Abstract
Long-term potentiation (LTP) and long-term depression (LTD) have both been studied as mechanisms of ocular dominance plasticity in the rat visual cortex. In a previous study, we suggested that a developmental increase in serotonin [5-hydroxytryptamine (5-HT)] might be involved in the decline of LTP, since 5-HT inhibited its induction. In the present study, to further understand the role of 5-HT in a developmental decrease in plasticity, we investigated the effect of 5-HT on the induction of LTD in the pathway from layer 4 to layer 2/3. LTD was inhibited by 5-HT (10 µM) in 5-week-old rats. The inhibitory effect was mediated by activation of 5-HT2 receptors. Since 5-HT also regulates the development of visual cortical circuits, we also investigated the role of 5-HT on the development of inhibition. The development of inhibition was retarded by chronic (2 weeks) depletion of endogenous 5-HT in 5-week-old rats, in which LTD was reinstated. These results suggest that 5-HT regulates the induction of LTD directly via activation of 5-HT2 receptors and indirectly by regulating cortical development. Thus, the present study provides significant insight into the roles of 5-HT on the development of visual cortical circuits and on the age-dependent decline of long-term synaptic plasticity.
Keywords: 5-HT, Development, GABA, Inhibition, LTD, Synaptic plasticity, Visual cortex
INTRODUCTION
Long-term synaptic plasticity has been studied as a mechanism of learning and memory [1,2], and experience-dependent cortical remodeling [3,4]. In the visual cortex, long-term synaptic plasticity has been studied to understand the underlying mechanism of ocular dominance (OD) plasticity, a well-studied example of experience-dependent cortical remodeling [5,6]. OD plasticity could not be induced well after the specific period of an early developmental stage called the 'critical period' [7]. The fact that long-term potentiation (LTP) and long-term depression (LTD) both decline after the critical period suggests a relevance of the relationship between long-term synaptic plasticity and OD plasticity [8,9].
Gamma-aminobutyric acid (GABA)-ergic inhibition constrains cortical activity in the normal functional range and sculpts neuronal activity patterns [10,11]. Inhibition also effectively blocks activation of N-methyl-D-aspartic acid receptor (NMDAR) [12] by preventing synaptic integration that is essential for removal of the Mg2+ block of NMDAR [13]. Since Ca2+ influx through NMDAR is an essential part of many forms of synaptic plasticity [14-16], inhibition could efficiently regulate the induction of plasticity. Strength of intracortical inhibition is an important determinant for visual cortical circuit formation [17]. It regulates the induction of OD plasticity and the timing of the critical period is also under the control of inhibitory development [18]. Since inhibitory circuits develop later than excitatory circuits during the critical period [19], it has been postulated that developmental increase in inhibition underlies the age-dependent decline of long-term synaptic plasticity in the visual cortex [20].
Serotonin [5-hydroxytryptamine (5-HT)] is an important neuromodulator that is known to regulate synaptic plasticity and neuronal development [21,22]. 5-HT is involved in OD plasticity [23] and also regulates synaptic plasticity in the visual cortex [24-26]. The developmental increase in 5-HT content has been correlated to an age-dependent decrease in LTP in the visual cortex [27]. These findings suggest important roles for 5-HT in the regulation of the critical period in visual cortical plasticity. In a previous study, we reported an age-dependent decline of both LTP and LTD during the critical period in the rat visual cortex and the decrease in long-term synaptic plasticity was closely related to the degree of inhibitory influence [28]. In another study, we confirmed the inhibitory effect of 5-HT on LTP induction [29]. However, the effect of 5-HT on LTD induction in the visual cortex is yet to be reported despite the known importance of LTD in OD plasticity [30]. Thus, we investigated the effect of 5-HT on LTD induction in the present study. Since 5-HT also is known to be an important developmental regulator [22], disruption of 5-HT signaling could affect the decline of LTD during the critical period. To address this issue, the effects of 5-HT depletion on the development of inhibition, as well as on the developmental decline of LTD, were investigated. The information on the roles of 5-HT in the development of inhibition and the induction of LTD could enrich our understanding of the nature of the developmental regulation of cortical plasticity.
