequential events: antigen delivery to antigen-presenting cells, migration of antigen-laden dendritic cells to draining lymph nodes, antigen presentation to circulating T-cells that enter the lymph nodes through the high endothelial venules, expansion of antigen-reactive T-cells in the lymph nodes, dissemination of the responding T-cells systemically to tumor deposits, and tumor cell destruction by activated tumor-reactive T-cells.
Vaccines may also fail because of poor immunogenicity of the included antigens. In fact, T-cell responses to some melanoma vaccines have been difficult to detect, despite observed regressions of metastatic tumor deposits. In one study, patients were vaccinated with a MAGE-A3 peptide, and regressions of one or more metastatic tumor deposits were observed in 7 of 25 patients receiving the full course of vaccines, but T-cell responses were not detectable in the blood in the original analyses.4 Subsequent analysis with very rigorous single-cell measures demonstrated T-cells reactive to this peptide in two responding patients,5 but the frequency of responding T-cells is far below the level expected for a therapeutic cytotoxic T-lymphocyte (CTL) response. The biologic relevance of these responses therefore remains uncertain.
Paradoxically, in some studies, induction of T-cell responses is not reliably associated with clinical tumor regression. In one study, vaccination with a modified gp100 peptide led to detectable CTL responses in the peripheral blood in over 90% of patients, but no clinical tumor regressions.6 In another arm of that study, in which patients were vaccinated in the same way but also were administered high-dose IL2, there were objective clinical tumor regressions in 41% of patients, but T-cell responses were observed in only a small minority of patients.6 It has been postulated that this discrepancy between immune response detected in the blood and tumor regression may be due to alterations of T-cell trafficking mediated by interleukin-2,7 but this hypothesis could not be addressed because T-cell responses were assessed only in the blood and not in other compartments.
A vaccine must be immunogenic to have a therapeutic effect, so the finding of a clinical benefit in the absence of clear evidence of immunogenicity may be attributable to random fluctuations in tumor size independent of vaccine effect. Alternatively, it may represent a vaccine effect that is not detectable in the peripheral blood. A T-cell response to vaccination may be difficult to detect in the peripheral blood because of dilution, depletion by trafficking to tumor deposits, or peripheral depletion due to other causes. If, however, the vaccine is immunogenic, the T-cell response should be detectable in the lymph node(s) draining the vaccine site. Thus, we hypothesized that evaluation of T-cell responses in the draining lymph node would permit a more sensitive measure of immunogenicity than evaluation of T-cell responses in the peripheral blood alone. Using sentinel node biopsy techniques, we have incorporated evaluation of a vaccine-draining lymph node (sentinel immunized node, SIN) in four clinical trials of melanoma vaccines. We have reported pilot immunologic data regarding these SIN, for five patients on one of these trials (UVA-Mel31).8 Herein we report the cumulative experience to date with SIN biopsy in 113 patients, with data on feasibility, success rate, and morphologic and immunologic findings. In addition, evaluation of immune responses in the SIN and in random nodes in the same patients permits evaluation of the hypothesis that the SIN is the primary site of the immune response to cutaneous vaccination.
PATIENTS AND METHODS
Patients
Patients were enrolled in four different melanoma vaccine trials, all of which have completed accrual. The Mel31 trial tested a mixture of four gp100 and tyrosinase-derived peptides, and included a tetanus helper peptide.9 The peptides were delivered pulsed on monocytes-derived dendritic cells (Arm A), or as an emulsion with granulocyte–macrophage colony-stimulating factor (GM-CSF) and Montanide ISA-51 adjuvant (arm B). Vaccines were given on days 0, 7, 14, 28, 35, and 42. Low-dose IL-2 was given daily on days 7 through 49. The SIN was harvested 6–9 days following the third vaccination. The Mel36 trial tested the same peptides used in the Mel31 trial.10 The peptides were delivered as an emulsion with GM-CSF and Montanide ISA-51 adjuvant. The vaccine schedule followed that of Mel31. Low-dose IL-2 was administered daily for 6 weeks (arm A at days 7–49; arm B at days 28–70). The SIN was harvested after the third immunization (on day 22). The Mel37 trial tested a vaccine that consisted of autologous melanoma cells plus GM-CSF in Montanide ISA-51 (manuscript in preparation). Vaccines were given over at least a 6-week period at weeks 0, 1, 2, 4, 5, and 6, with responders given additional vaccinations every 4 weeks for a maximum of nine vaccinations. Low-dose IL-2 was administered daily for 6 weeks following the second vaccination at week 1. The SIN was harvested 1 week following the third vaccination. The Mel39 trial was a two-arm trial with arm A receiving the same peptides tested in Mel31, and arm B receiving 12 peptides derived from tyrosinase, MAGE-A1, MAGE-A3, gp100, and NY-ESO-1, plus a tetanus helper peptide.11 The peptides were delivered as an emulsion with GM-CSF and Montanide ISA-51 adjuvant. The vaccine schedule followed that of Mel31. The SIN was harvested after the third immunization (on day 22). The patients on these trials all had advanced stage melanoma, ranging from high-risk resected melanoma (stage IIB, III, or IV; Mel36, Mel37, Mel39) to unresectable advanced melanoma (stage III or IV; Mel31, Mel37). For all of these trials, patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of at least 2. Patients were eligible for the trials only if they were on no other cytokine therapy or cytotoxic chemotherapy and if they were not immunosuppressed.
Vaccine Injection Methods
Patients were vaccinated at cutaneous sites, either subcutaneously (UVA-Mel31, arm 1: vaccinated with dendritic cells) or half intradermally and half subcutaneously. In all cases, patients received weekly cutaneous vaccinations in two different extremity vaccination sites for the first three vaccines. One of these two vaccination sites was considered the primary vaccination site (usually on an upper arm), and the other was considered a replicate vaccination site (usually on a thigh). The patients were vaccinated three additional times (weekly) at the primary vaccination site only. The replicate vaccination site was used to allow harvest of a lymph node draining that vaccination site. This node was identified using sentinel node technology and is referred to as the “sentinel immunized node” (SIN).
