Abstract
Tropical inhabitants are able to tolerate heat through permanent residence in hot and often humid tropical climates. The goal of this study was to clarify the peripheral mechanisms involved in thermal sweating pre and post exposure (heat-acclimatization over 10 days) by studying the sweating responses to acetylcholine (ACh), a primary neurotransmitter of sudomotor activity, in healthy subjects (n=12). Ten percent ACh was administered on the inner forearm skin for iontophoresis. Quantitative sudomotor axon reflex testing, after iontophoresis (2 mA for 5 min) with ACH, was performed to determine directly activated (DIR) and axon reflex-mediated (AXR) sweating during ACh iontophoresis. The sweat rate, activated sweat gland density, sweat gland output per single gland activated, as well as oral and skin temperature changes were measured. The post exposure activity had a short onset time (p<0.01), higher active sweat rate [(AXR (p<0.001) and DIR (p<0.001)], higher sweat output per gland (p<0.001) and higher transepidermal water loss (p<0.001) compared to the pre-exposure measurements. The activated sweat rate in the sudomotor activity increased the output for post-exposure compared to the pre-exposure measurements. The results suggested that post-exposure activity showed a higher active sweat gland output due to the combination of a higher AXR (DIR) sweat rate and a shorter onset time. Therefore, higher sudomotor responses to ACh receptors indicate accelerated sympathetic nerve responsiveness to ACh sensitivity by exposure to environmental conditions.
Keywords: Short-term heat-acclimatization, Sudomotor axon reflex, Active sweat gland, Single sweat gland output, Acetylcholine
INTRODUCTION
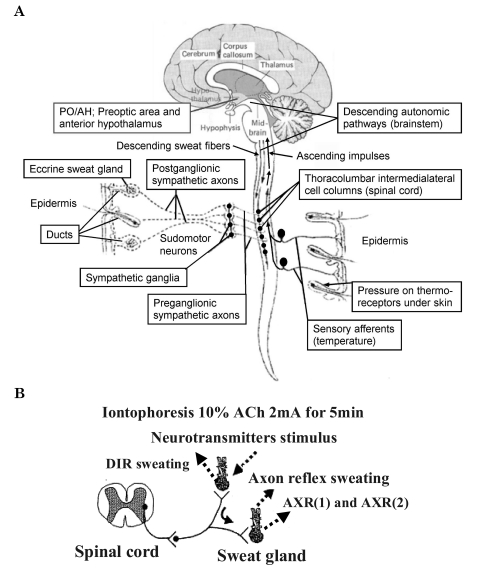
The effective heat acclimatization of the human body provides the thermoregulation needed to survive in extreme environmental conditions such as in high temperature environments. However, for persons less able to adapt to heat acclimatization the levels of vasodilatation and vasoconstriction are maintained without change in the physiological capabilities [1]. Heat resistance can improve by thermoregulation adaptation to the environment by exposure that can be repeated over a period of time [2]. Short term heat exposure reduces the initiation of sweating and increases the amount of sweating [3]. Environmental adaptation is controlled by the preoptic area and anterior hypothalamus (PO/AH), the receptive body of core temperature, and postganglionic sympathetic nerves in the peripheral nervous system. Acetylcholine (ACh) is the primary neurotransmitter involved in this process [4,5]. As shown in Fig. 1A, efferent sweat fibers originate in the hypothalamic preoptic sweat center, and descend through the ipsilateral brainstem and medulla to synapse with the intermediolateral cell column neurons [4]. The preganglionic fibers emerge from the anterior roots to reach (via the white rami communicantes) the chain ganglia.Unmyelinated postganglionic sympathetic class C fibers arise from the sympathetic ganglia to join the major peripheral nerves to reach the sweat glands, providing them with cholinergic innervation [4,5]. ACh induces direct sudomotor activity caused by the activation of muscarinic receptors and indirect sudomotor activity caused by activation of nicotine receptors (Fig. 1B). As shown in Fig. 1B, the peripheral motor neurons involved in perspiration mediate the activity of the muscarinic receptors and axon reflex activation caused by the nicotine receptors of the peripheral C fibers in the sympathetic nerves [4-8]. Consequently, direct and indirect reactions can be evaluated quantitatively (Fig. 1B) by ACh stimulation with the quantitative sudomotor axon reflex test (QSART) as a method for the quantification of the sudomotor reaction [4,9].
