Abstract
Visnagin (4-methoxy-7-methyl-5H-furo[3,2-g][1]-benzopyran-5-one), which is an active principle extracted from the fruits of Ammi visnaga, has been used as a treatment for low blood-pressure and blocked blood vessel contraction by inhibition of calcium influx into blood cells. However, the neuroprotective effect of visnagin was not clearly known until now. Thus, we investigated whether visnagin has a neuroprotective effect against kainic acid (KA)-induced neuronal cell death. In the cresyl violet staining, pre-treatment or post-treatment visnagin (100 mg/kg, p.o. or i.p.) showed a neuroprotective effect on KA (0.1 µg) toxicity. KA-induced gliosis and proinflammatory marker (IL-1β, TNF-α, IL-6, and COX-2) inductions were also suppressed by visnagin administration. These results suggest that visnagin has a neuroprotective effect in terms of suppressing KA-induced pathogenesis in the brain, and that these neuroprotective effects are associated with its anti-inflammatory effects.
Keywords: Neuroprotection, Kainic acid, Hippocampus, Visnagin, Cytokines
INTRODUCTION
Kainic acid (KA), which is an analog of the excitatory amino acid L-glutamate, elicits neuronal cell death followed by severe status epilepticus in the pyramidal layer of the hippocampal CA3 region [1] when KA is administered intracerebroventricularly (i.c.v.). It may be due to excessive activation of neurons by excitatory neurotransmitters (e.g. glutamate), which are massively released as a consequence of energy depletion and which result in excitotoxic neuron death [2,3]. Recent studies have demonstrated that the KA-induced neuronal death is associated with the activation of microglia and astrocytes in the hippocampus, and that these processes are induced by enhanced reactive oxygen species (ROS) production and cytokine expressions [4,5]. The microglia also can be detected using the complement receptor type 3 (OX-42) IR. The complement receptor type 3 is important in the adherence of neutrophils and monocytes to stimulated endothelium, and also in the phagocytosis of complement coated particles that raises the last step of the microglial activation [6]. Expressions, hypertrophy and proliferation of Glial fibrillary acidic protein (GFAP) specifically is found in astroglia, a cell type which is highly responsive to neurologic insults [7]. After brain injury, astrocytes undergo a number of cellular syntheses and release of a variety of growth factors and immunomodulatory cytokines [7]. In addition, recent studies have demonstrated that inflammatory and apoptotic processes contribute to the later stages of the damage induced by various brain injuries, and that these detrimentally affect neurologic outcome [5,8]. Among them, it has been reported well that pro-inflammatory mediators such as IL-1β, TNF-α, IL-6, and COX-2 can lead to deteriorative effect in the brain, and KA can trigger an aberrant inflammatory cytokine response by microglial cells and accelerated disease progression [4].
Visnagin (4-methoxy-7-methyl-5H-furo[3,2-g][1]-benzopyran-5-one) is an active principle extracted from the fruits of Ammi visnaga [9]. The fruit or its isolated active components have been used for the treatment of angina pectoris due to their peripheral and coronary vasodilator activity [10,11]. In isolated aorta, visnagin, and other related active principles present in these fruits such as visnadin and khellin inhibited vascular smooth muscle contractility, probably by acting at multiple sites to decrease the availability of Ca2+ required for activation [12-14]. Several reports have demonstrated that visnagin descents blood pressure and blocks blood vessel contraction as inhibiting calcium influx into cell [15]. However, the neuroprotective effect of visnagin on kainic acid (KA)-induced neuronal death has not been demonstrated until now although visnagin has been studied for therapeutic use for over 10 years.
In the present study, we explored the neuroprotective effects of visnagin in KA-induced neuronal cell death model. The peroral (p.o.) or intraperitoneal (i.p.) administration of visnagin remarkably suppresses hippocampal cell death, indicating that visnagin has a neuroprotective effect against KA-induced neuronal cell death.
METHODS
These experiments were approved by the Hallym University Animal Care and Use Committee. All procedures were conducted in accordance with the 'Guide for Care and Use of Laboratory Animals' published by the National Institutes of Health. Male ICR mice (MJ Co., Seoul, Korea) weighing 25~28 g were used for all the experiments.
