Abstract
The sleep homeostatic response significantly affects the state of anesthesia. In addition, sleep recovery may occur during anesthesia, either via a natural sleep-like process to occur or via a direct restorative effect. Little is known about the effects of isoflurane anesthesia on sleep homeostasis. We investigated whether 1) isoflurane anesthesia could provide a sleep-like process, and 2) the depth of anesthesia could differently affect the post-anesthesia sleep response. Nine rats were treated for 2 hours with ad libitum sleep (Control), sleep deprivation (SD), and isoflurane anesthesia with delta-wave-predominant state (ISO-1) or burst suppression pattern-predominant state (ISO-2) with at least a 1-week interval. Electroencephalogram and electromyogram were recorded and sleep-wake architecture was evaluated for 4 hours after each treatment. In the post-treatment period, the duration of transition to slow-wave-sleep decreased but slow wave sleep (SWS) increased in the SD group, but no sleep stages were significantly changed in ISO-1 and ISO-2 groups compared to Control. Different levels of anesthesia did not significantly affect the post-anesthesia sleep responses, but the deep level of anesthesia significantly delayed the latency to sleep compared to Control. The present results indicate that a natural sleep-like process likely occurs during isoflurane anesthesia and that the post-anesthesia sleep response occurs irrespective to the level of anesthesia.
Keywords: Isoflurane anesthesia, Sleep-wake architecture, Sleep deprivation, Slow wave sleep, Rat
INTRODUCTION
Although little is known about how the anesthetized state could modulate the mechanisms governing sleep homeostasis, the anesthetized state significantly affects sleep homeostasis, either by allowing processes normally associated with sleep to occur during anesthesia or by exerting a direct restorative effect [1-3]. Also, the anesthetic requirements are affected by sleep deprivation [4]. Furthermore, studies of specific brain regions in sleep-wake regulation have demonstrated that naturally occurring sleep and anesthesia share common neuronal substrates [5-7].
Sleep deprivation is common in patients in the intensive care unit [8] and can seriously deteriorate the condition of patients [9-11]. Frustratingly, advantageous homeostatic sleep response is difficult to obtain in an intensive care environment [2].
Cycles of sleep state accompanied by their typical electroencephalogram (EEG) patterns, such as a slow and large EEG pattern during non-rapid-eye-movement sleep (NREMS) and a fast and small EEG pattern during rapid-eye-movement sleep (REMS), occur during natural sleep, but they are not apparent during anesthesia. However, EEG patterns during anesthesia vary according to the anesthetic dose and the agent; various anesthetics induce slower and higher delta waves, and/or burst suppression.
In general, volatile anesthetics produce rapid anesthetic induction, easy alteration of anesthetic level, no delay in emergence and uneventful recovery from anesthesia. Furthermore, anesthesia can be safely maintained for relatively extended periods.
EEG aspects can also show similarities between anesthesia and sleep. Anesthetics induce similar bispectral index (BIS) values to those of natural sleep [12,13]. Additionally, slow wave sleep (SWS, also known as 'NREMS') empirically shows a similar EEG pattern upon visual inspection to the predominant delta wave state in anesthesia. As total sleep deprivation increases the total composition of sleep, selective REMS deprivation increases the REMS proportion during sleep recovery [14,15]. Isoflurane produces the EEG delta wave or burst suppression pattern according to the anesthetic depth. We explored whether 1) isoflurane anesthesia could provide a natural sleep-like process, and 2) the depth of anesthesia could differently affect post-anesthesia sleep response. Anesthetic depth was determined from the predominant EEG pattern: delta wave or burst-suppression pattern.
METHODS
Experimental animals
This experiment was approved by the Kyungpook National University Institutional Animal Care and Use Committee, and was carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (1996). Nine male Sprague-Dawley rats (Hyochang Science, Daegu, Korea) weighing 260 to 330 g at the time of surgery were used. Animals were individually housed in Plexiglas cages (28 cmW×42 cmD×18 cmH) throughout the study, and were maintained in a controlled environment: 22~24℃ ambient temperature and 12:12 hour light-dark cycle (lights on from 7:00 to 19:00). Commercial pellet food and tap water were available ad libitum except on the day of the experiment. The rats were sacrificed by overdose of CO2 inhalation after the completion of the experiments.