METHODS
Slice preparation
Visual cortical slices were prepared from Sprague-Dawley rats of either sex (postnatal 3 and 5 weeks, Orientbio Inc., Korea), housed under standard conditions (23±1℃, 12/12 hours light/dark cycle). Animal care and surgical procedures were approved by the Ethics Committee of the Catholic University of Korea and were consistent with the National Institutes of Health guideline for the care and use of laboratory animals. The brains were quickly removed after anesthetization with chloral hydrate (400 mg/kg, i.p.) and submerged in an ice-cold dissection medium. Coronal sections of the occipital cortex [400 µm in thickness for field potential (FP) recording and 300 µm in thickness for whole-cell recording] were prepared on a vibrotome (Campden Instruments, Leics, UK) and recovery was allowed in a storage chamber for 40 min at 37℃. The slices were maintained at room temperature prior to recording. The dissection and storage medium consisted of 125 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 2 mM MgSO4, 1.25 mM NaH2PO4, 25 mM NaHCO3, and 10 mM D-glucose, bubbled with 95% O2/5% CO2. The slices were transferred to the submerging chamber for recording and were superfused continuously with artificial cerebrospinal fluid (ACSF, 1.5~2 ml/min) containing 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1.25 mM NaH2PO4, 25 mM NaHCO3, and 10 mM D-glucose, bubbled with 95% O2/5% CO2 at 32~33℃.
Electrophysiological recording
The primary visual cortex was identified according to a rat brain atlas by Paxinos and Watson [31]. For FP recording, recording electrodes (2~3 MΩ), pulled from borosilicate glass pipettes (1B150F-4, World Precision Instruments, Inc., Sarasota, FL, USA) using a micropipette puller (MODEL P-97, Sutter instrument Co., Novato, CA, USA), were filled with ACSF and positioned in layer 2/3. FP was evoked by a brief square current pulse (0.1 or 0.2 msec) to the underlying layer 4 at a site in the middle of the cortex, using a concentric bipolar stimulating electrode (100 µm in diameter, SNE-100, David Kopf, CA, USA) and a constant current stimulus isolator (A 360, World Precision Instruments, Inc.). The amplitude of the negative FP peak was used as a measure of the evoked population excitatory synaptic current. A baseline response was obtained at 30-second intervals for at least 10 minutes with a stimulus intensity that yielded half-maximal FP amplitude. Low-frequency stimulation (LFS, 900 pulses at 1 Hz) was applied to induce LTD at the test stimulus intensity. The FP was recorded for 50 min after the application of LFS. The signals were amplified 1,000-fold, filtered between 0.1 and 3 kHz, digitized at 10 or 20 kHz (Digidata 1200A, Axon Instruments, Foster City, CA, USA), and then saved to a Pentium PC using the LTP Program (v2.3, www.ltp-program.com).
Whole-cell responses were recorded using a whole-cell patch-clamp recording technique with an EPC9 amplifier (HEKA Elektronik, Lambrecht, Germany) and Pulse 8.31 software (HEKA Elektronik). Patch electrodes (4~5 MΩ) were pulled from borosilicate glass and filled with a solution containing (in mM) 130 K-gluconate, 10 KCl, 4 Mg-ATP, 10 Na2-phosphocreatine, 0.3 Na3-GTP and 10 HEPES (pH 7.25 by KOH). Pyramidal neurons in layer 2/3 of the primary visual cortex were identified using IR-DIC video-microscopy with an upright microscope (BX51-WI fitted with a 40×/0.80NA water immersion objective, Olympus, Tokyo, Japan), and their regular spiking patterns were confirmed. Typical access resistance was 15~20 MΩ. Membrane potentials were not corrected for ~14-mV junction potential. Input resistance was measured by injection of a brief negative current pulse that evoked 5~15 mV of hyperpolarization. Membrane potential and current were both measured by switching between current clamp mode and voltage clamp mode. Excitatory events were measured at a holding potential of -75 mV in normal ACSF, and inhibitory events were subsequently recorded at a holding potential of 0 mV in the presence of the NMDAR antagonist, D-aminopentanoate (D-AP5, 50 µM), and the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) antagonist, 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 µM), by stimulation of layer 4. Data were filtered at 2.9 kHz, sampled at 20 kHz, and saved to the hard drive of a Pentium PC.
Depletion of 5-HT
5-HT was acutely depleted in the visual cortical slices by incubation of the slices in a solution containing para-chloroamphetamine (PCA) (10 µM) for 2 hours. For chronic depletion in vivo, PCA (8 mg/kg) was intraperitoneally injected into 3-week-old rats and the effect was investigated at 5 weeks of age.
Chemicals
D-AP5, DNQX, bicuculline, NAN-190, 2-methyl-5-hydroxytryptamine (2-me-5-HT), (±)-2,5-dimethoxy-4-iodoamphetamine (DOI), and ketanserin were purchased from Tocris (Bristol, UK). PCA, serotonin, (±)-8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT), mesulergine, and other chemicals were purchased from Sigma (St. Louis, MO, USA).
Statistical analysis
Data were expressed as the mean±SE. Group comparisons were performed using either a paired or an unpaired two-tailed Student's t-test unless otherwise specified. One-way ANOVA followed by a Tukey's post hoc test was also used (Systat v11, SYSTAT Software, Inc., Richmond, CA, USA). The level of significance was set at p<0.05.