Experimental animals
Male ICR mice (MJ Co., Seoul, Korea) weighing 25~28 g were used for all the experiments. Animals were housed 5 per cage in a room maintained at 22±0.5℃ with an alternating 12 hr light-dark cycle. Food and water were available ad libitum. The animals were allowed to adapt to the laboratory for at least 2 hr before testing and were only used once. To reduce variation, all experiments were performed during the light phase of the cycle (10:00~17:00).
Drug treatment and i.c.v. kainic acid injection
Visnagin was purchased from Acros organics. Visnagin was prepared following steps: (A) 1 g of decursinol was dissolved in 0.5 ml of ethanol plus 0.5 ml of polyethylene glycol 400 (B) Separately; 100 mg of sodium carboxymethylcellulose was dissolved in 9 ml of distilled water. (C) Finally, Solution (A) and Solution (B) were vigorously mixed. These solutions excluding visnagin were used as vehicle control. The KA (Sigma, USA) was dissolved in a phosphate buffer solution. The i.c.v. administrations of KA were performed following the procedure established by Laursen and Belknap [16]. Briefly, each mouse was injected at bregma with a 50 µl Hamilton microsyringe fitted with a 26-gauge needle that was inserted to a depth of 2.4 mm.
Cresyl violet staining method and histological analysis
Animals were sacrificed for the brain sample by perfusion at 1 day after KA administration. All perfusion procedures were worked in the fume hood. For perfusion, all mice were first deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p., Hanlim Pharm., Korea) and perfused intracardially with physiological saline followed with ice-cold phosphate-buffered 4% paraformaldehyde (pH 7.4). Whole brain was removed from the skull and postfixed in the same fixative for 4 hrs at 4℃. Then the brains were cryoprotected in 30% sucrose for 24 hrs at 4℃ and sectioned coronally (45 µm) on a freezing microtome and collected in cryoprotectant for storage at -20℃ until processed. Prepared sections were rinsed 3×10 min in PBS to remove cryoprotectant. Sections were mounted on microscope slides (Fisher, USA) and dried on air. The slides were soaked in cresyl violet working solution (0.02% buffer solution; 0.2% sodium acetate, 0.3% acetic acid) for 30 min. Then the sections were dehydrated through alcohol and xylene and coverslipped using Permount (Fisher, USA).
Histological analysis method in pyramidal layer of hippocampal CA3 region was performed following under procedures [17]. The number of cresyl violet-positive neurons was counted by two blinded observers at the same time using an image analyzing system equipped with a computer-based CCD camera (Olympus AX70, USA). The number of cresyl violet-positive neurons in the CA3 region of the hippocampus was counted in 3 sections in reference to the mouse atlas [18] for each animal [19]. Starting from the first section (interaural 2.10 mm, bregma -1.70 mm), counts were taken from at least three coronal sections at 0.135 mm increments. Thus, we could always perform neuronal counting of the same brain region and minimize any counting bias. The number of cresyl violet-positive neurons was compared to that of the control group of the same brain area from all animals. All experiments were conducted independently twice. The neuronal counting of the same group was combined for final analysis.
Isolation of total RNA
The entire process for isolation of total RNA was conducted three times independently. Finally, the animal number used for each group was nine. Three animals of each group were dissected for real time PCR analysis. Total cellular RNA was extracted from dissected hippocampus tissue using a rapid guanidine thiocyanate-watersaturated phenol/chloroform extraction procedure and subsequent precipitation with acidic sodium acetate [20]. Total cellular RNA in the aqueous phase was precipitated with ice-cold isopropyl alcohol. Isolated RNA samples were subjected to spectrophotometric analysis at 260 nm and 280 nm. The separated organic layer was extracted twice with an equal volume of sterilized (Millipore, USA) water and proteins were precipitated by adding two volumes of absolute ethanol to the water-extracted organic phase. The dried pellets were dissolved in a denaturing buffer (6 M guanidium chloride, 20 mM Tris-HCl [pH 8.0], and 1 mM EDTA).