Fig. 1.
(A) Possible functional pathway for sudomotor nerves under dynamometry stimulation: efferent sweat fibers originate centrally in the areas regulating sweating, the preoptic area and anterior hyopothalamus (PO/AH), and peripherally by ACh. The fibers descend through the ipsilateral brainstem and medulla to synapse with the intermediolateral cell column neurons. The preganglionic fibers emerge from the anterior roots to reach (via the white rami communicantes) the chain ganglia. Unmyelinated postganglionic sympathetic class C fibers arise from the sympathetic ganglia to join the major peripheral nerves to reach the sweat glands, providing them with cholinergic innervations (Figure modified from Vetrugno et al. 2003). (B) Diagram of the sudomotor axon reflex. The sudomotor axon reflex. Cholinergic agonists with neurotransmitters (ACh) applied through iontophoresis bind to muscarinic receptors causing local sweat production (DIR sweating). The cholinergic agonist simultaneously binds to nicotinic receptors on nerve terminals of sudomotor fibers and an impulse travels antidromically. At branch points this impulse travels orthodromically to a neighboring population of eccrine sweat glands causing an indirect axon mediated sweat response [AXR (1) and AXR (2) sweating].
The QSART is widely used clinically for the evaluation of sympathetic activation [9-14]. It is painless and there is no risk associated with the subcutaneous injection; it is a simple and convenient ion permeation method that can be used for evaluation. The results of QSART to acclimatized individuals over the long term have shown a reduction in the sweating response to stimuli [5,8,15,16]. The results of injection of ACh in native residents that move from warm areas to tropical areas and lived there for 2~12 years showed suppression of sweating sensitivity that depended on the extension of the period of acclimatization [6]. The sweating sensitivity increased based on the extension of the deacclimatization period [7]. The sweating sensitivity is the difference between the dynamic forces of the sweat glands and the sweat volume output from a single gland which is associated with induced threshold changes of the body depending on the climate conditions of the place of residence and the constant period of time living there [5,8]. Therefore, the dynamic forces of gland activity and the inhibition and suppression of single sweat gland output volume, depending on ACh sensitivity, influences the sweat volume of the whole body.
The goal of this study was to evaluate the rate of sweating due to the direct response, active sweat gland dynamic force and output of a single sweat gland by quantification of the reflex sudomotor onset time and sweating volume of the axon reflex response (AXR) at the point of stimulation using the QSART after ACh ionphoresis before and after acclimation over 10 days.
METHODS
Subjects
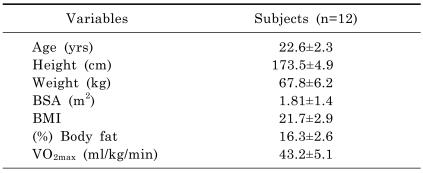
Following approval of the experimental protocol from the University of Soonchunhyang Research Committee and obtaining written informed consent normotensive participants volunteered for the study. Twelve male volunteers, 22.6 years of age, participated in this study. The physical characteristics of healthy subjects that were not athletes and participated in this experiment are shown in Table 1. The maximum oxygen consumption and heart rate were measured with a pulse sensor on the chest and an expired air gas analyzer (COSMED; Quark Pulmonary Function Testing Lung Volumes Module 2 ergo, Rome, Italy). The physical load (VO2max) was tested for maximal performance on one test of prolonged running on a treadmill (gradually increased from 2 to 16 Km/h) until the subject became exhausted.
Table 1.
Physical characteristics of subjects
Values are presented as the means±SD. BSA, body surface area; BMI, body mass index.
