Abstract
In the present work, the fruits of four Morus species, namely Morus alba (white mulberry), Morus nigra (black mulberry), Morus laevigata (large white fruit), and Morus laevigata (large black fruit), were analyzed for proximate composition, essential minerals, and antioxidant potentials. For this purpose, the ripe fruits were collected from the northern regions of Pakistan. The major nutritional components (moisture, ash, lipids, proteins, fibres, carbohydrates, and total sugar) were found to be in the suitable range along with good computed energy. Total dry weight, pH, and titratable acidity (percent citric acid) were (17.60±1.94)–(21.97±2.34) mg/100 g, (3.20±0.07)–(4.78±0.15), and (0.84±0.40)%–(2.00±0.08)%, respectively. Low riboflavin (vitamin B2) and niacin (vitamin B3) contents were recorded in all the fruits, while ascorbic acid (vitamin C) was in the range from (15.20±1.25) to (17.03±1.71) mg/100 g fresh weight (FW). The mulberry fruits were rich with regard to the total phenol and alkaloid contents, having values of (880±7.20)–(1650±12.25) mg/100 g FW and (390±.22)–(660±5.25) mg/100 g FW, respectively. Sufficient quantities of essential macro-(K, Ca, Mg, and Na) and micro-(Fe, Zn, and Ni) elements were found in all the fruits. K was the predominant element with concentration ranging from (1270±9.36) to (1731±11.50) mg/100 g, while Ca, Na, and Mg contents were (440±3.21)–(576±7.37), (260±3.86)–(280±3.50), and (24±3.51)–(360±4.20) mg/100 g, respectivly. The decreasing order of micro-minerals was Fe>Zn>Ni. The radical scavenging activity of methanolic extract of fruits was concentration-dependent and showed a correlation with total phenolic constituents of the respective fruits. Based on the results obtained, mulberry fruits were found to serve as a potential source of food diet and natural antioxidants.
Keywords: Mulberry fruits, Chemical composition, 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, Nutritional value, Pakistan
1. Introduction
Mulberry belongs to the genus Morus of the family Moraceae. It is an economically important plant being used for sericulture, as it is the sole food plant for the domesticated silkworm, Bombyx mori. The genus Morus, is widely distributed in Asia, Europe, North America, South America, and Africa, and is cultivated extensively in the eastern, central, and southern Asia for silk production. Mulberry is found from temperate and sub-tropical regions of the northern hemisphere, as well as in the tropics of the southern hemisphere, because it can grow in a wide variety of climatic, topographical, and soil conditions. They spread throughout all regions from the tropics to the sub-arctic and from sea level to altitudes as high as 4000 m. Mulberries are grown at considerably higher altitudes in the Himalaya-Hindu Kush region, while in Pakistan they are widely cultivated in northern regions (Elmacı and Altuğ, 2002; Darias-Martin et al., 2003; Arabshahi-Delouee and Urooj, 2007; Ercisli and Orhan, 2007).
Studies have been reported on the chemical composition and nutritional potentials of some mulberry species worldwide (Gerasopoulos and Stavroulakis, 1997; Elmacı and Altuğ, 2002; Darias-Martin et al., 2003; Arabshahi-Delouee and Urooj, 2007; Ercisli and Orhan, 2007). The deep colored mulberry fruits are rich in phenolic compounds, including flavonoids, anthocyanins, and carotenoids (Sass-Kiss, 2005; Zadernowski et al., 2005; Cieslik et al., 2006; Lin and Tang, 2007). Few species of mulberry were evaluated for their edible fruits (Morus alba, Morus indica, Morus nigra, and Morus laevigata), and timber (Morus laevigata and Morus serrata) (Hou, 1994). Black (M. nigra), red (M. rubra), and white mulberries (M. alba) are widespread in northern India, Pakistan, and Iran, where the tree and the fruits are known by the Persian-derived names toot (mulberry) or shahtoot (King’s or “Superior” mulberry). Shahtoot (M. laevigata), particularly the white variety, is a popular hybrid species in Pakistan. In Pakistan, shahtoot is highly appreciated for its delicious fruit, which is eaten fresh as well as in dried forms, and consumed in marmalades, juices, liquors, natural dyes, and cosmetics industries (Imran et al., 2007).
