Abstract
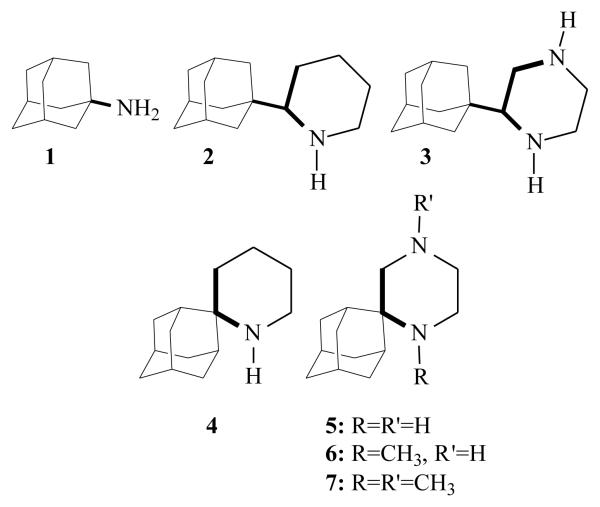
Spiro[piperidine-2,2′-adamantane] 4 is one of the most potent synthetic anti-influenza A aminoadamantanes or other cage structure amines tested so far. Based on previous results5h which demonstrate the boost of in-vitro potency by the presence of an additional amino group, we examined whether the incorporation of a second amino group into this heterocycle would increase the anti-influenza A virus activity. The new synthetic molecules 5 - 7 are capable of forming two hydrogen bonds within the receptor. We identified the diamino derivatives 5 and 6, which are active against influenza A H3N2 virus although less potent than amantadine and its equipotent spiropiperidine 4.
Keywords: amantadine, aminoadamantanes, piperazinones, spiropiperazines, influenza A virus
1. Introduction
Influenza presents a severe threat to public health. More casualties were inflicted in Europe in the 20th century by influenza than any other infectious disease.1 The 2009 pandemic influenza A virus of the subtype H1N1 although clinically has caused mainly mild disease similar to seasonal influenza, it has been responsible for severe disease and more than 10,000 fatalities were recorded during 2009.2,3 The ‘terrible experience’ of the 1918 flu pandemic,1 which killed approximately 600,000 people in the United States alone, was preceded by a mild ‘herald’ wave in the spring, and there is continuing concern that the pandemic H1N1 2009 virus might mutate into a more virulent form.
Amantadine 1 was the first anti-influenza virus A drugs which inhibited virus replication at micromolar concentrations.4 During the past twelve years we have synthesized many potent aminoadamantane derivatives.5 These compounds, in their protonated form, occlude the M2 protein ion channel pore6 and block its proton pump function7 in early and late endosomes,4 impairing a function critical for the virus replication.4,8
After several modifications in aminoadamantane structure some interesting findings are the following. The inclusion of the 1-aminoethyl pharmacophore group of rimantadine into a saturated heterocycle like piperidine resulting in 2 which is an active compound against influenza A virus. The effect of adding a second amino group in the piperidine ring, leading to piperazine 3, resulted in the retention of or an increase in the in vitro activity compared to amantadine.5h We have published that the spiropiperidine 45a,c (Scheme 1) is one of the most active anti-influenza A virus M2 agents ever synthesized after the discovery of amantadine and rimantadine.9 These findings triggered the synthesis of the spiropiperazine analogues 5 - 7 of spiropiperidine 4 (Scheme 1). It has been proposed that the amantadine 1-receptor complex is stabilized through formation of hydrogen bonds between the drug's ammonium group and the cluster formed by four acceptor groups of the tetrameric M2 receptor.6a,b The new spiropiperazine analogues 5 - 7 bear two amino groups and can possibly act through the formation of two hydrogen bonds with the acceptor group cluster. In order to test our hypothesis the spiropiperazine derivatives 5 - 7 were synthesized and their activity was evaluated against H3N2 influenza A viruses which continue to be responsible for annual epidemics.
Scheme 1.