Measurement and experimental procedure
All experiments were carried out in an automated climate chamber (24.0±0.5℃, relative humidity 40±3%, and <1 m/sec air velocity). The method used for acclimatization was immersing the lower body into warm water of 43℃ for 10 days [17]; QSART was used to test sweating activity before and after the exposure to acclimatization. The subjects were dressed in light clothing and rested quietly in the same environment for 60 min before the experiment, which was performed between 2~5 PM for all subjects. The apparatus used for the experiment was a ventilating capsule composed of two circular compartments directly in contact with the skin. The phenomenon of vasodilatation and sweating at the area directly stimulated by the axon reflex of the postganglionic sympathetic nerves in the skin causes sweating by muscarinic activity and the axon reflexivity of the nicotine active axon reflexes are indirectly stimulated. Consequently, the ACh mechanism was used to measure the sweating volume at the DIR stimulated area that acts on the sweat gland directly, which is at the outer rim of the cell, at a point stimulated by the 10% ACh solution. The central area at the capsule, with the ACh iontophoresis, measures the sweating volume of the AXR indirectly activated by the axon reflex. The ionphorisis results from the direct injections into the skin with the 10% ACh and the direct current of 2mA at the DIR site [5,6,15,16,18,19].
Two capsules were fixed at the center of the forearm with a rubber band to measure the sweating volume. The first capsule (capsule A) was located midway between the wrist and elbow, and the second capsule (capsule B) was located 10cm from capsule A. The space at the outer rim of capsule A was filled with 10% ACh solution (Ovisot, Daiichi Pharmaceutical Co., Ltd., Japan). A direct 2 mA current was applied for five minutes between the electrode (anode) of the ACh cell and a flat electrode (HV-BIGPAD, Omron, Kyoto) (cathode) placed at the surface of the skin on the forearm near to the wrist [5]. The production of sweat was measured at the outer most point [sweating directly activated, DIR (1)] and at the central point [axon flex mediated sweating, AXR (1)]. The sweating capsules A and B were changed after the electric current was applied for five minutes. The sweating activity was measured for an additional five minutes to evaluate the DIR (2) and AXR (2). The sweating activity was quantified for the start time of sweating for five minutes (AXR sweat onset-time, the resting pause for sweating after applying the current), sweat quantity, the area under the sweating curve, for 0~5 minutes AXR (1), 6~11 minutes AXR (2), and the areas of sweating of the forearm skin for DIR using a hygrometer-ventilated capsule. This equipment allowed for the determination of changes in the relative humidity of emitted gas by the hygrometer as a result of supplying nitrogen gas to the capsule at a constant speed of 0.3 l/min (H211, Technol Seven, Yokohama, Japan). The temperature was measured orally and on the skin near to the capsule, using a thermistor register (K-720, Technol Seven, Yokohama, Japan). Data was recorded automatically every five seconds on the rate of sweating and the oral temperature and the temperature of the subcutaneous skin [5,8,15,16,20]. The active sweat gland density of each subject was measured by the iodine-impregnated paper method during the last stage of the QSART recording; this is a method of calculation of the average sweat gland density (count/cm2) determined by scrubbing iodine-starch paper, on the subcutaneous skin, within 10~15 seconds of the stimulation of the forearm [5]. In addition, the secreted quantity of sweat, from an active single sweat gland, was obtained (µg/min/single gland). The output quantity was calculated from a single sweat gland involved in sweating for the DIR sweating quantity (mg/cm2/min) [5]. The direct attachment and derivation of the evaporated quantity of moisture (Transepidermal water loss: TEWL, µg/cm2/min) were used for the quantitative evaluation. The evaporated quantity from the skin was determined using the QSART with Tewameter (model No. TM 210; Courage and Khazaka, Germany) [5,21].
Statistical analysis
The SPSS statistical program for Windows (ver. 12.0) was used for the analysis of data. The average value and standard deviation (Mean±SD) were calculated before and after acclimatization. The paired Student's t-test was used for the statistical analysis and the level of significance was set at 5%.
RESULTS
The goal of this study was to quantify the reaction of muscarinic receptors directly stimulated for five minutes after QSART (2 mA for 5 min) with 10% ACh; a method reported previously [5,6,8]. A quantitative evaluation of the nicotine receptors was carried out. The rate of reaction of the muscarinic receptors was converted into the sweating volume as the DIR [6~11 minutes] stimulated directly by ACh after the stimulating current was discontinued. The DIR rate of sweating was converted into the standard density for the evaluation of the quantity of active sweat glands. The quantity of output from a single sweat gland was determined based on the active sweat gland density according to the DIR rate of sweating and used for the calculation of a single gland sweat volume.