RESULTS
5-HT inhibition on the induction of LTD
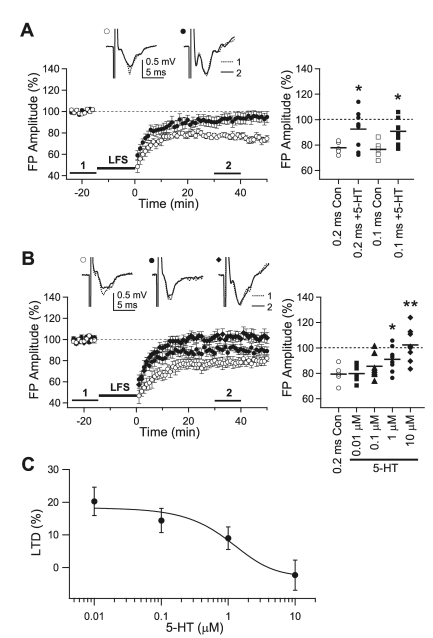
An increase in 5-HT content in the rat primary visual cortex during the critical period reportedly is important in the developmental decrease in LTP [27,29]. To further elucidate the role of 5-HT, the effect of 5-HT on LTD induction was investigated by bath application of 5-HT. In a previous report, we found that short-duration, high-intensity stimulation recruited more inhibition, and that LTD could not be induced in older animals with short-duration stimulation [28]. For example, LTD could be induced by 0.1-msec and 0.2-msec stimulation in 3-week-old rats, but only 0.2-msec stimulation could induce LTD in 5-week-old rats. Thus, in the present study, the 5-HT effect was studied with 0.1- and 0.2-msec stimulation in 3-week-old rats and with 0.2-msec stimulation in 5-week-old rats, because these experimental settings were LTD-inducing. In slices of 3-week-old rats, LFS with 0.1-msec stimulation induced LTD (76.6±3.1%, n=5), similar to that in our previous study [28]. LTD was diminished by the application of 10 µM 5-HT (90.7±3.1%, n=9, p<0.05). LTD induced with 0.2-msec stimulation (77.8±2.1%, n=5) also was inhibited by 10 µM 5-HT (93.0±5.0%, n=9, p<0.05) (Fig. 1A). In 5-week-old rats, LTD induced with 0.2-msec stimulation (79.4±3.3%, n=5) was completely inhibited by the application of 10 µM 5-HT (102.3±4.3%, n=8, p<0.01), while 0.1 µM 5-HT (85.6±3.7%, n=7, p<0.01 vs. baseline recording) and 1 µM 5-HT (91.0±3.5%, n=8, p<0.05 vs. baseline recording) exerted lesser inhibitory effects on the induction of LTD (Fig. 1B, C). The sigmoidal fit yielded a value for IC50 of 1.3 µM (Fig. 1C). Thus, 5-HT inhibited the induction of LTD in the pathway from layer 4 to layer 2/3 in the rat visual cortex. Since 10 µM 5-HT completely inhibited the induction of LTD in 5-week-old rats, this concentration was used to investigate the effect of 5-HT in later experiments.
Fig. 1.
Inhibitory effect of 5-HT on the induction of LTD. (A) Effect of 5-HT on the induction of LTD in 3-week-old rats. Low-frequency stimulation (LFS) with 0.2-msec square pulses was applied to induce LTD under normal ACSF (open circle, '0.2 ms Con,' n=5) and in the presence of 5-HT (10 µM, closed circle, '0.2 ms+5-HT,' n=9). LTD was also induced by stimulation with 0.1-msec square pulses (open square, '0.1 ms Con,' n=5; closed square, '0.1 ms+5-HT,' n=9; respectively). Right panel plots individual data (symbols) and averages (thick solid lines). *p<0.05 vs. 'Con' for each stimulus pulse duration. (B) LFS with stimulus pulse duration of 0.2-msec was applied to the slices from 5-week-old rats (open circle, '0.2 ms Con,' n=5). Concentration-dependent responses were obtained with four different concentrations of 5-HT at 0.01 µM (closed square, n=7), 0.1 µM (closed triangle, n=7), 1 µM (closed circle, n=8), and 10 µM (closed diamond, n=8). Right panel plots individual data (symbols) and averages (thick solid lines). *p<0.05 and **p<0.01 vs. '0.2 ms Con'. (C) Concentration-inhibition curve for the effect of 5-HT. The curve was fitted by sigmoid function (IC50=1.3 µM). Insets show average traces taken from a representative experiment at the indicated period.