Harvest of the SIN
Patients were injected intradermally 1 week (6–9 days) after the third vaccine with approximately 0.5 mCi technetium-99 sulfur colloid, distributed at multiple injection sites at the periphery of the vaccine site inflammatory reaction. Lymphoscintigraphy was performed, and the patient was brought to the outpatient Cancer Center clinic for excisional biopsy of the dominant sentinel node identified by this radiocolloid lymphatic mapping.
The patient was placed supine on a procedure table in a minor procedure room in the Cancer Center clinic. Early in the experience with this procedure, an intravenous line was placed, and the patients were administered low doses of midazolam for sedation just prior to the procedure. After multiple patient requests to have the biopsy performed without sedation and without an intravenous line, the routine was changed to exclude both procedures. This permitted patients to drive themselves home if desired, although most were accompanied by family.
The SIN was identified by transcutaneous evaluation of gamma counts using a standard handheld gamma probe (C-trak, Care Wise Medical Products Corp., Morgan Hill, CA). In those cases where more than one hot-spot lymph node was identified by lymphoscintigraphy and the gamma probe, the hot-test lymph node closest to the vaccine injection site was selected. Because this was a research procedure, no attempt was made to remove all hot lymph nodes, but only to remove a representative hot spot lymph node. In one case, the only SIN was in the iliac chain. In the interest of avoiding patient morbidity, that node was not removed for this research study.
Materials Used for SIN Biopsy
The procedure was performed by a surgical oncologist (C.L.S.) with an assistant to aid in operative exposure, who was either a nurse, a medical student, or a surgical resident. Both were fully gowned and gloved. The local anesthetic was 1% lidocaine with epinephrine and bicarbonate. A minor procedure tray was used, containing suture scissors, Metzenbaum scissors, Adson forceps, one other pair of forceps, several Crile clamps, a scalpel, a needle driver, and two shallow phrenic retractors. Electrocautery was not needed and not used. Occasionally a suture was used for hemostasis. The incisions were closed with a layer of running 3-0 polyglactin suture (Polysorb, US Surgical) in the subcutaneous tissue, and another layer of running deep dermal suture, plus a 4-0 Polysorb subcuticular stitch. Benzoin and steristrips were applied.
Harvest of Random Nonimmunized Nodes
For one of the clinical trials (Mel36), patients consented to removal of a nonradioactive node in addition to a SIN, when such a random node could be identified and removed without enlarging the incision and without substantial additional dissection. This was feasible in a minority of cases. In a few other cases, a nonsentinel node was removed incidentally, because it was attached to the SIN.
Informed Consent and Regulatory Procedures
The four clinical trials contributing to this study were performed with review and approval by the local Institutional Review Board (IRB) (Human Investigation Committee, University of Virginia), the Cancer Center Protocol Review Committee, and our General Clinical Research Center. In addition, all were performed under investigational new drug (IND) applications to the Food and Drug Administration (FDA) (IND nos. 7593, 8932, and 9847). All enrolled patients signed appropriate informed consent documents. More recently, patients also signed a separate informed consent document for the SIN biopsy on the day of that procedure, in addition to signing the original study consent that included the SIN biopsy.
Morphology and Measurements of the Lymph Nodes
Each lymph node, at the time of surgical resection, was measured in three dimensions (excluding surrounding adipose tissue), and these values were used to calculate a crude volume, as a product of the three dimensions. A central slice was cut from each lymph node after it was removed, and was submitted in formalin for formal histologic review, to rule out the presence of metastatic melanoma and for immunohistochemical stains and microscopic histologic evaluation.
The remainder of each lymph node was rendered into a single cell suspension by mechanical dissociation under sterile conditions in the Tissue Procurement Facility. The resulting lymphocyte suspensions were viably cryopreserved in 90% human AB serum (Sigma, St. Louis, MO) and 10% dimethyl sulfoxide (DMSO) (Sigma) and stored in liquid nitrogen.
Evaluation of Lymphocytes for T-Cell Responses to Defined Peptide Antigens
Peripheral blood lymphocytes were also collected from all patients before initiation of the vaccine regimen, each week prior to each vaccine, on the day of the SIN biopsy, and in follow-up after completion of the vaccines. Lymphocyte samples from these dates and also from the lymph nodes were thawed and evaluated simultaneously for T-cell responses to multiple defined peptide antigens. The primary measure of T-cell response was the ELISpot assay, in which the number of T-cells secreting interferon-gamma (IFNγ) in response to each tested peptide was enumerated.
Lymphocytes were stimulated with peptide as previously described.8 Culture medium consisted of RPMI-1640 (Life Technologies, Grand Island, NY) containing 10% heat-inactivated human AB serum (Sigma), 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Lymphocytes at 2 × 106 cells/ml were incubated with the peptide mixture used for immunization (40 μg/ml of each peptide) for 2 h at 37°C. The cells were then pelleted, resuspended in culture medium containing IL-2 (20 U/ml), and cultured for 14 days. The culture medium was replaced as needed.
These cell populations were then evaluated by ELISpot assay as previously described.8 Briefly, lymphocytes were plated at 10,000–150,000 cells per well in 96-well plates with equal numbers of antigen-presenting cells, which included C1R-A1 (HLA-A1+), C1R-A2 (HLA-A2+), C1R-A3 (HLA-A3+), and T2 (HLA-A2+). The cells were incubated with 10 μg/ml peptide for 2 h at 37°C and then washed to remove unbound peptide. As a negative control, lymphocytes were tested with antigen-presenting cells pulsed with an irrelevant peptide (from HIV or malarial proteins) or not pulsed with peptide. As a positive control, T-cells nonspecifically stimulated with 1 ng/ml phorbol myristate acetate (PMA) (Sigma) and 1 μM ionomycin (Sigma) were used.