Real-time PCR analysis
Expression of TNF-α, IL-1β, IL-6, COX-2 and GAPDH mRNA was evaluated by real-time PCR using QuantiTect™ SYBR® Green PCR Kit (Qiagen, Germany). All PCRs were performed in total volume of 20 µl using the QuantiTect™ SYBR® Green PCR Kit (Qiagen, Germany). Each reaction contained 1.5 µl of cDNA, 6.5 µl RNase-Free Water, each 1 µl of sense and antisense primer (20 µM) and 10 µl of 2X SYBR® Green PCR Master Mix (containing QuantiTect SYBR Green PCR buffer, dNTPs, SYBR Green I dye, ROX dye, and HotStarTaq DNA polymerase). After an initial denaturation step at 95℃ for 30 s, temperature cycling with a total of 40 cycles was initiated. Each cycle consisted of a denaturation phase at 95℃ for 30 s, an annealing phase at 60℃ for 30 s and an elongation phase at 72℃ for 30 s. Amplification was followed by melting curve analysis to verify the correctness of the amplification. A negative control with water instead of cDNA was run within every PCR to assess specificity of the reaction. To verify the accuracy of the amplification, PCR products were further analyzed on ethidium bromide-stained 2% agarose gel. For data analysis, Rotor-Gene 6000 Series Software 1.7 (Build 87) was used. Results are given as a ratio of the amount of TNF-α, IL-1β, IL-6, COX-2 mRNA to that of GAPDH mRNA. The following primers were used: TNF-α (NM_013693) sense: CAT CTT CTC AAA ATT CGA GTG ACA A, antisense: TGG GAG TAG ACA AGG TAC AAC CC; IL-1β (NM_008361) sense: TCT CGC AGC AGC ACA TCA, antisense: CAC ACA CCA GCA GGT TAT; IL-6 (NM_031168) sense: GAG GAT ACC ACT CCC AAC AGA CC, antisense: AAG TGC ATC ATC GTT GTT CAT ACA; COX-2 (AF344876) sense: TTC AAA AGA AGT GCT GGA AAA GGT, antisense: GAT CAT CTC TAC CTG AGT GTC TTT and GAPDH (NM_008084) sense: TCG TGG ATC TGA CGT GCC GCC TG, antisense: CAC CAC CCT GTT GCT GTA GCC GTA T. The entire process from dissection to RT-PCR was conducted three times independently. Finally, the animal number used for each group was nine. Each real time PCR result (independently three times) was quantified with GraphPad Prism Version 4.0 for Windows (GraphPad Software, USA) for statistical analysis.
Immunohistochemistry
Sections were cut with a cryostat at a thickness of 45 µm. Immuno-histochemical staining was performed with Elite ABC Kit (Vector Laboratories, USA). Sections were first rinsed with 0.1 M PBS three times for 10 min each, then pre-incubated in 0.1M PBS containing 1% BSA and 0.2% Triton X-100 for 30 min. After rinsing twice with 0.1 M PBS containing 0.5% BSA for 10~15 min each, sections were incubated with antibody against GFAP (1:50,000; Sigma, USA) and OX-42 (1:75,000; Accurate Chemical, USA) diluted with 0.1 M PBS containing 0.5% BSA and 0.05% sodium azide at 4℃. After overnight incubation, sections were rinsed and incubated with biotinylated secondary antibody 1:200 diluted with 0.1 M PBS containing 0.5% BSA for 1 hr at room temperature. After rinsing, the sections were incubated with ABC reagent 1:200 diluted with PBS for 1 hr at room temperature and then rinsed with PBS followed with 0.1 M phosphate buffer (PB). Finally sections were incubated in SIGMA FAST DAB kit (Sigma, USA), until the desired stain intensity developed. We standardized the lengths of DAB reaction time (10 min for all brain sections) to allow for uniform intensity of staining across the experimental groups. Sections were rinsed with 0.1 M PB, and then mounted to gelatin-coated slides, and dehydrated through alcohol and xylene. To quantify, we counted GFAP or OX-42 IR in each section. The animal number used for immuostaining was 5 per group.
Statistical analysis
Statistical analysis was carried out by one-way analysis of variance (ANOVA) with a Bonferroni post-hoc test using GraphPad Prism Version 4.0 for Windows (GraphPad Software, USA). p values less than 0.05 were considered to indicate statistical significance. All values were expressed as the mean±S.E.M.
RESULTS
The effect of visnagin administered orally or intraperitoneally on KA-induced neuronal death in the hippocampus
To determine a dose of visnagin showing a neuroprotective effect, we examined the dose-dependent effect (50, 80, 100 mg/kg, p.o.) of visnagin against KA toxicity. Visnagin was pretreated orally 20 min prior to KA injection. We observed that visnagin has a significant neuroprotective effect at 80 and 100 mg/kg, but not at 50 mg/kg (data not shown). In this study, visnagin was treated at the dose of 100 mg/kg showing a significant neuroprotective effect against KA toxicity.
