Abstract
Vascular NADPH oxidase plays a pivotal role in producing superoxide in endothelial cells and thus acts in the initiation and development of inflammatory cardiovascular diseases such as atherosclerosis. Epigallocatechin-3-gallate (EGCG), the major catechin derived from green tea, has multiple beneficial effects for treating cardiovascular disease but the effect of EGCG on the expression of vascular NADPH oxidase remains unknown. In this study, we investigated the mechanism(s) by which EGCG might inhibit the expression of subunits of NADPH oxidase, namely p47phox, p67phox and p22phox, induced by angiotensin II (Ang II) in human umbilical vein endothelial cells. Ang II increased the expression levels of p47phox, p67phox, and p22phox, but EGCG counteracted this effect on p47phox. Moreover, EGCG did not affect the production of reactive oxygen species induced by Ang II. These data suggest a novel mechanism whereby EGCG might provide direct vascular benefits for treating inflammatory cardiovascular diseases.
Keywords: EGCG, NADPH oxidase, Angiotensin II, ROS, HUVEC
INTRODUCTION
Atherosclerosis is a chronic systemic disease of the vasculature with an inflammatory component. Elevated vascular superoxide anion formation has been implicated in the initiation and progression of hypertension and atherosclerosis [1]. The production of reactive oxygen species (ROS) such as superoxide is increased by cytokines, peptide hormones and shear stress after vascular injury. The major source of ROS in vascular endothelial cells is NADPH oxidase (Nox). Nox4 produces mainly H2O2, while Nox1 generates mostly O2·- that is later converted to H2O2. Nox4 is responsible for basal H2O2 production, while O2·- production in nonstimulated and Ang II-stimulated cells depends on Nox1 [2]. ECs express all the components of Nox including gp91phox, p22phox, p47phox, p67phox, p40phox and the small G protein Rac1 [3].
Numerous studies have demonstrated that activation of the angiotensin II (Ang II) type 1 (AT1) receptor plays an important role in the pathogenesis of cardiovascular diseases. Ang II, an important stimulant for vascular NADPH oxidase, generates superoxide and other ROS, which stimulates IkappaB degradation and NF-kB activation. This activation was shown to enhance the expression of vascular cell adhesion molecule 1 involved in the early stages of atherosclerosis [4,5]. Ang II-induced ROS can cause peroxynitrite generation- and lipid oxidation. They also lead to the activation of redox-sensitive genes controlling chemotaxis- and the production of adhesion molecules, proinflammatory cytokines and matrix metalloproteinases, all of which are involved in the initiation and progression of atherosclerosis [6]. Moreover, overproduction of ROS leads to endothelial nitrous oxide synthase uncoupling, mitochondrial dysfunction and impaired antioxidant defenses resulting from the depletion of intracellular NADPH [7]. Recently, Ang II type 2 receptor upregulation reduced atherogenesis, possibly by modulating oxidative stress and the pro-inflammatory cascade, mediated via Akt-1 [8].
Flavonoids are polyphenolic compounds widely distributed in plants and their consumption might be associated with decreased risks for some chronic degenerative diseases in humans [9]. Epidemiological research in Japan has shown that green tea consumption can be protective against coronary atherosclerosis [10]. Recent lines of evidence have shown that epigallocatechin-3-gallate (EGCG), a flavanol that is enriched in green tea, reduces vascular adhesion molecule-1 expression [11,12] and monocyte chemotactic protein-1 production in vascular endothelium [13]. However, the effect of EGCG on the expression of NADPH oxidase, the major enzyme for ROS production in vascular endothelium, remains unknown. Specific inhibitors of NADPH oxidase might be helpful for the treatment of atherosclerosis.
In this study, we hypothesized that EGCG reduces the expression of NADPH oxidase and ROS production by Ang II in human umbilical vein endothelial cells (HUVECs).
METHODS
Materials
All antibodies for western blotting were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The culture medium was obtained from Invitrogen (Carlsbad, CA, USA). Ang II, EGCG and other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise specified.