Surgery for EEG electrode implantation
After at least seven days of habituation, EEG and electromyogram (EMG) recording electrodes were implanted in rats under general anesthesia (0.4 mg/kg of medetomidine and 60 mg/kg of ketamine, i.p.). Surgical procedures were similar to those used in the previous study [16]. In brief, after the rats showed no purposeful movement in response to tail pinching, they were secured in the stereotaxic apparatus. The scalp was additionally anesthetized with a subcutaneous injection of 2% lidocaine. Then, the midline of the scalp was incised and the skull surface was cleaned using a periosteal elevator and gauze. Four small holes were drilled bilaterally into the parietal bones (5.0 mm posterior and 2.5 mm lateral from bregma) and the interparietal bones (10.0 mm posterior and 1.25 mm lateral from bregma) without perforating the dura mater using a low-speed burr (ball diameter 0.8 mm). Gold-plated stainless steel screw electrodes (tip diameter 1.1 mm) were inserted into the holes. Two screw electrodes over the cerebellum (on the interparietal bone) served as reference and ground electrodes. Pins connected to screw electrodes with enamel-coated copper wires and EMG wires were arranged in 3×2 matrices and fixed over the skull with dental acrylic. For 3 days including the day of surgery, systemic antibiotics (Cephradine inj., Korea Schnell pharma Co., Ltd., Gyeonggido, Korea) and analgesics (Butophan inj., Myungmoon pharm. Co., Ltd., Seoul, Korea) were administered to prevent infection and pain during recovery.
Experimental procedures
General appearance and body weight of rats were examined every day, and only the rats in good condition with no body weight loss were used for the experiment. After at least one week of recovery, the animals were habituated to the experimental environment for 2 days. The recording chamber had a light source and an electric fan worked by direct current, which was used in the diurnal period to mask environmental sounds. A swivel and flexible tether cable system allowed the rats freely move. The ambient temperature in the recording cage was maintained at 22±1℃ for the Control and SD groups. On the day of the experiment, the rats were placed in a recording chamber at 10:00, and 2-hour habituation was allowed in the recording chamber. Recording was established from 12:00 to 18:00. The rats received treatments from 12:00 to 14:00, followed by 4-hour recovery. The first treatments were ad libitum sleep (group 'Control'), total sleep deprivation for 120 minutes (group 'SD'), delta wave-predominant level of isoflurane anesthesia for 90 minutes and 30 minutes washout (group 'ISO-1'), and burst-suppression state of isoflurane anesthesia for 90 minutes and 30 minutes washout (group 'ISO-2'). During the 4-hour recovery, no treatments were administered without EEG and EMG recording. All rats underwent the four above-mentioned trials and were assigned one of the four treatments according to a balanced crossover design with at least 1-week intervals.
Sleep deprivation and anesthesia
Total sleep deprivation was achieved by a gentle brush touch. When delta waves appeared on the computer screen, several gentle brush-rubs on the tail or chin were applied until the rats moved.
Anesthesia was achieved as follows: a cotton ball was soaked with 1.0 ml of isoflurane solution using a syringe and then placed in a transparent plastic jar (20 cm in the diameter, 20 cm in the height); the jar was then capped. After 1 minute, the rat was disconnected from the recording cable and put into the jar. Immediately after the rat lost its righting reflex, it was transferred to a face mask delivery system and the recording cable was re-connected. Loss of righting reflex was accomplished within 2 minutes in all rats. Isoflurane was delivered using a syringe infusion pump (Syringe infusion pump 22; Harvard Apparatus, South Natick, MA, USA) and a rodent ventilator (7025 Rodent ventilator; Ugo Basile, Italy). A glass syringe (Becton-Dickinson, NJ, USA) containing isoflurane solution was put in the infusion pump, and the infusion pump device and syringe were cooled with an ice-pack to keep isoflurane from over-evaporating due to heating. PE-50 tubing (length of 30 cm) was connected to the needle of the glass syringe, and the free end of the tubing was inserted in the gas-delivery tube of the ventilator. Isoflurane infusion rates were 0.12~0.13 ml/minute in ISO-1 group and 0.16~0.17 ml/minute in ISO-2 group with oxygen (2.5 l/minute). Ventilator setting was 50 strokes/minute and 8.0 ml of tidal volume. Rats breathed spontaneously and body temperature was maintained above 37.0℃ using a water blanket; during anesthesia, ambient temperature in the recording box was maintained at 25~26℃ using a heater located outside of the chamber.