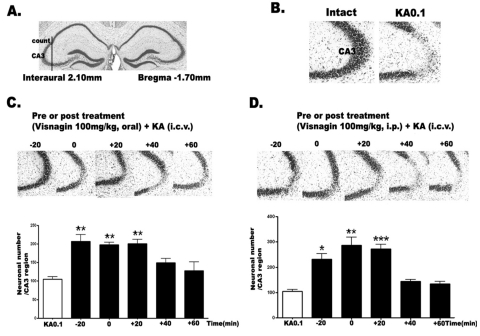
To examine the time course effect of visnagin on KA-induced neuronal death, we administered visnagin orally (p.o.) or intraperitoneally (i.p.) 20 min (-20 min) prior to KA (0.1 µg/5 µl, i.c.v.) injection or 0, 20 (+20), 40 (+40), 60 (+60) min after KA injection (0.1 µg/5 µl, i.c.v.). As shown in Fig. 1A, KA induced neuronal cell death in the pyramidal layer of the hippocampal CA3 region. When visnagin was treated before or after KA injection orally or intraperitneally, we observed that visnagin had neuroprotective effect on KA-induced neuronal cell death. However, delayed visnagin administration (+40 and +60 min) in both p.o. (Fig. 1B) and i.p. (Fig. 1C) did not show any neuroprotective effect on KA-induced neuronal death. The visnagin administered p.o. or i.p. at -20, 0, +20 min had similar neuroprotective effect on KA-induced neuronal death in the hippocampus.
Fig. 1.
The effect of visnagin administered orally or intraperitoneally on KA-induced neuronal death in the hippocampus. The location of the hippocampal CA3 region performing cresyl violet-positive neuronal count is indicated (A) referring Franklin [18]. I.c.v. kainic acid injecton induced neuronal cell death in the pyramidal cells in the CA3 region of hippocampus (B). Mice were administered visnagin orally (C) or intraperitoneally (D) 20 min (-20) prior to KA (0.1 µg/5 µl) injection or 0, 20 (+20), 40 (+40), 60 (+60) min after KA treatment (0.1 µg/5 µl). And then, the cresyl violet staining was performed at 1 day after KA. The vertical bars indicate the standard error of mean. *p<0.05, **p<0.01, ***p<0.001 (KA 0.1 vs other groups). The mice number of each group was 10.
The course alteration of proinflammatory cytokines mRNA on KA injection in the hippocampus
Previous studies suggest that inflammatory and apoptotic processes contribute to the later stages of the damage induced by brain injuries, and that these detrimentally affect neurologic outcome [5,21,22]. Therefore, we investigate the course alteration of proinflammatory cytokines mRNA on KA injection in the hippocampus. The mRNA levels of various proinflammatory markers such as IL-1β, TNF-α, IL-6, and COX-2 in the hippocampus were examined at 1, 3, 6, 12, 24 hrs after KA administration (0.1 µg/5 µl, i.c.v.).
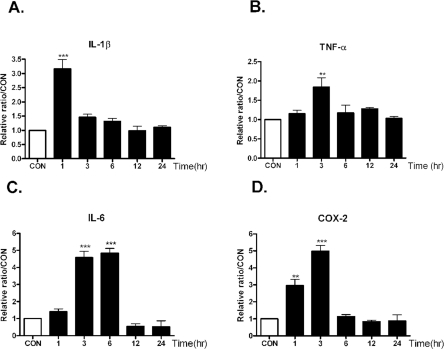
As shown in Fig. 2, intracerebroventricular injection with KA increased TNF-α and COX-2 mRNA levels, and the levels showed peak at 3 hrs after KA treatment. On the other hand, IL-1β mRNA level reached maximum level at 1hr after KA treatment, and IL-6 mRNA level showed peak level at 3 and 6 hrs after KA treatment. The increased cytokines mRNA level decreased gradually after peak level.
Fig. 2.
The time course alteration of proinflammatory cytokines mRNA on KA injection in the hippocampus. Alteration of IL-1β, TNF-α (B), IL-6 (C), and COX-2 (D) mRNA at 1, 3, 6, 12, 24 hrs after intracerebral (i.c.v.) KA (0.1 µg/5 µl) injectio was examined in the hippocampus. Control group (CON) was injected with i.c.v. PBS. The mice number of each group was 3. Experiments were conducted independently three times, giving a total of nine per group in the final statistical analysis. **p<0.01(CON vs other groups), ***p<0.001 (CON vs other groups).