5-HT receptor subtypes involved in the inhibition of LTD
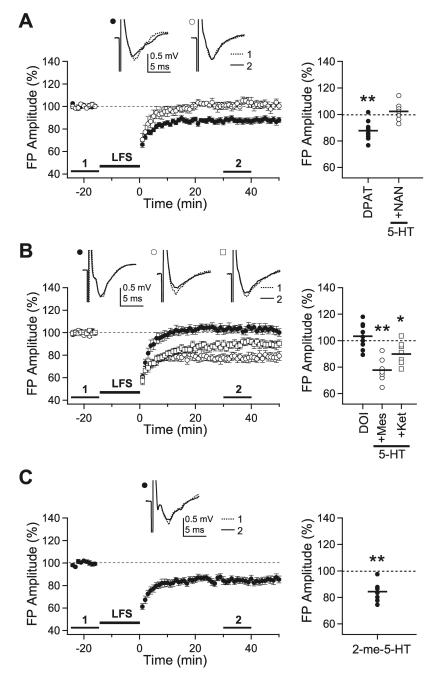
5-HT receptors consist of 7 different subtypes [32]. The involvement of 5-HT1A, 5-HT2, and 5-HT3 receptor subtypes was investigated in the present study, since these receptors are known to regulate cortical activity in the rat visual cortex [25,26,33]. All the experiments in this section were performed with 0.2-msec stimulation in slices of 5-week-old rats. The 5-HT1A receptor agonist, 8-OH-DPAT (10 µM), did not inhibit LTD (87.7±2.2%, n=10, p<0.01 vs. baseline recording) and co-application of the 5-HT1A receptor antagonist, NAN-190 (10 µM), with 5-HT (10 µM) did not affect the inhibitory effect of 5-HT on LTD (102.2±3.1%, n=7) (Fig. 2A). On the contrary, the 5-HT2 receptor agonist, DOI (10 µM), inhibited LTD (103.3±3.2%, n=9) and co-application of the 5-HT2 receptor antagonist, mesulergine (10 µM) (77.6±3.4%, n=7, p<0.01 vs. baseline recording), or ketanserin (10 µM) (89.8±2.9%, n=8, p<0.05 vs. baseline recording), with 5-HT (10 µM) blocked the inhibitory effect of 5-HT on LTD (Fig. 2B). The 5-HT3 receptor agonist, 2-me-5-HT (30 µM), had no effect on LTD induction (84.4±2.5%, n=8, p<0.01 vs. baseline recording) (Fig. 2C). These results suggest that activation of the 5-HT2 receptor mediates the inhibitory effect of 5-HT on the induction of LTD.
Fig. 2.
Effects of 5-HT receptor subtype-specific agonists and antagonists on the induction of LTD. LFS was applied with the stimulus duration of 0.2-msec to the slices from 5-week-old rats. (A) LFS was applied in the presence of 8-OH-DPAT (10 µM) (closed circle, 'DPAT,' n=10) or 5-HT (10 µM)+NAN-190 (10 µM) (open circle, '5-HT+NAN,' n=7). (B) LFS was applied in the presence of DOI (10 µM) (closed circle, 'DOI,' n=9), 5-HT (10 µM)+mesulergine (10 µM) (open circle, '5-HT+Mes,' n=7) or 5-HT (10 µM)+ketanserin (10 µM) (open square, '5-HT+Ket,' n=8). (C) LFS was applied in the presence of 2-me-5-HT (30 µM) (closed circle, '2-me-5-HT,' n=7). Insets show average traces taken from a representative experiment at the indicated period. Right panels plot individual data (symbols) and averages (thick solid lines). *p<0.05 and **p<0.01 vs. the baseline response.
Effect of 5-HT depletion on the induction of LTD
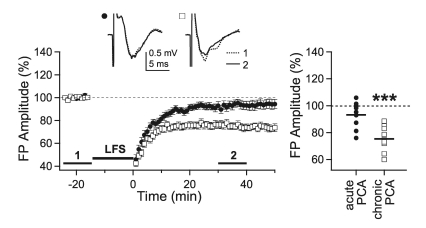
Previously, we reported that 5-HT content increased during the critical period in the visual cortex and that the depletion of endogenous 5-HT in visual cortical slices by 2-hour incubation of slices with PCA (10 µM) reinstated LTP in 5-week-old rats [29]. Thus, the effect of 5-HT depletion by incubation of slices with PCA was investigated to elucidate the role of endogenous 5-HT on LTD induction. In this experiment, LTD was induced by 0.1-msec stimulation, which induced no LTD in 5-week-old rats [28]. In contrast to LTP, LTD was not reinstated by acute depletion of 5-HT in slices of 5-week-old rats (93.1±3.3%, n=9, p=0.07 vs. baseline recording) (Fig. 3). Thus, acute depletion of endogenous 5-HT did not affect the induction of LTD in 5-week-old rats. Next, we investigated the effect of long-term depletion of 5-HT, since 5-HT has been known to regulate the development of visual cortical circuits [23]. To deplete 5-HT in vivo, PCA (8 mg/kg) was injected intraperitoneally in 3-week-old rats [34]. Then, the effect of 5-HT depletion was investigated at 5 weeks of age. LTD was readily induced by 2 weeks of chronic depletion of 5-HT in 5-week-old rats (75.2±2.8%, n=10, p<0.001 vs. baseline recording) (Fig. 3). This result indicates that LTD could be reinstated by chronic depletion of endogenous 5-HT.