The ELISpot assays were performed over a span of several years, and there were some improvements in the assay systems over time. Specifically, the earlier studies were done on plastic plates (Immulon 2 flat bottom plates, Dynatech, Chantilly, VA), but more recent ELISpot assays used filter-bottom MultiScreen plates (Millipore, Bedford, MA). Also, spots in early assays were read visually with a binocular microscope; more recently, an automated ELISpot plate reader (Biosys-Bioreader-3000, Karben, Germany) was used to count spots for all assays.
Criteria used for defining a positive response were consistent throughout this study.9–11 Results are reported as the number of peptide-reactive T-cells per 105 cells. The criteria for defining a positive response relay on the following definitions: Nvax is the number of T-cells responding to peptide in the vaccine; Nneg is the number of T-cells responding to the negative control (maximum of two negative controls: antigen-presenting cell alone or incubated with irrelevant peptide); and Rvax is the ratio Nvax/Nneg.
For the evaluation of responses of peripheral blood lymphocytes (PBL), a patient is considered to have a T-cell response to vaccination only if all the following criteria are met:
Nvax exceeds Nneg by >30 cells per 100,000 cells (corresponding to ~0.15% of CD8+ T-cells)
Rvax>2
(Nvax − 1 SD)>(Nneg + 1 SD)
Rvax after vaccination>2Rvax pre-vaccine (In the rare event of an unevaluable pre-vaccine PBL sample, a negative result after vaccine 1 was accepted as a surrogate for pre-vaccine response).
Observed fold increases <1 were converted to 1 to indicate no response. When the pre-vaccine PBL value was 0, this value was converted to 1 to avoid dividing by zero. Criterion 4 above was not applied to SIN.
Cell Lines
C1R-A1, C1R-A2, and C1R-A3 are human Epstein–Barr virus (EBV)-transformed B-lymphoblastoid cell lines that lack expression of their endogenous class I major histocompatibility complex (MHC) genes, but which have been transfected with and express the HLA-A*0101, HLA-A*0201, and HLA-A*0301 genes, respectively12 (provided by Peter Cresswell, Yale University, New Haven, CT). T2 is a mutant human T/B cell hybrid that lacks the transporter associated with antigen processing (TAP) but expresses the HLA-A*0201 gene13 (provided by Peter Cresswell). HLA typing was performed by clinical laboratories, by a microcytotoxicity assay on autologous lymphocytes, or by DNA typing using polymerase chain reaction (PCR) methods (One Lambda, Canoga Park, CA).
Peptide Vaccine Composition
All patients on trials Mel31 and Mel36, and half of patients on Mel39, received a vaccine comprising 100 μg each of the four melanoma peptides tyrosinase(240–251),14 tyrosinase(369–377),15 gp100(280–288),16 and gp100(17–25);17 and 190 μg HLA-DR-restricted tetanus helper peptide AQYIKANSKFIGITEL18 (Table 1). In addition, half of patients on UVA-Mel39 were vaccinated with a mixture of 12 peptides, including the four included in the earlier trials, plus tyrosinase(146–156),19 MAGE-A1(161–169),20 MAGEA3( 168–176),21 gp100(209–217),22 MAGE-A10(254–262),23 gp100(614–622),19 MAGE-A1(96–104),24 and NY-ESO-1(53–62) 25 (Table 1). These peptides have been previously reviewed.26
TABLE 1.
Peptides used in vaccines
| Class I MHC molecule | Source of peptide antigena | Sequence of peptide antigen | Mel31/Mel36b | Mel39b | Reference |
|---|---|---|---|---|---|
| HLA-A1 | Tyrosinase(240–251)c | DAEKSDICTDEY | • | • | 14 |
| Tyrosinase(146–156) | SSDYVIPIGTY | • | 19 | ||
| MAGE-A1(161–169) | EADPTGHSY | • | 20 | ||
| MAGE-A3(168–176) | EVDPIGHLY | • | 21 | ||
| HLA-A2 | Tyrosinase(369–377)d | YMDGTMSQV | • | • | 15 |
| gp100(209–217)e | IMDQVPFSV | • | 22 | ||
| gp100(280–288) | YLEPGPVTA | • | • | 16 | |
| MAGE-A10(254–262) | GLYDGMEHL | • | 23 | ||
| HLA-A3 | gp100(17–25) | ALLAVGATK | • | • | 17 |
| gp100(614–622) | LIYRRRLMK | • | 19 | ||
| MAGE-A1(96–104) | SLFRAVITK | • | 24 | ||
| NY-ESO-1(53–62) | ASGPGGGAPR | • | 25 |
The protein source and corresponding amino acid residues that give rise to the antigenic peptide are listed.
Mel31, Mel36, and Mel39 were clinical trials that utilized the designated peptides.
The synthetic peptide incorporated a serine (S) in place of the naturally occurring cysteine (C) at residue 244.
The synthetic peptide incorporated asparagine (N), the naturally occurring post-translational change that replaces aspartic acid (D) at residue 371.
The synthetic peptide incorporated methionine (M) in place of the naturally occurring threonine (T) at residue 210.