The effect of visnagin on pro-inflammatory cytokines increased by KA in the hippocampus
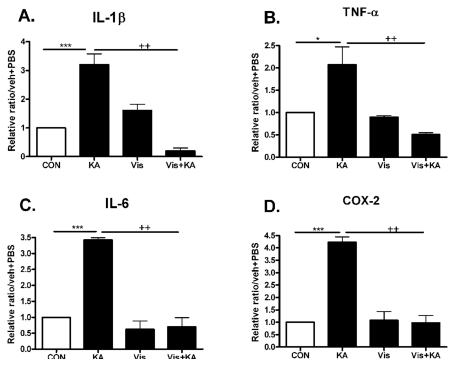
To investigate whether the neuroprotective effect of visnagin is accompanied by suppressions of proinflammatory markers such as IL-1β, TNF-α, IL-6, and COX-2 in the hippocampus, we observed an effect of visnagin on pro-inflammatory cytokines increased by KA in the hippocampus. The time point when pro-inflammatory cytokines peaked was refereed by Fig 2 result. The induction of IL-1β, TNF-α, IL-6, and COX-2 mRNA hippocampus were inhibited by visnagin pretreatment (Fig. 3). However, visnagin itself did not affect pro-inflammatory cytokines expression.
Fig. 3.
The effect of visnagin on proinflammatory cytokines increased by KA in the hippocampus. Alteration of -1β, TNF-α (B), IL-6 (C), and COX-2 (D) at 1 (A), 3 hrs (B, D), or 6 hrs (C) after intracerebroventricular (i.c.v.) KA injection was examined in the hippocampus. The increased IL-1β, TNF-α (B), IL-6 (C), and COX-2 (D) by KA was decreased by visnagin treated prior to 20 min. The mice number of each group was 3. Experiments were conducted independently three times, giving a total of nine per group in the final statistical analysis. *p<0.05, **p<0.01, ***p<0.001 (CON vs KA), ++p<0.01 (Vis vs Vis+KA). CON, vehicle (i.p.)+PBS (i.c.v.); KA, vehicle (i.p.)+KA (i.c.v.); Vis, Visnagin (i.p.)+PBS (i.c.v.).
The effect of visnagin on the GFAP, OX-42 expression induced by KA in the hippocampal CA3 region
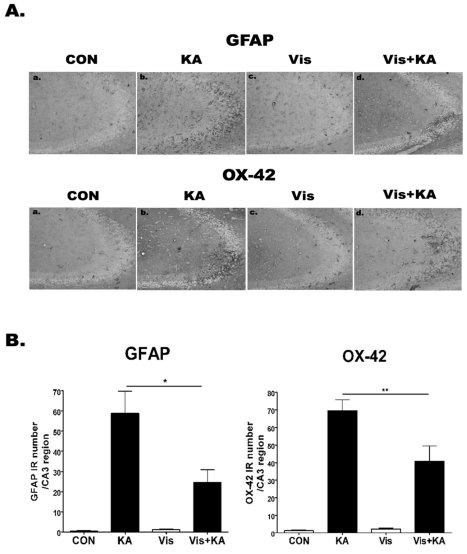
To investigate whether visnagin affects GFAP or OX-42 expression induced by KA, the activations of microglia and astrocytes were analyzed using anti-OX-42 (microglia marker) and anti-GFAP (astrocyte marker) antibodies, respectively (Fig. 4). In PBS treated control mice, both microglias and astrocytes having ramified morphology were barely detected. However, the number of anti-OX-42 cells and anti-GFAP cells was remarkably elevated at 1 day after i.c.v. KA treatment in the hippocampal CA3 region. In addition, visnagin administered orally at -20 min attenuated OX-42 and GFAP expression induced by KA.
Fig. 4.