AXR and DIR
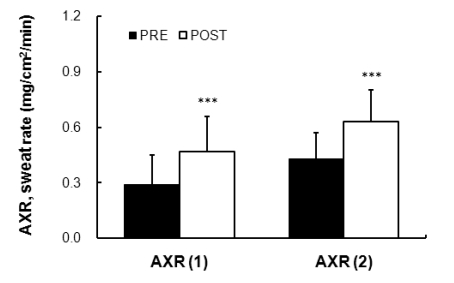
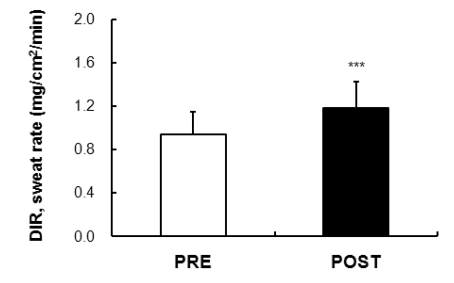
The measurements from the point of stimulation of the axon reflex by the QSART stimulus showed that a reduction in time after exposure (1.38±0.71 min) compared to before acclimatization to the exposure (1.68±0.65 min) for five minutes of AXR (1); the onset time was 0~5 minutes and the differences were significant (p<0.01). The AXR (1), that is, the sweat volume of indirect axon reflexes of nicotine receptors for five minutes, was increased after the exposure (0.47±0.19 mg/cm2/min) compared to the measures before the exposure (0.29±0.16 mg/cm2/min) (p<0.001). The AXR (2) significantly increased after the exposure (0.63±0.17 mg/cm2/min) compared to the acclimatization exposure (0.43±0.14 mg/cm2/min) (p<0.001) (Fig. 2). The evaluation of the total quantity associated with the sweating rate for five minutes, between 6~11 minutes after discontinuation of the stimulation, was calculated from the DIR rate of sweating (sweating due to the direct activation of muscarinic receptors). As suggested in Fig. 3, the DIR was significantly increased after exposure compared to before exposure to acclimatization (p<0.001).
Fig. 2.
Comparison of sweating activity between PRE (0.29±0.16 mg/cm2/min) and POST (0.47±0.19 mg/cm2/min) exposure (43℃ water immersion of leg, 60 min/day, during 10 days) in subjects with the AXR (1) sweat response. Comparison of sweating activity between PRE (0.43±0.14 mg/cm2/min) and POST (0.63±0.17 mg/cm2/min) exposure (43℃ water immersion of leg, 60 min/day, during 10 days) in subjects with the AXR (2) sweat response. AXR=axon reflex-mediated (indirectly activated) sweating during (nicotinic receptor mediated sweating activity), AXR (1)=0~5 min and AXR (2)=6~11 min. Values are presented as the means±SD. Statistical significance at ***p<0.001.
Fig. 3.
Comparison of the DIR (muscarinic receptor mediated sweating activity) sweat rate (directly activated 6~11 min) was 0.94±0.21 mg/cm2/min and 1.18±0.24 mg/cm2/min in the subjects PRE and POST exposure (43℃ water immersion of leg, 60 min/day, during 10 days), respectively. Values are presented as the means±SD. Statistical significance d at ***p<0.001.
Skin and oral temperature
The measurement of the skin temperature and oral temperature was performed at the same time. The temperature of the skin for five minutes with ACh stimulation before and after exposure to acclimatization was significantly increased with the five minutes of ACh stimulation (p<0.001). However, there was no significant change in the oral temperature after five minutes ACh stimulation. The temperature on the surface of skin was significantly increased at between 5~10 cm, at the center of the forearm, where the iontophoresis was performed (p<0.001). However, there was no significant temperature change in the skin at 10 cm above and below the stimulated areas.