Mulberry can grow in a wide range of climatic, topographical, and soil conditions, which can affect the chemical composition and nutritional status of plants. However, mulberry grown in Pakistan has not been subject to these kinds of studies. Therefore, the current study was undertaken to investigate the chemical composition, nutritional profile, and antioxidant potentials of various Morus species fruits [M. alba, M. nigra, and M. laevigata (large white and black fruits)].
2. Materials and methods
2.1. Reagents and chemicals
All reagents and chemicals used in this study were of analytical grade and were obtained from Merck (Darmstadt, Germany), unless stated otherwise.
2.2. Collection and pre-treatment of plant samples
The fruits of M. alba (white mulberry), M. nigra (black mulberry), M. laevigata (large white fruit), and M. laevigata (large black fruit) were collected from northern regions of Pakistan in April 2006. M. laevigata is usually called Pakistani variety. Samples were collected according to standard botanical field collection methodology (Humphry et al., 1993). For each sample, several fruits were harvested and pooled together. Plant fruits were wrapped in dark plastic bags to protect them from direct sunlight. Samples were transferred to laboratory soon after their collection. Genus and species of the plants were confirmed by comparison with herbarium reference materials at the Department of Botany, University of Peshawar, Pakistan. Before analyses, visible dirt and insect parts were removed from the fruits. Fruits were dried, pressed, and transferred to dark glass bottles and stored in a deep freezer until further analyses were performed. pH and titratable acidity were determined using fresh fruit samples shortly after their arrival to laboratory.
2.3. Proximate analysis
Plant materials were dried at 70 °C to a constant weight and moisture contents, ash, and crude fibre were determined by AOAC (2000) methods. Nitrogen content (N) of the sample was estimated by the method described by Kjeldahl (1983) and crude protein was calculated as N×6.25 (Imran et al., 2008), while total lipids from the samples were extracted with chloroform/methanol (2:1, v/v) and quantified gravimetrically (Christie, 1983). The amount of total carbohydrates was obtained by the difference between weight of the sample taken and sum of its moisture, ash, total lipid, protein, and fibre contents (Muler and Tobin, 1980). The calorific value was calculated by multiplying the values of total carbohydrate, lipid, and protein with the factors 4, 9, and 4, respectively. Then the products were summed and expressed in kilocalories (kcal) (Onyeike and Ikru, 1998; Imran et al., 2008).
2.4. Determination of total dry weight, pH, and titratable acidity
The total dry weight (TDW) was determined by AOAC (2000) methods, after the evaporation of moisture at 70 °C. For pH determination, mulberry fruits were squeezed for the extraction of juice and filtered. The pH measurements were made using a digital pH-meter (HI 8314, HANNA Instruments, Italy) calibrated with pH 4 and 7 buffers. Titratable acidity was measured by the titrimetric method (AOAC, 2000; Imran et al., 2008) and expressed as percent citric acid.
2.5. Determination of sugars and pectin contents
Extraction and determination of total water-soluble sugar, reducing sugar, and pectin were carried by AOAC (2000) methods. The non-reducing sugar was obtained by subtraction of reducing sugar from total water-soluble sugar.
2.6. Determination of vitamin, total phenol, and alkaloid contents
Vitamins (riboflavin and niacin) were determined as described by Okwu (2005), while ascorbic acid (vitamin C) was quantified using the spectrophotometric method reported by Hussain et al. (2008). Total phenolic contents of mulberry fruits were determined by the Folin-Ciocalteu method, as described elsewhere (Okwu, 2005; Khan et al., 2006) using tannic acid (TA) as a standard. The alkaloid contents were determined gravimetrically (Okwu, 2005).
2.7. Mineral analysis
The samples were dried in the oven (Gallenkamp, England) overnight at 110 °C. The finely ground samples (0.50 g) were taken and digested with 20 ml concentrated nitric acid. After adding 10 ml of perchloric acid, the contents were heated gently on a hot plate, followed by a vigorous heating till dryness (approximately 1–2 ml). This digestion technique made no attempt to dissolve any silicate-based material that may be present in the samples. After cooling, the digested samples were quantitatively transferred to a flask and diluted to 100 ml with deionized double distilled water, and then filtered. An atomic absorption spectrophotometer (Analyst 700, Perkin Elmer, USA), equipped with standard burner, air acetylene flame, and hollow cathode lamps as the radiation source, was used for the analyses of minerals (Hussain et al., 2005; Imran et al., 2007; 2008; Hussain and Khan, 2010).