Recording
Two channel EEG signals over the parietal cortex were measured monopolarly with respect to the reference electrode via a bioelectric amplifier (Model 3500, A-M System, Inc., Carlsborg, WA, USA). The EEG signals were amplified×10,000 and filtered over a range of 1 to 100 Hz. A 60-Hz notch filter was included. EMG signal was filtered over a range of 30 to 300 Hz and amplified by 10,000. They were displayed and sampled by an AD converter (DAQ Pad 6015, National Instruments Inc., Union City, CA, USA) controlled by custom-made program with LabView 7.0 (National Instruments Inc., Union City, CA, USA) at a sampling rate of 1 kHz; the data were saved to a personal computer.
Evaluation and data analysis
Sleep-wake state was scored manually with 10-second epochs of EEG and EMG signals, and each 10-second epoch was assigned to one episode of vigilance. Each vigilance state [wakefulness (W), transitional stage to slow wave sleep (tSWS), slow wave sleep (SWS) and rapid-eye-movement sleep (REMS)] was determined as follows: W, EEG theta rhythm (wave) with a phasic or tonic EMG; tSWS, EEG delta waves occupy less than 50% of the epoch and/or a sleep spindle and EMG tone is markedly reduced compared to W; SWS, slower and higher delta waves compared to light sleep appear and occupy more than 50% of the epoch with a minimal EMG tone; REMS, EEG theta waves of about 7 Hz and/or large amplitude spindles occupy all epoch and EMG is minimal.
Burst suppression is defined by EEG amplitude of <15 µV for >1.0 second and preceded by high amplitude bursts. Burst suppression ratio (BSR) is the percentage of the epoch (60-second) where the EEG is suppressed (silent EEG).
The recording period was divided into 2 periods: treatment period and post-treatment period. The treatment period is 12:00~14:00 in Control, 12:00~13:50 in SD group, and 12:00~13:30 in ISO-1 and ISO-2 groups, respectively. The post-treatment period is the recording time from 14:00 to 18:00. In the treatment period, anesthetized states were scored as Delta state in ISO-1 and Burst state in ISO-2. For evaluation of sleep-wake architecture in the treatment period and 4-hour post-treatment period in 30-minute blocks, three parameters were evaluated: 1) episode duration ratio: recording time spent in W, tSWS, SWS and REMS per recording time; 2) number of episodes: number of occurrences of each sleep-wake state per hour; 3) mean episode duration: mean duration of each episode.
In order to quantify the sleep pressure, the latency to 1 hour of sleep was calculated: the amount of time from 14:00 to the accumulation of a total of 360 epochs (i.e., 1 hour) scored as sleep (without regard to tSWS, SWS, REMS) [17].
Statistical analysis
All data were expressed as mean±S.D. The differences among the experimental groups during the treatment period, the post-treatment period and the latency to 1 hour of sleep were compared with one-way analysis of variance (ANOVA) followed by Bonferroni adjustment. A one-way ANOVA and two-factor repeated measures ANOVA followed by Bonferroni correction were included to identify differences between groups in the time course of the post-treatment period. Values of p<0.05 were considered significant.
RESULTS
BSR
BSR was 0.69±0.9% in ISO-1 group and 36.45±12.8% in ISO-2 group. No burst suppression was observed in Control and SD groups.
Total duration ratio (Fig. 1)
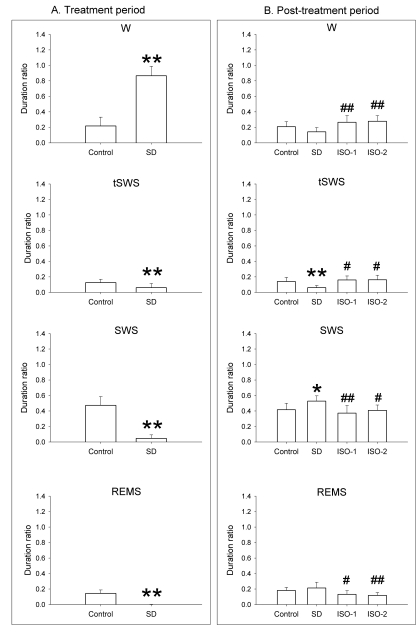
Fig. 1.