Active aminoadantane analogues having a piperidine and piperazine structure
2. Results and discussion
2.1 Synthetic chemistry
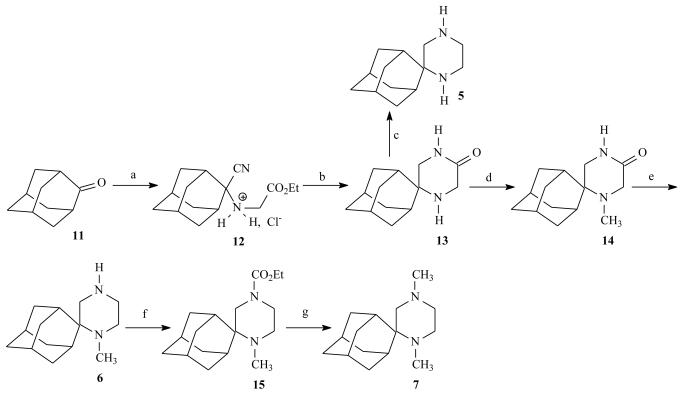
Spiropiperazinone 13 was the key structure for the synthesis of the novel aminoadamantane derivatives 5-7. In order to synthesize compound 13, convenient methods for the preparation of the α-amino nitriles 12 were needed (Scheme 2). The preferred synthetic routes leading to α-amino nitriles are still based on the Strecker reaction.10 The Strecker reaction conditions for preparing compound 12 are consistent with mixing the bulky ketone 11 with NaCN and NH2CH2COOEt·HCl respectively in a mixture of DMSO/water, and leaving the mixture to react at ambient temperature.
Scheme 2.
Reagents and Conditions: (a) NaCN, H2NCH2CO2Et·HCl, DMSO/H2O 29:1 (v/v), r.t., 48 h, and then HCl(g)/Et2O (80%); (b) (i) H2/PtO2, EtOH/HCl (g), 50 psi, r.t., 6.5 h (ii) H2O·Na2CO3 (71%); (c) LiAlH4, THF, Ar, r.t., 5 h (81%); (d) (i) CH2O(aq) 37%, MeOH, r.t., 3 h, and then NaCNBH3, r.t., 4 h at pH 6-7 (maintained by adding AcOH) (ii) 1 N NaOH to pH 8-9 (94%); (e) LiAlH4, THF, Ar, reflux, 7.5 h (96%); (f) ClCO2Et, Et3N, r.t., 24 h (62%); (g) LiAlH4, THF, Ar, r.t., 24 h and then reflux 1 h (quantitative).
Using these standard Strecker reaction conditions, the N-substituted α-amino nitrile 12 was afforded in 80% yield. Catalytic hydrogenation of the a-amino nitrile hydrochloride 12 over PtO2 followed by alkalization provided the desired spiropiperazinone 13 through cyclization of the intermediate diaminoester. The reductive methylation of 13 with NaBH4/CH2=O afforded the 1-Me piperiazinone 14. The piperazines 5 and 6 were obtained by means of a LiAlH4 reduction of 13 and 14 respectively. The dimethyl derivative 7 was obtained through the LiAlH4 reduction of the carbamate 15. The synthetic pathway depicted in Scheme 2 can be generalized for the synthesis of spiropiperazinones and spiropiperazines. Piperazinone rings have been used often in medicinal chemistry because of their structural similarity to constrained peptides, and numerous methods of ring assembly have been developed with the introduction of substitution at varying positions.11 Thus, we have successfully applied the methodology for the synthesis of the cyclooctane analogues (See Supplementary material).
2.2 Antiviral activity results
The potency of the new compounds 5 - 7 was examined in vitro against influenza A/Hong Kong/68 (H3N2) virus using a whole cell antiviral assay (plaque reduction assay), and was compared to the activity of amantadine 1 (Table 1). None of the compounds showed any significant toxicity up to 300 micromolar concentrations.
Table 1.