All of the peptides for Mel31 and UVA-Mel36 were synthesized and purified (>90%) by the Biomolecular Core Facility at the University of Virginia. Peptides for Mel39 were synthesized and purified by Multiple Peptide Systems (La Jolla, CA). Peptides for vaccination were vialed in aqueous salt solutions containing 75–80% lactated Ringer’s solution (Baxter) and sterile water, pH ~ 5.5 (5.0–7.0) and were filter-sterilized prior to aliquoting into sterile rubber-stoppered borosilicate glass vials. The mixtures of melanoma peptides were prepared as sterile aqueous solutions containing all 4 or 12 class I MHC-restricted peptides at 200 μg/ml each. The tetanus helper peptide was prepared as a separate sterile solution. All peptide solutions for vaccination were submitted to multiple quality-assurance studies including sterility (Clinical Microbiology Laboratory, University of Virginia), identity and purity as determined by mass spectrometry (Biomolecular Core Laboratory, University of Virginia), potency as determined by amino acid analysis to measure concentration (Biomolecular Core Laboratory, University of Virginia), general safety (Charles River Laboratories, Wilmington, MA), pyrogenicity (Charles River Laboratories), and stability as determined by mass spectrometry and amino acid analysis. Criteria for lot release were defined in INDs 7593 and 9847 and were in accordance with guidelines published in the Code of Federal Regulations.
In addition to the peptides used in the vaccine, irrelevant peptides YLKKIKNSL (malaria CSP334–342)27 and/or SLYNTVATL (HIV-gag p1777–85)28–30 were used in laboratory analyses.
RESULTS
Feasibility and Limitations of SIN Biopsy as an Outpatient Procedure
In four clinical trials, 119 SIN procedures were attempted or planned, with 116 SIN biopsies completed in 113 patients (Table 2). Three patients underwent SIN biopsy twice because of sequential enrollment in two of these trials. In three cases (2.5%) SIN biopsy was attempted but was aborted or failed. In one morbidly obese patient, the lymph node was too deep for removal in the clinic with available retractors. In one patient who was enrolled in a second vaccine trial, lymphoscintigraphy demonstrated a single lymphatic channel draining from the vaccine injection site to the site of the prior SIN biopsy scar, without a visualized lymph node. Presumably, the same lymph node was targeted and had already been removed. In the third case, lymphatic drainage was to an iliac lymph node, and removal of this was not attempted due to risk of greater morbidity.
TABLE 2.
Success and limitations of SIN biopsy
| Total number of SIN biopsy procedures attempted or planned | 119 | ||
| Number SIN biopsy procedures failed or aborted | 3 | ||
| Too deep for removal in clinic – morbidly obese | 1 | ||
| Failed due to prior SIN biopsy that took the same node | 1 | ||
| Aborted – drainage to iliac node, not attempted | 1 | ||
| Total number of SIN biopsy procedures completed | 116 | ||
| Number of patients with one successful SIN biopsy | 110 | ||
| Number of patients with two successful SIN biopsiesa | 3 |
Due to sequential enrollment into two of the clinical trials.
The mean age of patients at the time of the procedure was 55 years (range 25–82 years). On lymphoscintigraphy, the number of hot spots visualized ranged from 1 to 6 (mean 1.88, median 2). A total of 122 SIN were removed (mean 1.05/patient procedure, median 1, range 1–2). The vast majority of SIN biopsies were performed on inguinal lymph nodes (97%, Fig. 1), but three were left axillary lymph node biopsies in patients who had previously had bilateral inguinal node dissections or biopsies. Patients tolerated all of these procedures well under local anesthesia. Among those with inguinal SIN biopsies, 43 (37%) were performed on the right side, and 70 (60%) were performed on the left side.
FIG. 1.
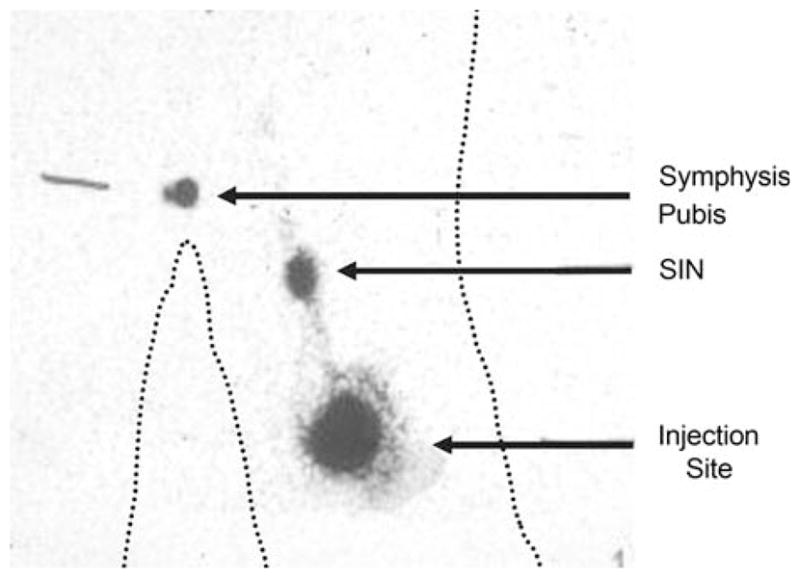
Identification of the SIN in the groin draining a cutaneous vaccine site in the thigh by Tc99-sulfur colloid and excision guided by a gamma probe. Technetium-labeled sulfur colloid was injected intradermally around the vaccine injection site reaction in the left thigh. The resulting gamma camera scan demonstrates the radio-activity at the injection site, the lymphatic channel draining toward the sentinel immunized node, and the hot spot representing the location of the SIN in the left groin. The location of the symphysis pubis was identified with a radioactive marker. The body contours are marked with dotted lines.
Harvest of Random Lymph Nodes
In four patients on Mel36, harvest of random lymph nodes (nonradioactive, in same basin as SIN) was performed intentionally as part of the research protocol on the same day as the harvest of the SIN. The protocol specified that the random lymph node would be removed only if this could be done through the same incision and with minimal additional dissection. These criteria were met in four cases. In six other patients, small lymph nodes removed incidentally at the time of the SIN biopsy were identified as nonradioactive and are also considered random lymph nodes. The gamma counts and crude lymph node volume were measured for each of these lymph nodes and were compared to the corresponding values for the SINs (Table 3). In addition, the total number of mononuclear cells obtained from each lymph node was recorded. The yield of cells for immunologic studies averaged 111 million per lymph node.
