Abstract
Objective: Angiogenic therapy is emerging as a potential strategy for the treatment of ischemic heart disease but is limited by a relatively short half-life of growth factors. Fibrin glue (FG) provides a reservoir for controlled-release of growth factors. The aim of this study was to evaluate the effects of basic fibroblast growth factor (bFGF) incorporating FG on angiogenesis and cardiac performance in a canine infarct model. Methods: Acute myocardial infarction was induced by ligation of the left anterior descending coronary artery (LAD). Group I (n=6) underwent ligation of LAD alone. In Group II, transmural channels were created in the infarct area (n=6). In Group III, non-transmural channels were created to locate FG cylinders containing bFGF (n=6). Eight weeks after operation, myocardial perfusion was assessed by single photon emission computed tomography, cardiac function by echocardiography, and vascular development by immunohistochemical staining. Results: Total vascular density and the number of large vessels (internal diameter ≥50 μm) were dramatically higher in Group III than in Groups I and II at eight weeks. Only the controlled-release group exhibited an improvement in regional myocardial perfusion associated with lower defect score. Animals in Group III presented improved cardiac regional systolic and diastolic functions as well as global systolic function in comparison with the other two groups. Conclusions: Enhanced and sustained angiogenic response can be achieved by controlled-release bFGF incorporating FG within transmyocardial laser channels, thus enabling improvement in myocardial perfusion and cardiac function.
Keywords: Angiogenesis, Basic fibroblast growth factor, Controlled release, Ischemic heart disease
1. Introduction
Therapeutic angiogenesis has emerged as a promising strategy for the treatment of patients with end-stage coronary artery disease who are not suitable candidates for surgical or percutaneous revascularization (Henry et al., 2003; 2007; Kastrup et al., 2005; Stewart et al., 2009). Basic fibroblast growth factor (bFGF), a potent mitogen, has the ability to induce endothelial and smooth muscle cell proliferation in vitro and elicit in vivo angiogenesis that includes the migration and proliferation of endothelial cells, vascular tube formation, and linkage to the preexisting vascular network (Beenken and Mohammadi, 2009). Considerable enthusiasm has arisen from numerous preclinical studies demonstrating improvements in perfusion, function, and vascularity utilizing bFGF-related protein therapy in a variety of animal models (Harada et al., 1994; Kawasuji et al., 2000). However, the translation of this experience into clinical practice has been disappointing for a few recurring reasons (Laham et al., 2000; Simons et al., 2002). One of the proposed problems is insufficient delivery of growth factors due to a short half-life in the body (Post et al., 2001).
Fibrin glue (FG), a natural component of the extracellular matrix, has been widely used as a tissue sealant in surgery (Currie et al., 2001; Karacal et al., 2007). Since fibrin sealant is lysed slowly, it can serve as a vehicle to slowly release various agents (Fasol et al., 1994; Kang et al., 1995). It is hypothesized that bFGF incorporating FG can be released at a steady rate and generate an effective concentration over a prolonged period of time, eliciting a controlled and sustained angiogenic response. The administration of FG containing bFGF directly into transmyocardial laser channels may have a synergistic angiogenic effect (Heilmann et al., 2003). In the present study, we used an FG carrier to evaluate whether the controlled-release bFGF in transmyocardial channels would further stimulate neovascularization and hereby resume cardiac perfusion and function in a canine infarct model.
2. Materials and methods
2.1. Preparation of bFGF incorporating FG
Human recombinant bFGF was supplied by Shuanghe Pharmaceutical Co., Ltd., Beijing, China, and the FG kit was obtained from Shanghai Xinxing Blood Product Research Institute, China. The FG used in this study is a two-component system that remains liquid after mixing for several seconds before solidifying into a solid gel matrix. Briefly, lyophilized human fibrinogen was dissolved in sterile 0.9% (w/v) sodium chloride at final concentration of 40 mg/ml (Solution I). Lyophilized human thrombin was further diluted in 40 mmol/L calcium chloride to obtain final concentration of 500 U/ml (Solution II). For bFGF incorporating fibrin glue, 35 μg of bFGF was added to 0.5 ml of Solution II at a final concentration of 70 μg/ml (bFGF-thrombin, Solution III). The 0.5 ml of Solutions I and III were prepared for the following procedure.
2.2. Animal model and grouping
All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research of China and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences of China (National Institutes of Health Publication 85-23, revised 1985). A total of 18 adult male mongrel dogs weighing 15 to 20 kg (provided by Weitonglihua Experimental Animal Center, Beijing, China) were studied. They were fed a normal diet and housed individually under controlled environmental conditions (20–25 °C room temperature, 55%–65% relative humidity). The animals were anesthetized with intravenous administration of thiopental sodium (15 mg/kg) and then maintained with ketamine, incubated and mechanically ventilated (Newport NMI Wave E200). A small left thoracotomy through the 5th intercostal space was performed to expose the heart using a sterile technique. The left anterior descending coronary artery (LAD), distal to its first diagonal branch, was isolated and partially ligated with a 3.0 polypropylene suture for ischemic preconditioning for 20 min, with changes in pressure and electrocardiography (ECG) observed and lidocaine (1 mg/kg) injected intravenously when necessary. It was then completely ligated to establish an acute myocardial infarction model.