Anti-influenza virus A (H3N2) activity of amantadine 1 and some aminoadamantane analogues 5 - 7a in MDCKb
Abbreviations and notes:
Amantadine 1 was tested as hydrochloride salt, 5, 7 as dimaleate and 6 as monomaleate salts
influenza A H3N2 (A/Hong Kong/68
Concentration required to reduce virus-induced cytopathic effect in MDCK (Madin-Darby canine kidney) cells by 50%
Data are shown as mean ± SD of three independent determinations.
Compounds 5, 6 were found to be inhibitors of H3N2 influenza A virus replication. No significant antiviral effect was observed against the amantadine resistant influenza A/WSN/33 (H1N1) strain.
We recall that in order to inhibit virus replication the prototype amantadine drug 1, existing mostly in its protonated form even at neutral pH,12 must first be solvated in the lipid bilayer13 prior to the blockage of the M2 proton pump inside the acidic endosomes. Recent experimental studies predicted that amantadine 1 anchors inside M2 protein pore; in the binding site the adamantyl group fits the lipophilic pocket above Val 27 and the ammonium group is H-bonded to the hydroxyl groups of Ser 31 or with waters of the polar pore connected to the polar groups like Ser31 hydroxyl groups or His37 imidazole groups. Thus, the in vitro activity of an aminoadamantane analogue results from its favorable hydrogen bonding and van der Waals interaction with the M2 receptor; compounds 5 - 7 act through their diprotonated form at the low pH environment of endosomes and are capable of forming two hydrogen bonds.
The most active agent was compound 5 with EC50 values of 8.58 μM, although less active than amantadine 1. N-Methylation reduced the activity probably by hampering the hydrogen bonding ability of the ligand (compound 6). It was found that spiropiperidine 4 was equipotent to amantadine 1 and piperidine 2 was 2-fold less active. It is interesting to consider the effect of the second amino group to the potency when the structure changes from 2 → 3 and 4 → 5. Piperazine 3 was more active than piperidine 2 and 2-fold more potent than amantadine 1 whereas spiropiperazine 5 is three times less active than spiropiperidine 4 or amantadine 1.
The biological activity, i.e., the EC50 value (Table 1), of 5 or 6 is exerted by the fraction of their in vitro effective drug concentration corresponding to the concentration of the molecules that bind acceptor groups inside the M2 pore after passing the membrane barriers.13 Thus, the EC50 values of the compounds are due to a compromise between their membrane penetration ability and binding affinity properties.
Judging that similar changes occurred in the membrane penetration ability when the structure changes from 2 → 3 and 4 → 5, the enhanced activity of 3 compared to that of 5 could be interpreted in terms of additional hydrogen bonding interactions although each of these H-bonds can be considered to be weaker compared to that in the parent piperidine due to the presence of the second amino group. Since 5 could also form two hydrogen bonds with the receptor, its diminished potency could be explained in terms of an incorrect binding orientation of this molecule.
3. Experimental
3.1 Chemistry
3.1.1 General
Melting points were determined using a Büchi capillary apparatus and are uncorrected. IR spectra were recorded on a Perkin-Elmer 833 spectrometer. 1H NMR spectra were recorded on a Bruker MSL 400 at 400 MHz using CDCl3 as solvent and TMS as internal standard. 13C NMR spectra were recorded on a Brucker AC 200 spectrometer at 50 MHz, using CDCl3 as solvent and TMS as internal standard. Carbon multiplicities were established by the DEPT experiments. The 2D NMR techniques (HMQC and COSY) were used for the elucidation of the structures of some derivatives. All solvents were carefully dried before use. Thin layer chromatography was performed on TLC precoated silica gel 60 F254 plates (layer thickness 0.2mm). Column chromatography purification was carried out on silica gel 60 (70-230 mesh). Microanalyses were carried out by the Service Central de Microanalyses (CNRS) France, and the results obtained had a maximum deviation of ± 0.4% from the theoretical value.