TABLE 3.
Sentinel immunized nodes and random nodes compared clinicallya
| Lymph node source | Statistic | Gamma counts (10 s) | Lymph node volume (mm3) | Number of cells (106) |
|---|---|---|---|---|
| SIN (N = 122) | Mean | 7,598 | 1,422 | 111 |
| Median | 3,792 | 1,080 | 65 | |
| (Range) | (327–50,387) | (196–4,590) | (3–692) | |
| Random nodes (N = 10) | Mean | 50 | 494 | 33 |
| Median | 29 | 212 | 18 | |
| (Range) | (1–222) | (125–2,080) | (4–124) |
These data represent the recorded data for maximal gamma counts over 10 seconds (N = 106), LN volume (N = 107), and cell counts (N = 122) for SIN samples, as well as maximal gamma counts (N = 8), LN volume (N = 8), and cell counts (N = 10) for random node samples.
Histologic Evaluation of the Lymph Nodes
The SINs were evaluated on hematoxylin and eosin (H & E) sections by a surgical pathologist. None contained metastatic melanoma, nor did any of the random lymph nodes contain evidence of melanoma. The lymph nodes were evaluated further by immunohistochemical stains for dendritic cell infiltrates using S100, CD23, and CD1a antibodies (Fig. 2). S100+ cells routinely stained very strongly and were clustered in the paracortical areas of the lymph node. CD1a+ cells were more lightly stained and routinely were fewer in number, but in the same general distribution as the S100+ cells (not shown). CD23 stains DC and B cells, and prominent cell clusters in the paracortical areas were observed in most SIN (not shown). It was possible to measure the extent of DC infiltration of nodes by use of image analysis software, dividing the cross-sectional area of S100+ cells by the total cross-sectional area of the node (excluding the fatty hilum); these values varied widely from 1.7% to 19.6% in five patients evaluated on arm A (peptides delivered on DC) and eight patients evaluated on arm B (peptides delivered with GM-CSF and Montanide ISA-51 of UVA-Mel31) (data not shown).
FIG. 2.
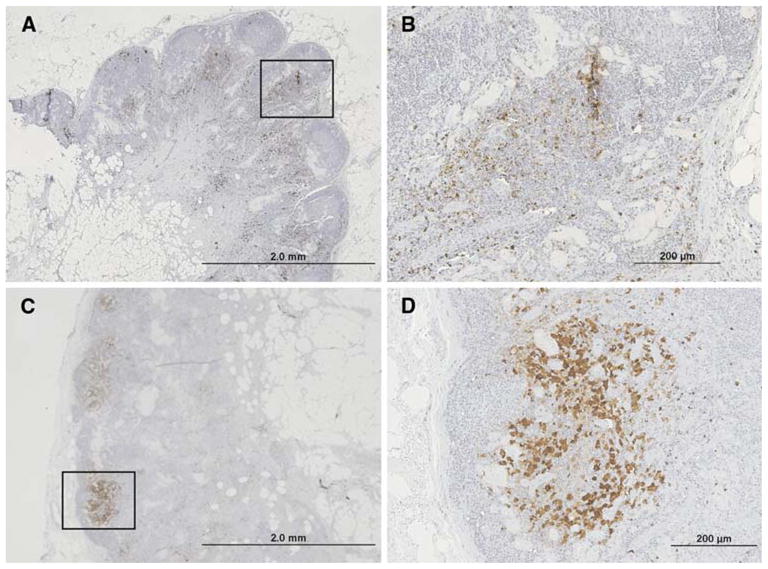
Immunohistochemical identification and quantification of S100+ dendritic cells in two different SINs. A central slice of each immunized lymph node was preserved in formalin and evaluated by immunohistochemical stains for S100+ dendritic cells. The proportion of the cross-sectional area occupied by DC ranged from about 2% to 20%. Examples are shown for patient VMM226 (A, B) with 6.6% of the lymph node containing S100+ dendritic cells and for VMM193 (C, D) with 2.7% of the lymph node containing S100+ dendritic cells. Histologically, the lymph node from patient VMM226 (A) had reactive features, whereas the lymph node from patient VMM193 (B) did not have reactive features. (A) and (C) were at an original magnification of 20× with the boxed areas approximately corresponding to (B) and (D); (B) and (D) were at an original magnification of 100×.
Peptide-Reactive T-Cell Responses in the SIN
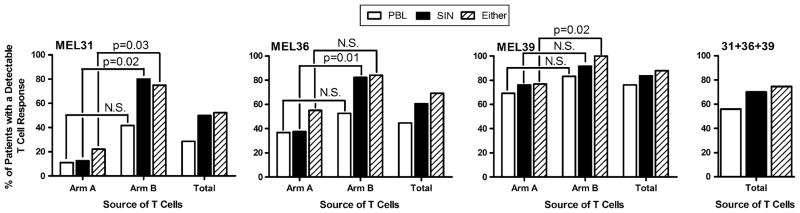
Data on the immune responses detected in the PBL and SIN have been reported in detail separately for each of the four studies (9–11 and manuscript in preparation). Individual and combined summary data from Mel31, Mel36, and Mel39 (N = 110) are shown in Fig. 3. For Mel31 and Mel36 T-cell responses were more frequently observed in the SIN obtained from arm B from each study in comparison with arm A from each study, while the fraction of patients with T-cell responses was equivalent for both arms A and B of Mel39. Evaluation of the SIN alone detected T-cell responses 25% more often than were detected in PBL (70% versus 56%); combined evaluation of the SIN and the PBL detected immunogenicity 33% more often than in PBL alone (74.5% versus 56%). Thus, one-quarter to one-third of T-cell responses would have been missed if only the PBL were evaluated in these studies.
FIG. 3.