Cell culture
HUVECs were obtained from Clonetics (Walkersville, MD, USA). They were grown in medium 199 containing 0.1 mg/ml heparin, 25 µg/ml endothelial cell growth factor (Biomedical Technologies: Stoughton, MA, USA), 2 mM L-glutamine, 100 U/ml penicillin G, 100 µg/ml streptomycin and 20% fetal bovine serum (FBS). The medium was renewed every two days until confluence, when cells were subcultured at a 1:3 ratio and then cultured in an atmosphere of 95% air and 5% CO2 at 37℃.
Western blot analysis
HUVEC cultures were starved for 12 h and treated with the desired drugs for desired times. Cells were lysed in ice-cold buffer (20 mM Tris-HCl pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM Na3VO4, 1 mM PMSF and 1 µg/ml leupeptin). The lysates were sonicated and centrifuged (12,000 rpm, 20 min). Protein concentration was measured by the Bradford method. Equal amounts of protein (10 µg) were run on 12% SDS-PAGE and blotted onto polyvinylidene difluoride membranes. These were incubated with goat polyclonal antibodies (1:1,000) for subunits of NADPH oxidase. Secondary antigoat antibodies and enhanced chemiluminescence (ECL) Plus reagent kits (Amersham: Little Chalfont, Buckinghamshire, UK) were used for detection and subsequently exposed to ECL hyperfilms.
Detection of ROS
The cells were starved in phenol red-free M199 medium containing 1% FBS for 12 h and stimulated with Ang II and 2',7'-dichlorofluorescein diacetate for 2 h. Fluorescence signals were quantified (Molecular Devices: Sunnyvale, CA, USA).
Statistical analysis
Results are shown as the means±SEM from at least three independent experiments. Statistical significance between the means was assessed by one-way ANOVA followed by Turkey's multiple comparison test; p<0.05 was taken as statistically significant.
RESULTS
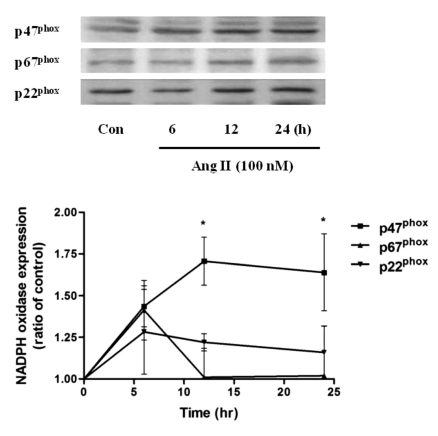
Ang II increased the levels of p47phox, p67phox and p22phox in HUVECs
To determine whether the expression of NADPH oxidase, the major enzyme for ROS production in vascular endothelium, would be affected by Ang II, HUVECs were treated with Ang II. Ang II (100 nM) increased the expression of p47phox, a non heme-containing, regulatory subunit of NADPH oxidase, in a time-dependent manner; the levels peaked at 12 h and were sustained until 24 h. However, p67phox, a non heme-containing, regulatory subunit of NADPH oxidase, levels peaked at 6 h and returned to basal levels at 12 h after Ang II treatment; p22phox, a heme-containing, catalytic subunit of NADPH oxidase, levels peaked at 6 h and decreased with time but were above basal levels at 24 h after Ang II treatment (Fig. 1). Thus, Ang II increases the protein level of endothelial NADPH oxidase subunits. However, the changes observed in p67phox, p22phox protein level are contrast to p47phox.
Fig. 1.
Effect of Ang II treatment (100 nM, 0~24 h) on the expression levels of NADPH oxidase subunits p47phox, p67phox and p22phox in HUVECs. Summary data are shown as the mean±SEM. *p<0.05 by Turkey's multiple comparison test.
