Abstract
Lake Tanganyika comprises a cichlid species flock with substrate-breeding and mouthbrooding lineages. While sexual selection via mate choice on male mating color is thought to boost speciation rates in mouthbrooding cichlids, this is not the case in substrate-breeding lamprologines, which mostly form stable pairs and lack sexual dichromatism. We present a comprehensive reconstruction of the evolution of the cichlid tribe Lamprologini, based upon mtDNA sequences and multilocus nuclear DNA (AFLP) markers. Twelve mtDNA clades were identified, seven of which were corroborated by the AFLP tree. The radiation is likely to have started about 5.3 MYA, contemporarily with that of the mouthbrooding C-lineage, and probably triggered by the onset of deep-water conditions in Lake Tanganyika. Neither the Congo- nor the Malagarazi River species form the most ancestral branch. Several conflicts in the mtDNA phylogeny with taxonomic assignments based upon color, eco-morphology and behavior could be resolved and complemented by the AFLP analysis. Introgressive hybridization upon secondary contact seems to be the most likely cause for paraphyly of taxa due to mtDNA capture in species involving brood-care helpers, while accidental hybridization best explains the para- or polyphyly of several gastropod shell breeders. Taxonomic error or paraphyly due to the survival of ancestral lineages appear responsible for inconsistencies in the genera Lamprologus and Neolamprologus.
Keywords: Molecular phylogeny, Speciation, Hybridization, Cichlid fishes, Adaptive radiation
1. Introduction
Lake Tanganyika, the oldest of the East African Great Lakes, comprises by far the greatest diversity of cichlid fishes in terms of morphology, ecology and behavior. Its cichlid species flock includes two substrate-breeding and several mouthbrooding lineages, subdivided into 12–16 tribes (Poll, 1986; Takahashi, 2003; Salzburger, 2009), which are largely supported by comparative morphological and molecular phylogenetic data (reviewed in Koblmüller et al., 2008a). In consequence, Lake Tanganyika contains a polyphyletic conglomerate of lineages which evolved in parallel under the same abiotic influences from a handful of ancient species that once colonized the emerging lake some 9–12 million years ago (MYA; Cohen et al., 1993). Several new lineages formed via adaptive radiation in the lake itself, probably about 5–6 MYA when the proto-lakes fused into one single deep lake with tropical clearwater conditions (Tiercelin and Mondeguer, 1991; Salzburger et al., 2002a; Koblmüller et al., 2008a; but see Genner et al., 2007), and only two lineages colonized the lake at a later stage (Klett and Meyer, 2002; Koch et al., 2007).
With currently more than 80 species described from Lake Tanganyika (Koblmüller et al., 2008a), the lamprologine cichlids dominate Lake Tanganyika’s cichlid fauna, comprising about 40% of the lake’s species. They are all substrate breeders and form the sister group of an equally diverse lineage of mouthbrooding species, termed the C-lineage (Salzburger et al., 2002a; Clabaut et al., 2005; Koblmüller et al., 2008a). Lamprologines have colonized most lacustrine habitats, but most often live in the littoral zone. Although considered a single tribe (Poll, 1986), lamprologine cichlids encompass tremendous morphological, ecological and behavioral diversity. With respect to size and ecology, some species are large semi-pelagic predators, several are medium sized invertebrate pickers or herbivores, and some are small enough to fit into and live in empty gastropod shells (Sato and Gashagaza, 1997). In fact, shell-breeding represents a highly successful evolutionary strategy, which was suggested to have arisen multiple times during the radiation of the lamprologines (Sturmbauer et al., 1994; Koblmüller et al., 2007a). Remarkably, eight additional lamprologine species are found in the Congo River (Schelly and Stiassny, 2004), and at least one species occurs in the Malagarazi River (De Vos et al., 2001; Schelly et al., 2003). In terms of social organization, some species form pairs (Nakano and Nagoshi, 1990; Sturmbauer et al., 2008) or harems (Yanagisawa, 1987; Walter and Trillmich, 1994; Awata et al., 2006; Matsumoto and Kohda, 2007), exhibit sex-role-reversed polyandry (Yamagishi and Kohda, 1996) or are organized in family clans in which the elder offspring contribute to brood care and defense (Taborsky and Limberger, 1981; Taborsky, 1984; Awata et al., 2005; Heg et al., 2005; Heg and Bachar, 2007). Furthermore, several species exhibit alternative male reproductive phenotypes (Sato et al., 2004; Katoh et al., 2005; Ota and Kohda, 2006) and genetic parentage analyses demonstrated helper parasitism in two cooperatively breeding species (Dierkes et al., 1999, 2005; Dierkes et al., 2008; Awata et al., 2005), sneaker fertilization in a gastropod shell breeder (Katoh et al., 2005), unrelated offspring in the nests of another shell-breeding species (Sunobe and Munehara, 2003) and exceptionally high levels of multiple paternity in a socially monogamous species with biparental nest defense (Sefc et al., 2008).
While the monophyly of Poll’s tribe Lamprologini is firmly established (Sturmbauer et al., 1994; Stiassny, 1997; Takahashi et al., 1998; Salzburger et al., 2002a), the intra-tribal relationships and taxonomy remain problematic and in need of revision. Morphology-based and molecular approaches have consistently been incongruent, resolving most genera as polyphyletic in molecular phylogenies (Sturmbauer et al., 1994; Salzburger et al., 2002b; Schelly et al., 2006; Day et al., 2007; Koblmüller et al., 2007a; Nevado et al., 2009). The most thorough morphology-based analysis was carried out by Stiassny (1997), in which the “ossified group of lamprologines” was defined in congruence with a mitochondrial phylogeny (Sturmbauer et al., 1994). Ossified group lamprologines posses a sesamoid bone within the labial ligament (see Fig. 3 in Schelly et al., 2006), which is unique among cichlids and perhaps even Perciformes. The definition of the ossified group also highlights the inadequacy of current lamprologine classification, with representatives scattered among the genera Lamprologus, Neolamprologus, Lepidiolamprologus, and Altolamprologus. More recently, Takahashi (2003) used a partially different suite of morphological characters to examine relationships among Tanganyikan cichlids, but did not recover the ossified group as a monophyletic assemblage within 10 lamprologine species representing the Lamprologini in a broader taxonomic spectrum. A combined molecular and morphological study of Schelly et al. (2006) addressed the evolution of the genus Lepidiolamprologus within the ossified group, and suggested the exclusion of Lepidiolamprologus cunningtoni and the inclusion of Neolamprologus meeli, Neolamprologus hecqui, Neolamprologus boulengeri and Neolamprologus variostigma plus two undescribed species – one of which has been recently described as Lepidiolamprologus mimicus (Schelly et al., 2007) – in the “two-pore” Lepidiolamprologus-clade. Neolamprologus lemairii, which was suggested to be another potential member of the Lepidiolamprologus-clade due to its possession of two pores in the neurocranial lateral line foramina, was resolved outside the clade (Schelly et al., 2006).
As substrate breeders lacking sexual dichromatism the lamprologine cichlids are an interesting test case for the driving forces of speciation, contrasting with the mostly brightly colored males in the maternally mouthbrooding C-lineage (Clabaut et al., 2005). Sexual selection based upon female mate choice on male mating color, which was suggested to be a major driving force of rapid speciation (Terai et al., 2006; Seehausen et al., 2008), can be ruled out as driving force of diversification in lamprologines. On the other hand, lamprologines show other traits, which might be subject to sexual selection such as brood-care helping behavior and size dimorphism. Indeed, previous molecular phylogenetic studies on lamprologines have resulted in surprising findings: While the monophyly of genera defined by Poll (1986) was often corroborated in the mouthbrooding Tanganyikan lineages analyzed so far (Brandstätter et al., 2005; Duftner et al., 2005; Koblmüller et al., 2004, 2005, 2007b; Salzburger et al.; 2002a; Sturmbauer and Meyer, 1993), the taxonomy of the Lamprologini appeared to be much more in conflict with five of Poll’s (1986) seven genera being para- or polyphyletic (Sturmbauer et al., 1994; Schelly et al., 2006; Day et al., 2007; Koblmüller et al., 2007a; Nevado et al., 2009). While the relatively few cases of paraphyly or polyphyly of the genera within the Tropheini, Ectodini and Limnochromini were most likely the result of ancient incomplete lineage sorting or convergent evolution of similar morphologies, repeated interspecific hybridization was suggested to be the more likely cause in the Lamprologini (Salzburger et al., 2002b; Schelly et al., 2006; Koblmüller et al., 2007a). Due to the great degree of genetic divergence among conspecifics of Neolamprologus marunguensis belonging to different major mtDNA lineages, hybridization was put forward as the most probable explanation, in congruence with the results based on nuclear DNA data (Salzburger et al., 2002b). The same was found for Lepidiolamprologus nkambae (Schelly et al., 2006) and in several gastropod shell-breeding species (Koblmüller et al., 2007a; Nevado et al., 2009). This problem was not adequately addressed by the most recent mitochondrial DNA phylogeny of Day et al. (2007), requiring further analyses including nuclear DNA markers.