3.1.2 Synthesis of adamantane derivatives
N-[[2-cyano(tricyclo[3.3.1.13,7]dec-2-yl)]glycine ethyl ester Hydrochloride (12)
The hydrochloride salt 12 was prepared by adding ethereal HCl to a solution of the free amino nitrile10 under ice cooling. The white precipitate was filtered off, washed with cold ether (4×25 mL) and dried; mp 65 - 67 °C (dec) (partial decomposition at 50 - 64 °C and melting with full decomposition at 65 - 67 °C).
Spiro[piperazine-2,2′-tricyclo[3.3.1.13,7]decan]-5-one (13)
To a solution of the amino nitrile hydrochloride 12 (2.1 g, 7.02 mmol) in abs ethanol (150 mL), PtO2 (210 mg) and ethanolic HCl (3.5 mL) were added and the mixture was hydrogenated for 6.5 h under 50 psi at ambient temperature. The catalyst was filtered off, washed with portions of ethanol (3×15mL), and the filtrate was evaporated in vaccuo. The solid residue was dissolved in water (35 mL) and the aqueous solution was washed with ether (25 mL) and made alkaline with solid Na2CO3. The resulting insoluble solid was extracted with chloroform (3 × 25 mL) and the combined organic extracts were dried (Na2SO4) and evaporated in vacuo. The solid residue was chromatographated on silica gel column using CH2Cl2 - MeOH mixtures 40:1 and then 15:1 as eluents to afford pure crystalline piperazinone 13. Yield: 1.10 g, 71%; mp 180 - 182 °C (CH2Cl2 - Et2O); IR (Nujol): ν(N-H) 3310, 3210 ν(C=O) 1670 cm−1; 1H-NMR (CDCl3, 400 MHz) δ (ppm) 1.45 (br s, 1H, 1-H), 1.52 (br d, 2H, J= 12.8 Hz, 4′e, 9′e -H), 1.61-1.95 (complex m, 10H, 1′, 3′, 5′, 6′, 7′, 8′, 10′-H), 2.12 (br d, 2H, J= 12.4Hz, 4′a, 9′a-H), 3.39 (s, 2H, 3-H), 3.42 (s, 2H, 6-H), 6.88 (br s, 1H, 4-H); 13C-NMR (CDCl3, 50 MHz) δ (ppm) 27.5, 27.6 (5′,7′-C), 31.9 (4′,9′-C), 32.5 (1′,3′-C), 33.5 (8′,10′-C), 38.2 (6′-C), 44.2 (6-C), 50.0 (3-C), 53.2 (2,2′-C), 170.7 (5-C). Anal. (C13H20N2O) C, H, N.
1-Methylspiro[piperazine-2,2′-tricyclo[3.3.1.13,7]decan]-5-one (14)
To a stirring solution of piperazinone 13 (1.0 g, 4.54 mmol) in methanol (26 mL), aqueous formaldehyde 37 % (2 mL, 25.0 mmol) was added dropwise. The solution was stirred for 3 h at ambient temperature and then NaCNBH3 (485 mg, 7.72 mmol) was added in one portion. The reaction mixture was stirred for 20 min and then the pH was adjusted to 6-7 by the addition of acetic acid. Stirring was continued for 4 h at ambient temperature with the occasional addition of acetic acid to maintain a neutral pH. Solvent was evaporated and the residue was treated with water (40 mL) and NaOH 1N until the pH was adjusted to 8-9. The mixture was extracted with dichloromethane (3 × 30 mL) and the combined organic extracts were washed with water (2 × 25 mL) and dried (Na2SO4). The solvent was evaporated in vacuo to remain a TLC pure white solid. Yield: 1.0 g, 94%; mp 224 - 226 °C (CH2Cl2 - n-pentane); IR (Nujol): ν(N-H) 3188 ν(C=O) 1677 cm−1; 1H-NMR (CDCl3, 400 MHz, 290 K) δ (ppm) 1.30-2.22 (complex m, 14H, 1′, 3′, 4′, 5′, 6′, 7′, 8′, 9′,10′-adamantane H), 2.33 (s, 3H, 1-CH3), 2.75-3.10 (br s, 1H, 6-H), 3.20-3.38 (br s, 1H, 3-H), 3.52-3.80 (br s, 2H, 3,6-H), 6.94 (br s, 1H, 4-H); 13C-NMR (CDCl3, 50 MHz, 290 K) δ (ppm) 26.9, 27.6 (5′,7′-C), 29.5, 32.6 (1′,3′-C), 31.4 (4′,9′-C), 33.5 (8′,10′-C), 36.0 (1-CH3), 38.1 (6′-C), 41.6 (3-C), 52.5 (6-C), 56.3 (2,2′-C), 171.4 (5-C). Anal. (C14H22N2O) C, H, N.