Thirty minutes after ligation, the dogs were randomized into three groups. Group I (n=6) served as blank control and had acute myocardial infarction alone. In Group II (n=6), transmural channels were made in the infarct area at the density of 1 channel/cm2; an average of 11 (range 10–12) channels were made in each heart. Potassium-titanyl-phosphate (KTP) double-frequency Nd:YAG (neodymium-doped yttrium aluminium garnet; Nd:Y3Al5O12) laser (Laser Technology and Engineering Research Institute, Huazhong University of Science and Technology, Wuhan, China) was used. In Group III (n=6), non-transmural channels (10–12 channels per heart) were created in the infarct territory. A total of 0.5 ml of Solutions I and III were delivered through a double pump applicator, which holds the two components in separate syringes and provides simultaneous mixing and delivery. Notably, previous studies showed no significant angiogenic response in animals treated with FG, suggesting that the angiogenic effects result entirely from the component contained in FG (Fasol et al., 1994). After hemodynamic stability the pericardium and chest were closed, and the dogs were allowed to recover.
2.3. Myocardial perfusion imaging
Single photon emission computed tomography (SPECT) imaging with technetium-99m methoxyisobutylisonitrile (99mTc-MIBI) was conducted eight weeks after the surgery. Dogs were anesthetized with intravenous 3% (w/v) sodium pentobarbital (20–30 mg/kg) and were placed in the dorsal position in an SPECT scanner (Millennium VG, GE, Wisconsin, USA). SPECT images were acquired at 15 min after intravenous injection of 99mTc-MIBI [(244.2±37.0) MBq]. Briefly, projection data were acquired over 180° (right anterior oblique 45° to left posterior oblique 45°) using a 64×64 matrix in a 20% energy window (centered on the 140-keV X-ray photopeak), 30 s per 6° frame (totally 30 frames). All SPECT images were batch-reconstructed using standard filtered back-projection (order 4, cutoff 0.28×Nyquist frequency) without attenuation or scatter correction.
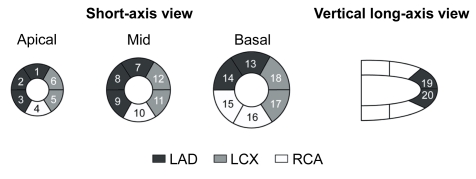
For each dog, the myocardial perfusion SPECT images were visually scored using a Cedars-Sinai 20-segment (CS-20) model (Fig. 1) and a 0–4 scale (0=normal uptake, 1=mildly reduced uptake, 2=moderately reduced uptake, 3=severely reduced uptake, and 4=no uptake), as previously described (Germano et al., 2000). Two independent observers, who were blinded for related information, interpreted the images, and each segment was scored by consensus. The defect score was calculated as the sum of the perfusion defect. For each animal, a summed defect score in CS-20 and LAD territory (1, 2, 3, 7, 8, 9, 13, 14, 19, 20) and the number of segments (defect score >1) in CS-20 were calculated (Fig. 1).
Fig. 1.
Cedars-Sinai 20-segment model used for semiquantitative visual scoring of perfusion (Germano et al., 2000)
LAD: left anterior descending coronary artery; LCX: left circumflex coronary artery; RCA: right coronary artery
2.4. Echocardiographic assessment of cardiac function
Left ventricular (LV) function was evaluated by transthoracic echocardiography using a Sequoia 256 system (ACUSON, California, USA) with a 3.0–5.5 MHz transducer. The following parameters were derived from the M-mode tracing: thickness of interventricular septum (IVS) and left ventricular anterior wall (LVAW) during systole and diastole to analyze regional wall thickening (ΔT), and motion amplitude (MA) and fractional shortening (FS) of LVAW. The variables measured on two-dimensional (2D) echocardiography were the left ventricular end-systolic dimension (LVESD) and the left ventricular end-diastolic dimension (LVEDD). LV ejection fraction (EF), stroke volume (SV), cardiac output (CO), and fractional area contraction (FAC) were calculated using the Simpson biplane method. Regional wall movement score index (WMSI) was calculated according to the guidelines of the American Society of Echocardiography (Bourdillon et al., 1989). Based on a standard 16-segment model, wall motion was graded as follows: 1=normal; 2=hypokinetic (reduced systolic wall thickening); 3=akinetic (absent systolic wall thickening); or 4=dyskinetic (outward systolic wall motion). The systolic peak velocity (s) and the early-to-late diastolic peak velocity ratio (e/a) of the mitral valve (MV) and LVAW were determined by pulsed Doppler and pulsed Doppler tissue imaging (DTI). Echocardiograms were interpreted in a blinded manner by a cardiologist with expertise in echocardiography.
2.5. Histological study
After the SPECT and echocardiograph were performed, the dogs were euthanized and the hearts were excised. For dogs in Groups II and III, three infarct portions were cut off and fixed in 10% (v/v) buffered formalin. The transmural sections (3-μm) of the LV were embedded in paraffin stained with hematoxylin-eosin (H&E). Blood vessels were highlighted by staining endothelial cells for factor VIII-related antigen (Santa Cruz, California, USA) by applying the streptavidin-biotin peroxidase complex (SABC) method. Angiogenesis was assessed by counting the number of microvessels, including capillaries (diameter <20 mm) and arterioles (diameter ≥20 μm and <100 μm with tunica media), which were defined as round or elliptical structures with a clearly-defined lumen lined by cells staining positively to factor VIII. Three randomly selected high power fields (hpfs) from each portion (400×) were examined by two pathologists who were blinded to the grouping. The average number of the vessels in one portion was used for the assessment of vascular density. The same fields were selected to calculate microvessels with diameter ≥50 μm using an HPIAS-2000 image analysis system (Qianping Company of Imaging Technology of Tongji Medical College, Wuhan, China). Finally, the area of positive expression and average absorbance of factor VIII were analyzed at 200× field with the HPIAS-2000 image analysis system. Three fields were randomly selected from each portion for quantitative analysis.