Duration ratio of each episode to total recording time in rats during the treatment period and the post-treatment period. In the treatment period, the rats were given ad libitum sleep, sleep deprivation, and delta wave-predominant state or burst suppression pattern-predominant state of isoflurane anesthesia ('Control', 'SD', 'ISO-1' and 'ISO-2' groups, respectively) during the treatment period. In the treatment period, ISO-1 and ISO-2 groups received 1.5-hour anesthesia followed by 30-minute washout, and the duration ratio of the delta-predominant state was 0.91 (0.1) and burst suppression-predominant state was 0.95 (0.1). Vigilance stages during 4-hour post-treatment period following 2-hour treatments were scored as 4 episodes using electroencephalogram and electromyogram: wakefulness (W), transition to slow-wave-sleep (tSWS), slow-wave-sleep (SWS) and rapid-eye-movement sleep (REMS). Data were expressed as mean (SD), n=9 per group. Data were analyzed by one-way ANOVA followed by a Bonferroni test. *p<0.05 and **p<0.01 vs. Control group, and #p<0.05 and ##p<0.01 vs. SD group.
In the treatment period, SD group (0.87±0.12) showed a significantly increased W time and a significantly decreased sleep time compared to the Control group (0.22±0.11).
In the post-treatment period, no significant difference in sleep-wake parameters was observed between Control and isoflurane treatment groups. Bonferroni test revealed ISO-1 and ISO-2 groups had a significantly longer W duration ratio than the SD group (p<0.01). Duration ratio for tSWS in ISO-1 and ISO-2 groups was significantly higher than in the SD group, whereas that in the SD group was significantly decreased compared to Control. SWS of SD group was significantly enhanced compared to Control; in contrast, SWS of ISO-1 and ISO-2 groups were significantly lower than that of the SD group. ISO-1 and ISO-2 groups had significantly lower values of REMS than SD group.
Number of episodes/hour (Fig. 2)
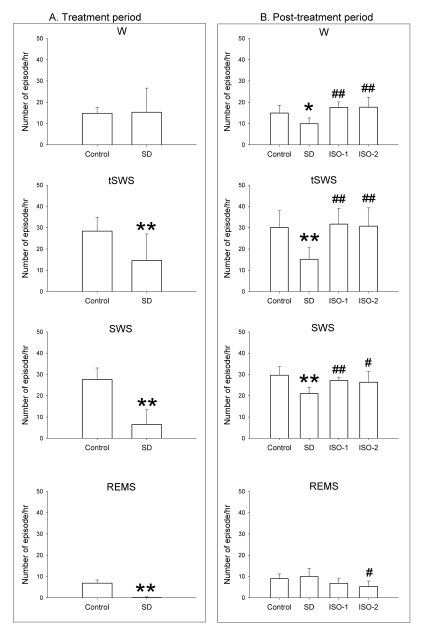
Fig. 2.
Number of episodes per hour in rats during the treatment period and the post-treatment period. The rats were given ad libitum sleep, sleep deprivation, and delta wave-predominant state or burst suppression pattern-predominant state of isoflurane anesthesia ('Control', 'SD', 'ISO-1' and 'ISO-2' groups, respectively) during the treatment period. In the treatment period, delta predominant state occurred at 2.72 (2.7) and burst suppression predominant state occurred at 0.85 (0.5). Vigilance stages during 4-hour post-treatment period following 2-hour treatments were scored as 4 episodes using electroencephalogram and electromyogram: wakefulness (W), transition to slow-wave-sleep (tSWS), slow-wave-sleep (SWS) and rapid-eye-movement sleep (REMS). Data were expressed as mean (SD), n=9 per group. Data were analyzed by one-way ANOVA followed by a Bonferroni test. *p<0.05 and **p<0.01 vs. Control group, and #p<0.05 and ##p<0.01 vs. SD group.
In the treatment period, sleep deprivation significantly decreased all vigilance values, except at W of SD group, when compared to Control. The values for Delta state and Burst state were 2.72±2.7 and 0.85±0.5, respectively.
In the post-treatment period, all vigilance states between Control and isoflurane treatment groups showed no statistically significant differences. SD group showed a significant decrease for W, tSWS and SWS when compared to the Control group, whereas ISO-1 and ISO-2 groups showed a significant enhancement of the same vigilance states compared to the SD group. For REMS, only the ISO-2 group showed a significant decrease compared to the SD group.
Mean episode duration (Fig. 3)
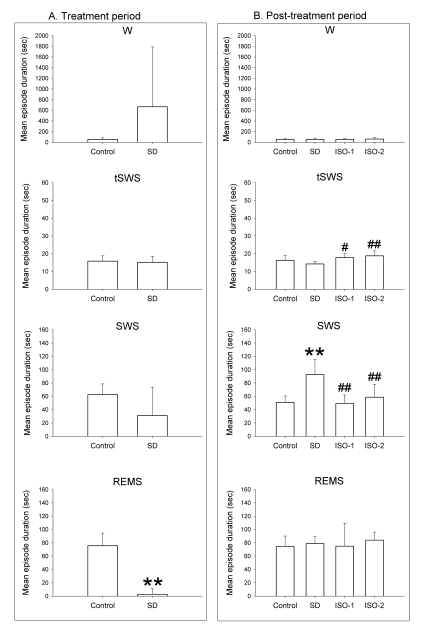
Fig. 3.