2.8. DPPH radical scavenging activity
The dried fruit samples were ground and extracted with methanol thrice, and the extracts were combined. The crude extracts obtained were filtered and evaporated in a vacuum to dryness. The potential antioxidant activity of the methanolic extracts was assessed on the basis of the scavenging activity of the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical (Khan et al., 2006; Arabshahi-Delouee and Urooj, 2007). Briefly, 1 ml of a 1 mmol/L methanolic solution of DPPH was mixed with 5 ml of extract solution in methanol (containing 20–100 μg of dried extract). The mixture was then vortexed vigorously, and left for 30 min at room temperature in the dark. The absorbance was measured at 517 nm using ultraviolet (UV)-visible spectrophotometer (SP-3000, Plus Optima, Japan). The activity was expressed as percentage of DPPH scavenging relative to control (5 ml methanol and 1 ml DPPH solution) using the following equation: DSA (%)=(Acon−Asam)×100%/Acon, where DSA is DPPH scavenging activity, A con is absorbance of control, and A sam is absorbance of sample.
2.9. Statistical analysis
Results were expressed as mean±standard deviation (SD) of three separate determinations. The data were statistically analyzed using the statistical program (Origin Version 5.1). The significant differences between means were calculated by a one-way analysis of variance (ANOVA) using Duncan’s multiple-range test at P<0.05 (Imran et al., 2008).
3. Results and discussion
3.1. Proximate composition
The proximate composition of mulberry fruits is given in Table 1. The moisture contents were in the range of (78.03±3.22) to (82.40±3.85) g/100 g fresh weight (FW). The lowest value was recorded for M. laevigata (large black fruit) and the highest for M. nigra, while the rest of the values were in this range. The moisture contents of the mulberry species (Turkish origin) were 71.5% to 74.6% (Ercisli and Orhan, 2007). The ash contents ranged between (0.46±0.06) g/100 g dry weight (DW) [M. laevigata (large white fruit)] and (0.87±0.12) g/100 g DW [M. laevigata (large black fruit)]. In all the studied fruit samples, the total lipid contents were in the range of (0.48±0.11) to (0.71±0.07) g/100 g DW. The highest value was found for M. laevigata (large white fruit) and the lowest was for M. alba. In the case of protein contents, M. laevigata (large black fruit) showed the highest value [(1.73±0.10) g/100 g DW], followed by M. laevigata (large white fruit), M. alba, and M. nigra (Table 1). The crude fibre contents of the fruit samples varied widely between (0.57±0.14) and (11.75±1.21) g/100 g DW. M. nigra contained an exceptionally high content of fibres, while the rest of the samples showed a comparatively small amount of fibers. These results showed that M. nigra could be a good source of fibres. The total carbohydrate contents showed slight variations among the studied fruits samples. The carbohydrate concentration was found to be in the range from (13.83±1.20) g/100 g DW (M. nigra) to (17.96±1.54) g/100 g DW [M. laevigata (large black fruit)]. The calorific value, calculated on a dry weight basis, ranged between (64.11±2.45) and (84.22±4.00) kcal/100 g. M. laevigata (large black fruit) was found to have a high calorific value (Table 1). The overall results showed that the fruit samples could be a potential source of lipids, proteins, fibres, carbohydrates, and hence energy. However, some variations were observed in the studied parameters; therefore, for food purposes the fruits should be selected as per demand. The results are in good agreement with the reported literature (Duke and Ayensu, 1985; Ikhtiar and Alam, 2007; Imran et al., 2007).
Table 1.