2.6. Statistical analysis
SPSS 15.0 (SPSS Science, Chicago, IL, USA) for Windows was used for statistical analysis and all data were presented as mean±standard deviation (SD). One-way analysis of variance (ANOVA) was used followed by Student-Newman-Keuls test for multiple comparisons. Values of P<0.05 were considered significant.
3. Results
Two (one in Group I; one in Group II) of 18 animals died on 22 and 34 d after surgery, respectively. There were no deaths related to transmyocardial laser penetration and/or administration of bFGF incorporating FG. All other animals survived until euthanasia without postoperative complications and were used in the analysis (Group I, n=5; Group II, n=5; Group III, n=6).
3.1. Myocardial perfusion
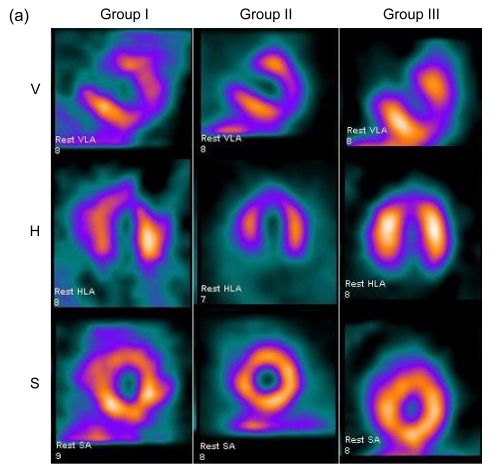
SPECT images were divided into three different axes: short-axis images, vertical-axis images, and horizontal-axis images (Fig. 2a). Eight weeks after surgery, the control group showed a characteristic reduced perfusion corresponding to the occluded distal LAD: radioactivity of LVAW displayed degraded distribution. There was no apparent improvement of perfusion in Group II. Images of Group III typically demonstrated improved myocardial blood perfusion in three axes.
Fig. 2.
Myocardial perfusion analysis
(a) Representative SPECT images of myocardial perfusion in three different groups. Group I demonstrated persistence of the ligation-induced LVAW perfusion defect. Group II exhibited slight improvement of blood perfusion. Group III exhibited large improvement of blood perfusion (V: vertical long axis; H: horizontal long axis; S: short axis); (b) Defect scores in 20 segments and LAD territory; (c) The number of segments with defect score >1. Values are expressed as mean±SD. * P<0.05 vs. Group I; # P<0.05 vs. Group II
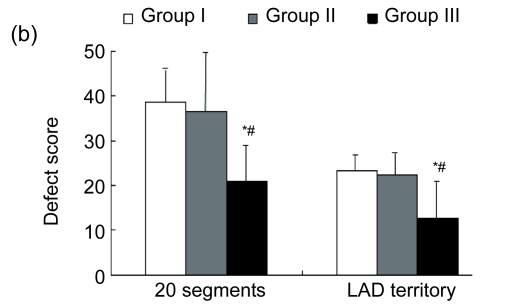
There were no significant differences in the baseline defect score among the three groups (P>0.05). Regional defect scores were different after eight weeks. Group III demonstrated significantly lower total regional defect score in CS-20 and LAD territory compared with Groups I (P<0.05) and II (P<0.05) (Fig. 2b). In addition, there were less segments with a defect score >1 in Group III than in Groups I (P<0.05) and II (P<0.05) (Fig. 2c).
3.2. Cardiac function
Echocardiographic assessment of cardiac function demonstrated significant benefits in the controlled-release group vs. Groups I and II (Table 1). Eight weeks after the operation, the thickness of IVS during systole was significantly greater in Group III than in Groups I and II (P<0.05). ΔT was higher in Group III compared to Group I (P<0.05). Similarly, a much greater thickness of LVAW during diastole and systole was seen in the controlled-release group (P<0.05 in comparison with Groups I and II). Moreover, Group III exhibited enhanced MA and higher FS than the other two groups. All of the above data showed no significant difference between Groups I and II. Although no significant differences were found in LVEDD (P=0.997) and LVESD (P=0.127) among groups, EF, SV, CO, FAC, and WMSI as indexes of global systolic function revealed marked improvement in Group III. Compared to Groups I and II, SV and CO were higher and WMSI was lower in Group III (P<0.05). Importantly, DTI showed that in the sustained-release group, the e/a of LVAW was higher than that in Groups I and II (P<0.05). However, no significant differences were seen in e/a of MV in the three groups (P=0.301).
Table 1.