Spiro[piperazine-2,2′-tricyclo[3.3.1.13,7]decane] (5)
To a stirring suspension of LiAlH4 (690 mg, 18.0 mmol) in dry THF (30 mL), piperazinone 13 (800 mg, 3.60 mmol) was added portionwise under ice cooling and argon atmosphere. The reaction mixture was stirred at ambient temperature for 5 h (TLC monitoring, CH2Cl2 - MeOH 15:1) and then water and NaOH 20% w/v was added under ice cooling. The inorganic precipitate was filtered off, washed with warm THF and the filtrate was evaporated in vacuo. The oily residue was dissolved in ether and the organic solution was dried (Na2SO4) and evaporated to afford the pure oily piperazine 5, which is sensitive to air and light. Yield: 610 mg, 81%; IR (Nujol): ν(N-H) 3301, 3276 cm−1; 1H-NMR (CDCl3, 400 MHz) δ (ppm) 1.47 (br d, 2H, J ≈ 13 Hz, 4′e,9′e-H), 1.57-1.92 (complex m, 12H, 1,4,1′, 3′, 5′, 6′, 7′, 8′, 10′-H), 1.95 (br d, 2H, J ≈ 13 Hz, 4′a,9′a-H), 2.75 (s, 4H, 5,6-H), 2.87 (s, 2H, 3-H); 13C-NMR (CDCl3, 50 MHz) δ (ppm) 27.6, 27.9 (5′,7′-C), 31.8 (4′,9′-C), 32.6 (1′,3′-C), 33.2 (8′,10′-C), 38.7 (6′-C), 40.4, 46.9 (5,6-C), 53.2 (3-C), 53.7 (2,2′-C). Hydrochloride: mp > 260 °C (MeOH-Et2O). Dimaleate: mp 161 - 163 °C (MeOH - Et2O); 1 H-NMR (CD3OD, 400 MHz) δ (ppm) 1.76-2.15 (complex m, 12H, 1′, 3′, 4′e, 5′, 6′, 7′, 8′, 9′e, 10′-H), 2.26 (s, 2H, 4′a, 9′a-H), 3.46 (d, 4H, J = 4.2 Hz, 5, 6-H), 3.65 (d, 2H, J = 4.5 Hz, 3-H), 5.19, 5.25 (brs + brs, 6H, 4xCOOH, 1, 4-H), 6.28 (s, 4H, 2xCH=CH); 13C-NMR (CD3OD , 100 MHz) δ (ppm) 27.8, 28.2 (5′,7′-C), 31.5 (4′,9′-C), 32.3 (1′,3′-C), 33.4 (8′,10′-C), 37.3, 41.8 (5, 6-C), 38.9 (6′-C), 48.2 (3-C), 61.1 (2,2′-C), 136.0 (2xCH=CH), 170.8 (2xCOOH). Anal. (C21H30N2O8) C, H, N.