Mean episode duration during total recording time in rats during the treatment period and the post-treatment period. In the treatment period, the rats were given ad libitum sleep, sleep deprivation, and delta wave-predominant state or burst suppression pattern-predominant state of isoflurane anesthesia ('Control', 'SD', 'ISO-1' and 'ISO-2' groups, respectively). In the treatment period, the values of the delta-predominant state was 3006 (2448.1) and that of the burst suppression-predominant state was 4701.43 (1365.4). Vigilance stages during the 4-hour post-treatment period following 2-hour treatments were scored as 4 episodes using electroencephalogram and electromyogram: wakefulness (W), transition to slow-wave-sleep (tSWS), slow-wave-sleep (SWS) and rapid-eye-movement sleep (REMS). Data were expressed as mean (SD), n=9 per group. Data were analyzed by one-way ANOVA followed by a Bonferroni test. **p<0.01 vs. Control group, and #p<0.05 and ##p<0.01 vs. SD group.
In the treatment period, the mean episode durations for Delta state and Burst state were 3,006.0±2,448.1 and 4,701±1,365.4 seconds, respectively.
In the post-treatment period, ANOVA revealed that values for W and REMS between groups were not significantly different (F=0.272, p=0.85 at W; F=0.36, p=0.783 at REMS). tSWS in ISO-1 and ISO-2 groups persisted significantly longer than in SD group. Mean duration of SWS in SD group was significantly longer than in Control group, whereas SWS in ISO-1 and ISO-2 groups was significantly lower than in the SD group.
The latency to 1 hour of sleep (Fig. 4)
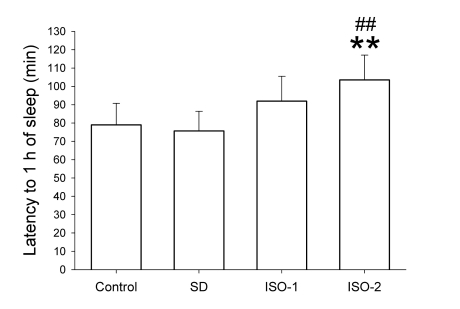
Fig. 4.
The latency to 1 hour of sleep in rats after ad libitum sleep, sleep deprivation and isoflurane anesthesia according to groups. The rats were given ad libitum sleep, sleep deprivation, and delta wave-predominant state or burst suppression pattern-predominant state of isoflurane anesthesia (group 'Control', 'SD', 'ISO-1' and 'ISO-2', respectively) during the treatment period. Vigilance stages during the 4-hour post-treatment period following 2-hour treatments were scored as 4 episodes using electroencephalogram and electromyogram: wakefulness (W), transition to slow-wave-sleep (tSWS), slow-wave-sleep (SWS) and rapid-eye-movement sleep (REMS). In order to quantify the sleep pressure, the latency to 1-hour of sleep was calculated: the amount of time from 14:00 to the accumulation of total of 360 epochs (i.e., 1 hour) scored as sleep (without regard to the tSWS, SWS, REMS). Data were expressed as mean (SD bar), n=9 per group. Data were analyzed by one-way ANOVA followed by a Bonferroni test. **p<0.01 vs. Control group, and ##p<0.01 vs. SD group.
The latency to 1 hour of sleep values were 79.0±11.7, 75.7±10.7, 92.0±13.5 and 103.6±13.5 minutes in Control, SD, ISO-1 and ISO-2 groups, respectively. One-way ANOVA revealed that treatments statistically affected the latency to 1 hour of sleep (F=10.39 and p<0.001), and Bonferroni test demonstrated that ISO-2 group had a sleep-delaying effect relative to Control and SD groups.