In the present study we aim to resolve the phylogeny of the Lamprologini more fully by including previously omitted taxa and by applying two different sets of molecular markers - sequences of the mitochondrial NADH dehydrogenase subunit 2 (ND2) genes and a set of 623 AFLP (Amplified Fragment–Length Polymorphism) loci. Furthermore, we attempt to provide a temporal framework for the diversification of this cichlid tribe by applying a Bayesian relaxed molecular clock model to estimate divergence times. Our study includes 72 of the 81described species, plus 5 undescribed species and thus represents the most comprehensive taxon sampling so far. To elucidate the relationships of lake endemics to riverine taxa, our analysis includes two species from the Congo River and another riverine species, Neolamprologus devosi (Schelly et al., 2003), which is endemic to the Malagarazi River. To minimize potential confusion due to inadequate generic assignment against the current state of knowledge, we follow Stiassny’s (1997) classification, in which the Lamprologini comprise eight genera: Altolamprologus Poll, 1986, Chalinochromis Poll, 1974, Julidochromis Boulenger, 1898, Lamprologus Schilthuis, 1891, Lepidiolamprologus Pellegrin, 1904, Neolamprologus Colombe and Allgayer, 1985, Telmatochromis Boulenger, 1898, and Variabilichromis Colombe and Allgayer, 1985. In addition, we follow Schelly et al. (2006) by naming all members of the “two-pore” Lepidiolamprologus-clade N. meeli, N. hecqui, N. boulengeri and N. variostigma as Lepidiolamprologus, and by treating L. cunningtoni as member of the still problematic genus Neolamprologus.
2. Materials and methods
2.1. Taxonomic sampling and DNA extraction
Our study is based on 161 specimens belonging to the cichlid tribe Lamprologini. The complete ND2 gene (1047 bp) was obtained from 91 individuals representing 77 lamprologine species plus 13 outgroup taxa (four species of the tribe Eretmodini plus nine species of the C-lineage sensu Clabaut et al., 2005; based on Salzburger et al., 2002a and Koblmüller et al., 2008a). AFLP data were obtained from 80 individuals representing 42 lamprologine species (71 individuals) plus nine outgroup taxa (two representatives of the tribe Eretmodini plus seven individuals representing three species of the C-lineage). Due to insufficient DNA quality not all species included in the mitochondrial dataset could be included in the AFLP data. Note that only a few representatives of the ossified group lamprologines have been used for AFLP fingerprinting because a previous study (Koblmüller et al., 2007a) explicitly dealt with the phylogenetic relationships (mtDNA + AFLPs) within the ossified group lamprologines. Most of the specimens were sampled during several field expeditions from 1995 to 2007, and some additional samples were obtained from the aquarium trade (Appendix A). Voucher specimens are stored at the Department of Zoology, University of Graz, Austria and the Royal Africa Museum in Tervuren, Belgium. For all specimens, fin clips were taken and preserved in 99% ethanol. For DNA extraction we applied a proteinase K digestion followed by protein precipitation with ammonium acetate.
2.2. mtDNA analysis
Polymerase chain reaction (PCR), purification of PCR products, and sequencing followed the protocol described in Duftner et al. (2005). The primers used for PCR and sequencing were Met, ND2.2A, Trp (Kocher et al., 1989) and ND2.T-R (Duftner et al., 2005) for ND2. DNA fragments were purified with SephadexTM G-50 (Amersham Biosciences) following the manufacturer’s instruction and subsequently visualized on an ABI 373 or an ABI 3130xl automated sequencer (Applied Biosystems). All sequences are available from GenBank under the accession numbers listed in the Appendix A.
DNA sequences were aligned by eye using the Sequence Navigator software (Applied Biosystems). To assess the overall phylogenetic signal we performed a likelihood-mapping analysis (Strimmer and von Haeseler, 1997), using TREE–PUZZLE 5.1 (Schmidt et al., 2002). Phylogenetic inference was based on neighbor joining (NJ), maximum parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI).
NJ, MP and ML were performed in PAUP∗ version 4.0b10 (Swofford, 2002). To assess the degree of saturation of transition (ti) and transversion (tv) mutations at each codon position, we plotted the number of mutations against pairwise uncorrected p-distances (not shown). Based on the estimated ti/tv ratio inferred from these pairwise comparisons we derived a weighting scheme for a weighted MP analysis: 30:2 for third codon positions of two and three fold degenerate amino acids, 5:2 for third codon positions of four fold degenerate amino acids, 30:24 for second codon positions and 30:12 for first codon positions. C/T substitutions at the first codon position of leucine were treated as a fifth base and were down-weighted to the same weight as transitions at third codon positions. For NJ and ML analysis, we applied the best-fitting substitution model selected by Modeltest 3.06 (Posada and Crandall, 1998): TrN + I + G (Tamura and Nei, 1993) with nucleotide frequencies A = 0.2640, C = 0.3656, G = 0.1220, T = 0.1484; proportion of invariable sites (I) = 0.3834; gamma shape parameter (α) = 1.1482; and R-matrix A ↔ G, A ↔ T, C ↔ G, G ↔ T = 1.0000, A ↔ G = 20.2677, C ↔ T = 6.7919. Heuristic tree searches under MP and ML criteria applied random addition of taxa and TBR branch swapping (1000 replicates for MP and 100 replicates for ML). Statistical support for the resulting topologies was assessed by bootstrapping (1000 pseudo-replicates for NJ and MP) and quartet puzzling (25,000 random quartets for ML). For BI, performed in MrBayes 3.0b4 (Huelsenbeck and Ronquist, 2001), data were partitioned by codon position. Rate heterogeneity was set according to a gamma distribution with six rate categories (GTR model; Yang, 1994) for each data partition. Posterior probabilities were obtained from Metropolis-coupled Markov chain Monte Carlo simulations (2 independent runs; 10 chains with 3 million generations each; chain temperature: 0.2; trees sampled every 100 generations). A 50% majority-rule consensus tree was constructed after a 1 million generation burn-in to allow likelihood values to reach stability (chain stationarity and run parameter convergence were checked using Tracer v1.4; Rambaut and Drummond, 2008). To assess whether the topologies obtained by the different tree building algorithms differed significantly we performed Shimodaira–Hasegawa (SH) tests (Shimodaira and Hasegawa, 1999; full optimization; 1000 bootstrap replicates) in PAUP∗.
Despite some inherent problems (e.g. Pulquério and Nichols, 2007), time estimates based on molecular data do provide an approximate framework to put diversification events in a temporal context. Divergence times were estimated within a Bayesian MCMC framework in the program BEAST v1.4.8 (Drummond and Rambaut, 2007), including the above-mentioned taxa plus additional representatives of the tribe Haplochromini (including additional taxa of the tribe Tropheini, which is nested within the Haplochromini (see Salzburger et al., 2005; Koblmüller et al., 2008b)), and Boulengerochromis microlepis, Bathybates leo, Hemibates stenosoma and Telotrematocara macrostoma as outgroups, justified by previously published evidence regarding the phylogenetic relationships among Lake Tanganyika’s cichlid tribes (reviewed in Koblmüller et al., 2008a). The inclusion of these additional taxa was necessary due to a lack of potential calibration points within the Lamprologini. Divergence times were calculated as 95% highest posterior density (HPD) intervals on a time-measured phylogeny. We employed the SRD06 two-partition codon-specific rates model of nucleotide evolution, which has fewer parameters than the GTR + I + G model, but has been shown to provide a better fit for protein coding sequence data (Shapiro et al., 2006) and implemented a relaxed molecular clock with log-normally autocorrelated rates among branches (Drummond et al., 2006). We used default settings for all priors except for the tree prior which was set to Yule Process (speciation) as suggested by Drummond et al. (2007). Monophyly of clades was not enforced, except for those used as calibration points, and operators were auto-optimized. As calibration points we applied the age of 5–6 MY for the formation of a truly lacustrine habitat with clear- and deep-water conditions in Lake Tanganyika (Tiercelin and Mondeguer, 1991; Lezzar et al., 1996; Cohen et al., 1997), constraining: (i) the time of the most recent common ancestor (MRCA) of the C-lineage encompassing the majority of mouthbrooding Lake Tanganyika cichlid tribes (Clabaut et al., 2005), and (ii) the time for the MRCA of the tribe Lamprologini, assuming that the onset of their diversification, the “primary radiation” in Lake Tanganyika, was coincident with that of the C-lineage (Salzburger et al., 2002a; Koblmüller et al., 2008a); (iii) a minimum age of 1.1 MY for the split of the Congo River lamprologines, based on the time window for a Lukuga-connection between Lake Tanganyika and the Congo system (Lezzar et al., 1996; Cohen et al., 1997); (iv) a maximum age of 0.57–1 MY for the split between the utaka and mbuna cichlids, based on the age of the refilling of Lake Malawi (Delvaux, 1995; Sturmbauer et al., 2001). A preliminary run was used for parameter optimization. The final analysis was run for 107 generations, with trees sampled every 1000 generations. The log file was analyzed using Tracer v1.4 (Rambaut and Drummond, 2008) to determine the appropriate burn-in (106 generations), which was discarded from the log and tree file. The post burn-in effective sample sizes (ESSs) for all parameters were >300, indicating that the log file accurately represented the posterior distribution (Kuhner, 2009). Divergence times were calculated from the post burn-in results and TreeAnnotator v1.4.8 (a module in the BEAST program package) was used to compute a maximum-clade-credibility tree, which was visualized in FigTree v1.2.2 (Rambaut, 2009). In order to check whether the posterior probabilities were actually affected by the data, we also ran analyses in which BEAST only sampled from the prior distribution (Drummond et al., 2006). To test for the effect of calibration points on divergence time estimates, we performed two additional analyses with different combinations of these calibration points (Table 2). Recently, alternative age estimates for the East African cichlid radiations have been proposed (Genner et al., 2007). Applying these calibration points for the Lake Tanganyika radiation yielded age estimates that are at odds with geology-based lake history data (see Koblmüller et al., 2008a, b). To evaluate the alternatives, we applied two alternative calibration schemes: We conducted a second analysis in which we assumed an alternative age estimate of 1.1–3.5 MY for the split of the Congo River lamprologines, based on the time window for a Lukuga-connection between Lake Tanganyika and the Congo system and constrained the primary Tanganyika radiation (in our case the MRCA of the C-lineage) to an age of 9–12 million years. This was based on the assumption that the primary Tanganyika radiation had taken place at the onset of the Lake formation 9–12 MYA as a consequence of allopatric diversification in a series of small shallow lakes in the area currently occupied by the Congo River system and Lake Tanganyika (Tiercelin and Mondeguer, 1991; Cohen et al., 1993). This assumption implies that the secondary radiation observed in most Lake Tanganyika cichlid tribes (Koblmüller et al., 2004, 2005, 2007a,b, 2010; Duftner et al., 2005) coincided with the formation of a real lacustrine habitat with deep-water conditions about 5–6 MYA, compatible with the fossil calibrated diversification scenario discussed by Genner et al. (2007). As a third alternative calibration, we applied a normally distributed prior with a mean of 30 MYA (S.D., 3.0 MY; to account for some degree of uncertainty in this age estimate) for the MRCA of the C-lineage, as was proposed by Genner et al. (2007) based on a presumed Gondwanan origin of the family Cichlidae.