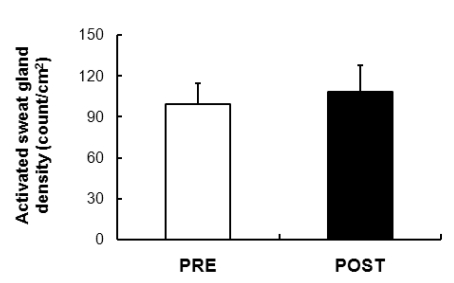
Activated sweat gland density
The active sweat gland density of the unit area, based on the quantity of sweating derived from the rate of sweating due to the direct inducement reaction (direct activation of muscarinic receptor) to ACh as shown in Fig. 4 was determined. The results showed a tendency to be increase after exposure compared to before exposure.
Fig. 4.
Comparison of the active sweat gland density was 99.2±15.61 count/cm2 and 108.4±19.44 count/cm2 in the subjects PRE and POST exposure (43℃ water immersion of leg, 60 min/day, during 10 days), respectively. Values are presented as the means±SD.
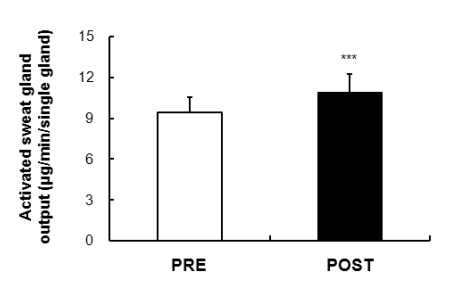
Activate sweat gland output
The output volume of active sweat glands shown in Fig. 5 suggests the quantity of a single sweat gland output derived from the rate of sweating caused by direct activation of muscarinic receptors. A significant increase was observed after exposure compared to before exposure to acclimatization in the output volume from a single sweating gland (p<0.001).
Fig. 5.
Comparison of the active sweat output per gland was 9.45±1.08 µg/min/single gland and 10.93±1.33 µg/min/single gland in the subjects by PRE and POST exposure (43℃ water immersion of leg, 60 min/day, during 10 days), respectively. Values are presented as the means±SD. Statistical significance at ***p<0.001.
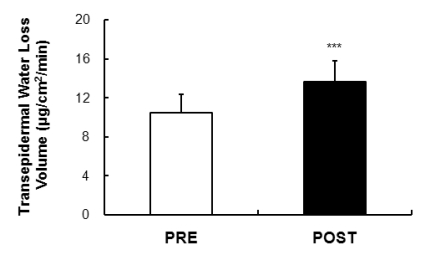
Transepidermal water loss volume
The results of moisture evaporation near the AXR/DIR for the five minutes of stimulation (5 minutes in between 0~5 minutes), between 6~11 minutes after stimulation, is shown in Fig. 6. The evaluation was of the AXR (sweating due to the direct activation of nicotine receptors) and the results showed a statistically significant difference after exposure compared to before exposure (p<0.001).
Fig. 6.
Comparison of the sweating activity (volume of skin evaporative loss) was 10.51±1.91 µg/cm2/min and 13.67±2.12 µg/cm2/min in the subjects PRE and POST exposure (43℃ water immersion of leg, 60 min/day, during 10 days), respectively. Values are presented as the means±SD. Statistical significance at ***p<0.001.
DISCUSSION
Sweating caused by the axon reflex before and after exposure was evaluated with 10 days of exposure, immersing the lower body into warm water at 43℃ for 60 minutes a day. The active sweat gland density and single sweat gland output volume as well as the evaporated quantity were evaluated quantitatively. The immediate sweating increased; however, after repeated exposure acclimatization occurred. The increase in the volume of sweat allows the body to compensate for the increase in body temperature [22]. The reduction of the Na+ concentration reduces the loss of Na+ due to sweating [23,24]. Short term acclimatization occurs with an exposure of one to two hours a day and can be complete between a few days and by the second week of exposure [25,26]. However, discontinuation of exposure to acclimatization generally stabilizes over a period of time from one week to one month [25]. The sweating reaction to short term and long term acclimatization is different. Both the activity of the nervous system associated with sweating and sweat gland reactivity are involved in the sweating reaction during short term acclimatization.