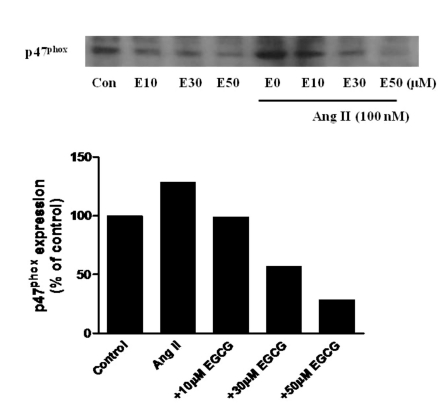
Effect of EGCG on the expression of p47phox induced by Ang II in HUVECs
To determine whether Ang II-stimulated p47phox, a non heme-containing, regulatory subunit of NADPH oxidase, expression would be affected by EGCG, HUVECs were pretreated for 0.5 h with 0~50 µM EGCG prior to treatment with Ang II (100 nM) for 24 h. Increasing concentrations of EGCG inhibited Ang II-induced p47phox expression (Fig. 2). Thus, EGCG, a major catechin obtained from green tea leaf, decreases the protein level of p47phox in a concentration-dependent manner.
Fig. 2.
Effect of EGCG on p47phox expression in HUVECs treated with Ang II (100 nM) for 24 h, 30 min after treatment with EGCG (10~50 µM).
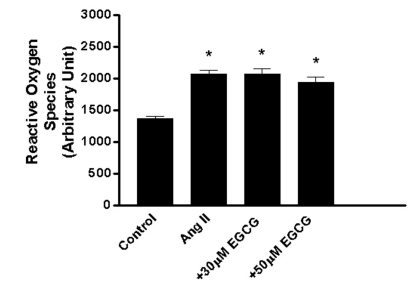
Effect of EGCG on the production of ROS induced by Ang II in HUVECs
To determine whether Ang II-induced ROS production would be affected by EGCG, HUVECs were pretreated with EGCG prior to treatment with Ang II. Ang II (100 nM) increased ROS production (151.5±4.2%, n=12, p<0.05) compared with control (no treatment) and this was not prevented by 30~50 µM EGCG (151.0±6.6%, n=12, p<0.05; 141.3±6.3%, n=12, p<0.05, respectively; Fig. 3). These data suggest that EGCG decreases the protein level of p47phox, a non heme-containing, regulatory subunit of NADPH oxidase, without affecting ROS.
Fig. 3.
Effect of EGCG (30~50 µM) on ROS induced by Ang II (100 nM) for 24 h in HUVECs. EGCG was pretreated 30 min before Ang II stimulation. Bars represent the mean±SEM. *p<0.05 by Turkey's multiple comparison test.
DISCUSSION
Numerous lines of evidence suggest that increased oxidative stress causes cardiovascular diseases. ROS such as superoxide and H2O2 produce vascular inflammation, resulting in atherosclerosis. Vascular NADPH oxidases are predominant sources of superoxide in the vasculature [14]. NADPH oxidase is composed of two essential membrane-bound components, gp91phox/Nox2 and p22phox, which compose flavocytochrome b558- and four cytosolic components, p47phox, p67phox, p40phox, and the small G protein rac1/2 [15]. Among the four cytosolic components of NADPH oxidase, p47phox is important for vascular ROS production [16]. Moreover, Nox4, a homologue of gp91phox/Nox2, might function as the major catalytic component of an endothelial NADPH oxidase [17]. The NADPH oxidases, Nox4 and Nox2, are major sources of ROS in endothelial cells and are implicated both in vasodilator dysfunction [18].
A peptide hormone, Ang II, a major stimulus for vascular NADPH oxidase, also plays an important role in atherosclerosis. AT 1 receptor enhanced the activation of nuclear factor NF-kappaB, which stimulated the production of proinflammatory cytokines. Activation of AT 1 receptor via inducible cyclooxygenase promoted biosynthesis of matrix metalloproteinases [19]. Ang II increased superoxide production in endothelial cells from wild-type mice, but not in those from p47(phox-/-) knockout mice [20]. Candesartan, an AT 1 receptor antagonist, reduced NADPH activity, attenuated the diabetes-induced over-expression of p22phox and led to a substantial improvement of endothelium-dependent vasodilatation in Sprague Dawley rats [21]. Irbesartan, an AT 1 receptor antagonist, attenuated atherosclerosis, and this effect was partly related to the inhibition of oxidative stress and inflammatory signal transduction pathways in high cholesterol-diet apolipoprotein E knock-out mice [22]. Ang II upregulated the expression levels of Nox4 and p22phox and enhanced Nox4 translocation to the cell membrane in human endothelial cells [23].