Echocardiographic evaluation of cardiac regional and global functions
| Parameter | Group I (n=5) | Group II (n=5) | Group III (n=6) | P value |
| IVS (M-mode) | ||||
| Thickness at diastole (cm) | 0.37±0.05 | 0.39±0.05 | 0.47±0.10 | 0.078 |
| Thickness at systole (cm) | 0.52±0.07 | 0.58±0.06 | 0.75±0.19*# | 0.028 |
| ΔT (%) | 41.88±3.14 | 49.10±5.53 | 56.93±7.76* | 0.005 |
| LVAW (M-mode) | ||||
| Thickness at diastole (cm) | 0.36±0.04 | 0.38±0.07 | 0.51±0.09*# | 0.009 |
| Thickness at systole (cm) | 0.51±0.08 | 0.56±0.07 | 0.81±0.16*# | 0.002 |
| ΔT (%) | 40.99±6.04 | 51.08±11.9 | 57.70±10.09 | 0.053 |
| MA (cm/s) | 0.26±0.08 | 0.39±0.05 | 0.58±0.17*# | 0.002 |
| FS (%) | 23.84±4.54 | 33.42±5.49 | 46.54±10.42*# | 0.001 |
| LVEDD (cm) | 3.27±0.07 | 3.26±0.12 | 3.26±0.10 | 0.997 |
| LVESD (cm) | 2.25±0.09 | 2.21±0.06 | 2.13±0.12 | 0.127 |
| EF (%) | 47.60±7.54 | 55.18±1.96* | 60.68±4.76* | 0.007 |
| SV (ml) | 11.98±1.71 | 13.12±0.64 | 16.16±1.65*# | 0.002 |
| CO (L/min) | 1.48±0.14 | 1.59±0.10 | 1.86±0.11*# | 0.001 |
| FAC (%) | 32.12±4.29 | 37.38±4.83 | 43.76±8.85* | 0.040 |
| WMSI | 22.20±1.79 | 20.60±0.55* | 17.00±0.71*# | <0.001 |
| LVAW (DTI) | ||||
| s (cm/s) | 0.86±0.14 | 1.18±0.24* | 1.36±0.24* | 0.010 |
| e (cm/s) | 0.74±0.15 | 1.04±0.25 | 0.96±0.20 | 0.095 |
| a (cm/s) | 0.83±0.19 | 0.91±0.11 | 0.60±0.11*# | 0.016 |
| e/a | 0.90±0.13 | 1.17±0.35 | 1.58±0.26*# | 0.006 |
| MA (cm/s) | 0.22±0.03 | 0.38±0.05* | 0.54±0.09*# | <0.001 |
| MV (DTI) | ||||
| e (cm/s) | 0.76±0.16 | 0.70±0.14 | 0.72±0.14 | 0.809 |
| a (cm/s) | 0.67±0.12 | 0.58±0.13 | 0.47±0.06* | 0.034 |
| e/a | 1.20±0.44 | 1.26±0.37 | 1.58±0.38 | 0.301 |
| HR (bpm) | 122±5 | 121±4 | 118±5 | 0.463 |
Values are expressed as mean±SD
P<0.05 vs. Group I
P<0.05 vs. Group II
IVS: interventricular septum; ΔT: regional thickening; LVAW: left ventricular anterior wall; MA: motion amplitude; FS: fractional shortening; LVEDD: left ventricular end-diastolic dimension; LVESD: left ventricular end-systolic dimension; EF: ejection fraction; SV: stroke volume; CO: cardiac output; FAC: fractional area contraction; WMSI: regional wall movement score index; DTI: Doppler tissue imaging; s: systolic peak velocity; e: early diastolic peak velocity; a: late diastolic peak velocity; e/a: early-to-late diastolic peak velocity ratio; MV: mitral valve; HR: heart rate; bpm: beat per minute
3.3. Vascular density
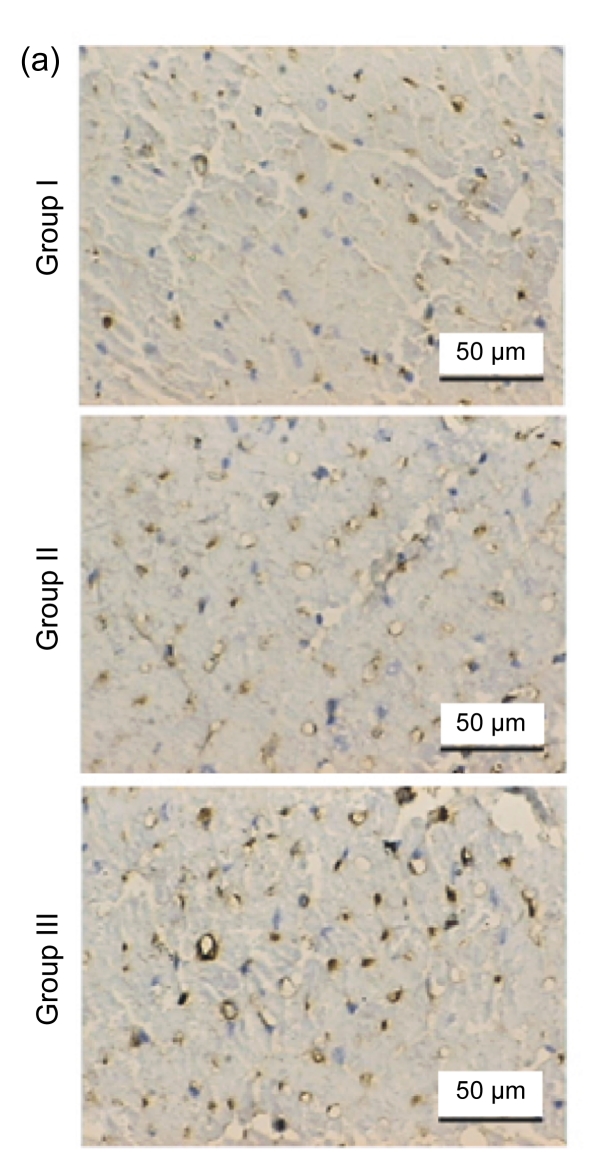
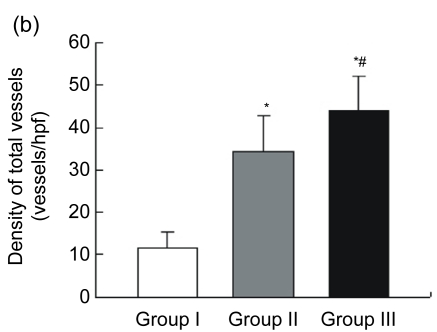
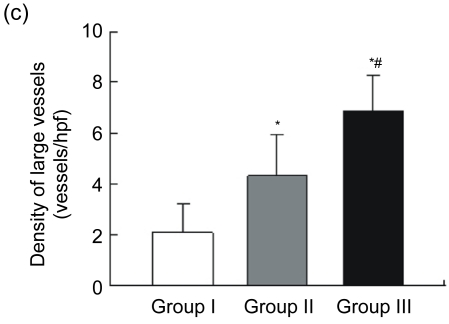
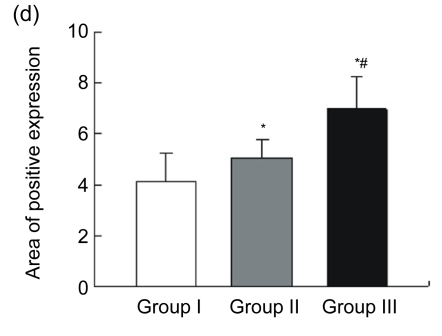
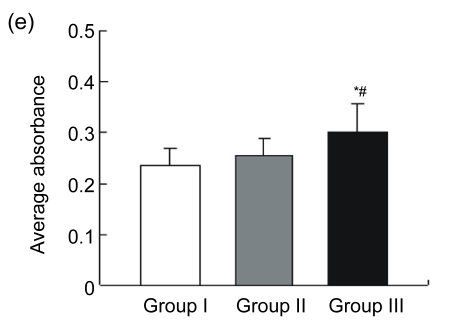
Blood vessels were highlighted by immunostaining for factor VIII (Fig. 3a). The endothelial cell dyed brown, single or clustered, was counted as one vessel. Total vascular density was dramatically higher in Group III [n=18, (44.00±8.01) vessels/hpf] compared to Group I [n=15, (11.47±3.89) vessels/hpf, P<0.05] and Group II [n=15, (34.27±8.51) vessels/hpf, P<0.05] (Fig. 3b). A pathological image analysis system revealed that the density of large vessels, whose internal diameter (measured in the shortest direction) was at least 50 μm in the infarct area, was also higher in the controlled-release group [(6.89±1.41) vessels/hpf] than both Groups I [(2.07±1.16) vessels/hpf, P<0.05] and II [(4.33±1.63) vessels/hpf, P<0.05] (Fig. 3c). Quantitative analysis showed that the area of positive expression as well as average absorbance of factor VIII was greatly higher in Group III (Figs. 3d and 3e).
Fig. 3.