Proximate composition of Morus species fruits
| Plant name | Moisture (g/100 g FW) | Ash (g/100 g DW) | Lipid (g/100 g DW) | Protein (g/100 g DW) | Fibre (g/100 g DW) | TC (g/100 g DW) | E (kcal/100 g DW) |
| Morus alba | 81.72±2.25 | 0.57±0.11 | 0.48±0.11 | 1.55±0.30 | 1.47±0.15 | 14.21±1.01 | 67.36±3.22 |
| Morus nigra | 82.40±3.85 | 0.50±0.08 | 0.55±0.06 | 0.96±0.16 | 11.75±1.21 | 13.83±1.20 | 64.11±2.45 |
| Morus laevigata (large white fruit) | 81.48±1.87 | 0.46±0.06 | 0.71±0.07 | 1.57±0.19 | 0.57±0.14 | 15.21±1.32 | 73.51±3.46 |
| Morus laevigata (large black fruit) | 78.03±3.22 | 0.87±0.12 | 0.60±0.03 | 1.73±0.10 | 0.81±0.12 | 17.96±1.54 | 84.22±4.00 |
FW: fresh weight; DW: dry weight; TC: total carbohydrates; E: energy value. Values (mean±SD) in the same column are not significantly different at P<0.05
3.2. Total dry weight, pH, and titratable acidity
The TDW, pH, and titratable acidity of the fruit samples are presented in Table 2. The TDWs of the fruits of Morus species were in the range of (17.60±1.94) mg/100 g (M. nigra) to (21.97±2.34) mg/100 g [M. laevigata (large black fruit)]. The TDWs of mulberry species were 24.9% to 29.5% (Ercisli and Orhan, 2007). The pH of the fruit juice of Morus species was acidic, ranging from 3.20±0.07 to 4.78±0.15. M. laevigata (large black fruit) has higher pH value while pH values for the rest of the fruits were close to each other. pH values of M. nigra fruit juice ranged in 3.60–3.80 (Turkish origin) (Elmacı and Altuğ, 2002) and 3.10–3.36 (Spanish origin) (Darias-Martin et al., 2003), which are in good agreement with the present work. However, a slightly higher pH range (3.52–5.60) was reported previously (Ercisli and Orhan, 2007). The titratable acidity of the fruits of M. laevigata (large white fruit) was found to be (0.84±0.40)% citric acid and M. alba was (2.00±0.08)% citric acid, while the rest were in between these values, as shown in Table 2. The values for the titratable acidity, found in the present study, are in good agreement with the reported data (Elmacı and Altuğ, 2002; Ercisli and Orhan, 2007); however, they are lower than the results of Darias-Martin et al. (2003).
Table 2.
Total dry weight, pH, and titratable acidity of Morus species fruits
| Plant name | TDW (mg/100 g) | pH | TA (% citric acid) |
| Morus alba | 18.28±2.21 | 3.35±0.02 | 2.00±0.08 |
| Morus nigra | 17.60±1.94 | 3.68±0.04 | 1.51±0.14 |
| Morus laevigata (large white fruit) | 18.52±1.26 | 3.20±0.07 | 0.84±0.40 |
| Morus laevigata (large black fruit) | 21.97±2.34 | 4.78±0.15 | 1.46±0.12 |
TDW: total dry weight; TA: titratable acidity. Values (mean±SD) in the same column are not significantly different at P<0.05
3.3. Sugar and pectin contents
The sugar and pectin contents of the selected fruit samples are given in Table 3. M. laevigata (large white fruit) was found to have a higher amount of sugar [(10.89±1.91) g/100 g FW], while the lower value was found for M. nigra [(6.64±1.12) g/100 g FW]. A similar trend was also observed for the reducing sugar. In case of non-reducing sugar, lower quantity was found in M. laevigata (large black fruit) [(1.56±0.17) g/100 g FW] and higher value was recorded for M. laevigata (large white fruit) [(1.78±1.21) g/100 g FW] (Table 3). The results showed that the sugar contents of Morus species of Pakistani origin were found to be low as compared to M. nigra of Turkish origin (11.3%–16.2%) (Elmacı and Altuğ, 2002). This may be due to the different environmental and geological conditions. However, the presence of sugar in the fruits may encourage their use as sugar sources in different food recipes. The pectin was detected only in M. nigra [(0.76±0.03) g/100 g FW], while the rest of the fruit samples were found to be devoid of this compound (Table 3).
Table 3.