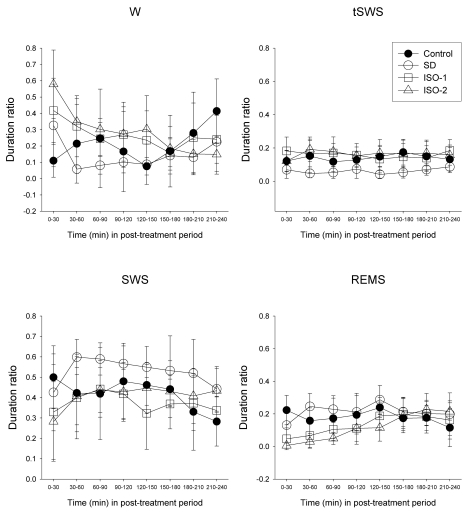
Time course of duration ratio in post-treatment period
Repeated measures ANOVA revealed that treatment (F=6.78, p=0.001 at W; F=8.54, p<0.001 at tSWS; F=5.97, p=0.003 at SWS; F=6.03, p=0.003 at REMS) and time course (F=4.69, p<0.001 at W; F=2.72, p=0.010 at SWS; F=7.47, p<0.001 at REMS) were significant, except at tSWS (F(7, 203)=0.58, p=0.774). Bonferroni test indicated that no vigilance states showed statistically significant differences between Control group and isoflurane treatment groups. Sleep deprivation significantly decreased tSWS (p=0.009) and increased SWS (p=0.039) compared to Control. All vigilance states in ISO-1 and ISO-2 groups were significantly different compared to SD group (Fig. 5).
Fig. 5.
Time course of the duration ratio at each episode in rats during the treatment period and the post-treatment period. The rats were given ad libitum sleep, sleep deprivation, and delta wave-predominant state or burst suppression pattern-predominant state of isoflurane anesthesia ('Control', 'SD', 'ISO-1' and 'ISO-2' groups, respectively) during the treatment period. Vigilance stages during the 4-hour post-treatment period following 2-hour treatments were scored as 4 episodes using electroencephalogram and electromyogram: wakefulness (W), transition to slow-wave-sleep (tSWS), slow-wave-sleep (SWS) and rapid-eye-movement sleep (REMS). Data were expressed as mean (SD), n=9 per group. Data were analyzed by two-factor repeated measures ANOVA followed by a Bonferroni test. Statistical results are not marked in the figure but are described in the text.
One-way ANOVA followed by Bonferroni test (Fig. 5) indicated that the duration ratio of the initial 30-minute W values in ISO-1 and ISO-2 groups was significantly decreased compared to Control (p=0.02 and p<0.001, respectively) and the following 30-minute data were significantly decreased compared to SD group (p=0.021 and p=0.012, respectively). Duration ratio of tSWS in ISO-1 and/or ISO-2 groups was significantly decreased at most analytical times compared to that in SD group. Slow-wave-sleep values among all groups were not significantly different except at one analytical time. Duration ratio of REMS in ISO-1 and/or ISO-2 groups during initial 1.5 hour was significantly decreased compared to Control and SD groups. In particular, REMS values in ISO-2 showed a significant decrease compared to the Control group during the initial 1.5 hour (p<0.05).
DISCUSSION
The present results demonstrate that there is no sleep rebound following anesthesia, and that isoflurane anesthesia may substitute for sleep irrespective of the level of anesthesia. In addition, the dominant EEG state during isoflurane anesthesia was not a cause of the selective sleep-wake episode difference, but the ISO-2 level of anesthesia tended to extend the latency to 1 hour of sleep.