Immunohistochemical staining and quantitative analysis eight weeks after surgery
(a) Representative images of myocardial sections depicting factor VIII-related antigen (brown). The microvessels are more numerous and larger in Group III than in Groups I and II; (b) The density of total vessels per high power field (hpf) in the infarct area; (c) The density of large vessels (internal diameter ≥50 μm) per hpf in the infarct area; (d) The area of positive expression in the three groups; (e) The average absorbance among three groups. * P<0.05 vs. Group I; # P<0.05 vs. Group II
4. Discussion
The present study demonstrates that the administration of bFGF incorporating FG directly into transmyocardial laser channels within an infarcted myocardium, results in greatly increased microvessels and significant improvement in regional myocardial perfusion. Importantly, this improvement is associated with enhanced cardiac regional and global function. Hence, these data indicate that controlled-release bFGF promote more efficient neovascularization.
The mechanical-stimulation and pharmacologic-stimulation of additional angiogenesis, in response to myocardial ischemia, have been tried for the treatment of patients with otherwise intractable angina pectoris. Despite favorable results with angiogenic growth factors such as bFGF, the optimal delivery of angiogenic growth factors remains problematic. Systemic or intracoronary delivery, while preferable owing to ease of use and clinical applicability, does not achieve adequate concentrations in the myocardium to maximize efficacy (Laham et al., 1999). Furthermore, such delivery methods for therapeutic angiogenesis have been incriminated in the proatherogenic effects, which may lead to plaque expansion or instability (Kornowski et al., 2000a). Delivery of bFGF via direct myocardial injection can assist in the avoidance of high levels of circulating angiogenic activity but is confined to single administration and angioma. Recently, FG has been examined as a drug or growth-factor delivery vehicle, acting as an important material for tissue engineering. Particularly, fibrin plays an active role through specific receptor-mediated interactions with cells of the blood and vessel walls. These result in fibrin-specific responses of endothelial cells including adhesion and spreading, migration, and angiogenesis, which may be interrelated with the potent stimulation of the same responses by bFGF (Sahni et al., 2006). This concept is supported by in vitro studies demonstrating that fibrin clots are an excellent matrix for bFGF-stimulated angiogenesis (DeBlois et al., 1994; Brown et al., 1996). The bFGF binds specifically and with high affinity to fibrinogen prior to the polymerization of the fibrin (Sahni et al., 1998). The rate of bFGF release from FG can be controlled according to concentrations of fibrinogen and thrombin (Jeon et al., 2005). Chawla et al. (1999) employed an angiogenic strategy that utilizes enhanced vascular endothelial growth factor (VEGF) in an FG in two patients with critical limb ischemia. The results angiographically demonstrated the growth of new blood vessels after administration of the VEGF and FG composite. The FG aids in the slow release of the growth factor and thus prolongs its availability, sustaining angiogenesis. On the basis of the above findings, we speculated that the controlled-release bFGF using FG might induce an enhanced angiogenic response and thus promote cardiac perfusion and function.