Table 2.
Testing the influence of particular calibration points on posterior estimates of divergence times, with mean rate and coefficient of variation for each scenario.
| Calibrations used | MVhL-clade⁎ | Posterior estimates of calibration points |
Mean rate (×10−3) | Coefficient of variation | |||
|---|---|---|---|---|---|---|---|
| C-lineage | Lake Malawi flock | Lamprologini | Congo/Lake Tanganyika | ||||
| C-lineage (5–6)/LM (0.57–1)/Lamprologini (5–6)/Congo (1.1–3.5) | 7.42 (6.42–8.58) | 5.51 (5.06–6.00) | 0.92 (0.78–1.00) | 5.28 (5.00–5.76) | 1.68 (1.18–2.15) | 15.97 (13.91–17.97) | 0.34 (0.26–0.43) |
| 5.39 (5.00–5.89) | 0.84 (0.62–1.00) | 5.53 (5.07–6.00) | 1.69 (1.10–2.77) | ||||
| C-lineage (5–6)/LM (0.57–1)/Congo (1.1–3.5) | 6.72 (5.57–8.00) | 5.35 (5.00–5.86) | 0.90 (0.74–1.00) | 4.55 (3.68–5.52) | 1.50 (1.10–1.91) | 17.94 (14.63–21.12) | 0.32 (0.24–0.41) |
| 5.43 (5.00–5.92) | 0.84 (0.62–1.00) | 1.77 (1.10–2.94) | |||||
| C-lineage (5–6)/LM (0.57–1) | 6.75 (5.57–8.11) | 5.35 (5.00–5.87) | 0.90 (0.74–1.00) | 4.57 (3.61–5.54) | 1.50 (1.01–2.02) | 17.87 (14.55–21.20) | 0.33 (0.25–0.42) |
| 5.41 (5.00–5.91) | 0.84 (0.62–1.00) | ||||||
| C-lineage (9–12)/Congo (1.1–3.5) | 13.06 (10.37–16.10) | 10.14 (9.00–11.65) | 2.34 (1.40–3.40) | 8.87 (6.90–11.06) | 2.82 (2.10–3.50) | 9.19 (7.27–11.20) | 0.33 (0.24–0.41) |
| 10.35 (9.00–11.80) | 2.12 (1.10–3.30) | ||||||
| C-lineage (30) | 38.00 (27.89–49.01) | 29.07 (23.21–35.12) | 6.83 (4.00–10.12) | 25.96 (18.49–33.58) | 8.50 (5.49–11.99) | 3.17 (2.33–4.06) | 0.33 (0.24–0.41) |
We ran three independent analyses in BEAST using combinations of different sets of calibration points (uniform priors): (i) 5–6 MY for the most recent common ancestor (MRCA) of the cichlids assigned to the C-lineage (encompassing the majority of mouthbrooding Lake Tanganyika cichlid tribes; Clabaut et al., 2005), assuming that this “primary radiation” (Salzburger et al., 2002a) coincided with the formation of a truly lacustrine habitat with deep-water conditions in Lake Tanganyika (Tiercelin and Mondeguer, 1991; Lezzar et al., 1996; Cohen et al., 1997); (ii) a maximum age of 0.57–1 MY for the split between the utaka and mbuna cichlids, based on the age of the refilling of Lake Malawi (Delvaux, 1995; Sturmbauer et al., 2001); (iii) an age of 5–6 MYA for the MRCA of the tribe Lamprologini, assuming that the onset of their diversification also coincided with the formation of a truly lacustrine habitat with deep-water conditions in Lake Tanganyika; (iv) a minimum age of 1.1 MY for the split of the Congo-lamprologines, based on the time window for a Lukuga-connection between Lake Tanganyika and the Congo system (Lezzar et al., 1996; Cohen et al., 1997). In a fourth run we assumed an age of 9–12 MY for the MRCA of the C-lineage, compatible with the fossil calibrated diversification scenario discussed by Genner et al. (2007). In a fifth run we applied a normally distributed prior with a mean of 30 MYA (S.D., 3.0 MY) for the MRCA of the C-lineage, based on Genner et al.’s (2007) Gondwanan origin scenario for the family Cichlidae.
In order to check whether the posterior probabilities were actually affected by the data, we also ran analyses in which BEAST only sampled from the prior distribution (only for analyses with at least two calibration points). Estimates based on priors only are given below the estimates using the data.
Age estimates are indicated in MY. Numbers in parentheses represent the 95% highest posterior density (HPD) interval.
The MVhL clade includes the C-lineage, the Lamprologini and the Eretmodini (Takahashi et al., 2001).
2.3. AFLP analysis
Restriction digestion, pre-selective and selective PCR followed the protocol described in Egger et al. (2007). For selective amplification we used the following eight primer combinations: EcoRI-ACA/MseI-CAA, EcoRI-ACA/MseI-CAG, EcoRI-ACA/MseI-CAC, EcoRI-ACA/MseI-CAT, EcoRI-ACT/MseI-CAA, EcoRI-ACT/MseI-CAG, EcoRI-ACT/MseI-CAC, EcoRI-ACT/MseI-CAT. Selective PCR products were visualized on an ABI 3100xl automated sequencer (Applied Biosystems) along with an internal size standard (GeneScan-500 ROX, Applied Biosystems).
Raw fragment data were analyzed using GENEMAPPER v.3.7 (Applied Biosystems). We found automated scoring problematic with respect to fragments that showed gradual intensity differences between samples. Because any detection threshold has to be arbitrary in such cases, presence or absence of peaks (assumed to represent homologous fragments) was scored by eye within a range of 100–500 bp (only clearly distinguishable and unambiguous peaks were scored) and assembled as a binary matrix in GENEMAPPER. In a few cases, fragments were scored as missing data when character states could not be determined unambiguously. It is clear that manual scoring yields a lower number of marker bands than automated scoring, but data quality is substantially improved by this approach. Matrices from the different primer combinations were combined into one data set yielding a data matrix of 80 individuals × 623 loci.
A NJ tree based on Nei and Li’s (1979) distances was constructed in PAUP∗ and bootstrap values from 1000 pseudo-replicates were used as a standard measure of confidence in the reconstructed tree topology.
3. Results
3.1. mtDNA phylogeny
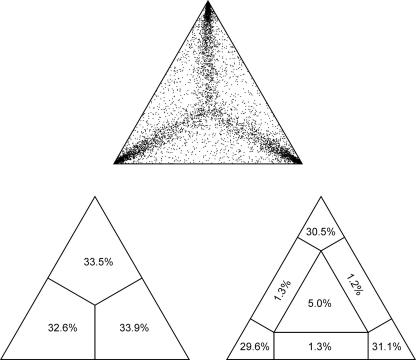
Likelihood mapping yielded 91.2% fully resolved quartets (Fig. 1), indicating a strong phylogenetic signal in the dataset. Pairwise sequence divergence (uncorrected p-distance) between lamprologine species ranged from 0.2% to 13.4%.
Fig. 1.
Results from the likelihood mapping analysis of the ND2 dataset, indicating the presence of a strong phylogenetic signal.
Whereas NJ, ML and BI analyses yielded highly congruent results with only minor differences with respect to the tree building algorithm used, SH tests revealed a significantly lower likelihood value for the MP topology (Table 1). Only the best tree (BI) – based on the likelihood criterion – is shown in Fig. 2.
Table 1.
Comparison of alternative phylogenetic hypotheses.
| Tree | −ln L | Δ−ln L | P |
|---|---|---|---|
| NJ | 14248.05672 | 39.89702 | 0.157 |
| MP | 14358.34803 | 150.18833 | <0.001 |
| ML | 14209.70277 | 1.54307 | 0.815 |
| BI | 14028.15970 | Best |
Shimodaira–Hasegawa (SH) tests (Shimodaira and Hasegawa, 1999) were used to determine whether NJ, MP, ML and BI topologies differed significantly under a maximum likelihood criterion.