The effect of visnagin on the GFAP, OX-42 expression induced by KA in hippocampal CA3 region. OX-42 and GFAP immunoreactivities (A) in the hippocampus were examined at 24 hr after i.c.v. injection of KA (0.1 µg/5 µl). Animals (N=5 per each group) were pretreated orally with either PBS or visnagin (100 mg/kg) 20 min prior to KA (0.1 µg/5 µl). To quantify, we counted GFAP or OX-42 IR in each section (B). CON (a): vehicle (i.p.)+PBS (i.c.v.), KA (b): vehicle (i.p.)+KA (i.c.v.), Vis (c): Visnagin (i.p.)+PBS (i.c.v.), Vis+KA (d): Visnagin (i.p.)+KA (i.c.v.). Antibody against OX-42 and GFAP was used at 1:10,000 dilution for immunostaining. *p<0.05 (KA vs Vis+KA), **p<0.01 (KA vs Vis+KA).
DISCUSSION
There is an accumulating body of evidence which suggests that inflammation contributes to brain damage occurring after acute injury, and that it detrimentally affects neurological outcome [5,8]. In addition, under inflammatory conditions, free oxygen radicals, nitric oxide, and inflammatory cytokines produced by activated microglial cells seem to cause neuronal damage. Because the hippocampus is densely populated with microglia and is one of the most sensitive and malleable regions of the brain [23], it is speculated that excessive production of inflammatory cytokines in the hippocampus would be associated with KA toxicity. In the present study, we observed that pre- or post-visnagin administered orally (p.o.) or intraperitoneally (i.p.) inhibited KA-induced neuronal cell death in the hippocampal CA3 region. Visnagin not only inhibited microglial and astroglial activation but also attenuated the inflammatory marker expressions concomitantly, suggesting that visnagin exerts its neuroprotective effects via an anti-inflammatory mechanism in KA model.
Visnagin has been shown to relax KCl-and noradrenaline induced contractions in guinea-pig aortic strips to a similar extent [13] and this vasorelaxant effect was explained by the inhibition of Ca2+ entry into vascular smooth muscle cells. During excitotoxicity, glutamate concentrations increase in the synapse leads to intracellular Ca2+ concentration increase, activating a cell death pathway [2,3,24]. Thus, it is speculated that visnagin may have a neuroprotective effect against KA toxicity by inhibiting Ca2+ influx. Although inhibition of visnagin on Ca2+ entry into hippocampal neuron was not confirmed in the present study, it may be one mechanism on the neuroprotective effect of visnagin. In regard to its anti-inflammatory effect, we also observed whether visnagin attenuated inflammatory markers against KA toxicity in the hippocampus. It appears that khellin extracts have some antimicrobial activity; this might be attributable to both the khellin and visnagin constituents, which both seem to have antifungal, antibacterial, and antiviral activity [25]. In addition, several reports have demonstrated that drugs or compounds having an anti-inflammatory effect can show a neuroprotective effect [4,26,27]. Thus, we examined the effect of visnagin on proinflammatory mediators expression induced by KA.
Recently, it has been demonstrated that proinflammatory cytokines have established potent pro-excitatory actions [28-31]. Collectively these reports show that proinflammatory cytokines simultaneously facilitate excitatory glutamatergic pathways while concurrently reducing inhibitory GABAergic transmission. Thus it is speculated that the combinatory synergic effect of these cytokines increased by KA and known facilitatory action of KA on excitatory neurotransmission may lead to neuronal cell death in the hippocampus. Thus, the inhibitory effect of visnagin on cytokines expression showing synergic effect on neuronal cell death by KA may explain the neuroprotective effect of visnagin. It has been reported that COX-2 inhibitors may protect the brain against neurodegenerative diseases [32]. In addition, COX-2 specific inhibitors have a neuroprotective effect in models of focal [33] and global brain ischemia [34]. Furthermore, reactive oxygen species are generated by COX-2 activity. Oxidative stress has been demanded to induce neurodegeneration in a variety of disease states [35]. Prostaglandins, which are the product of cyclooxygenase metabolism, can produce injury by inflammatory and vascular mechanisms, as well as directly lead to apoptosis in some cell types [36]. Furthermore, it has been reported that antioxidative effects were also observed in the case of visnagin [37]. Thus, it is speculated that COX-2 inhibition and the antioxidative effect of visnagin may be another mechanism explaining the neuroprotective effect on KA toxicity.