Sprouting angiogenesis is the best-understood mechanism of new blood vessel growth and involves the formation of new capillaries in response to ischemia or other stimuli. Arteriogenesis involves the widening of existing arteries and formation of collateral vessels and is capable of restoring a large volume of blood flow to a distal ischemic tissue (Phelps and Garcia, 2009). In a previous study, Yamamoto et al. (2000) injected bFGF into transmyocardial channels in a pig model of chronic myocardial ischemia. The results showed that bFGF increased total vascular density by almost 40% over that observed in the control group. However, the number of large vessels (internal diameter ≥50 μm) was doubled by the addition of bFGF. These data suggested that bFGF further enhances angiogenesis in ischemic myocardium mainly by increasing the size (collateral remodeling) but not the total number of vessels (Yamamoto et al., 2000). In contrast, our histological investigation showed that total vascular density in the controlled-release group increased 28% over that in Group II, and this was associated with a 59% increase in the number of large vessels (internal diameter ≥50 μm). This indicates that, except for angiogenesis, FG containing bFGF may induce arteriogenesis. Intramyocardial controlled-release bFGF seems to be more effective in producing a apparent and stable vasculature.
Previous clinical trial using percutaneous direct myocardial revascularization in patients with chronic refractory myocardial ischemia showed no significant improvement from nuclear perfusion imaging (Kornowski et al., 2000b). Another animal study found improvements in myocardial perfusion and regional and global contractile reserve six months after transmyocardial laser revascularization (TMR) in a porcine model of hibernating myocardium (Hughes et al., 1999). Our quantitative analysis using SPECT demonstrated a significantly increased perfusion in all segments as well as LAD territory eight weeks after the operation, compared with reversed changes happened in the control group. Notably, whereas the number of microvessels increased in both Groups II and III, better perfusion was rendered only by Group III. As mentioned above, slow-release bFGF further stimulated arteriogenesis, which has the ability to increase the lumen by growth. On the other hand, in addition to its mitogenic activity, bFGF has the ability to protect the myocardium acutely from tissue loss and dysfunction (House et al., 2003; Jiang et al., 2004). Accordingly, controlled-release bFGF enabled itself to work over a long period in situ, which induced rich angiogenesis and good collateral formation, while increasing regional blood flow around bFGF-injected sites.
It is expected that enhanced angiogenesis and improved perfusion will preserve LV function, because LV functional loss may be attributable to insufficient blood flow that accompanies significant ischemic injury. In the present study, Group III exhibited significantly increased regional wall thickening and MA, which may be the result of improved local perfusion of hibernating myocardium adjacent to the infarct zone. However, no evident improvements were seen in Group II. This indicates LV regional systolic function benefits more from sustained-release bFGF in the transmyocardial laser channels. Choi et al. (2006) recently demonstrated that intramyocardial injection of pCK-VEGF165 improved wall thickening and wall motion, but no benefit of EF was seen. Despite inconspicuous changes in LVEDD and LVESD, eight weeks after bFGF administration in our study, cardiac global systolic function (as represented by EF, SV, CO, and WMSI) was strikingly improved. These results suggest that bFGF-impregnated FG had no significant effects on ventricular remodeling process, although myocardial perfusion and cardiac function were markedly improved. The negative results may be ascribed to a short follow-up period (Sutton and Sharpe, 2000).
There are few experimental and clinical trials regarding the effect of controlled-release bFGF on cardiac diastolic function. Furthermore, Iwakura et al. (2003) presented that Tau, the time constant of isovolumic-relaxation, as an index of global diastolic function, was significantly smaller after intramyocardial injection of bFGF-impregnated gelatin hydrogel. We herein evaluated cardiac diastolic function in detail using a DTI technique. The e/a of LVAW was markedly higher in Group III, but no significant differences were found with respect to e/a of MV, suggesting controlled-release bFGF enables the improvement of cardiac regional diastolic function. The global diastolic function was not restored, probably owing to incomplete improvement of myocardial ischemia after angiogenic therapy.
Several modalities of controlled-release system with various vehicles have been evaluated. Lopez et al. (1997) reported that the sustained release of bFGF with alginate microsphere results in a significant improvement in myocardial function in the presence of chronic myocardial ischemia. Since the alginate is a poorly biodegradable polysaccharide, it may be difficult to control the carrier degradation. In contrast, an accumulating body of evidence exists that slow-release bFGF incorporated into a biodegradable gelatin hydrogel can promote growth of microvessels and improve LV function (Iwakura et al., 2003; Shao et al., 2006). In consistence with these researchers, our study group investigated the effects of a new controlled-release system utilizing FG containing bFGF on cardiac performance, which achieved encouraging results.