Patients on the three vaccine trials Mel31, Mel36, and Mel39 were evaluated for evidence of T-cell response in the PBL and in the SIN. The proportion of patients with T-cell responses to at least one peptide in the PBL, in the SIN, or in either the PBL or SIN are presented from the individual trials as well as for the three trials combined (N = 110). The Mel31, Mel36, and Mel37 trials each had two study arms (A and B) which are described in the “Patients and Methods” section. Results are reported for the individual arms of each study, for each study as a whole (“Total”), and the combined results of all three studies (“31 + 36 + 39”). Statistical significance was determined with Fisher’s exact test. N.S., not significant.
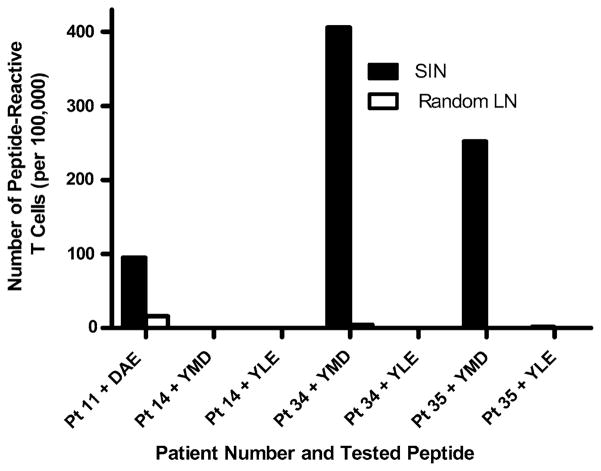
For those four patients from whom a SIN and a random lymph node were prospectively collected in accord with the protocol, T-cell responses were measured in parallel using lymphocytes from the SIN and from the random lymph node after one in vitro sensitization with a mixture of four melanoma peptides from gp100 and tyrosinase (DAEKSDICTDEY, YLEPGPVTA, YMDGTMSQV, and ALLAVGATK) (Fig. 4). T-cell responses were observed in three of the four patients, and in all three of those patients there were T-cell responses in the SIN, but none in the random lymph nodes.
FIG. 4.
T-cell response in SIN is specific, and is not evident in a random node from the same basin (Mel36). Lymphocytes from a SIN and from a random, nonradioactive node were sensitized in vitro once with the four melanoma peptide mixture, then assayed by ELIspot assay for reactivity to each of the peptides relevant for the patient’s HLA type. Patients 11, 14, 34, and 35 were studied. Patient 11 was HLA-A1+ and reactivity was tested to the tyrosinase peptide DAEKSDICTDEY. The other three patients were HLA-A2+ and reactivity was tested to the gp100 and tyrosinase peptides YLEPGPVTA and YMDGTMSQV. The reactivity from the SIN is shown in the solid black bars. Reactivity from the random nodes is shown in the white empty bars. Reactivity was negative for patient 14, and reactivity to YLEPGPVTA was negative in all three cases evaluated.
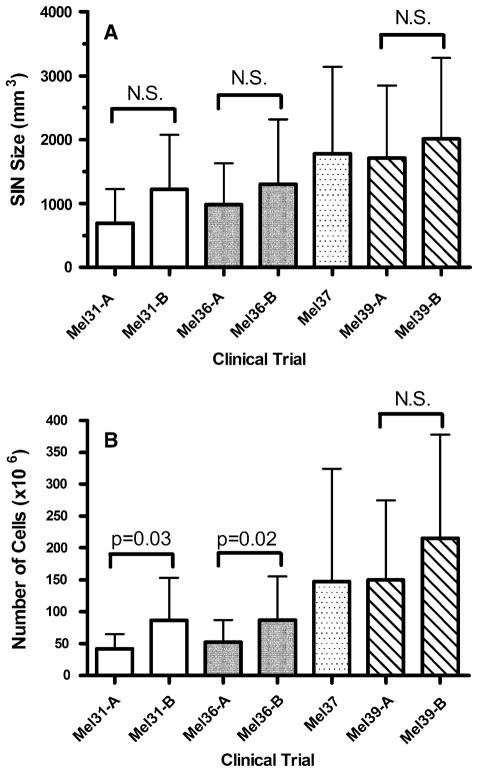
Lymph Node Volume and Cell Yield
The SIN obtained from Mel31, Mel36, Mel37, and Mel39 were analyzed for both cell size and number of cells obtained (Fig. 5). Although there is an apparent trend towards the SIN obtained on arm B of each of the two-armed trials being larger than the SIN obtained on arm A, this trend does not reach statistical significance. This same relationship is observed for cell number, although for this parameter arm B of Mel31 and Mel36 is significantly different that arm A. For both Mel31 and Mel36 the increased cell number in arm B is associated with these same arms having a significantly higher fraction of patients with a detectable T-cell response than is observed in arm A (Fig. 3).
FIG. 5.
SIN size and cell yield by clinical trial study arm. Mel31-A and Mel31-B are two arms of the same study, while Mel36-A and Mel36-B are two arms of a second study and Mel39-A and Mel39-B are two of a third study. The Mel37 trial did not have separate arms. (A) The mean calculated volume of the SIN±1 standard deviation (SD). (B) The mean number of cells obtained from each SIN±1 SD for patients on each arm of the trials are plotted as open bars. The Mann–Whitney test was used to determine statistical significance. N.S., not significant.
DISCUSSION
Optimization of cancer vaccines will be aided by comprehensive immune monitoring in multiple lymphoid compartments. Critical compartments for immune monitoring include: (a) the lymph node as the site of T-cell response induction, (b) the circulating PBL, and (c) the sites of primary and metastatic tumor. Cancer vaccine clinical trials meant to induce T-cell-mediated immunity are evaluated routinely by measuring the immune response in the PBL but not the other two compartments. The present report summarizes our experience with harvesting SINs as a means of immune monitoring.