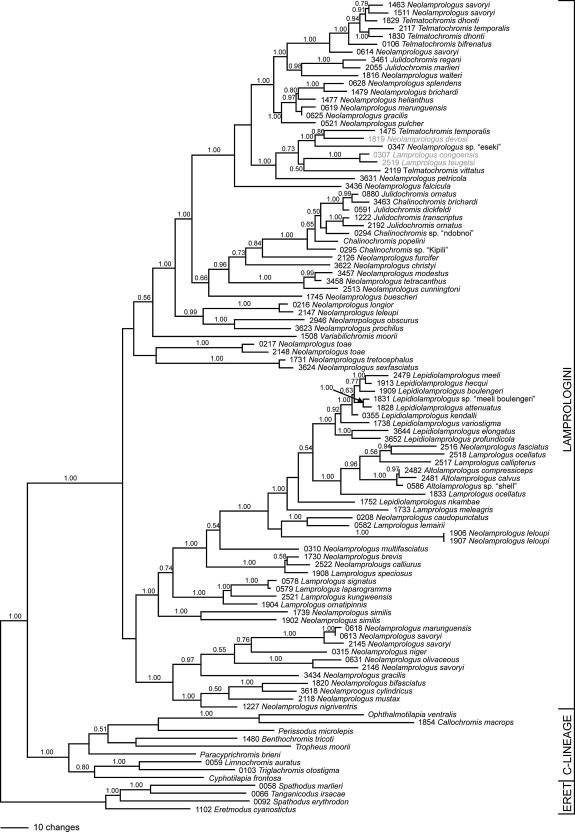
Fig. 2.
Bayesian 50% majority-rule consensus tree of the ND2 dataset. Only posterior propabilities >0.50 are shown. Riverine lamprologine taxa are shaded in grey.
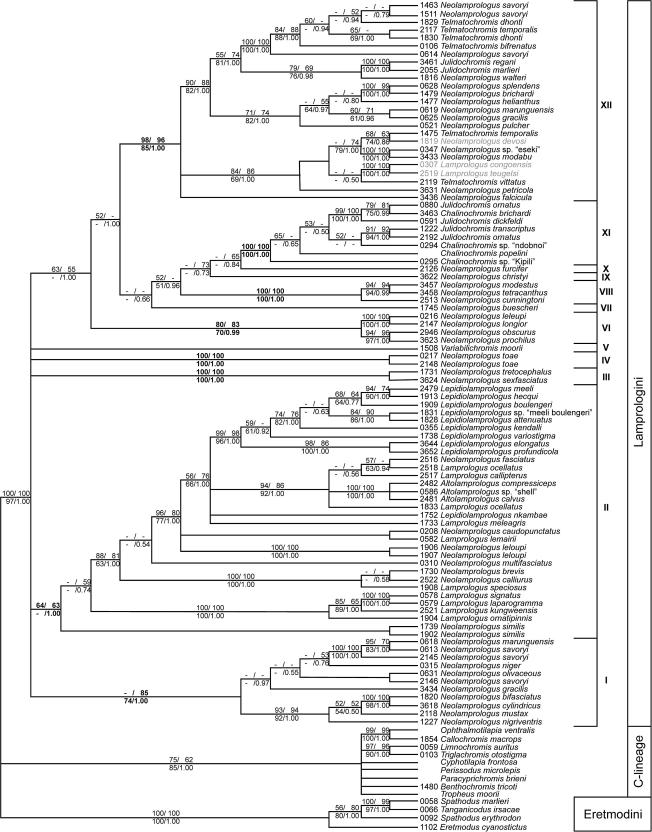
Fig. 3 shows a 75% majority-rule consensus tree based on the NJ, MP, ML and BI topologies. We present a 75% majority rule and not a strict consensus tree because of the significantly deviating MP topology (see above), which is equivalent to the strict consensus of the outcome of the remaining three tree building algorithms. Of the 1047 bp of ND2, 561 bp showed variation and 449 bp were parsimony- informative. The maximum parsimony analysis resulted in 62 most parsimonious trees (weighted tree length = 15,691 steps; CI excluding uninformative sites = 0.3994; RI = 0.6920; trees not shown). Neighbor-joining, maximum likelihood and Bayesian inference yielded the same tree topology. Our new phylogeny is fully compatible with earlier works (Sturmbauer et al., 1994; Schelly et al., 2006; Day et al., 2007; Koblmüller et al., 2007a). All four tree building algorithms revealed strong support for the monophyly of the Lamprologini. Within the Lamprologini, 12 clades emerged: clade I comprising two subclades, one with Neolamprologus nigriventris, Neolamprologus mustax, Neolamprologus cylindricus, and the second subclade with Neolamprologus gracilis, Neolamprologus savoryi, Neolamprologus olivaceous, Neolamprologus niger and N. marunguensis (clade I in Fig. 3); clade II comprising the very species-rich ossified group with Neolamprologus similis as its most ancestral split, followed by a subclade with Lamprologus ornatipinnis, Lamprologus kungweensis, Lamprologus laparogramma and Lamprologus signatus, another subclade with the Lamprologus speciosus, Neolamprologus calliurus and Neolamprologus brevis, a subclade with Neolamprologus multifasciatus, the subclade with Neolamprologus leloupi, the subclade with Lamprologus lemairii and Neolamprologus caudopunctatus, the subclade comprising Lamprologus meleagris, the subclade with L. nkambae, the subclade containing Lamprologus ocellatus, three species of Altolamprologus, Lamprologus callipterus and Neolamprologus fasciatus, and the subclade comprising the members of the “two-pore” Lepidiolamprologus-clade Lepidiolamprologus profundicola, Lepidiolamprologus elongatus, Lepidiolamprologus variostigma, Lepidiolamprologus kendalli, Lepidiolamprologus attenuatus, L. sp. nov. “hecqui-boulengeri”, Lepidiolamprologus boulengeri, Lepidiolamprologus hecqui and Lepidiolamprologus meeli (clade II); clade III comprising Neolamprologus tretocephalus and Neolamprologus sexfasciatus; clade IV with Neolamprologus toae; clade V with Variabilichromis moorii; clade VI with Neolamprologus prochilus, Neolamprologus obscurus, Neolamprologus leleupi and Neolamprologus longior, clade VII comprising Neolamprologus buescheri, clade VIII with Neolamprologus cunningtoni, Neolamprologus tetracanthus and Neolamprologus modestus, clade IX with Neolamprologus furcifer; clade X with Neolamprologus christyi; clade XI comprising four Chalinochromis species with Julidochromis ornatus, Julidochromis transcriptus and Julidochromis dickfeldi in paraphyletic grouping; and the species-rich clade XII with five subclades, the first with Neolamprologus falcicula (clade XII.1), the second containing Neolamprologus petricola, Telmatochromis vittatus, the two Congo River species Lamprologus teugelsi and Lamprologus congoensis, Neolamprologus mondabu, N. sp. nov. “eseki”, the Malagarazi River species N. devosi and Telmatochromis temporalis (clade XII.2), the third subclade comprising the brood-care helper species Neolamprologus pulcher, N. gracilis, N. marunguensis, Neolamprologus helianthus, Neolamprologus brichardi and Neolamprologus splendens (clade XII.3), the fourth subclade with Neolamprologus walteri, Julidochromis marlieri and Julidochromis regani (clade XII.4), and the fifth subclade comprising various genotypes of N. savoryi, Telmatochromis bifrenatus, Telmatochromis dhonti, and T. temporalis (clade XII.5). Fig. 2b depicts the Bayesian tree; NJ and ML yielded highly similar topologies. The branching order of the 12 clades recovered was as follows: Clades VI–XII were always resolved as monophylum (VI, (VII, (VIII, (IX, (X, (XI, XII))))). This monophylum – albeit with slightly different branching order (VI, (X, (IX, (VIII, (XI, XII))))) – was also found in MP (trees not shown), when N. buescheri (clade VII) was excluded (it was placed inside clade I in MP).
Fig. 3.
75% Majority-rule consensus tree of the NJ, ML, BI and strict consensus of 62 most parsimonious trees, representing the phylogenetic relationships of the Lamprologini based on the complete ND2 gene. Only bootstrap values >50 and posterior probabilities >0.50 are shown. Roman numbers indicate major, well supported mtDNA lineages (corresponding bootstrap values and posterior probabilities are printed in bold lettering). Riverine lamprologine taxa are shaded in grey.