However, there are still some limitations for our study. First, the acute myocardial infarction model in the present study does not represent chronic myocardial ischemia that is more often seen in the clinical setting. However, this method can be applied as an additional therapeutic regimen to those patients who are not suitable for percutaneous or surgical revascularization after acute infarction. Second, this study did not evaluate the use of FG without bFGF as an additional control group, although previous studies have demonstrated no extra angiogenic response with FG alone (Fasol et al., 1994). Third, we used just one administration regimen of bFGF. Therefore, further studies are warranted to clarify the dose-effect relationship and to subsequently obtain the optimal efficacy.
5. Conclusions
The present study showed that controlled-release bFGF incorporating FG in transmyocardial channels could augment angiogenesis, improve myocardial perfusion, and preserve cardiac regional and global function. The synergistic approach may maximize the benefit of therapeutic angiogenesis and provide a novel strategy for patients with ischemic heart disease.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81070166), and the Scientific Research Common Program of Beijing Municipal Commission of Education (No. KM201010025020), China
References
- 1.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourdillon PD, Broderick TM, Sawada SG, Armstrong WF, Ryan T, Dillon JC, Fineberg NS, Feigenbaum H. Regional wall motion index for infarct and noninfarct regions after reperfusion in acute myocardial infarction: comparison with global wall motion index. J Am Soc Echocardiogr. 1989;2(6):398–407. doi: 10.1016/s0894-7317(89)80041-0. [DOI] [PubMed] [Google Scholar]
- 3.Brown KJ, Maynes SF, Bezos A, Maguire DJ, Ford MD, Parish CR. A novel in vitro assay for human angiogenesis. Lab Invest. 1996;75(4):539–555. [PubMed] [Google Scholar]
- 4.Chawla PS, Keelan MH, Kipshidze N. Angiogenesis for the treatment of vascular diseases. Int Angiol. 1999;18(3):185–192. [PubMed] [Google Scholar]
- 5.Choi JS, Kim KB, Han W, Kim DS, Park JS, Lee JJ, Lee DS. Efficacy of therapeutic angiogenesis by intramyocardial injection of pCK-VEGF165 in pigs. Ann Thorac Surg. 2006;82(2):679–686. doi: 10.1016/j.athoracsur.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001;108(6):1713–1726. doi: 10.1097/00006534-200111000-00045. [DOI] [PubMed] [Google Scholar]
- 7.DeBlois C, Cote MF, Doillon CJ. Heparin-fibroblast growth factor-fibrin complex: in vitro and in vivo applications to collagen-based materials. Biomaterials. 1994;15(9):665–672. doi: 10.1016/0142-9612(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 8.Fasol R, Schumacher B, Schlaudraff K, Hauenstein KH, Seitelberger R. Experimental use of a modified fibrin glue to induce site-directed angiogenesis from the aorta to the heart. J Thorac Cardiovasc Surg. 1994;107(6):1432–1439. [PubMed] [Google Scholar]
- 9.Germano G, Kavanagh PB, Waechter P, Areeda J, van Kriekinge S, Sharir T, Lewin HC, Berman DS. A new algorithm for the quantitation of myocardial perfusion SPECT. I: technical principles and reproducibility. J Nucl Med. 2000;41(4):712–719. [PubMed] [Google Scholar]
- 10.Harada K, Grossman W, Friedman M, Edelman ER, Prasad PV, Keighley CS, Manning WJ, Sellke FW, Simons M. Basic fibroblast growth factor improves myocardial function in chronically ischemic porcine hearts. J Clin Invest. 1994;94(2):623–630. doi: 10.1172/JCI117378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heilmann CA, Attmann T, von Samson P, Gobel H, Marme D, Beyersdorf F, Lutter G. Transmyocardial laser revascularization combined with vascular endothelial growth factor 121 (VEGF121) gene therapy for chronic myocardial ischemia–do the effects really add up? Eur J Cardiothorac Surg. 2003;23(1):74–80. doi: 10.1016/S1010-7940(02)00718-2. [DOI] [PubMed] [Google Scholar]
- 12.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107(10):1359–1365. doi: 10.1161/01.CIR.0000061911.47710.8A. [DOI] [PubMed] [Google Scholar]
- 13.Henry TD, Grines CL, Watkins MW, Dib N, Barbeau G, Moreadith R, Andrasfay T, Engler RL. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol. 2007;50(11):1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 14.House SL, Bolte C, Zhou M, Doetschman T, Klevitsky R, Newman G, Schultz Jel J. Cardiac-specific overexpression of fibroblast growth factor-2 protects against myocardial dysfunction and infarction in a murine model of low-flow ischemia. Circulation. 2003;108(25):3140–3148. doi: 10.1161/01.CIR.0000105723.91637.1C. [DOI] [PubMed] [Google Scholar]
- 15.Hughes GC, Kypson AP, St. Louis JD, Annex BH, Coleman RE, DeGrado TR, Donovan CL, Lowe JE, Landolfo KP. Improved perfusion and contractile reserve after transmyocardial laser revascularization in a model of hibernating myocardium. Ann Thorac Surg. 1999;67(6):1714–1720. doi: 10.1016/S0003-4975(99)00317-3. [DOI] [PubMed] [Google Scholar]