The technique of lymphoscintigraphy for lymphatic mapping is well described and has been used for decades. It has been adapted for identification and harvest of sentinel nodes for staging primary melanomas, which is now standard practice.31,32 We have used the same techniques for identification and harvest of a sentinel node draining a site of cutaneous vaccination. The procedure can be even less morbid than a staging sentinel node biopsy because only one node is needed. On average, 111 million lymphocytes are obtained from a single SIN, an amount which is adequate for several assays of immune function.
SIN harvest is well tolerated as a minor procedure with straight local anesthesia. Thus, this procedure can be employed by any surgeon experienced in sentinel node biopsy techniques, and is limited only by local IRB approval, informed patient consent, and logistic and cost issues. We were able to perform the procedure without an IV, and without an anesthesiologist in a minor treatment room, in our clinic which was a minimal cost. The technetium-labeled sulfur colloid injection is standard in most hospitals. The main cost of lymphoscintigraphy is the cost of the lymphoscintigram and its interpretation by a nuclear medicine radiologist. The procedure can be done without a lymphoscintigram, but inclusion of a radiologist as a co-investigator in these studies facilitated the use of the technique. We avoided blue dye for these studies because the SIN was easily localized, almost always, just below the subcutaneous fascia. Blue dye in this setting would leave blue-stained skin in some patients and may be associated with anaphylaxis in some patients.33,34
The timing chosen for harvest of the SIN was 1 week (6–9 days) after the third vaccination. The vast majority of patients were vaccinated on a Wednesday, and the nodes were harvested on a Tuesday or a Thursday the following week. Murine and human studies suggest that, after a single immunization, epidermal Langerhans cells migrate and mature to draining nodes within hours, peaking at 2–3 days, with peak T-cell accumulation in the draining nodes at day 5–10.35–37 Here we harvested a SIN 1 week after the third vaccine in an effort to identify the peak time of T-cell accumulation. It is possible, however, that the peak may be slightly earlier or later, or may be at a steady state after several vaccines. Because the T-cell response is readily detectable in most patients when evaluating the SIN lymphocytes, we have continued to use this approach, but further investigation may be warranted to optimize the timing of SIN harvest.
We hypothesized that lymph nodes draining sites of cutaneous vaccination can be identified by sentinel node biopsy techniques, and the data from over 100 patients support this hypothesis. Furthermore, we hypothesized that evaluation of T-cell responses in lymph nodes draining a vaccine site is a more sensitive measure of immunogenicity than evaluation limited to the peripheral blood. As shown in Fig. 3, T-cell responses to defined peptides were detected 25% more often in the SIN than in the PBL, and combined evaluation of the PBL and SIN increases sensitivity of detecting immunogenicity to nearly 75%.
A fundamental assumption behind harvest of the SIN is that this lymph node is the site of the primary cellular immune response to cutaneous vaccination. There are ample studies in murine and human systems that support this assumption.35–37 The data further support this assumption by demonstrating that the immune responses detected to peptide vaccines are detectable only in the SIN and not in a random lymph node (Fig. 4). We have also shown that T-cell responses in a SIN were detected at a high level, when they were either absent or at low level in both blood and a tumor involved node prior to vaccination. 8 The data presented herein support continued evaluation of the SIN for monitoring T-cell responses to vaccination. Our own ongoing studies with SIN include the evaluation of T-cell phenotype and function, as well as the functional characterization of dendritic cells.
It is of interest that some patients have T-cell responses in the SIN that are not detectable in the PBL, even at multiple time points.8,9 This may be due to depletion of antigen-reactive T-cells from the peripheral blood by trafficking to tumor, by activation-induced cell death, or by other immunosuppressive functions of the tumor or the host. Having found that T-cell responses are detectable in the majority of patients to a 4- or 12-peptide vaccine preparation used in these studies, future work should attempt to dissect out further the mechanisms for interference with establishment and maintenance of systemic immunity to cancer antigens. This will require evaluation of T-cells in both SIN and blood, as well as those infiltrating tumor deposits. The process of optimizing vaccines likely will benefit from the immunologic information that may be obtained by evaluation of T-cell responses and dendritic cell activity in the SIN.
In summary, we conclude that: (1) the SIN is a primary site of cellular immune responses to cutaneous vaccination; (2) T-cell responses to tumor vaccines are more readily detectable in the SIN than in the peripheral blood; (3) cells from the SIN can be evaluated by flow cytometry or by immunohistochemically for expression of dendritic cell or T-cell activation markers; (4) evaluation of T-cell dissemination from the SIN to the periphery may aid in understanding failures or successes of tumor vaccines; (5) SIN may be useful in characterizing immune responses to cancer vaccines, and in optimizing them; and (6) surgeons should take advantage of their unique access to this technique for immune monitoring of cancer vaccine trials.