Fig. 3.
Effect of 5-HT depletion on the induction of LTD. Slices from 5-week-old rats were incubated in PCA (10 µM)-containing solution for 2 hours to deplete 5-HT acutely and then LFS was applied with 0.1-msec stimulation duration (closed circle, 'acute PCA,' n=9). To investigate the effect of chronic depletion of 5-HT, LFS was applied to the slices from 5-week-old rats, in which 5-HT was depleted for 2 weeks by intraperitoneal injection of PCA (8 mg/kg) (open square, 'chronic PCA,' n=10). Insets show average traces taken from a representative experiment at the indicated periods. Right panel plots individual data (symbols) and averages (thick solid lines). ***p<0.001 vs. the baseline response.
Effect of chronic 5-HT depletion in the developing visual cortex
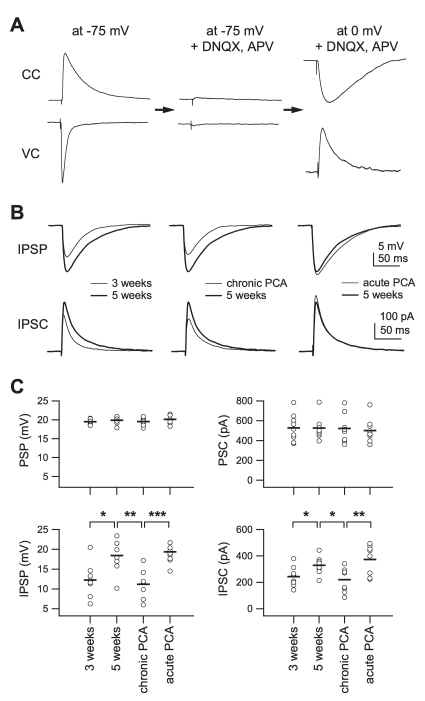
To understand the effect of chronic depletion of 5-HT on the induction of LTD, we investigated the effect of 5-HT depletion on the development of inhibition, since development of inhibition is an important determinant of the critical period in the visual cortex [18,20]. From a functional point of view, an increase in inhibition relative to excitation is more important than the development itself [35]. Thus, the relative development of inhibition compared to excitation was assessed after chronic depletion of 5-HT. To this end, we evoked 20 mV of depolarization from the membrane potential of -75 mV in layer 2/3 pyramidal cells by stimulation of the underlying layer 4 under normal ACSF. Then, DNQX (20 µM) and D-AP5 (50 µM) were applied to eliminate the excitatory components and positive current was injected to depolarize the membrane potential to 0 mV in order to maximize the inhibitory postsynaptic potentials (IPSPs) and currents (IPSCs) (Fig. 4A). These components were mediated by GABA receptor type A, since bath application of the GABAA receptor antagonist, bicuculline (10 µM), abolished them (data not shown). IPSCs and IPSPs, evoked by the stimulus intensity evoking 20 mV of depolarization at -75 mV, were measured in slices from 3-week-old rats ('3 weeks,' n=8), 5-week-old rats ('5 weeks,' n=8), 5-week-old rats which were injected with PCA at 3 weeks of age ('chronic PCA,' n=8), and 5-week-old rats in which endogenous 5-HT was acutely depleted by incubation with PCA for 2 hours ('acute PCA,' n=8) (Fig. 4B, C). Input resistance decreased during development from '3 weeks' (141.3±9.2 MΩ) to '5 weeks' (94.5±4.7 MΩ, p<0.001) and PCA treatment did not affect input resistance (89.5±6.9 MΩ for 'chronic PCA;' 97.2±9.1 MΩ, for 'acute PCA,' p=0.16). Whole-cell currents for a depolarization of 20 mV were similar among the groups (528.2±45.2 pA, 526.8±47.9 pA, 523.1±60.2 pA, and 500±39.1 pA for '3 weeks,' '5 weeks,' 'chronic PCA,' and 'acute PCA,' respectively, p=0.93 by ANOVA) (Fig. 4C, upper right panel). Stimulus currents applied to evoke 20 mV of depolarization also were similar among groups (128.3±8.7 µA, 148.5±7.7 µA, 144.5±5.2 µA, and 150.7±8.9 µA for '3 weeks,' '5 weeks,' 'chronic PCA,' and 'acute PCA', respectively, p=0.194 by ANOVA). To the contrary, IPSPs were increased from 12.1±1.5 mV in '3 weeks' to 18.4±1.5 mV in '5 weeks' (p<0.05), and this increase was completely reverted to that of '3 weeks' in 'chronic PCA' (11.1±1.4 mV, p=0.63 vs. '3 weeks'). The IPSPs for 'acute PCA' (19.3±1 mV) were similar to '5 weeks' and much greater than for 'chronic PCA' (p<0.001) (Fig. 4C, lower left panel). The IPSCs showed similar results (242.8±26.9 pA, 328.6±23.9 pA, 220.5±29.6 pA, and 373.5±39.8 pA for '3 weeks,' '5 weeks,' 'chronic PCA,' and 'acute PCA,' respectively) (Fig. 4C, lower right panel). These results indicate that an increase in the inhibitory to excitatory (I/E) ratio by endogenous 5-HT might be involved in the developmental decline of the induction of LTD in the rat visual cortex.