1-Methylspiro[piperazine-2,2′-tricyclo[3.3.1.13,7]decane] (6)
To a stirring suspension of LiAlH4 (1.25 g, 32.9 mmol) in dry THF (62 mL), piperazinone 14 (1.50 g, 6.40 mmol) was added portionwise under ice cooling and argon atmosphere. The reaction mixture was refluxed for 7.5 h (TLC monitoring, CH2Cl2 - MeOH 15:1). After cooling to ambient temperature the reaction mixture was quenched in exactly the same way described in 5 to afford the TLC pure oily 1-methyl piperazine 6. Yield: 1.35 g, 96% (after some hours on air 1-methyl piperazine 6 was solidified); IR (Nujol): ν(N-H) 3316 cm−1; 1H-NMR (CDCl3, 400 MHz, 265 K) δ (ppm) 1.30 (br d, 1H, J ≈ 12 Hz, 9′e-H), 1.38 (br d, 1H, J ≈ 12 Hz, 4′e-H), 1.45-1.93 (complex m, 10H, 4, 3′, 5′, 6′, 7′, 8′, 10′-H), 1.96 (br d, 1H, J ≈ 12 Hz, 9′a-H), 2.15 (br d, 1H, J ≈ 12 Hz, 4′a-H), 2.26-2.40 (m, 2H, 5a, 6e-H), 2.32 (s, 3H, 1-CH3), 2.54 (br s, 1H, 1′-H), 2.68 (d, 1H, J ≈ 13 Hz, 3a-H), 2.90-3.08 (m, 2H, 3e, 5e-H), 3.29 (br t, 1H, J = 13.7 Hz, 6a-H); 13C-NMR (CDCl3, 50 MHz, 265 K) δ (ppm) 26.9, 27.3 (5′,7′-C), 28.1, 32.5 (1′,3′-C), 31.4, 31.6 (4′,9′-C), 32.7, 32.9 (8′,10′-C), 33.7 (1-CH3), 38.3 (5-C), 38.4 (6′-C), 42.0 (3-C), 47.9 (6-C), 57.0 (2,2′-C). Maleate: mp 161 - 163 °C (dec) (EtOH - Et2O); Anal. (C18H28N2O4) C, H, N. Semifumarate: mp 204 - 206 °C (dec) (MeOH - Et2O); Anal. (C16H26N2O2) C, H.
1,4-Dimethylspiro[piperazine-2,2′ricyclo[3.3.1.13,7] decane] (7)
To a stirring solution of piperazine 6 (650 mg, 2.95 mmol) and triethylamine (1.20 g, 11.8 mmol) in dry ether (15 mL) was added dropwise a solution of ethyl chloroformate (532 mg, 4.90 mmol) in dry ether (5 mL) under ice cooling. The resulting mixture was stirred for 24 h at ambient temperature and then water (15 mL) was added and the mixture was extracted with ether (2 × 10 mL). The combined organic extracts were washed with water (5 × 15 mL) and dried (Na2SO4). After solvent removal the oily residue was chromatographated on silica gel column with CH2Cl2 - MeOH 20:1 as eluent to afford pure carbamate 15 as a viscous oil. Yield: 535 mg, 62%; IR (Nujol): ν(C=O) 1700 cm−1. Hydrochloride: mp 225 - 227 °C (EtOH-Et2O); Anal. (C17H29ClN2O2) C, H, N.
A solution of carbamate 15 (600 mg, 2.05 mmol) in dry THF (5 mL) was added to a stirring suspension of LiAlH4 (390 mg, 10.3 mmol) in dry THF (10 mL) under ice cooling and argon atmosphere. The reaction mixture was stirred at ambient temperature for 24 h and then was gently refluxed for 1 h (TLC monitoring, CH2Cl2 - MeOH 20:1). After cooling to ambient temperature the reaction mixture was quenched in exactly the same way described in 5 to afford the TLC pure oily 1,4-dimethyl piperazine 7. Yield: 480 mg, quantitative; 1H-NMR (CDCl3, 400 MHz) δ (ppm) 1.37 (br d, 2H, J ≈ 11 Hz, 4′e,9′e-H), 1.53-1.88 (complex m, 10H, 3, 3′, 5′, 6′, 7′, 8′, 10′-H), 2.06 (br d, 1H, J = 11.5 Hz, 9′a-H), 2.14-2.30 (m, 3H, 5, 4′a-H ), 2.16 (s, 3H, 4-CH3), 2.26 (s, 3H, 1-CH3), 2.38-2.58 (m, 2H, 1′, 6e-H), 2.86 (br d, 1H, J = 12 Hz, 3-H), 3.36 (br t, J ≈ 13 Hz, 6a-H); 13C-NMR (CDCl3, 50 MHz) δ (ppm) 27.2, 27.6 (5′,7′-C), 29.8, 32.9 (1′,3′-C), 31.7, 32.2 (4′,9′-C), 33.2 (8′,10′-C), 33.7 (1-CH3), 38.7 (6′-C), 47.2 (4-CH3), 47.9 (5-C), 48.3 (6-C), 52.0 (3-C), 58.5 (2,2′-C). Dimaleate: mp 151 - 153 °C (EtOH - Et2O); Anal. (C23H34N2O8) C, H, N.