In the present study, the time course of duration ratio did not show significant changes between both isoflurane anesthesia groups and the Control group. This is somewhat different from the study by Tung and co-workers [3]. They noted a significant decrease in NREMS and REMS during the first 4-hour period after a 30-minute washout period following 11.5-hour of sedative infusion with propofol. The dissimilar results were likely caused by the rapid washout of isoflurane. Moreover, in the present study, mean values for W and tSWS in both isoflurane-treated groups initially increased, but those for SWS and REMS decreased in the same time period. However, W enhancement and REMS reduction persisted for about 150 minutes although it was not significant. Of course, sleep disturbance by isoflurane was assumed, but because tSWS and SWS were already recovered to control value, this assumption is unlikely. We hypothesized that burst and suppression pattern during anesthesia could substitute for REMS. REMS reduction following isoflurane anesthesia had been presumed to be caused by a sufficient production of REMS during anesthesia, because generally total sleep deprivation results in a total sleep rebound and a selective-REMS deprivation leads to a proportional REMS enhancement during recovery [2,14]. In the present study, two-factor repeated measures failed to show significant REMS reduction, and there was no correlation between REMS duration ratio and BSR (r=-0.25, p=0.367). Therefore, we concluded that burst and suppression produced by isoflurane anesthesia do not selectively substitute for REMS episodes. [0]In contrast, one-way ANOVA showed meaningful results, including a significant W increase and REMS decrease in ISO-1 and/or ISO-2 groups during the initial post-treatment period compared to Control and/or SD groups without significant SWS change. Especially, REMS values in ISO-2 were consistently and significantly decreased during the initial 1.5-hour compared to the Control and SD groups. That is, W enhancement and REMS reduction indicate a sleep-like process, and the REMS-like process may occur during isoflurane anesthesia. These results suggest that isoflurane anesthesia could substitute for total sleep, and furthermore that the level of isoflurane anesthesia that produces predominant burst-suppression has a REMS-selective-substitution effect. The homeostatic sleep response after sleep deprivation includes increases in the intensity and amount of non-rapid-eye-movement sleep (NREMS) and REMS [18]. Tung and co-workers showed that a recovery process from sleep deprivation may occur during the anaesthetized state by comparing the NREMS and REMS distribution in rats given 6-hour propofol anesthesia to that in rats allowed to sleep ad libitum following a 24-hour period of sleep deprivation [2]. The sleep response patterns during recovery in both groups were similar, and no delayed rebound effects were observed in either group for 72 hours after sleep deprivation. However, because the present study design was different from theirs, whether sleep rebound was provided during isoflurane anesthesia was not clear. Nevertheless, if a sleep-like process did not occur during anesthesia, the subsequent sleep response should be confronted with a virtual sleep rebound as in the SD group, but the present results showed no such rebound. Thus, a natural sleep-like process seems to occur in the period of isoflurane anesthesia.
Previous results indicated that general anesthesia itself is unlikely to play a major role in disrupting sleep-wake architecture [19-21]. Moot and Knill [20] demonstrated that SWS was moderately suppressed and Stage 2 sleep reciprocally slightly increased in the first post-anesthetic night, but there were no significant changes in the sleep onset latency, the total quantity of sleep, or the proportion of REMS. Although the previous results do not mean isoflurane anesthesia could substitute for sleep, at least they indicated that anesthesia with isoflurane leads to only a modest and transient alteration in nocturnal sleep. Abdominal surgery under isoflurane anesthesia suppressed SWS and nearly eliminated REMS on the first night after surgery, and on subsequent nights REMS gradually increased to greater than the preoperative amount, furthermore REMS was intensified [19]. In the present study, no parameters during the sleep recovery period were affected by anesthesia when compared to Control. Thus, the previously observed REMS elimination on the first night may be not caused by isoflurane. However, previous studies differed from our experiment in their purpose and in the time-points of anesthesia. In particular, because the anesthesia was included during the natural waking period, no sleep deprivation or sleep-like process was needed. In contrast, we treated the rats during their natural sleep period and did not apply the surgical intervention as in the previous study [3].
Burst suppression predominant state of anesthesia significantly delayed the latency to accumulation of 1 hour of sleep. The time course of the duration ratio showed that REMS in the ISO-2 and ISO-1 groups was nearly absent in the initial sleep recovery period, and REMS subsequently gradually increased but still had a lower value than in the other group. Therefore the significantly delayed latency to 1 hour of sleep in the ISO-2 group was caused by an initial prolonged suppression of REMS and transient SWS, and this initial response was similar to previous results [3,19]. Latency to 1 hour of sleep might indicate that deeper isoflurane anesthesia produces deeper sleep.
In a differing view on similar results, a previous report insisted that isoflurane may contribute to postoperative sleep disturbance (POSD), but ketamine may decrease POSD [22]. In fact, the sleep response after anesthesia is open to various interpretations [3]. Nevertheless, previous studies assume that sleep and anesthesia may share common neural substrates [2-7]. A ketamine dose evoking a light level of anesthesia produced patterns of Fos expression in the CNS that were similar to those in wakefulness [5]. Therefore, the previous results might be analyzed in a different way, namely, that a natural sleep-like process could be induced during isoflurane anesthesia and a natural wakefulness-like process could be provided during ketamine anesthesia. On the other hand, isoflurane anesthesia itself may not contribute to the development of POSD.
Although the EEGs from the ISO-1 and ISO-2 groups during isoflurane anesthesia (i.e., treatment period) were also recorded, sleep and wake stages during the treatment period could not be scored, especially SWS and REMS during anesthesia. No criteria for determination of SWS and REMS during anesthesia were found, and scoring was abandoned during the treatment period.