Sugars and pectin contents of Morus species fruits
| Plant name | Total sugar (g/100 g FW) | Reducing sugar (g/100 g FW) | Non-reducing sugar (g/100 g FW) | Pectin (g/100 g FW) |
| Morus alba | 7.55±1.01a | 5.90±0.92c | 1.65±0.11d | ND |
| Morus nigra | 6.64±1.12a | 4.94±0.73c | 1.70±0.15d | 0.76±0.03 |
| Morus laevigata (large white fruit) | 10.89±1.91b | 8.11±1.10b | 1.78±1.21d | ND |
| Morus laevigata (large black fruit) | 8.18±1.83b | 6.62±0.97b | 1.56±0.17d | ND |
FW: fresh weight; ND: not detected. Values (mean±SD) followed by the same superscript in each column are not significantly different at P<0.05
3.4. Vitamin, total phenol, and alkaloid contents
Table 4 shows vitamin, total phenol, and alkaloid contents of the studied fruit samples. The selected water-soluble vitamins, namely riboflavin (vitamin B2), niacin or nicotinic acid (vitamin B3), and ascorbic acid (vitamin C) were analyzed. The results showed low levels of riboflavin in the samples, which varied between (0.040±0.000) mg/100 g FW (M. nigra) and (0.088±0.001) mg/100 g FW (M. alba). A variable amount of niacin was recorded in the Morus species, ranging from (0.40±0.03) to (3.10±0.60) mg/100 g FW. Again, a higher quantity of niacin was found in M. alba. In previous studies, low concentrations of riboflavin and niacin were reported (Duke and Ayensu, 1985; Okwu, 2005). The ascorbic acid contents were found to range from (15.20±1.25) mg/100 g FW (M. alba) to (17.03±1.71) mg/100 g FW [M. laevigata (large black fruit)]. The ascorbic acid contents of Turkish mulberry fruits (M. alba, M. nigra, and M. rubra) were 19.4 to 22.4 mg/100 ml (Ercisli and Orhan, 2007). Variation in concentrations of water-soluble vitamins was also reported for other plant species (Okwu, 2005; Imran et al., 2007; Hussain et al., 2008).
Table 4.
Vitamin, total phenol, and alkaloid contents of Morus species fruits
| Plant name | Riboflavin (mg/100 g FW) | Niacin (mg/100 g FW) | Ascorbic acid (mg/100 g FW) | Total phenols# (mg/100 g FW) | Alkaloid (mg/100 g FW) |
| Morus alba | 0.088±0.001 | 3.10±0.60 | 15.20±1.25 | 1650±12.25 | 660±5.25 |
| Morus nigra | 0.040±0.000 | 1.60±0.10 | 15.37±0.89 | 880±7.20 | 630±5.93 |
| Morus laevigata (large white fruit) | 0.055±0.002 | 0.40±0.03 | 16.35±1.80 | 1300±8.53 | 390±3.22 |
| Morus laevigata (large black fruit) | 0.072±0.003 | 0.85±0.11 | 17.03±1.71 | 1100±9.65 | 630±4.95 |
FW: fresh weight
Tannic acid equivalents. Values (mean±SD) in the same column are not significantly different at P<0.05
Regarding the total phenolic contents of mulberry fruits, variable amounts of these constituents were found as shown in Table 4. The high quantity of total phenols was found in M. alba [(1650±12.25) mg/100 g FW], while the low content was recorded in M. nigra [(880±7.20) mg/100 g FW]. M. laevigata (large white fruit) and M. laevigata (large black fruit) were also different significantly in their total phenolic contents. The total phenolic contents were (1515.9±5.7) mg gallic acid equivalents (GAE)/100 g FW in Chinese origin mulberries (Lin and Tang, 2007) and 181–1422 mg GAE/100 g FW in Turkish origin mulberries (Ercisli and Orhan, 2007), which are comparable with the present study. However, the lower amount of total phenols was reported by Okwu (2005) and Khan et al. (2006). The variation of phenolic compounds in the fruits depends on many factors, such as degree of maturity at harvest, genetic differences, and environmental conditions during fruit development. In red-colored fruits, phenols increase during the last ripening stage, due to the maximal accumulation of anthocyanins and flavonols (Gerasopoulos and Stavroulakis, 1997; Zadernowski et al., 2005). Phenols possess a wide spectrum of biological activities (Al-Bayati and Al-Mola, 2008) and the results showed that mulberry fruits could be good sources of these natural constituents.
The alkaloid contents of mulberry fruits were in the range from (390±3.22) mg/100 g FW [M. laevigata (large white fruit)] to (660±5.25) mg/100 g FW (M. alba), as shown in Table 4. M. nigra and M. laevigata (large black fruit) contained the same amount of alkaloid, i.e., 630 mg/100 g FW. The alkaloid contents of two Nigerian medicinal plants were (0.36±0.10) mg/100 g FW (Garcinia kola Heckel) and (0.28±0.20) mg/100 g FW (Aframomum melegueta) (Okwu, 2005), which are much lower than those of the present study. The overall results show that the selected Morus species could the potential sources of natural vitamins, phenols, and alkaloids.