3.2. AFLP phylogeny
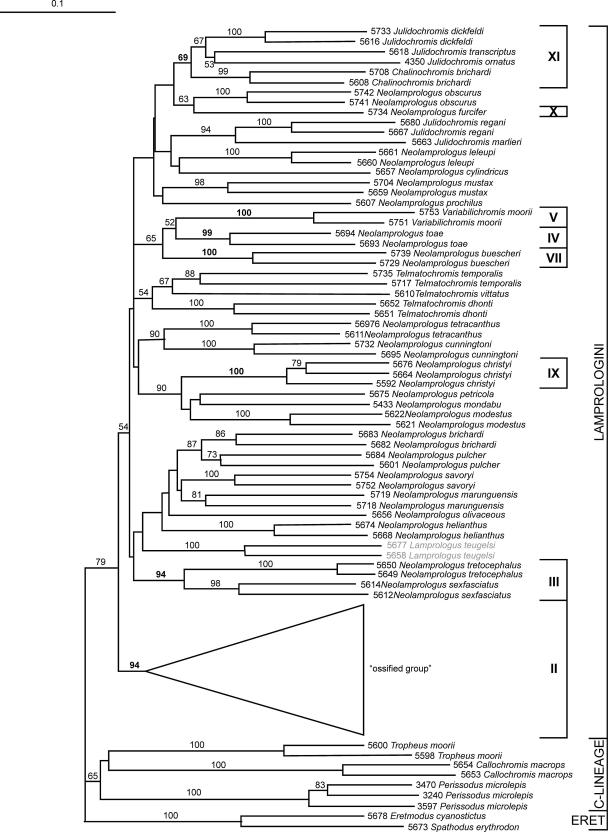
For 80 taxa (representing 47 species) AFLPs could successfully be amplified. Seven out of the 12 clades corresponded to those identified on the basis of mtDNA sequences, as depicted in Fig. 4: clade II comprising the ossified group; clade III comprising N. tretocephalus and N. sexfasciatus; clade IV with N. toae; clade V with V. moorii; clade VII comprising N. buescheri; clade IX with N. furcifer; clade X with N. christyi; and clade XI comprising four Chalinochromis species with J. ornatus, J. transcriptus and J. dickfeldi. In the species-rich clade XII with five subclades, three of the five subclades were retained: subclade XII.3 comprising N. helianthus, N. olivaceous, N. savoryi, N. marunguensis, N. brichardi and N. pulcher, was retained as well as subclade XII.4 with J. marlieri and J. regani, and subclade XII.5 comprising all analyzed individuals of the three species of the genus Telmatochromis, T. bifrenatus, T. dhonti, and T. temporalis. Subclade XII.1 with N. falcicula was not corroborated due to insufficient DNA quality, subclade XII.2 represented by N. petricola, N. mondabu and the Congo River species L. teugelsi, was split into two clades. Clade VI represented by N. prochilus, N. obscurus and N. leleupi was split into three separate clades, clade VIII with N. cunningtoni, N. tetracanthus and N. modestus was split into one clade comprising N. cunningtoni and N. tetracanthus, a second with N. modestus. In summary, the AFLP tree was highly compatible with the mtDNA phylogeny by corroborating 7 of the 12 clades with good bootstrap support. The remaining 5 mtDNA clades were subdivided. As in the mtDNA phylogeny, most branches between clades were short. In contrast to the mtDNA tree, however, all species recovered as polyphyletic in the mtDNA phylogeny were monophyletic in the AFLP tree. Moreover, the genus Telmatochromis, which was scattered in the mtDNA tree, was monophyletic in the AFLP analysis, as were the Neolamprologus species with brood-care helper behavior of clade XII.3. The paraphyletic placement of the genus Julidochromis was retained, corroborating the close relationship of J. ornatus, J. transcriptus and J. dickfeldi with the genus Chalinochromis.
Fig. 4.
NJ tree (Nei and Li distances Nei and Li, 1979)) based on 623 AFLP loci. Only bootstrap values >50 are shown. Riverine lamprologine taxa are shaded in grey.
3.3. Mitochondrial phylo-chronology
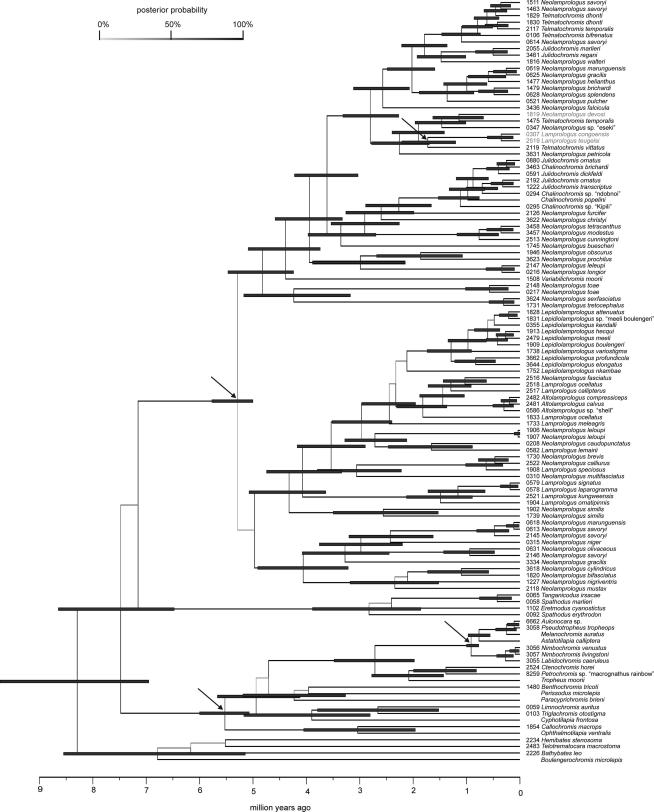
The chronogram for the radiation of the lamprologine cichlids resulting from a relaxed molecular clock analysis with BEAST and applying four calibration points ((i) MRCA of the C-lineage; (ii) MRCA of the Malawi cichlid species flock; (iii) MRCA of the Lamprologini; (iv) time window for the colonization of the Congo system via the Lukuga River) is shown in Fig. 5 (chronograms based on alternative sets of calibration points are shown in Supplementary Fig. 1–4). For all calibration nodes in all analyses, mean posterior estimates were close to prior node ages (but usually with smaller HPD intervals), indicating that the combinations of calibration points are reasonably concordant (Sanders and Lee, 2007). The mean rate covariance was slightly negative in all analyses, with its 95% HPD interval spanning zero (not shown), indicating that branches with fast and slow substitution rates are next to each other, meaning that there is no strong evidence for autocorrelation of rates in the phylogeny (Drummond et al., 2006). All calibration sets yielded similar significant but not exceptionally high levels of rate heterogeneity among lineages (mean coefficients of branch rate variation, 0.32–0.34; Table 2), thus justifying the use of a relaxed clock model (Drummond et al., 2007). Mean substitution rates varied between 0.01597 and 0.01794 substitutions per site per MY among the analyses assuming that the primary radiation (Salzburger et al., 2002a) coincided with the formation of a real lacustrine habitat 5–6 MYA (Table 2). Analyses assuming ages of 9–12 and 30 MY for the diversification of the C-lineage yielded mean substitution rates of 0.00919 and 0.00317 substitutions per site per MY, respectively (Table 2). We emphasize that our divergence time estimates should be regarded as approximate, because the 95% HPD intervals of adjacent nodes are large and often overlapping.
Fig. 5.
A chronogram of the diversification of the Lamprologini based on complete ND2 sequences, assuming that the diversification of both the C-lineage and the Lamprologini started in the course of the so-called “primary radiation”, which was proposed to have coincided with the formation of tropical clearwater habitat with deep-water conditions 5–6 MYA (Salzburger et al., 2002a); assuming a maximum age of 0.57–1 MY for the Lake Malawi cichlid species flock, based on the age of the refilling of Lake Malawi (Delvaux, 1995; Sturmbauer et al., 2001); and assuming an age of 1.1–3.5 MY for the split of the Congo-lamprologines, based on the time window for a Lukuga-connection between Lake Tanganyika and the Congo system (Lezzar et al., 1996; Cohen et al., 1997). Chronograms for alternative diversification scenarios are shown in Supplementary Fig. 1–4. Divergence time estimates are represented as the mean node height of the 95% highest posterior density (HPD) interval from a BEAST maximum-clade-credibility tree. Grey bars span the 95% HPD for each well supported node. No bars are assigned to nodes with low posterior probability. Calibration points are marked by arrows. Riverine lamprologine taxa are shaded in grey.
Assuming that the diversification of both the C-lineage and the Lamprologini started in the course of the so-called “primary radiation”, which was proposed to have coincided with the formation of tropical clearwater habitat with deep-water conditions 5–6 MYA (Salzburger et al., 2002a), the Lamprologini were estimated to have diverged from the Eretmodini and the C-lineage approximately 7.5 MYA. The onset of diversification within the Lamprologini was estimated to ∼5.3 MYA. Note that this estimate was heavily constrained by applying a uniform prior of 5–6 MYA for this particular node. In the course of this first cladogenesis event at the base of the lamprologine radiation, clades I and II (ossified group), as well as the ancestor of clades III–XII emerged. The ancestor of the species poor clades III (N. toae) and IV (N. sexfasciatus + N. tretocephalus) branched off ∼4.8 MYA and the split between these two lineages happened ∼4.2 MYA. The next clade to diverge was clade V (V. moorii) at ∼4.4 MYA, followed by clade VI ∼4 MYA. The split between the ancestor of clades VII–XI and clade XII happened ∼3.7 MYA. The mostly species poor lineages VII–XI, diverged in subsequent order ∼3.4, ∼2.9, ∼2.6 and ∼2.3 MYA. The most recent common ancestor (MRCA) of the Chalinochromis/Julidochromis species in clade XI dates back to ∼1.2 MYA. The split of the Congo River lamprologines from the closet related Lake Tanganyika species (T. vittatus) was estimated at ∼1.7 MYA, which lies well within the possible time window of 1.1–3.5 MYA for the connection of Lake Tanganyika with the Congo system (Lezzar et al., 1996; Cohen et al., 1997). N. devosi, which occurs in the Malagarazi River, diverged from its Lake Tanganyika sister taxon ∼1.2 MYA. Taking together this series of cladogenesis events, the radiation of the Lamprologini seems to have happened more gradually than that of Lake Tanganyika’s mouthbrooding cichlid lineages (Salzburger et al., 2002a; Sturmbauer et al., 2003; Brandstätter et al., 2005; Koblmüller et al., 2004, 2005, 2007b, 2010; Duftner et al., 2005). Estimates without prior constraints on the MRCA of the Lamprologini revealed only slightly more recent age estimates for splits within the Lamprologini, but confidence intervals were largely overlapping with the results of the analysis in which we constrained the MRCA of the Lamprologini to an age of 5–6 MYA. Likewise, using a prior on the time window for a possible colonization of the Congo system via the Lukuga River had little effect on the resulting age estimates. The alternative calibrations, congruent with Genner et al.’s (2007) fossil calibrated and Gondwanan based scenarios for cichlid diversification yielded roughly two and five times older node ages, respectively (Table 2; Supplementary Figs. 3 and 4).