Catechin, a flavanol derived from green tea, possesses antioxidant, antiangiogenesis and antiproliferation activities to the prevention and treatment of cardiovascular diseases [24]. EGCG, the major component of green tea catechins, is known to have many effects that will translate to vascular benefits including suppression of vascular hypertrophy [25]. EGCG decreased the risk of cardiovascular disease by reducing inflammatory markers in rats fed an atherogenic diet [26]. EGCG inhibited endothelin-1-stimulated generation of C-reactive protein in vascular smooth muscle cells, which relieved the inflammatory response and oxidative stress by blocking ROS signals and producing an antiatherosclerotic effect [27]. EGCG inhibited cytosolic subunits of NADPH oxidase from translocating into membrane suggesting inhibition of NADPH oxidase activity in cutaneous mastocytoma cells [28]. Flavonoids prevented oxidative stress in activated monocytes. These inhibitory effects may involve downregulation of PKC-dependent NADPH oxidase pathway, phosphorylation of p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated protein kinase in human monocytic THP-1 cells [29].
In this study, we investigated whether EGCG could reduce Ang II-mediated NADPH oxidase expression in HUVECs. Ang II increased the levels of subunits of NADPH oxidase, namely p47phox, p67phox and p22phox. However, EGCG prevented the p47phox, non heme-containing, regulatory subunit of NADPH oxidase, expression induced by Ang II in a concentration-dependent manner. This novel finding for the effects of green tea/EGCG is complemented by findings that the expressional suppression of NADPH oxidase might be an important component of the vascular protective effect of resveratrol, an important antioxidant found in grapes and wine [30]. Moreover, dietary flavonoid, quercetin prevented Ang II-induced endothelial dysfunction by inhibiting the overexpression of p47phox and the subsequent increased O2·- production, resulting in increased nitric oxide bioavailability [31]. Quercetin and catechin regulated S100B-activated oxidant stress-sensitive pathways through blocking p47phox protein expression in human monocytes [32]. Together, these results show that flavonoids have a common inhibitory effect on NADPH oxidase expression in a number of different cell types in a variety of conditions.
To understand the potential mechanism(s) for EGCG attenuation of p47phox expression by Ang II, we confirmed that ROS attenuation in HUVECs induced by EGCG led to an inhibition of p47phox expression. Our data demonstrated that EGCG did not affect ROS induced by Ang II. However, EGCG was effective in reducing CoCl2-derived ROS [33]. Ang II activated p38 MAPK and increased ROS in the vasculature [34]. Therefore, an inhibition of NADPH oxidase expression by EGCG may be insufficient to affect ROS production in HUVECs. And an interference on ROS by EGCG may not be necessary for an inhibition of endothelial NADPH oxidase expression.
In conclusion, the antiatherosclerotic activity of EGCG is likely not associated with decreased ROS production although there may be some benefit through inhibiting the expression of NADPH oxidase induced by Ang II.
ACKNOWLEDGEMENTS
This work was supported by the research grants of the Chungbuk National University in 2008.