3.5. Mineral composition
The mineral composition of mulberry fruits is shown in Table 5. All the selected essential elements (K, Ca, Na, Mg, Fe, Zn, and Ni) were detected in four fruit samples; however, their concentrations were found to be varied among the species and even between the same species. As can be seen from Table 5, K was predominant element. Among the macro-minerals (K, Ca, Na, and Mg), the concentration of K was varied from (1270±9.36) mg/100 g (M. nigra) to (1731±11.50) mg/100 g (M. alba), while M. laevigata containing (1650±8.86) and (1644±13.10) mg/100 g for large white and black fruits, respectively. The concentrations of Ca, Na, and Mg were (440±3.21) mg/100 g [M. laevigata (large white fruit)] to (576±7.37) mg/100 g [M. alba and M. laevigata (large black fruit)], (260±3.86) mg/100 g [M. laevigata (large white fruit)] to (280±3.50) mg/100 g (M. alba), and (240±3.90) mg/100 g [M. alba, M. nigra, M. laevigata (large black fruit)] to (360±4.20) mg/100 g [M. laevigata (large white fruit)], respectively. Among the essential micro-minerals, Fe was present in sufficient amount in M. nigra [(77.6±1.98) mg/100 g] followed by M. alba [(73.0±2.60) mg/100 g], M. laevigata (large black fruit) [(63.6±1.96) mg/100 g] and M. laevigata (large white fruit) [(48.6±2.32) mg/100 g]. The high level of Fe might be of nutritional importance, especially in those parts of the world where anaemia and Fe deficiency are relatively rampant. Similarly, Zn was found in the range of (50.20±1.93) mg/100 g (M. alba) to (59.20±2.25) mg/100 g (M. nigra), while a narrow range of Ni contents was recorded, ranging from (1.20±0.05) to (2.20±0.15) mg/100 g (Table 5). The over all decreasing order of mineral elements in the studied fruits was K>Ca>Na>Mg>Fe>Zn>Ni, except M. laevigata (large white fruit), in which Mg was found in an amount higher than Na, and Zn higher than Fe. The mineral composition of mulberry fruits (M. alba, M. rubra, and M. nigra) grown in Turkey was: K [1141 (834–1668) mg/100 g], Ca [139 (132–152) mg/100 g], Mg [109 (106–115) mg/100 g], Na [60 (59–61) mg/100 g], Fe [4.3 (4.2–4.5) mg/100 g], and Zn [3.1 (2.8–3.2) mg/100 g] (Ercisli and Orhan, 2007). Okwu (2005) reported much lower amounts of these elements in the studied plants. The present study showed a higher mineral profile as compared with previously reported data (Ercisli and Orhan, 2007; Okwu, 2005). Thus, existence of sufficient quantities of essential minerals in the studied fruits, which may act as better supplements of these elements by including them in daily diet, allows one to easily meet a reasonable amount of the daily requirements.
Table 5.
Mineral composition of Morus species fruits
| Plant name | K (mg/100 g) | Ca (mg/100 g) | Na (mg/100 g) | Mg (mg/100 g) | Fe (mg/100 g) | Zn (mg/100 g) | Ni (mg/100 g) |
| Morus alba | 1731±11.50a | 576±7.37a | 280±3.50a | 240±3.90a | 73.0±2.60a | 50.20±1.93a | 2.20±0.15a |
| Morus nigra | 1270±9.36b | 470±6.95b | 272±5.32a | 240±3.51a | 77.6±1.98a | 59.20±2.25a | 1.60±0.11a |
| Morus laevigata (large white fruit) | 1650±8.86c | 440±3.21b | 260±3.86a | 360±4.20b | 48.6±2.32b | 53.40±2.43a | 1.20±0.05a |
| Morus laevigata (large black fruit) | 1644±13.10c | 576±4.85a | 264±3.37a | 240±2.90a | 63.6±1.96a | 50.80±1.22a | 1.80±0.09a |
Values (mean±SD) followed by the same superscript in each column are not significantly different at P<0.05
3.6. DPPH radical scavenging activity
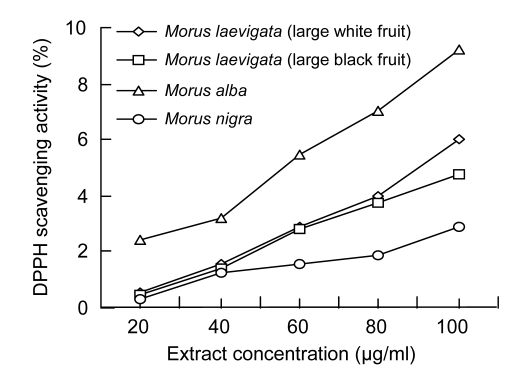