Several earlier reports showed microglia expressing many inflammatory mediators after inflammatory injury [38,39]. Recently, it has been reported well that activated microglia is a significant source of redundant extracellular glutamate that induces excitotoxic neuronal death [40]. In addition, there is a report that excitatory amino acids released by microglia are suggested to compose the major determinant of neurotoxicity rather than reactive oxygen intermediates and cytokines [41]. Furthermore, Liang et al. [42] have demonstrated that astrocytes prevented excito-neurotoxicity by the reduction of exogenous glutamate whereas microglia did not, and conversely, activated microglia released an excess of glutamate that induced excitotoxic neuronal death. Taken together, it is speculated that activated glial cells induced by KA may be involved in increasing extracellular glutamate concentration finally as regulating glutamate release each other, or induce synergic effect on KA toxicity. Although we can not confirm that visnagin plays important roles as glutamate scavenger or glutamate receptor blocker, it seems that visnagin contributes to partly attenuate glutamate release as inhibiting gliosis.
We also observed that visnagin co-treatment (at 0 hr, p.o.) with KA injection produced a similar protective effect with Pre- or post-treatment of visnagin in the CA3 region. This result suggests that in addition to the anti-inflammatory effects, visnagin may also be effective during the acute damage process. First, direct inhibition of Ca2+ influx into neurons may be one possibility as mentioned above [13] although the underlying mechanism remains to be explored in detail. Second, the antioxydative effect of visnagin may be another candidate on co-treatment effect [37]. However, further study should be conducted to elucidate these hypotheses.
In conclusion, based on all this information, we speculate that the neuroprotective effects of visnagin in vivo are the results of multiple mechanisms, and that one of these may be associated with the suppression of inflammatory processes.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A081028), Priority Research Centers Program through the National Research Foundation of Korea (NRF, 2009-0094072) and Grant (2009K001254) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology.
ABBREVIATIONS
- KA
kainic acid
- GFAP
glial fibrillary acidic protein
- IR
immunoreactivity
- IL-1β
interleukin-1beta
- TNF-α
tumor necrosis factor-alpha
- IL-6
interleukin-6
- COX-2
cyclooxygenase-2
References
- 1.Sperk G. Kainic acid seizures in the rat. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 2.Beal MF. Mechanisms of excitotoxicity in neurologic diseases. Faseb J. 1992;6:3338–3344. [PubMed] [Google Scholar]
- 3.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 4.Jin Y, Lim CM, Kim SW, Park JY, Seo JS, Han PL, Yoon SH, Lee JK. Fluoxetine attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Brain Research. 2009;1281:108–116. doi: 10.1016/j.brainres.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 5.Penkowa M, Florit S, Giralt M, Quintana A, Molinero A, Carrasco J, Hidalgo J. Metallothionein reduces central nervous system inflammation, neurodegeneration, and cell death following kainic acid-induced epileptic seizures. J Neurosci Res. 2005;79:522–534. doi: 10.1002/jnr.20387. [DOI] [PubMed] [Google Scholar]
- 6.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 7.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 8.Kim JB, Yu YM, Kim SW, Lee JK. Anti-inflammatory mechanism is involved in ethyl pyruvate-mediated efficacious neuroprotection in the postischemic brain. Brain Research. 2005;1060:188–192. doi: 10.1016/j.brainres.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Smith E, Pucci LA, Bywater WG. Crystalline Visnagan. Science. 1952;115:520–521. doi: 10.1126/science.115.2993.520. [DOI] [PubMed] [Google Scholar]
- 10.Anrep GV, Barsoum GS, Kenawy MR, Misrahy G. Ammi Visnaga in the treatment of the anginal syndrome. Br Heart J. 1946;8:171–177. [PMC free article] [PubMed] [Google Scholar]
- 11.Anrep GV, Barsoum GS, Kenawy MR. The pharmacological actions of the crystalline principles of Ammi Visnaga Linn. J Pharm Pharmacol. 1949;1:164–176. doi: 10.1111/j.2042-7158.1949.tb12395.x. [DOI] [PubMed] [Google Scholar]