4. Discussion
Perhaps the most intriguing question connected to the diversification of the Lamprologini is the role of sexual selection. While this is considered highly important in mouthbrooding cichlid lineages (Dominey, 1984; Seehausen, 2000; Terai et al., 2006; Seehausen et al., 2008; Salzburger, 2009), substrate-breeding species with pair-formation lack many features associated with sexual selection as a diversifying force. Unlike haplochromines and several other mouthbrooding cichlid tribes, lamprologine cichlids never display pronounced sexual dichromatism upon which sexual selection via mate choice might act (Schluter and Price, 1993; Gray and McKinnon, 2007), indicating that color is not their basis for mate choice. However, while mating color can be ruled out as an important factor facilitating “proper” mate choice and driving reproductive isolation and speciation in this group, this may not be the case for other male-specific traits such as larger body size and territory quality and size. In fact, sexual size dimorphism is quite frequently observed in lamprologines, with males typically slightly larger than females, so that positive selection on male body size facilitating territorial defense seems likely (see e.g. Schütz et al., 2006; Maan and Taborsky, 2008). However, the most pronounced cases of sexual size dimorphism are observed in some obligatory and facultative gastropod shell breeders (L. callipterus, L. lemairii, L. ornatipinnis, N. calliurus, N. fasciatus). In L. callipterus, L. lemairii (some females appear to reach maturity at a body size small enough to spawn in gastropod shells) and N. fasciatus, females are small enough to fit in a Neothauma shell, while males are too large to enter the shells and thus have to release their sperm over the shell entrance. In L. callipterus an alterative reproductive tactic evolved, with dwarf males that are even smaller than females and thus able to enter the shells to fertilize the eggs, even in the presence of the large territorial male. The point may be that male color traits can be pushed by diversifying sexual selection which is not the case for male fitness traits, so that selection acting on those cannot easily lead to two novel species in sympatry. However, divergent selection acting on polymorphic and naturally selected traits relevant for differential trophic specialization, as described for Central American cichlids (Barluenga and Meyer, 2004), may as well have promoted (sympatric) speciation in lamprologines.
Sexual selection can also act in social context, to produce signals for intraspecific communication. This is the case in lamprologines with brood-care helper behavior which often show individual color markings on the head or body. The N. pulcher group and perhaps the genus Julidochromis may serve as examples. The subclade comprising the N. pulcher group with species exhibiting sophisticated brood-care helping behavior is monophyletic according to our AFLP phylogeny (Fig. 4) and species-rich. All species display distinct color patterns on the head which are species-specific, albeit variable enough that individuals can be distinguished (Hert, 1985; Balshine-Earn and Lotem, 1998; Frostman and Sherman, 2004). It thus seems likely that the “head mask” facilitates social interactions and is thus subject to sexual selection. As most of these species are allopatric, maintenance and evolution of color in this group may be similar to the pattern found in the maternal mouthbrooding genus Tropheus, for which about 120 distinctly colored populations or sister species were described. As in the N. pulcher group, no sexual color dimorphism exists in Tropheus, and most color morphs are allopatric, so that divergent selection in sympatry seems unlikely as driving force of speciation (Egger et al., 2010). Taken together, disruptive sexual selection does not seem to be frequent in the Lamprologini, albeit other diversifying selective processes in social context might exist, in addition to those in the context of natural selection (see also Verburg and Bills, 2007; Takahashi et al., 2009). These might help to explain why the Lamprologini are the only non-mouthbrooding cichlid lineage which has formed a species flock with substantial diversity in sympatry.
Several lamprologine species show various degrees of population genetic structure and/or geographic variation in morphology (e.g. Duftner et al., 2006; Koblmüller et al., 2007c; Risch and Snoeks, 2008; Nevado et al., 2009) or form geographic sister species (Duftner et al., 2007), pointing to the influence of ecological specialization on the dispersal ability promoting allopatric speciation. Apparent correlation between habitat specialization and dispersal ability and degree of genetic structure and species richness has been inferred not only for lamprologines but other Lake Tanganyika cichlids as well (Taylor et al., 2001; Stiver et al., 2004; Duftner et al., 2006, 2007; Koblmüller et al., 2007c; Koblmüller et al., 2009; Sefc et al., 2007; Nevado et al., 2009; Takahashi et al., 2009; Wagner and McCune, 2009). Another peculiarity of the lamprologine cichlids is the high frequency of interspecific gene flow for a variety of reasons discussed below. Hybridization is also likely to be involved in speciation in particular lamprologine lineages (Salzburger et al., 2002a; Schelly et al., 2006; Koblmüller et al., 2007a), contributing to the species richness of this tribe. Remarkably, on the other hand, it seems that the hybridization events in the history of several species did not wipe out the involved species.
4.1. Phylogenetic implications
Our re-analysis is fully compatible with previous works (Sturmbauer et al., 1994; Schelly et al., 2006; Day et al., 2007; Koblmüller et al., 2007a), but adds several new groupings. Considering that the inconsistent placement of N. buescheri in the mitochondrial MP tree was most likely caused by long-branch-attraction (Hendy and Penny, 1989), the 79 species analyzed were grouped into 12 clades (named clades I–XII in this study) which can be further grouped into 6 major mtDNA lineages, as clades VI–XII are likely to be monophyletic. Just as in previous analyses, the Congo River lamprologines were not recovered as sister clade to the lake endemics. Strikingly, N. devosi from the Malagarazi River was most closely related to a lake endemic (T. temporalis), although it was grouped in the same subclade of clade XII as its Congo River allies, together with N. petricola and N. sp. “eseki”. Thus, the inclusion of the Malagarazi-lamprologine corroborates our hypothesis that the most ancestral lineages of the tribe Lamprologini lived in the lake proper (Sturmbauer et al., 1994) and that all riverine species diverged from their lake allies about 1.7 and 1.2 MYA. The possibility that the most ancient branch of the Lamprologini lived in the Proto-Malagarazi-Congo River and seeded the lacustrine radiation can be ruled out. Instead, lamprologines apparently colonized the Congo system via the Lukuga River, Lake Tanganyika’s only outflow, in the time window of 1.1–3.5 MYA during which this connection between Lake Tanganyika and the Congo system was open (Lezzar et al., 1996; Cohen et al., 1997). In our new phylogeny, para- or polyphyly of the genera Lamprologus, Neolamprologus, Julidochromis, Chalinochromis and Telmatochromis is evident, but most likely for different reasons which are discussed below. We should note that we consider the genus Lepidiolamprologus as revised according to Schelly et al. (2006).
Concerning the mtDNA – based groupings, clade I comprises two subclades, the first including N. mustax, N. nigriventris, N. cylindricus and Neolamprologus bifasciatus. Its second subclade – except for N. niger – exclusively comprises species with complex social system in which elder offspring contributes to brood care and territorial defense (Kuwamura, 1997). These species are N. gracilis, N. savoryi, N. olivaceous and N. marunguensis. In the mtDNA phylogeny three of them (N. gracilis, N. savoryi and N. marunguensis) are para- or polyphyletic with respect to their clade assignment, in that additional individuals were grouped in a subclade of clade XII, as previously shown for N. marunguensis (Salzburger et al., 2002b). Recent reciprocal gene flow, at least in one ancestral species, is further substantiated by this new evidence. The nuclear multilocus tree based on AFLP suggests monophyly of these taxa in the hypothetical species tree, with additional information from the mtDNA tree suggesting a past hybridization or introgression event twisting the tree, or less likely ancient incomplete lineage sorting. Clade II was already defined by Sturmbauer et al. (1994) as a highly diverse species group adapted to a variety of truly lacustrine ecological niches and named the “ossified group” on morphological grounds by Stiassny (1997). This clade also contains the predatory genus Lepidiolamprologus, which was recently revised by Schelly et al. (2006). The ossified group – lamprologines comprise perhaps the greatest ecological and behavioral diversity in that they contain both the largest predatory Lepidiolamprologus and the smallest cichlid species breeding in gastropod shells. Here we note great taxonomic incongruence within certain gastropod shell breeders, namely the sister species pair N. multifasciatus and N. similis, and the L. ocellatus species-assemblage (L. ocellatus, L. meleagris and L. speciosus), which on morphological and behavioral grounds must be grouped together. This issue is treated in detail in a separate paper, so that we briefly refer to our hypothesis of repeated hybridization events which was discussed elsewhere (Koblmüller et al., 2007a). Clade III represents a new lineage formed by two eco-morphologically similar species, N. sexfasciatus and N. tretocephalus. These genetically close sister taxa are the only descendents of an ancient lineage. Likewise, clade IV comprises N. toae only as monotypic representative of another ancient lineage. The same applies to clade V which comprises V. moorii. This taxon was suggested as the most ancestral split in the pioneering molecular work of Sturmbauer et al. (1994), but now allies with 5 other ancient lineages. Clade VI was also identified earlier to comprise N. longior, and is now extended to also contain N. leleupi, N. prochilus and N. obscurus. Clade VII again is monotypic comprising N. buescheri only. As discussed above, this species jumps inside clade I in MP, most likely due to long-branch attraction (Hendy and Penny, 1989). Clade VIII comprises N. cunningtoni (formerly Lepidiolamprologus; see Schelly et al., 2006), sister to N. modestus and N. tetracanthus. Clade IX comprises N. christyi only. We note that a highly similar species, tentatively named Neolamprologus sp. “eseki” is grouped in clade XII. Clade X is also monotypic with N. furcifer only. Clade XI comprises three species of the genus Chalinochromis plus J. ornatus, J. transcriptus and J. dickfeldi, but excluding J. marlieri and J. regani which are grouped in clade XII. Finally, clade XII comprises a highly complex and eco-morphologically as well as behaviorally heterogeneous array of taxa. The first subclade (clade XII.1) comprises N. falcicula only, the second is comprised by N. petricola, the two Congo River lamprologines L. congoensis and L. teugelsi, sister to (T. vittatus (N. sp. “eseki” (N. devosi (T. temporalis)))). It should be noted that one additional haplotype of T. temporalis is placed in paraphyly.