Fig. 4.
Effect of chronic 5-HT depletion on the development of the inhibitory network. 5-HT was depleted by single intraperitoneal injection of PCA (8 mg/kg) at 3 weeks of age and slices were taken at 5 weeks of age. (A) Experimental sequences. 20 mV of postsynaptic potential (PSP) and corresponding postsynaptic current (PSC) were recorded at -75 mV of membrane potential (left traces). Bath application of DNQX (20 µM) and APV (50 µM) eliminated PSP and PSC (center traces). At 0 mV of membrane potential, isolated inhibitory postsynaptic potential (IPSP) and current (IPSC) were recorded (right traces). CC: current clamp mode; VC: voltage clamp mode. (B) Averaged IPSPs (upper traces) and IPSCs (lower traces) showing differences between groups. Left traces: IPSPs and IPSCs recorded from slices of 3-week-old (thin line, n=8) and 5-week-old rats (thick line, n=8). Center traces: from slices of 5-week-old (thick line) and 5-week-old PCA-injected (thin line, n=8) rats. Right traces: from slices of 5-week-old rats (thick line) and slices of 5-week-old rats in which 5-HT was depleted by PCA incubation for 2 hours (thin line, n=8). (C) Individual data (symbols) and averages (thick lines) for PSP, PSC, IPSP and IPSC for each of the groups indicated in the lower panels. *p<0.05, **p<0.01, and ***p<0.001 between groups linked by lines.
DISCUSSION
In the present study, we investigated the effects of 5-HT on the induction of LTD in the rat visual cortex. In 5-week-old rats, LTD was completely inhibited by 10 µM 5-HT. Although activation of both 5-HT1A and 5-HT2 receptors were required to inhibit the induction of LTP [29], activation of the 5-HT2 receptor alone was sufficient to block the induction of LTD. Reportedly, 5-HT1A receptors exert an inhibitory effect on LTP via the suppression of NMDAR [25,36]. Since LTD was induced with less Ca2+ than LTP [37], it is conceivable that the Ca2+ influx through NMDAR might have been enough to induce LTD even though NMDAR was partially blocked by activation of the 5-HT1A receptor. Alternatively, Ca2+ from other sources, such as voltage-gated Ca2+ channels, could have been involved in the induction of LTD under these conditions. In any case, activation of 5-HT1A receptors would not be necessary for the inhibitory effect of 5-HT on LTD. In 3-week-old rats, the inhibitory effect of 5-HT on LTD was much weaker than in 5-week-old rats. This result suggests that the mechanism underlying 5-HT2 receptor-mediated inhibition of LTD might develop at 3 to 5 weeks of age. GABAergic inhibition is one of the most plausible candidates as a target of 5-HT2 receptor-mediated modulation, since inhibition increases during the critical period [19] and is an important regulator of the induction of synaptic plasticity [18]. In addition, it was suggested that the inhibitory effect of 5-HT2 receptors on LTP might be mediated by the modulation of inhibitory influences [26], since 5-HT preferentially depolarizes inhibitory neurons [38]. A detailed mechanism for the action of the 5-HT2 receptor on inhibitory neurotransmission remains to be addressed.