- 12.Duarte J, Perez-Vizcaino F, Torres AI, Zarzuelo A, Jimenez J, Tamargo J. Vasodilator effects of visnagin in isolated rat vascular smooth muscle. Eur J Pharmacol. 1995;286:115–122. doi: 10.1016/0014-2999(95)00418-k. [DOI] [PubMed] [Google Scholar]
- 13.Rauwald HW, Brehm O, Odenthal KP. The involvement of a Ca2+ channel blocking mode of action in the pharmacology of Ammi visnaga fruits. Planta Medica. 1994;60:101–105. doi: 10.1055/s-2006-959426. [DOI] [PubMed] [Google Scholar]
- 14.Ubeda A, Tejerina T, Tamargo J, Villar A. Effects of khellin on contractile responses and 45Ca2+ movements in rat isolated aorta. J Pharm Pharmacol. 1991;43:46–48. doi: 10.1111/j.2042-7158.1991.tb05447.x. [DOI] [PubMed] [Google Scholar]
- 15.Duarte J, Torres AI, Zarzuelo A. Cardiovascular effects of visnagin on rats. Planta Medica. 2000;66:35–39. doi: 10.1055/s-2000-11108. [DOI] [PubMed] [Google Scholar]
- 16.Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986;16:355–357. doi: 10.1016/0160-5402(86)90038-0. [DOI] [PubMed] [Google Scholar]
- 17.Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd ed. San Diego: Academic Press; 1997. [Google Scholar]
- 19.Kwon MS, Seo YJ, Choi SM, Choi HW, Jung JS, Park SH, Suh HW. The differential effects of single or repeated restraint stress on kainic acid-induced neuronal death in the hippocampal CA3 region: the role of glucocorticoid and various signal molecules. J Neurochem. 2007;103:1530–1541. doi: 10.1111/j.1471-4159.2007.04865.x. [DOI] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 21.Kim SW, Yu YM, Piao CS, Kim JB, Lee JK. Inhibition of delayed induction of p38 mitogen-activated protein kinase attenuates kainic acid-induced neuronal loss in the hippocampus. Brain Research. 2004;1007:188–191. doi: 10.1016/j.brainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Weiss JH, Sensi SL, Koh JY. Zn(2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 23.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 24.White BC, Sullivan JM, DeGracia DJ, O'Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 25.Hudson J, Towers GHN. Phytomedicines as antivirals. Drugs Future. 1999;24:295–300. [Google Scholar]
- 26.Cho IH, Kim SW, Kim JB, Kim TK, Lee KW, Han PL, Lee JK. Ethyl pyruvate attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J Neurosci Res. 2006;84:1505–1511. doi: 10.1002/jnr.21052. [DOI] [PubMed] [Google Scholar]
- 27.Yoo KY, Hwang IK, Kim JD, Kang IJ, Park J, Yi JS, Kim JK, Bae YS, Won MH. Antiinflammatory effect of the ethanol extract of Berberis koreana in a gerbil model of cerebral ischemia/reperfusion. Phytother Res. 2008;22:1527–1532. doi: 10.1002/ptr.2527. [DOI] [PubMed] [Google Scholar]
- 28.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 29.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Cheng Q, Malik S, Yang J. Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther. 2000;292:497–504. [PubMed] [Google Scholar]
- 32.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 33.Nogawa S, Zhang F, Ross ME, Iadecola C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–2755. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci U S A. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 36.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 37.Aboul-Enein HY, Kladna A, Kruk I, Lichszteld K, Michalska T. Effect of psoralens on Fenton-like reaction generating reactive oxygen species. Biopolymers. 2003;72:59–68. doi: 10.1002/bip.10285. [DOI] [PubMed] [Google Scholar]
- 38.Buttini M, Appel K, Sauter A, Gebicke-Haerter PJ, Boddeke HW. Expression of tumor necrosis factor alpha after focal cerebral ischaemia in the rat. Neuroscience. 1996;71:1–16. doi: 10.1016/0306-4522(95)00414-9. [DOI] [PubMed] [Google Scholar]
- 39.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 40.Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76:846–854. doi: 10.1046/j.1471-4159.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- 41.Piani D, Spranger M, Frei K, Schaffner A, Fontana A. Macrophage-induced cytotoxicity of N-methyl-D-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. Eur J Immunol. 1992;22:2429–2436. doi: 10.1002/eji.1830220936. [DOI] [PubMed] [Google Scholar]
- 42.Liang J, Takeuchi H, Doi Y, Kawanokuchi J, Sonobe Y, Jin S, Yawata I, Li H, Yasuoka S, Mizuno T, Suzumura A. Excitatory amino acid transporter expression by astrocytes is neuroprotective against microglial excitotoxicity. Brain Research. 2008;1210:11–19. doi: 10.1016/j.brainres.2008.03.012. [DOI] [PubMed] [Google Scholar]