Concerning the AFLP tree, it turned out that it was surprisingly compatible with the mtDNA phylogeny, corroborating 7 of the 12 clades with good bootstrap support. The remaining 5 clades were subdivided. As in the mtDNA phylogeny, most branches between the clades were short, corroborating that their diversification was rapid and established early in the lamprologine radiation. However, the AFLP data were able to shed light on several problematic placements in the mtDNA phylogeny: Contrasting with the mtDNA tree, all species which were placed para- or polyphyletically by mtDNA resulted as monophyletic in the AFLP tree. The genus Telmatochromis, which was scattered almost all over the mtDNA tree, turned out as monophyletic in the AFLP analysis, likewise the Neolamprologus species with brood-care helper behavior within clade XII.3. Interestingly, the paraphyletic placement of the genus Julidochromis was retained, corroborating the close relationship of J. ornatus, J. transcriptus and J. dickfeldi with the genus Chalinochromis and a somewhat more distant relationship to J. marlieri and J. regani. This paraphyly is most likely not due to ancient incomplete lineage sorting, given the large number of partially well supported nodes between the two Julidochromis clades (McCracken and Sorensen, 2005), but rather indicates convergent evolution of the particular Julidochromis phenotype with its characteristic body coloration. Also, the form of the genital papilla that clearly differs between the representatives of the two clades rejects a rather close relationship. The genital papilla of Chalinochromis on the other hand is very similar to that of J. dickfeldi, J. ornatus and J. transcriptus, further supporting the inferred phylogenetic relationships. Another aspect might be an ancient hybridization event among ancestral species of these two lineages, which might explain their partial morphological similarity despite long separate evolution afterward (Salzburger et al., 2002b).
4.2. Para- or polyphyletic taxa
While in some genera para- or polyphyly seems to be most likely the result of ancient ancestral polymorphism, it may be due to introgressive hybridization in others. A third alternative may be inadequate taxonomic assignment due to lack of diagnostic synapomorphic characters, causing genera defined by plesiomorphic traits. Previous studies (Sturmbauer et al., 1994; Stiassny, 1997; Schelly et al., 2006) diagnosed such taxonomic problems for the genera Lamprologus, Neolamprologus and Lepidiolamprologus. Our study confirms these problems and adds the genera Julidochromis and Chalinochromis to the list. Two cases of strong incompatibility of the mtDNA phylogeny with taxonomic assignments were found in the brood-care helper species N. marunguensis with its behavioral and morphological allies and the predatory species L. nkambae with its sister species L. elongatus and L. kendalli. As these taxa were resolved with their allies in the nuclear DNA tree, ancient incomplete lineage sorting or introgressive hybridization was taken into account, and both studies opted in favor of introgressive hybridization as the more likely reason (Salzburger et al., 2002b; Schelly et al., 2006). In many cases, however, it is impossible to rule out one of the two options, as both scenarios are realistic and might even be involved during the diversification of a lineage.
We found more problematic placements in the mtDNA phylogenies suggesting para- or polyphyly of species, similar to that observed in N. marunguensis (Salzburger et al., 2002b): These concerned N. savoryi, N. gracilis, T. temporalis and T. dhonti, as well as a case of complete mtDNA exchange in all L. callipterus of the northern basin of Lake Tanganyika after introgression of N. fasciatus mtDNA (Nevado et al., 2009). While it seems possible to argue in favor of ancient incomplete lineage sorting of an ancestral polymorphism in the cases of generic para- or polyphyly (as seen in Telmatochromis and Julidochromis), introgression and subsequent mtDNA capture seems a more likely explanation for the paraphyly within species (as seen in N. marunguensis, N. savoryi, and N. gracilis; see also Salzburger et al., 2002b; Schelly et al., 2006 and Nevado et al., 2009 for more detailed arguments). The additional cases presented here of reticulation among taxa with brood-care helper behavior spread over clades I and XII highlight once more that introgressive hybridization is more frequent in the lamprologines than in any other tribe studied.
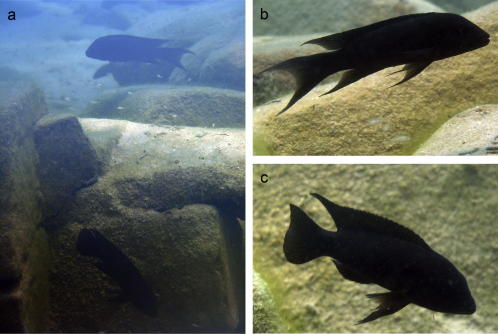
Considering the high frequency of trans-specific gene flow one must ask for possible reasons. Unlike all other Tanganyikan lineages except for B. microlepis they are substrate breeders forming stable pairs or harems. Unlike riverine substrate breeders of the Tilapiine or Pelvicachromine lineages, several species display complex social behavior. Moreover, several social species form arrays of allopatric sister species which most likely evolved in allopatry. These species seem to remain reproductively coherent for long time spans, so that lake-level fluctuations may connect previously disjunct species and cause introgression or hybridization as discussed in detail in Salzburger et al. (2002b). The following reticulations are suggested from the closest sister group relationships within and among the two mtDNA clades: N. savoryi with N. marunguensis; N. savoryi and T. temporalis; N. savoryi and T. dhonti; N. savoryi and T. bifrenatus; N. gracilis and N. marunguensis and/or N. savoryi. These cases have in common that the close relatedness of the introgressed mtDNA genotypes to populations or sister species of the donor species argues against ancestral polymorphism. A second array of lamprologine species living in close connection to each other concerns gastropod shell dwellers. They often live and spawn in extremely close vicinity, e.g. in L. callipterus nests, so that they are at higher risk for accidental cross-species fertilizations. That such hybridization cases are extremely rare can be ruled out, as (F1-) hybrids were repeatedly observed in L. callipterus nests (Aibara, pers. comm., Koblmüller et al., 2007a) as were heterospecific breeding pairs in other lamprologine species (Fig. 6). We thus suggest that substrate breeders with complex social behavior, as adaptation to a life in densly packed species communities, may be more susceptible to trans-specific gene flow than maternal mouthbrooders.
Fig. 6.
Hetero-specific breeding pair (Neolamprologus christyi × N. modestus) guarding its fry near the Kalambo estuary in the southeast of Lake Tanganyika; (a) the pair at the breeding site; and the two parents (b) N. christyi and (c) N. modestus. The most obvious difference between the two species is the shape of the caudal fin.
Concerning the third alternative, para- or polyphyly of species due to inadequate taxon sampling or lack of synapomorphic characters, two reasons can be put forward. The first would involve taxonomic error to be corrected in a revision including molecular phylogenies and additional synapomorphic characters, whatever traits they might be. The second reason is a much more intriguing one, in that evolution will necessarily produce para- or even polyphyletic taxa, when new species evolve strikingly novel features within a homogeneous clade, as exemplified by the evolution of birds within a clade of dinosaurs. In analogy, the genera Lamprologus or Neolamprologus may as well be considered as a plesiomorphic bauplan from which multiple sub-lineages with novel traits branched off. Given the scenario of multifold subdivision of rocky shores by ecological barriers, local innovation and speciation should prevail in the Lamprologini, as e.g. in the Tropheini for which parallel evolution of genus-diagnostic dentition types was also suggested (Sturmbauer et al., 2003; Koblmüller et al., 2010). Molecular phylogenies will be useful to trace the ancestral states of various clades, to identify the ancestral generic features.
An interesting point for discussion is the validity of paraphyletic genera. Should it turn out that the morphological features defining the genus Neolamprologus were originally apomorphic traits to be repeatedly modified in various sub-lineages by further innovation, one could argue to retain this genus name for all now plesiomorphic species and revise all genera with novel traits in monophyletic genera defined by certain apomorphic features. In this way necessary taxonomic adjustments can be carried out, but a “novel genus inflation” avoided.