3.2 Antiviral activity evaluation: plague reduction assay in MDCK cells
Pre-plated MDCK cells were pre-treated for 5 h with 100, 33, and 11 μM of the compounds in DMEM, 0.3% BSA and 1 μg/mL trypsin. After 5 h, cells were washed twice with PBS and infected with 100 plaque forming units of either WSN (amantadine resistant mutant virus) or HK68 (influenza H3N2 A/Hong Kong/68), by adding the appropriate virus dilution in 0.3% BSA/PBS (200 μL in a 6-well plate). After 1 h cells were washed with PBS twice and solid media of each compound was added and left for 10 minutes. Then cells were placed at 37 °C for 40 h and plaques were counted.
Supplementary Material
Acknowledgements
This research activity was supported by a research grant from the University of Athens, Greece (ELKE Account KA: 70/4/3238) and by grants from the NIH to CFB including R21AI085306 and P01AI058113 (García-Sastre).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Synthesis and characterization of the cyclooctane analogues of compounds 5 - 7.
References and Notes
- 1.Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Nature. 437(2005):889–893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 2.Wang TT, Palese P. Cell. 137(2009):983–985. doi: 10.1016/j.cell.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. Science. 325(2009):484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. EMBO J. 4(1985):3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hay AJ. Semin. Virol. 3(1992):21–30. [Google Scholar]; (c) Pinto LH, Holsinger LJ, Lamb RA. Cell. 69(1992):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]; (d) Grambas S, Hay AJ. Virology. 190(1992):11–18. doi: 10.1016/0042-6822(92)91187-y. [DOI] [PubMed] [Google Scholar]
- 5.For some representative publications see: Kolocouris A. Vol. 48. National Documentation Center, Vas. Constantinou; Athens, Greece: 1995. Ph.D Thesis. Kolocouris N, Foscolos GB, Kolocouris A, Marakos P, Pouli N, Fytas G, Ikeda S, De Clercq E. J. Med. Chem. 37(1994):2896–2902. doi: 10.1021/jm00044a010. Kolocouris N, Kolocouris A, Foscolos GB, Fytas G, Neyts J, Padalko E, Balzarini J, Snoeck R, Andrei G, De Clercq E. J. Med. Chem. 39(1996):3307–3318. doi: 10.1021/jm950891z. Stamatiou G, Kolocouris A, Kolocouris N, Fytas G, Foscolos GB, Neyts J, De Clercq E. Bioorg. Med. Chem. Lett. 11(2001):2137–2142. doi: 10.1016/s0960-894x(01)00388-2. Zoidis G, Kolocouris N, Foscolos GB, Kolocouris A, Fytas G, Karayannis P, Padalko E, Neyts J, De Clercq E. Antiviral Chem. Chemother. 14(2003):153–164. doi: 10.1177/095632020301400305. Stamatiou G, Foscolos GB, Fytas G, Kolocouris A, Kolocouris N, Pannecouque C, Witvrouw M, Padalko E, Neyts J, De Clercq E. Bioorg. Med. Chem. 11(2003):5485–5492. doi: 10.1016/j.bmc.2003.09.024. Stylianakis I, Kolocouris A, Kolocouris N, Fytas G, Foscolos GB, Padalko E, Neyts J, De Clercq E. Bioorg. Med. Chem. Lett. 13(2003):1699–1703. doi: 10.1016/s0960-894x(03)00231-2. Tataridis D, Fytas G, Kolocouris A, Fytas C, Kolocouris N, Foscolos GB, Padalko E, Neyts J, De Clercq E. Bioorg. Med. Chem. Lett. 17(2007):692–696. doi: 10.1016/j.bmcl.2006.10.092. Kolocouris A, Spearpoint P, Martin SR, Hay AJ, López-Querol M, Sureda FX, Padalko E, Neyts J, De Clercq E. Bioorg. Med. Chem. Lett. 18(2008):6156–6160. doi: 10.1016/j.bmcl.2008.10.003.