Free radicals, which are involved in the process of lipid peroxidation, are considered to play a major role in numerous chronic pathologies, such as cancer and cardiovascular diseases. A compound with radical reducing power may serve as a potential antioxidant (Khan et al., 2006; Arabshahi-Delouee and Urooj, 2007). The DPPH radical has been widely used to evaluate the free radical scavenging ability of various natural products and has been accepted as a model compound for free radicals (Arabshahi-Delouee and Urooj, 2007; Khan et al., 2006). Radical scavenging activity of methanolic fruit extracts was determined using different concentrations (20–100 µg/ml) of each extract as shown in Fig. 1. The radical scavenging activity was determined in a concentration-dependent manner and showed a linear correlation (r=0.987) with concentration. The extract of M. alba showed highest radical scavenging activity, significantly different (P<0.01) than the other fruits, followed by M. laevigata (large white fruit), M. laevigata (large black fruit), and M. nigra. The radical scavenging activity for M. nigra was significantly lower (P<0.01) than that of the other fruits. A similar trend was observed in the case of their phenolic contents (Table 4). Low radical scavenging activity was also reported previously; however, no correlation was found between radical scavenging activity and the total phenols (Khan et al., 2006). A strong correlation between free radical scavenging and the phenolic contents has been reported for mulberry (M. indica L., Indian origin) leaves (Arabshahi-Delouee and Urooj, 2007), cereals (Peterson et al., 2001), fruits (Gao et al., 2000), beverages (Fogliano et al., 1999), and culinary herbs (Zheng and Wang, 2001).
Fig. 1.
DPPH scavenging activity of methanolic extracts of Morus species fruits
Percentage DPPH scavenging capacity is relative to control
4. Conclusions
In the present study, analytical investigations were carried out to ascertain the chemical composition, minerals profile, nutritional values, and antioxidant potentials of Morus species of Pakistan. The results showed that all the studied mulberry fruits were nutritionally rich and may be useful in a balanced diet. Higher phenolic contents with antioxidant activity further increase their nutritive as well as phytomedicinal potentials. The current work is the first of its kind, providing new reference data and also public awareness regarding consuming these unconventional fruits. The present study is also a step towards the standardization of these fruits as potential healthy foods, which may also be used in food and pharmaceutical industries.
References
- 1.Al-Bayati FA, Al-Mola HF. Antibacterial and antifungal activities of different parts of Tribulus terrestris L. growing in Iraq. J Zhejiang Univ-Sci B. 2008;9(2):154–159. doi: 10.1631/jzus.B0720251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AOAC (Association of Official Analytical Chemists) Official Methods of Analysis International. 17th Ed. Washington, DC: AOAC; 2000. [Google Scholar]
- 3.Arabshahi-Delouee S, Urooj A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007;102(4):1233–1240. doi: 10.1016/j.foodchem.2006.07.013. [DOI] [Google Scholar]
- 4.Christie WW. Aliphatic and Related Natural Product Chemistry. 2nd Ed. Oxford: Pergamon Press; 1983. Lipids. [Google Scholar]
- 5.Cieslik E, Greda A, Adamus W. Contents of polyphenols in fruit and vegetables. Food Chem. 2006;94(1):135–142. doi: 10.1016/j.foodchem.2004.11.015. [DOI] [Google Scholar]
- 6.Darias-Martin J, Lobo-Rodrigo G, Hernandez-Cordero J, Diaz-Diaz E, Diaz-Romero C. Alcoholic beverages obtained from black mulberry. Food Technol Biotechnol. 2003;41(2):173–176. [Google Scholar]
- 7.Duke JA, Ayensu ES. Medicinal Plants of China. Algonac, MI: Reference Publications Inc.; 1985. [Google Scholar]
- 8.Elmacı Y, Altuğ T. Flavour evaluation of three black mulberry (Morus nigra) cultivars using GC/MS, chemical and sensory data. J Sci Food Agric. 2002;82(6):632–635. doi: 10.1002/jsfa.1085. [DOI] [Google Scholar]