- 16.Iwakura A, Fujita M, Kataoka K, Tambara K, Sakakibara Y, Komeda M, Tabata Y. Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Heart Vessels. 2003;18(2):93–99. doi: 10.1007/s10380-002-0686-5. [DOI] [PubMed] [Google Scholar]
- 17.Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005;105(3):249–259. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Jiang ZS, Srisakuldee W, Soulet F, Bouche G, Kardami E. Non-angiogenic FGF-2 protects the ischemic heart from injury, in the presence or absence of reperfusion. Cardiovasc Res. 2004;62(1):154–166. doi: 10.1016/j.cardiores.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Kang SS, Gosselin C, Ren D, Greisler HP. Selective stimulation of endothelial cell proliferation with inhibition of smooth muscle cell proliferation by fibroblast growth factor-1 plus heparin delivered from fibrin glue suspensions. Surgery. 1995;118(2):280–287. doi: 10.1016/S0039-6060(05)80335-6. [DOI] [PubMed] [Google Scholar]
- 20.Karacal N, Cobanoglu U, Ambarcioglu O, Kutlu N. The effect of fibrin glue on fat graft survival. J Plast Reconstr Aesthet Surg. 2007;60(3):300–303. doi: 10.1016/j.bjps.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, Botker HE, Dudek D, Drvota V, Hesse B, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45(7):982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 22.Kawasuji M, Nagamine H, Ikeda M, Sakakibara N, Takemura H, Fujii S, Watanabe Y. Therapeutic angiogenesis with intramyocardial administration of basic fibroblast growth factor. Ann Thorac Surg. 2000;69(4):1155–1161. doi: 10.1016/S0003-4975(99)01557-X. [DOI] [PubMed] [Google Scholar]
- 23.Kornowski R, Fuchs S, Leon MB, Epstein SE. Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation. 2000;101(4):454–458. doi: 10.1161/01.cir.101.4.454. [DOI] [PubMed] [Google Scholar]
- 24.Kornowski R, Baim DS, Moses JW, Hong MK, Laham RJ, Fuchs S, Hendel RC, Wallace D, Cohen DJ, Bonow RO, et al. Short- and intermediate-term clinical outcomes from direct myocardial laser revascularization guided by Biosense left ventricular electromechanical mapping. Circulation. 2000;102(10):1120–1125. doi: 10.1161/01.cir.102.10.1120. [DOI] [PubMed] [Google Scholar]
- 25.Laham RJ, Garcia L, Baim DS, Post M, Simons M. Therapeutic angiogenesis using basic fibroblast growth factor and vascular endothelial growth factor using various delivery strategies. Curr Interv Cardiol Rep. 1999;1(3):228–233. [PubMed] [Google Scholar]
- 26.Laham RJ, Chronos NA, Pike M, Leimbach ME, Udelson JE, Pearlman JD, Pettigrew RI, Whitehouse MJ, Yoshizawa C, Simons M. Intracoronary basic fibroblast growth factor (FGF-2) in patients with severe ischemic heart disease: results of a phase I open-label dose escalation study. J Am Coll Cardiol. 2000;36(7):2132–2139. doi: 10.1016/S0735-1097(00)00988-8. [DOI] [PubMed] [Google Scholar]
- 27.Lopez JJ, Edelman ER, Stamler A, Hibberd MG, Prasad P, Caputo RP, Carrozza JP, Douglas PS, Sellke FW, Simons M. Basic fibroblast growth factor in a porcine model of chronic myocardial ischemia: a comparison of angiographic, echocardiographic and coronary flow parameters. J Pharmacol Exp Ther. 1997;282(1):385–390. [PubMed] [Google Scholar]
- 28.Phelps EA, Garcia AJ. Update on therapeutic vascularization strategies. Regen Med. 2009;4(1):65–80. doi: 10.2217/17460751.4.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Post MJ, Laham R, Sellke FW, Simons M. Therapeutic angiogenesis in cardiology using protein formulations. Cardiovasc Res. 2001;49(3):522–531. doi: 10.1016/S0008-6363(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 30.Sahni A, Odrljin T, Francis CW. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J Biol Chem. 1998;273(13):7554–7559. doi: 10.1074/jbc.273.13.7554. [DOI] [PubMed] [Google Scholar]
- 31.Sahni A, Khorana AA, Baggs RB, Peng H, Francis CW. FGF-2 binding to fibrin(ogen) is required for augmented angiogenesis. Blood. 2006;107(1):126–131. doi: 10.1182/blood-2005-06-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao ZQ, Takaji K, Katayama Y, Kunitomo R, Sakaguchi H, Lai ZF, Kawasuji M. Effects of intramyocardial administration of slow-release basic fibroblast growth factor on angiogenesis and ventricular remodeling in a rat infarct model. Circ J. 2006;70(4):471–477. doi: 10.1253/circj.70.471. [DOI] [PubMed] [Google Scholar]
- 33.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105(7):788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 34.Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, Della Siega A, Bilodeau L, Burton JR, Proulx G, et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. 2009;17(6):1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto N, Kohmoto T, Roethy W, Gu A, DeRosa C, Rabbani LE, Smith CR, Burkhoff D. Histologic evidence that basic fibroblast growth factor enhances the angiogenic effects of transmyocardial laser revascularization. Basic Res Cardiol. 2000;95(1):55–63. doi: 10.1007/s003950050008. [DOI] [PubMed] [Google Scholar]