Depletion of 5-HT in slices by 2-hour incubation with 10 µM PCA was enough to reinstate LTP in 5-week-old rats [29]. However, LTD was not reinstated by the same acute treatment. By contrast, prolonged depletion of 5-HT by intraperitoneal injection of PCA [34,39] reinstated LTD, which might have resulted from the retarded development of inhibition. As mentioned, the late maturation of the inhibitory circuit is one of the proposed mechanisms for the decline of long-term synaptic plasticity during the critical period [20]. Although we did not directly evaluate excitatory development, since 20-mV depolarization was evoked by similar amplitudes of stimulus currents among groups, we predicted that excitatory circuits might not be significantly affected by 5-HT depletion. Thus, these results support the crucial involvement of postnatal development of inhibitory circuits on the age-dependent decline of long-term synaptic plasticity. However, the causal relationship between retarded development of inhibition and reinstatement of LTD was uncertain in this experiment. The effect of pharmacologic agents which enhance GABAergic inhibition, such as benzodiazepine [40], on LTD induction in 5-HT depleted slices would help explain the causal relationship.
In addition, since our experimental protocol measured inhibitory strength relative to excitation, it could be interpreted that inhibitory development was relatively retarded compared to excitatory development by depletion of 5-HT. To the best of our knowledge, this is the first evidence that 5-HT preferentially controls the development of inhibition over excitation. The mechanism underlying 5-HT-mediated modulation of neuronal development is largely unknown. Because brain-derived neurotrophic factor (BDNF) mediates the effect of 5-HT on neuronal development [41,42], and BDNF is an important regulator of the maturation of inhibition [43], 5-HT might regulate the development of inhibitory circuits through the modulation of BDNF signaling. Studies on the effect of 5-HT depletion on BDNF signaling would help address this issue.
In contrast to the present study, 5-HT facilitated the induction of long-term synaptic plasticity in cats [24,44] and in adult rats [45]. Since 5-HT receptor distribution is heterogeneous in various species and brain regions [32], 5-HT could affect various cellular functions differently in different conditions. More importantly, the effect of 5-HT on the induction of plasticity could change during development, since expression patterns of 5-HT receptors change during development [46,47]. Thus, further studies on the effects of 5-HT in older animals would promote a more thorough understanding of the roles of 5-HT.
Results of the present study suggest that 5-HT could inhibit LTD either directly via the activation of the 5-HT2 receptor or indirectly by regulating the development of inhibition. Since 5-HT content increases during the critical period in the visual cortex, these results suggest important roles for 5-HT in a developmental decrease in long-term synaptic plasticity and in the timing of the critical period. These findings also provide a framework for understanding the interplay between 5-HT and the development of inhibition in the visual cortex, both of which have been known to regulate the induction of long-term synaptic plasticity and OD plasticity.
ACKNOWLEDGEMENTS
This study was supported by Grant No. R01-2003-000-10656-0 from the Basic Research Program of the Korea Science and Engineering Foundation.
ABBREVIATIONS
- 5-HT
5-hydroxytryptamine
- AMPAR
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- ACSF
artificial cerebrospinal fluid
- BDNF
brain-derived neurotrophic factor
- D-AP5
D-aminopentanoate
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- FP
field potential
- GABA
gamma-aminobutyric acid
- IPSC
inhibitory postsynaptic current
- IPSP
inhibitory postsynaptic potential
- LTD
long-term depression
- LTP
long-term potentiation
- NMDAR
N-methyl-D-aspartic acid receptor
- OD
ocular dominance
- PCA
para-chloroamphetamine
References
- 1.Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 4.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 5.Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J Neurobiol. 1999;41:83–91. doi: 10.1002/(sici)1097-4695(199910)41:1<83::aid-neu11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 8.Perkins ATt, Teyler TJ. A critical period for long-term potentiation in the developing rat visual cortex. Brain Res. 1988;439:222–229. doi: 10.1016/0006-8993(88)91478-3. [DOI] [PubMed] [Google Scholar]
- 9.Dudek SM, Friedlander MJ. Developmental down-regulation of LTD in cortical layer IV and its independence of modulation by inhibition. Neuron. 1996;16:1097–1106. doi: 10.1016/s0896-6273(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 10.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 11.Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Luhmann HJ, Prince DA. Control of NMDA receptor-mediated activity by GABAergic mechanisms in mature and developing rat neocortex. Brain Res Dev Brain Res. 1990;54:287–290. doi: 10.1016/0165-3806(90)90152-o. [DOI] [PubMed] [Google Scholar]
- 13.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 14.Perkel DJ, Petrozzino JJ, Nicoll RA, Connor JA. The role of Ca2+ entry via synaptically activated NMDA receptors in the induction of long-term potentiation. Neuron. 1993;11:817–823. doi: 10.1016/0896-6273(93)90111-4. [DOI] [PubMed] [Google Scholar]