ABBREVIATIONS
- EGCG
epigallocatechin-3-gallate
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate-oxidase
- HUVEC
human umbilical vein endothelial cell
- Ang II
angiotensin II
- MAPK
mitogen-activated protein kinase
- ROS
reactive oxygen species
References
- 1.Rueckschloss U, Duerrschmidt N, Morawietz H. NADPH oxidase in endothelial cells: impact on atherosclerosis. Antioxid Redox Signal. 2003;5:171–180. doi: 10.1089/152308603764816532. [DOI] [PubMed] [Google Scholar]
- 2.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NADPH oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Schmeisser A, Garlichs CD, Plotze K, Damme U, Mugge A, Daniel G. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res. 1999;44:215–222. doi: 10.1016/s0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 5.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappa B activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 6.Wassmann S, Nickenig G. Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J Hypertens Suppl. 2006;24:S15–S21. doi: 10.1097/01.hjh.0000220402.53869.72. [DOI] [PubMed] [Google Scholar]
- 7.Gao L, Mann GE. Vascular NADPH oxidase activation in diabetes: a double-edged sword in redox signaling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 8.Hu C, Dandapat A, Chen J, Liu Y, Hermonat PL, Carey RM, Mehta JL. Over-expression of angiotensin II type 2 receptor (agtr2) reduces atherogenesis and modulates LOX-1, endothelial nitric oxide synthase and heme-oxygenase-1 expression. Atherosclerosis. 2008;199:288–294. doi: 10.1016/j.atherosclerosis.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 10.Sasazuki S, Kodama H, Yoshimasu K, Liu Y, Washio M, Tanaka K, Tokunaga S, Kono S, Arai H, Doi Y, Kawano T, Nakagaki O, Takada K, Koyanagi S, Hiyamuta K, Nii T, Shirai K, Ideishi M, Arakawa K, Mohri M, Takeshita A. Relation between green tea consumption and the severity of coronary atherosclerosis among Japanese men and women. Ann Epidemiol. 2000;10:401–408. doi: 10.1016/s1047-2797(00)00066-1. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig A, Lorenz M, Grimbo N, Steinle F, Meiners S, Bartsch C, Stangl K, Baumann G, Stangl V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced vcam-1 expression and monocyte adhesion to endothelial cells. Biochem Biophys Res Commun. 2004;316:659–665. doi: 10.1016/j.bbrc.2004.02.099. [DOI] [PubMed] [Google Scholar]
- 12.Chae YJ, Kim CH, Ha TS, Hescheler J, Ahn HY, Sachinidis A. Epigallocatechin-3-O-gallate inhibits the angiotensin II-induced adhesion molecule expression in human umbilical vein endothelial cell via inhibition of MAPK pathways. Cell Physiol Biochem. 2007;20:859–866. doi: 10.1159/000110446. [DOI] [PubMed] [Google Scholar]
- 13.Ahn HY, Xu Y, Davidge ST. Epigallocatechin-3-O-gallate inhibits TNFα-induced monocyte chemotactic protein-1 production from vascular endothelial cells. Life Sciences. 2008;82:964–968. doi: 10.1016/j.lfs.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Niemiec P, Zak I. Vascular NADPH oxidases-role in the pathogenesis of atherosclerosis. Postepy Biochem. 2005;51:1–11. [PubMed] [Google Scholar]
- 15.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 16.Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med. 2002;32:1116–1122. doi: 10.1016/s0891-5849(02)00789-x. [DOI] [PubMed] [Google Scholar]
- 17.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NADPH oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 18.Dworakowski R, Alom-Ruiz SP, Shah AM. NADPH oxidase-derived reactive oxygen species in the regulation of endothelial phenotype. Pharmacol Rep. 2008;60:21–28. [PubMed] [Google Scholar]
- 19.Skultetyova D, Filipova S, Riecansky I, Skultety J. The role of angiotensin type 1 receptor in inflammation and endothelial dysfunction. Recent Pat Cardiovasc Drug Discov. 2007;2:23–27. doi: 10.2174/157489007779606130. [DOI] [PubMed] [Google Scholar]