In conclusion, isoflurane anesthesia may restore sleep homeostasis because neither level of isoflurane anesthesia resulted in sleep-deprivation. Although neither level of anesthesia clearly disturbed the normal sleep-wake architecture in the post-treatment period, a significant delay in the latency to 1-hour of sleep and a decrease in the time course of the duration ratio in the ISO-2 group suggested that a deeper level of isoflurane anesthesia could disturb or delay the restoration of normal sleep-wake architecture.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-H00015) and by BK21 Project.
ABBREVIATIONS
- EEG
electroencephalogram
- EMG
electromyogram
- NREMS
non-rapid-eye-movement sleep
- W
wakefulness
- tSWS
transitional to slow-wave sleep
- SWS
slow-wave sleep
- REMS
rapid eye movement sleep
References
- 1.Tung A, Mendelson WB. Anesthesia and sleep. Sleep Med Rev. 2004;8:213–225. doi: 10.1016/j.smrv.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Tung A, Bergmann BM, Herrera S, Cao D, Mendelson WB. Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology. 2004;100:1419–1426. doi: 10.1097/00000542-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Tung A, Lynch JP, Mendelson WB. Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesth Analg. 2001;92:1232–1236. doi: 10.1097/00000539-200105000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Tung A, Szafran MJ, Bluhm B, Mendelson WB. Sleep deprivation potentiates the onset and duration of loss of righting reflex induced by propofol and isoflurane. Anesthesiology. 2002;97:906–911. doi: 10.1097/00000542-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Nelson LE, Franks N, Maze M, Chamberlin NL, Saper CB. Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol. 2008;508:648–662. doi: 10.1002/cne.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–818. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 9.Chen HI, Tang YR. Sleep loss impairs inspiratory muscle endurance. Am Rev Respir Dis. 1989;140:907–909. doi: 10.1164/ajrccm/140.4.907. [DOI] [PubMed] [Google Scholar]
- 10.Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun. 2002;16:503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 11.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 12.Sleigh JW, Andrzejowski J, Steyn-Ross A, Steyn-Ross M. The bispectral index: a measure of depth of sleep? Anesth Analg. 1999;88:659–661. doi: 10.1097/00000539-199903000-00035. [DOI] [PubMed] [Google Scholar]
- 13.Tung A, Lynch JP, Roizen MF. Use of the BIS monitor to detect onset of naturally occurring sleep. J Clin Monit Comput. 2002;17:37–42. doi: 10.1023/a:1015404803637. [DOI] [PubMed] [Google Scholar]
- 14.Maloney KJ, Mainville L, Jones BE. c-Fos expression in dopaminergic and GABAergic neurons of the ventral mesencephalic tegmentum after paradoxical sleep deprivation and recovery. Eur J Neurosci. 2002;15:774–778. doi: 10.1046/j.1460-9568.2002.01907.x. [DOI] [PubMed] [Google Scholar]
- 15.Sapin E, Lapray D, Berod A, Goutagny R, Leger L, Ravassard P, Clement O, Hanriot L, Fort P, Luppi PH. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS ONE. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang HS, Kim JY, Kim SH, Lee MG. Role of dopamine receptors on electroencephalographic changes produced by repetitive apomorphine treatments in rats. Korean J Physiol Pharmacol. 2009;13:147–151. doi: 10.4196/kjpp.2009.13.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisor JP, Edgar DM, Yesavage J, Ryan HS, McCormick CM, Lapustea N, Murphy GM., Jr Sleep and circadian abnormalities in a transgenic mouse model of Alzheimer's disease: a role for cholinergic transmission. Neuroscience. 2005;131:375–385. doi: 10.1016/j.neuroscience.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Borbely AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14:171–182. doi: 10.1016/0166-4328(84)90186-4. [DOI] [PubMed] [Google Scholar]
- 19.Knill RL, Moote CA, Skinner MI, Rose EA. Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology. 1990;73:52–61. doi: 10.1097/00000542-199007000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Moote CA, Knill RL. Isoflurane anesthesia causes a transient alteration in nocturnal sleep. Anesthesiology. 1988;69:327–331. doi: 10.1097/00000542-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Kavey NB, Ahshuler KZ. Sleep in herniorrhaphy patients. Am J Surg. 1979;138:683–687. doi: 10.1016/0002-9610(79)90348-9. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi S, Kushikata T, Matsuki A. Effects of isoflurane and ketamine on sleep in rabbits. Psychiatry Clin Neurosci. 2001;55:239–240. doi: 10.1046/j.1440-1819.2001.00840.x. [DOI] [PubMed] [Google Scholar]