The production of sweat increased before and after acclimatization (38℃ of temperature in rectum was maintained for 9 days), but there was no change in the association of the production and quantity of sweating illustrating the reactivity of the central nerve stimulus on the sweat glands [27].
The results of this study showed the acceleration of the peripheral sympathetic nervous system caused by thermal exposures for 10 days. The accelerated sweating reaction is considered advantageous in terms of energy consumption by in vivo metabolism, an enhanced sweating capability caused by stimulation of the peripheral nervous system for thermoregulation. These results provide evidence for the observation that vasodilatation is accompanied by secretion of the sweat glands [27-29]. The increase in the sweat volume due to short term acclimatization is associated with the process of thermoregulation that is necessary for short term acclimatization. In addition, the enhanced sensitivity of the sweat gland appears to be due to increases in indirect AXR, the direct rate of sweating of the DIR, active sweat gland density and the output volume from individual sweat glands.
The results of this study show the effects of the autonomic nervous system on thermoregulation, which depends on the adaptation of acclimatization. The physical adaptation changes associated with peripheral sweating were confirmed by the results of this study. However, t the central nervous activity associated with sweating activity requires further study.
ACKNOWLEDGEMENTS
The authors extend their thanks to the subjects whose participation made this study possible.
ABBREVIATIONS
- QSART
quantitative sudomotor axon reflex test
- ACh
acetylcholine
- AXR
axon reflex-mediated
- DIR
directly activated
References
- 1.Wilkerson WJ, Young RJ, Melius JM. Investigation of a fatal heat stroke. Am Ind Hyg Assoc J. 1986;47:A493–A494. [PubMed] [Google Scholar]
- 2.Nadel ER, Pandolf KB, Roberts MF, Stojwijk JA. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol. 1974;37:515–520. doi: 10.1152/jappl.1974.37.4.515. [DOI] [PubMed] [Google Scholar]
- 3.Kuno Y. Human Perspiration. Springfield: Charles C Thomas; 1956. pp. 287–291. [Google Scholar]
- 4.Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256–270. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- 5.Lee JB, Bae JS, Matsumoto T, Yang HM, Min YK. Tropical Malaysians and temperate Koreans exhibit significant differences in sweating sensitivity in response to iontophoretically administered acetylcholine. Int J Biometeorol. 2009;53:149–157. doi: 10.1007/s00484-008-0197-9. [DOI] [PubMed] [Google Scholar]
- 6.Bae JS, Lee JB, Matsumoto T, Timothy O, Min YK, Yang HM. Prolonged residence of temperate natives in the tropics produces a suppression of sweating. Pflugers Arch. 2006;453:67–72. doi: 10.1007/s00424-006-0098-x. [DOI] [PubMed] [Google Scholar]
- 7.Lee JB, Bae JS, Lee MY, Yang HM, Min YK, Song HY, Ko KK, Kwon JT, Matsumoto T. The change in peripheral sweating mechanisms of the tropical Malaysian who stays in Japan. J Therm Biol. 2004;29:743–747. [Google Scholar]
- 8.Lee JB. Heat acclimatization in hot summer for ten weeks suppress the sensitivity of sweating in response to iontophoretically-administered acetylcholin. Korean J Physiol Pharmacol. 2008;12:349–335. doi: 10.4196/kjpp.2008.12.6.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Illigens BM, Gibbons CH. Sweat testing to evaluate autonomic function. Clin Auton Res. 2009;19:79–87. doi: 10.1007/s10286-008-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eishi K, Lee JB, Bae SJ, Takenaka M, Katayama I. Impaired Sweating Function in Adult Atopic Dermatitis. Br J Dermatol. 2002;147:683–688. doi: 10.1046/j.1365-2133.2002.04765.x. [DOI] [PubMed] [Google Scholar]