- 6.(a) Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, Tereshko V, Nanda V, Stayrook S, DeGrado W. Nature. 451(2008):596–599. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cady SD, Schmidt-Rohr K, Wang J, Soto CS, DeGrado WF, Hong M. Nature. 463(2010):689–692. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Duff KC, Gilchrist PJ, Saxena AM, Bradshaw JP. Virology. 202(1994):287–293. doi: 10.1006/viro.1994.1345. [DOI] [PubMed] [Google Scholar]
- 7.(a) Wang C, Takeuchi K, Pinto LW, Lamb RA. J. Virol. 1993;67:5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chizhmakov IV, Geraghty FM, Ogden DC, Hayhurst A, Antoniou M, Hay AJ. J. Physiol. 494(1996):329–336. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciampor F, Bayley PM, Nermut MV, Hirst EMA, Sugrue RJ, Hay AJ. Virology. 188(1992):14–24. doi: 10.1016/0042-6822(92)90730-d. [DOI] [PubMed] [Google Scholar]
- 9.(a) Aldrich PE, Hermann EC, Meier WE, Paulshock M, Prichard WW, Snyder JA, Watts JC. J. Med. Chem. 14(1971):535–543. doi: 10.1021/jm00288a019. [DOI] [PubMed] [Google Scholar]; (b) Lundahl K, Schut J, Schlatmann JLMA, Paerels GB, Peters A. J. Med. Chem. 15(1972):129–132. doi: 10.1021/jm00272a003. [DOI] [PubMed] [Google Scholar]; (c) Van Hes R, Smit A, Kralt T, Peters A. J. Med. Chem. 15(1972):132–136. doi: 10.1021/jm00272a004. [DOI] [PubMed] [Google Scholar]; (d) de la Cuesta E, Ballesteros P, Trigo GG. J. Pharm. Sci. 73(1984):1307–1309. doi: 10.1002/jps.2600730933. [DOI] [PubMed] [Google Scholar]; (e) Martinez AG, Vilar ET, Fraile AG, de la Moya Cerero S, Herrero MER, Ruiz PM, Subramanian LR, Gancedo AG. J. Med. Chem. 38(1995):4474–4477. doi: 10.1021/jm00022a012. [DOI] [PubMed] [Google Scholar]
- 10.Fytas C, Zoidis G, Fytas G. Tetrahedron. 64(2008):6749–6754. [Google Scholar]
- 11.See for example: Beshore DC, Dinsmore CJ. Org. Lett. 2002;4:1201–1204. doi: 10.1021/ol025644l. Petasis NA, Patel ZD. Tetrahedron Lett. 2000;41:9607–9611. Rübsam F, Mazitschek R, Giannis A. Tetrahedron. 56(2000):8481–8487.
- 12.pKa is ~ 10.5 for amantadine 1. Graton J, Laurence C, Berthelot M, Le Questel J-Y, Besseau F, Raczynska ED. J. Chem. Soc. Perkin Trans. II. 1999:997–1001.
- 13.Mason RP, Rhodes DG, Herbette LG. J. Med. Chem. 34(1991):869–877. doi: 10.1021/jm00107a001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.