- 20.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorenkamp M, Riad A, Stiehl S, Spillmann F, Westermann D, Du J, Pauschinger M, Noutsias M, Adams V, Schultheiss HP, Tschope C. Protection against oxidative stress in diabetic rats: role of angiotensin AT(1) receptor and beta 1-adrenoceptor antagonism. Eur J Pharmacol. 2005;520:179–187. doi: 10.1016/j.ejphar.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Yao R, Cheng X, Chen Y, Xie JJ, Yu X, Liao MY, Ding YJ, Tang TT, Liao YH. Molecular mechanisms of irbesartan suppressing atherosclerosis in high cholesterol-diet apolipoprotein E knock-out mice. Int J Cardiol. 2010;139:113–122. doi: 10.1016/j.ijcard.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez E, Rodino-Janeiro BK, Ucieda-Somoza R, Gonzalez-Juanatey JR. Pravastatin counteracts angiotensin II-induced upregulation and activation of NADPH oxidase at plasma membrane of human endothelial cells. J Cardiovasc Pharmacol. 2010;55:203–212. doi: 10.1097/FJC.0b013e3181ce5f5a. [DOI] [PubMed] [Google Scholar]
- 24.Cooper R, Morré DJ, Morré DM. Medicinal benefits of green tea: Part 1. Review of noncancer health benefits. J Altern Complement Med. 2005;11:521–528. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, Song HJ, Yun SH, Chae YJ, Kim CH, Ha TS, Sachinidis A, Ahn HY, Davidge ST. Inhibition of angiotensin II-induced vascular smooth muscle cell hypertrophy by different catechins. Korean J Physiol Pharmacol. 2005;9:117–123. [Google Scholar]
- 26.Ramesh E, Geraldine P, Thomas PA. Regulatory effect of epigallocatechin gallate on the expression of C-reactive protein and other inflammatory markers in an experimental model of atherosclerosis. Chem Biol Interact. 2010;183:125–132. doi: 10.1016/j.cbi.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Wang CJ, Liu JT, Guo F. (-)-epigallocatechin gallate inhibits endothelin-1-induced C-reactive protein production in vascular smooth muscle cells. Basic Clin Pharmacol Toxicol. 2010;107:669–675. doi: 10.1111/j.1742-7843.2010.00557.x. [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa H, Wakano K, Kitani S. Inhibition of NADPH oxidase subunits translocation by tea catechin EGCG in mast cell. Biochem Biophys Res Commun. 2007;362:504–509. doi: 10.1016/j.bbrc.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Wu CH, Wu CF, Huang HW, Jao YC, Yen GC. Naturally occurring flavonoids attenuate high glucose-induced expression of proinflammatory cytokines in human monocytic THP-1 cells. Mol Nutr Food Res. 2009;53:984–995. doi: 10.1002/mnfr.200800495. [DOI] [PubMed] [Google Scholar]
- 30.Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60(Suppl 4):111–116. [PubMed] [Google Scholar]
- 31.Sanchez M, Lodi F, Vera R, Villar IC, Cogolludo A, Jimenez R, Moreno L, Romero M, Tamargo J, Perez-Vizcaino F, Duarte J. Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin II in rat aorta. J Nutr. 2007;137:910–915. doi: 10.1093/jn/137.4.910. [DOI] [PubMed] [Google Scholar]
- 32.Huang SM, Wu CH, Yen GC. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol Nutr Food Res. 2006;50:1129–1139. doi: 10.1002/mnfr.200600075. [DOI] [PubMed] [Google Scholar]
- 33.Crispo JA, Ansell DR, Piche M, Eibl JK, Khaper N, Ross GM, Tai TC. Protective effects of polyphenolic compounds on oxidative stress-induced cytotoxicity in PC12 cells. Can J Physiol Pharmacol. 2010;88:429–438. doi: 10.1139/y09-137. [DOI] [PubMed] [Google Scholar]
- 34.Bao W, Behm DJ, Nerurkar SS, Ao Z, Bentley R, Mirabile RC, Johns DG, Woods TN, Doe CP, Coatney RW, Ohlstein JF, Douglas SA, Willette RN, Yue TL. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol. 2007;49:362–368. doi: 10.1097/FJC.0b013e318046f34a. [DOI] [PubMed] [Google Scholar]