- 11.Low PA. Clinical Autonomic Disorders. 2nd ed. Philadelphia: Lippincott-Raven; 1997. Laboratory evaluation of autonomic function; pp. 179–208. [Google Scholar]
- 12.Low PA. Evaluation of sudomotor function. Clin Neurophysiol. 2004;115:1506–1513. doi: 10.1016/j.clinph.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Maeda A, Yamanouchi H, Lee JB, Katayama I. Oral prednisolone improved acetylcholine-induced sweating in Sjogen's syndrome related anhidrosis. Clin Rheumatol. 2000;19:396–397. doi: 10.1007/pl00011177. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Aravinda KP, Lim EC, Chan YH, Deng C, Huang XF, Wilder-Smith EP. A static handgrip method for distal quantitative sweat measurements. Neuroscience Letters. 2007;421:229–233. doi: 10.1016/j.neulet.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Lee JB, Othman T, Lee JS, Quan FS, Choi JH, Yang HM, Min YK, Matsumoto T, Kosaka M. Sudomotor modifications by acclimatization of stay in temperate Japan of Malaysian native tropical subjects. Jan J Tropical Medicine Hygiene. 2002;30:295–299. [Google Scholar]
- 16.Lee JB, Matsumoto T, Bea JS, Choi JH, Yang HM, Min YK. Economical Sweating Function in Africans: Quantitative Sudomotor Axon Reflex Test. Korean J Physiol Pharmacol. 2004;8:21–25. [Google Scholar]
- 17.Lee JB, Kosaka M, Othman T, Matsumoto T, Kaneda E, Yamauchi M, Taimura A, Ohwatari N, Nakase Y, Makita S. Evaluation of the applicability of infrared and thermistorthermometry in thermophysiology research. Trop Med. 1999;41:133–142. [Google Scholar]
- 18.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983;14:573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 19.Lee JB, Bae JS, Shin YO, Kang JC, Matsumoto T, Toktasynovna AA, Alipov Gabit K, Kim WJ, Min YK, Yang HM. Long-term tropical residency diminishes central sudomotor sensitivities in male subjects. Korean J Physiol Pharmacol. 2007;11:233–237. [Google Scholar]
- 20.Lee JB, Matsumoto T, Othman T, Kosaka M. Suppression of the sweat gland sensitivity to acetylcholine applied iontophoretically in tropical africans compared to temperate japanese. Trop Med. 1997;39:111–121. [Google Scholar]
- 21.Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164–178. doi: 10.1111/j.1600-0536.1990.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 22.Lind AR, Bass DE. Optimal exposure time for development of acclimatization to heat. Fed Proc. 1963;22:704–708. [PubMed] [Google Scholar]
- 23.Hori S, Tanaka N. Adaptive change in physiological response of the men to heat induced by heat acclimatization and physical training. Jan J Tropical Medicine Hygiene. 1993;21:193–199. [Google Scholar]
- 24.Nielsen B, Strange S, Christensen NJ, Warberg J, Saltin B. Acute and adaptive responses in humans to exercise in a warm humid environment. Pflugers Arch. 1997;434:49–56. doi: 10.1007/s004240050361. [DOI] [PubMed] [Google Scholar]
- 25.Pandolf BK. Time course of heat acclimation and its decay. Int J Sports Med. 1998;19:S157–S160. doi: 10.1055/s-2007-971985. [DOI] [PubMed] [Google Scholar]
- 26.Sawaka MN. Thermoregulatory responses to acute exercise heat stress and acclimation. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, Section 4. Environmental Physiology. New York: Oxford University Press; 1996. pp. 157–185. [Google Scholar]
- 27.Sugenoya J, Ogawa T, Jmai K, Ohnishi N, Natsume K. Cutaneous vasodilatation responses synchronize with sweat expulsions. Eur J Appl Physiol Occup Physiol. 1995;71:33–40. doi: 10.1007/BF00511230. [DOI] [PubMed] [Google Scholar]
- 28.Kenney WL, Munce TA. Aging and human temperature regulation. J Appl Physiol. 2003;95:2598–2603. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- 29.Scremin G, Kenney WL. Aging and the skin blood flow response to the unloading of baroreceptors during heat and cold stress. J Appl Physiol. 2004;96:1019–1025. doi: 10.1152/japplphysiol.00928.2003. [DOI] [PubMed] [Google Scholar]