- 9.Ercisli S, Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (M. nigra) mulberry fruits. Food Chem. 2007;103(4):1380–1384. doi: 10.1016/j.foodchem.2006.10.054. [DOI] [Google Scholar]
- 10.Fogliano V, Verde V, Randazzo G, Ritieni A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agric Food Chem. 1999;47(3):1035–1040. doi: 10.1021/jf980496s. [DOI] [PubMed] [Google Scholar]
- 11.Gao X, Ohlander M, Jeppsson N, Björk L, Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem. 2000;48(5):1485–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- 12.Gerasopoulos D, Stavroulakis G. Quality characteristics of four mulberry (Morus sp.) cultivars in the area of Chania, Greece. J Sci Food Agric. 1997;73(2):261–264. doi: 10.1002/(SICI)1097-0010(199702)73:2<261::AID-JSFA724>3.0.CO;2-S. [DOI] [Google Scholar]
- 13.Hou YJ. Mulberry Breeding. Hangzhou, China: Sericulture Department, Zhejiang Agriculture University; 1994. (in Chinese) PhD Thesis. [Google Scholar]
- 14.Humphry CM, Clegg MS, Keen CL, Grivetti LE. Food diversity and drought survival. The Hausa example. Int J Food Sci Nutr. 1993;44(1):1–16. doi: 10.3109/09637489309017417. [DOI] [Google Scholar]
- 15.Hussain I, Khan H. Investigation of heavy metals content in medicinal plant, Eclipta alba L. J Chem Soc Pak. 2010;32(1):28–33. [Google Scholar]
- 16.Hussain I, Khan L, Mehmood T, Khan I, Ullah W, Khan H. Effect of heavy metals on the growth and development of Silybum marianum, in various polluted areas of Peshawar, Pakistan. J Chem Soc Pak. 2005;27(4):367–373. [Google Scholar]
- 17.Hussain I, Khan L, Marwat GA, Ahmed N, Saleem M. Comparative study of vitamin C contents in fruits and medicinal plants. J Chem Soc Pak. 2008;30(3):406–409. [Google Scholar]
- 18.Ikhtiar K, Alam Z. Nutritional composition of Pakistani wheat varieties. J Zhejiang Univ-Sci B. 2007;8(8):555–559. doi: 10.1631/jzus.2007.B0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imran M, Talpur FN, Jan MS, Khan A, Khan I. Analysis of nutritional components of some wild edible plants. J Chem Soc Pak. 2007;29(5):500–508. [Google Scholar]
- 20.Imran M, Khan H, Hassan SS, Khan R. Physicochemical characteristics of various milk samples available in Pakistan. J Zhejiang Univ-Sci B. 2008;9(7):546–551. doi: 10.1631/jzus.B0820052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan T, Ahmad M, Khan R, Khan H, Ejaz A, Choudhary MI. Evaluation of phytomedicinal potentials of selected plants of Pakistan. Am Lab. 2006;38(9):20–22. [Google Scholar]
- 22.Kjeldahl J. Determination of protein nitrogen in food products. Encyc Food Agric. 1983;28:757–765. [Google Scholar]
- 23.Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101(1):140–147. doi: 10.1016/j.foodchem.2006.01.014. [DOI] [Google Scholar]
- 24.Muler HG, Tobin G. Nutrition of Food Processing. London: Croom Helm Ltd.; 1980. [Google Scholar]
- 25.Okwu DE. Phytochemicals, vitamins and mineral contents of two Nigerian medicinal plants. Int J Mol Med Adv Sci. 2005;1(4):375–381. [Google Scholar]
- 26.Onyeike EN, Ikru PR. Preliminary investigation of proximate composition of heat processed cashew nut seed (Anacardium occidentale) flours. J Appl Sci Environ Manage. 1998;1(1):27–30. [Google Scholar]
- 27.Peterson DM, Emmons CLL, Hibbs AH. Phenolic antioxidants and antioxidant activity in pearling fractions of oat groats. J Cereal Sci. 2001;33(1):97–103. doi: 10.1006/jcrs.2000.0347. [DOI] [Google Scholar]
- 28.Sass-Kiss A. Differences in anthocyanin and carotenoid content of fruits and vegetables. Food Res Int. 2005;38(8-9):1023–1029. doi: 10.1016/j.foodres.2005.03.014. [DOI] [Google Scholar]
- 29.Zadernowski R, Naczk M, Nesterowicz J. Phenolic acid profiles in some small berries. J Agric Food Chem. 2005;53(6):2118–2124. doi: 10.1021/jf040411p. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49(11):5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]