- 15.Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 17.Burchfiel JL, Duffy FH. Role of intracortical inhibition in deprivation amblyopia: reversal by microiontophoretic bicuculline. Brain Res. 1981;206:479–484. doi: 10.1016/0006-8993(81)90551-5. [DOI] [PubMed] [Google Scholar]
- 18.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozas C, Frank H, Heynen AJ, Morales B, Bear MF, Kirkwood A. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J Neurosci. 2001;21:6791–6801. doi: 10.1523/JNEUROSCI.21-17-06791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 22.Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- 23.Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995;7:1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 24.Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- 25.Edagawa Y, Saito H, Abe K. 5-HT1A receptor-mediated inhibition of long-term potentiation in rat visual cortex. Eur J Pharmacol. 1998;349:221–224. doi: 10.1016/s0014-2999(98)00286-6. [DOI] [PubMed] [Google Scholar]
- 26.Edagawa Y, Saito H, Abe K. The serotonin 5-HT2 receptor-phospholipase C system inhibits the induction of long-term potentiation in the rat visual cortex. Eur J Neurosci. 2000;12:1391–1396. doi: 10.1046/j.1460-9568.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 27.Edagawa Y, Saito H, Abe K. Endogenous serotonin contributes to a developmental decrease in long-term potentiation in the rat visual cortex. J Neurosci. 2001;21:1532–1537. doi: 10.1523/JNEUROSCI.21-05-01532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang HJ, Cho KH, Kim HS, Hahn SJ, Kim MS, Rhie DJ. Age-dependent decline in supragranular long-term synaptic plasticity by increased inhibition during the critical period in the rat primary visual cortex. J Neurophysiol. 2009;101:269–275. doi: 10.1152/jn.90900.2008. [DOI] [PubMed] [Google Scholar]
- 29.Kim HS, Jang HJ, Cho KH, Hahn SJ, Kim MJ, Yoon SH, Jo YH, Kim MS, Rhie DJ. Serotonin inhibits the induction of NMDA receptor-dependent long-term potentiation in the rat primary visual cortex. Brain Res. 2006;1103:49–55. doi: 10.1016/j.brainres.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 30.Yoon BJ, Smith GB, Heynen AJ, Neve RL, Bear MF. Essential role for a long-term depression mechanism in ocular dominance plasticity. Proc Natl Acad Sci U S A. 2009;106:9860–9865. doi: 10.1073/pnas.0901305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1997. [Google Scholar]
- 32.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 33.Roerig B, Nelson DA, Katz LC. Fast synaptic signaling by nicotinic acetylcholine and serotonin 5-HT3 receptors in developing visual cortex. J Neurosci. 1997;17:8353–8362. doi: 10.1523/JNEUROSCI.17-21-08353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey JA, McMaster SE, Yunger LM. P-Chloramphetamine: Selective neurotoxic action in brain. Science. 1975;187:841–843. doi: 10.1126/science.47181. [DOI] [PubMed] [Google Scholar]
- 35.Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex. 2010;20:456–467. doi: 10.1093/cercor/bhp114. [DOI] [PubMed] [Google Scholar]
- 36.Edagawa Y, Saito H, Abe K. Stimulation of the 5-HT1A receptor selectively suppresses NMDA receptor-mediated synaptic excitation in the rat visual cortex. Brain Res. 1999;827:225–228. doi: 10.1016/s0006-8993(99)01300-1. [DOI] [PubMed] [Google Scholar]
- 37.Kimura F, Tsumoto T, Nishigori A, Yoshimura Y. Long-term depression but not potentiation is induced in Ca(2+)-chelated visual cortex neurons. Neuroreport. 1990;1:65–68. doi: 10.1097/00001756-199009000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Foehring RC, van Brederode JF, Kinney GA, Spain WJ. Serotonergic modulation of supragranular neurons in rat sensorimotor cortex. J Neurosci. 2002;22:8238–8250. doi: 10.1523/JNEUROSCI.22-18-08238.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haidkind R, Eller M, Kask A, Harro M, Rinken A, Oreland L, Harro J. Increased behavioural activity of rats in forced swimming test after partial denervation of serotonergic system by parachloroamphetamine treatment. Neurochem Int. 2004;45:721–732. doi: 10.1016/j.neuint.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Campo-Soria C, Chang Y, Weiss DS. Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol. 2006;148:984–990. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Juric DM, Miklic S, Carman-Krzan M. Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res. 2006;1108:54–62. doi: 10.1016/j.brainres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 44.Kirkwood A. Serotonergic control of developmental plasticity. Proc Natl Acad Sci U S A. 2000;97:1951–1952. doi: 10.1073/pnas.070044697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 46.D'Amato RJ, Blue ME, Largent BL, Lynch DR, Ledbetter DJ, Molliver ME, Snyder SH. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci U S A. 1987;84:4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dyck RH, Cynader MS. Autoradiographic localization of serotonin receptor subtypes in cat visual cortex: transient regional, laminar, and columnar distributions during postnatal development. J Neurosci. 1993;13:4316–4338. doi: 10.1523/JNEUROSCI.13-10-04316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]