Acknowledgments
This study was funded by NIH R01 CA57653 and CA78519 to CLS. The work was supported also by Schering–Plough Research Institute, Argonex, Inc., Chiron Corporation, the Human Immune Therapy Center at the University of Virginia (funded by the Cancer Research Institute), the Cancer Center Support Grant (NIH P30CA44579) at the University of Virginia (Tissue Procurement Facility, FACS Core, Biomolecular Core), the Pratt Fund at the University of Virginia, and the General Clinical Research Center at the University of Virginia (M01 RR00847). We acknowledge the assistance of Dr. Kyo U. Chu for performing one of the SIN biopsies; of Mark Ruffa, Michael Hanshew, and Melanie Mayer for their assistance in tissue procurement; of Robyn Fink, Priscilla Merrill, and Carmel Nail in patient care and accrual on these trials; and of Kelly Clore for assistance in manuscript preparation.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 3.Slingluff CL, Chianese-Bullock KA, Bullock TNJ, et al. Immunity to melanoma antigens: from self-tolerance to immunotherapy. Adv Immunol. 2006;90:243–95. doi: 10.1016/S0065-2776(06)90007-8. [DOI] [PubMed] [Google Scholar]
- 4.Marchand M, van Baren N, Weynants P, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–30. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Coulie PG, Karanikas V, Colau D, et al. A monoclonal cytolytic T-lymphocyte response observed in a melanoma patient vaccinated with a tumor-specific antigenic peptide encoded by gene MAGE-3. Proc Natl Acad Sci USA. 2001;98:10290–5. doi: 10.1073/pnas.161260098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163:1690–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Yamshchikov GV, Barnd DL, Eastham S, et al. Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. Int J Cancer. 2001;92:703–11. doi: 10.1002/1097-0215(20010601)92:5<703::aid-ijc1250>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, et al. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–26. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, et al. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol. 2004;22:4474–85. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 11.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–95. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 12.Storkus WJ, Salter RD, Cresswell P, et al. Peptide-induced modulation of target cell sensitivity to natural killing. J Immunol. 1992;149:1185–90. [PubMed] [Google Scholar]
- 13.Anderson KS, Alexander J, Wei M, et al. Intracellular transport of class I MHC molecules in antigen processing mutant cell lines. J Immunol. 1993;151:3407–19. [PubMed] [Google Scholar]
- 14.Kittlesen DJ, Thompson LW, Gulden PH, et al. Human melanoma patients recognize an HLA-A1-restricted CTL epitope from tyrosinase containing two cysteine residues: implications for tumor vaccine development. J Immunol. 1998;160:2099–106. [PubMed] [Google Scholar]
- 15.Skipper JC, Hendrickson RC, Gulden PH, et al. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527–34. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox AL, Skipper J, Chen Y, et al. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–9. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 17.Skipper JC, Kittlesen DJ, Hendrickson RC, et al. Shared epitopes for HLA-A3-restricted melanoma-reactive human CTL include a naturally processed epitope from Pmel-17/gp100. J Immunol. 1996;157:5027–33. [PubMed] [Google Scholar]
- 18.Slingluff CL, Jr, Yamshchikov G, Neese P, et al. Phase I trial of a melanoma vaccine with gp100(280–288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–24. [PubMed] [Google Scholar]
- 19.Kawakami Y, Robbins PF, Wang X, et al. Identification of new melanoma epitopes on melanosomal proteins recognized by tumor infiltrating T lymphocytes restricted by HLA-A1, -A2, and -A3 alleles. J Immunol. 1998;161:6985–92. [PubMed] [Google Scholar]
- 20.Traversari C, van der Bruggen P, Luescher IF, et al. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992;176:1453–7. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaugler B, Van den Eynde B, van der Bruggen P, et al. Human geneMAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–30. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkhurst MR, Salgaller ML, Southwood S, et al. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–48. [PubMed] [Google Scholar]
- 23.Huang LQ, Brasseur F, Serrano A, et al. Cytolytic T lymphocytes recognize an antigen encoded by MAGE-A10 on a human melanoma. J Immunol. 1999;162:6849–54. [PubMed] [Google Scholar]
- 24.Chaux P, Luiten R, Demotte N, et al. Identification of five MAGE-A1 epitopes recognized by cytolytic T lymphocytes obtained by in vitro stimulation with dendritic cells transduced with MAGE-A1. J Immunol. 1999;163:2928–36. [PubMed] [Google Scholar]
- 25.Wang RF, Johnston SL, Southwood S, et al. Recognition of an antigenic peptide derived from tyrosinase-related protein-2 by CTL in the context of HLA-A31 and -A33. J Immunol. 1998;160:890–7. [PubMed] [Google Scholar]
- 26.Renkvist N, Castelli C, Robbins PF, et al. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum-Tirouvanziam U, Servis C, Habluetzel A, et al. Localization of HLA-A2.1-restricted T cell epitopes in the circumsporozoite protein of Plasmodium falciparum. J Immunol. 1995;154:3922–31. [PubMed] [Google Scholar]
- 28.Johnson RP, Trocha A, Yang L, et al. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–21. [PubMed] [Google Scholar]
- 29.Goulder PJ, Sewell AK, Lalloo DG, et al. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)-identical siblings with HLA-A*0201 are influenced by epitope mutation. J Exp Med. 1997;185:1423–33. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brander C, Hartman KE, Trocha AK, et al. Lack of strong immune selection pressure by the immunodominant, HLA-A* 0201-restricted cytotoxic T lymphocyte response in chronic human immunodefficiency virus-1 infection. J Clin Invest. 1998;101:2559–66. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 32.Sondak VK, Zager JS, Messina JL, et al. Sentinel lymph node biopsy as the standard of care for cutaneous melanoma. Clin Adv Hematol Oncol. 2007;5:483–90. [PubMed] [Google Scholar]
- 33.Cimmino VM, Brown AC, Szocik JF, et al. Allergic reactions to isosulfan blue during sentinel node biopsy–a common event. Surgery. 2001;130:439–42. doi: 10.1067/msy.2001.116407. [DOI] [PubMed] [Google Scholar]
- 34.Leong SP, Donegan E, Heffernon W, et al. Adverse reactions to isosulfan blue during selective sentinel lymph node dissection in melanoma. Ann Surg Oncol. 2000;7:361–6. doi: 10.1007/s10434-000-0361-x. [DOI] [PubMed] [Google Scholar]
- 35.Macatonia SE, Knight SC, Edwards AJ, et al. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–67. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosato A, Zambon A, Macino B, et al. Anti-L-selectin monoclonal antibody treatment in mice enhances tumor growth by preventing CTL sensitization in peripheral lymph nodes draining the tumor area. Int J Cancer. 1996;65:847–51. doi: 10.1002/(SICI)1097-0215(19960315)65:6<847::AID-IJC23>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Yoshizawa H, Chang AE, Shu S. Specific adoptive immunotherapy mediated by tumor-draining lymph node cells sequentially activated with anti-CD3 and IL-2. J Immunol. 1991;147:729–37. [PubMed] [Google Scholar]