4.3. Timing and pace of the lamprologine radiation
Age estimates based on molecular phylogenetic trees are based on a series of assumptions and require that four general conditions be met: (i) an accurate and well supported tree that resolves the important nodes in the phylogeny; (ii) reliable calibration points that provide upper and lower bounds for the nodes of interest; (iii) molecular clock methods that account for substitution rate heterogeneity within and across lineages; and (iv) a broad taxon sampling that includes the entire diversity in lineages (Soltis et al., 2002). Unfortunately, reliable calibration points for estimating divergence times in the East African cichlid species flocks are scarce, with no fossils available from these lacustrine species flocks. Thus, estimation of divergence times in East African cichlids has mainly relied on the assumption that the onset of these spectacular intralacustrine radiations coincided with the formation of a real lacustrine habitat in Lake Tanganyika and the refilling of Lakes Malawi and Victoria following their desiccation (Cohen et al., 1993, 1997; Delvaux, 1995; Johnson et al., 1996; Lezzar et al., 1996; Stager and Johnson, 2008). Only recently, alternative age estimates for the East African cichlid species flocks have been proposed based on independent external calibration points (Genner et al., 2007; Schwarzer et al., 2009) resulting in considerably older divergence time estimates. However, these recent developments are not free of pitfalls and in particular difficult to reconcile with the biologic characteristics of the Lake Tanganyika cichlid species flock, such that these calibration methods are quite problematic (for a detailed discussion see Koblmüller et al., 2008a, b). The chronograms based on these alternative calibrations are shown in Supplementary Figs. 3 and 4. We decided to use the following calibration points (for justification see Koblmüller et al., 2008a, b): The establishment of a real lacustrine habitat in Lake Tanganyika with clear- and deep-water conditions 5–6 MYA (Tiercelin and Mondeguer, 1991; see also Salzburger et al., 2002), the time window for the Congo River Lamprologus to leave the lake via the Lukuga (Lezzar et al., 1996; Cohen et al., 1997), and the age of the Lake Malawi species flock (Delvaux, 1995; Sturmbauer et al., 2001). There are ongoing analyses of a sediment core of the Lake Malawi deep basin at a depth of 650 m, which will without doubt result in a more precise estimate for the onset of lacustrine conditions in Lake Malawi after a dryup or near-dryup (Andrew Cohen, personal communication).
Contrasting the general diversification patterns observed in the mouthbrooding Lake Tanganyika cichlid tribes (Salzburger et al., 2002a; Sturmbauer et al., 2003; Brandstätter et al., 2005; Koblmüller et al., 2004, 2005, 2007b, 2010; Duftner et al., 2005), the Lamprologini diversified more gradually with no clear bursts of speciation evident (also see Day et al., 2008), albeit a concentration of cladogenetic events about 2.5–3 MYA coincided with a period of increased diversification rates in the mouthbrooding cichlid lineages. Many species seem to be rather old, whereas recent speciation (<1 MYA) predominantly occurred among the brood-care helper cichlids, in the Julidochromis assemblage and (mainly) the shell-breeding members of the genus Lepidiolamprologus. The ecological and behavioral characteristics of these species point to a reduced dispersal capacity, implying high degrees of population structure, as has already been demonstrated for the N. brichardi/N. pulcher complex (Stiver et al., 2004; Duftner et al., 2007) and an increased potential for allopatric speciation. Indeed, most members of these groups are allopatrically distributed and some occur only along very short stretches of shoreline, indicating that ecological specialization resulted in increased diversification rate in these groups.
4.4. Taxonomic implications
A revision of the species and genera of the Lamprologini is needed and we hope to contribute valuable information for future assignments. Clearly, this work needs to be based on the original descriptions of Boulenger (1898) and Max Poll’s insightful revisions (1956, 1986), as well as on more recent work of others (Stiassny, 1991, 1997; Colombe and Allgayer, 1985; Takahashi, 2003; Schelly and Stiassny, 2004; Schelly et al., 2006). As discussed by Stiassny (1992), meristic and morphometric measurements, osteology and dentition alone will not suffice to achieve the goal, as too many characters are homoplastic. Concerning the genus Neolamprologus, its description was based on the assumption that the Congo River species branched ancestrally to the rest, so that Lamprologus was retained in those species only, except for L. callipterus. A viable way might be to re-assign the genus name Lamprologus to most Neolamprologus species. As L. congoensis is the type species of the genus Lamprologus, this would be a viable scenario. The type species of the genus Neolamrologus is N. tetracanthus. Thus, when keeping the genus Neolamprologus, priority must be given to the clade also containing N. tetracanthus (clade VIII), also including the species N. modestus and N. cunningtoni, (plus N. christy, N. mondabu and N. petricola on the basis of nuclear DNA data). Naming all members of the ossified group a new genus name Stiassnia as recently suggested by Day et al. (2007), will not solve the problem, but cause further confusion, as perfectly valid genera such as Lepidiolamprologus (Schelly et al., 2006, 2007) and Altolamprologus (but excluding N. fasciatus; contrasting Day et al., 2007; see also Koblmüller et al., 2007a, and Nevado et al., 2009) would be subsumed, neglecting the huge eco-morphological and behavioral diversity of the ossified group.
Considering molecular traits and brood-care helping behavior as synapomorphic traits, the N. brichardi assemblage including N. pulcher, N. helianthus, N. marunguensis, N. olivaceous and N. sarvori, may be a good candidate group for a new genus, as would be the clade comprising N. tetracanthus, N. cunningtoni, N. christyi, N. petricola, N. modestus and N. mondabu. Concerning Julidochromis, a split in one group retaining the genus name (J. marlieri and J. regani), and a new genus comprising all Chalinochromis and J. ornatus and J. dickfeldi is suggested by molecular data and the shape of the male genital papilla. This could be achieved by revising the latter species as Chalinochromis.
Acknowledgments
For help with species identifications, we thank Jos Snoeks (MRAC, Tervuren, Belgium) and Melanie Stiassny. For providing tissues or for assistance in the field, we thank Heinz Büscher, Lothar Seegers, Roger Bills, Alex Chilala, Cyprian Kapasa, Harris Phiri, and the team at the Mpulungu Station of the Department of Fisheries, Ministry of Agriculture, and Cooperatives, Republic of Zambia. Christian Sturmbauer, Nina Duftner and Stephan Koblmüller were supported by the Austrian Science Foundation (Grants P15239, P17680 and P17968). Robert Schelly was supported by the AMNH Axelrod Fund and a grant from the American Cichlid Association. Walter Salzburger was supported by the Swiss National Science Foundation and the European Research Council (ERC), Starting Grant ‘INTERGENADAPT’. Walter Salzburger, Stephan Koblmüller, and Nina Duftner got further support from the Austrian Academy of Sciences (DOC and DOC-FFORTE (women in research and technology) fellowships) and the University of Graz (Stephan Koblmüller and Nina Duftner).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ympev.2010.06.018.
Appendix A
List of samples used for mtDNA and AFLP analyses, with sampling locality and coordinates (if known) of lamprologine taxa and GenBank accession numbers for the ND2 gene.
| Species | SampleID | Sampling locality | Coordinates | GenBank Acc. No. ND2 | AFLPs |
|---|---|---|---|---|---|
| Altolamprologus calvus | 2481 | Nakaku | S 8°40’, E 30°54’ | EF191108 | − |
| 3899 | Kapembwa | S 8°37’, E 30°51’ | - | + | |
| 4099 | Chaitika | S 8°34’, E 30°47’ | - | + | |
| Altolamprologus compressiceps | 2482 | Nakaku | S 8°40’, E 30°54’ | EF191105 | − |
| 3900 | Kalambo Lodge | S 8°37’, E 31°37’ | - | + | |
| 3988 | Kalambo | S 8°36’, E 31°11’ | - | + | |
| Altolamprologus sp. “shell” | 0586 | Sumbu | S 8°31’, E 30°29’ | EF191107 | − |
| Chalinochromis brichardi | 3463 | Ulwile | S 7°27’, E 30°34’ | HM623820 | |
| 5608 | Kalambo Lodge | S 8°37’, E 31°37’ | - | + | |
| 5708 | Kalambo Lodge | S 8°37’, E 31°37’ | - | + | |
| Chalinochromis popelini | Aquarium trade | U07244 | − | ||
| Chalinochromis sp. “kipili” | 0295 | Aquarium trade | HM623802 | − | |
| Chalinochromis sp. “ndobnoi” | 0294 | Aquarium trade | HM623801 | − | |
| Julidochromis dickfeldi | 0591 | ? | HM623790 | − | |
| 5616 | ? | - | + | ||
| 5733 | ? | - | + | ||
| Julidochromis marlieri | 2055 | Kalambo | S 8°36’, E 31°11’ | HM623819 | − |
| 5663 | ? | - | + | ||
| Julidochromis ornatus | 0880 | Nkondwe Island | S 7°23’, E 30°33’ | HM623791 | − |
| 2192 | Kasakalawe | S 8°47’, E 31°05’ | EF191082 | − | |
| 4350 | Kasakalawe | S 8°47’, E 31°05’ | - | + | |
| Julidochromis regani | 3461 | Masaka | S 5°02’, E 29°46’ | HM623818 | − |
| 5667 | Kaseke | - | + | ||
| 5680 | Kaseke | - | + | ||
| Julidochromis transcriptus | 1222 | ? | HM623792 | − | |
| 5599 | Gombe | - | + | ||
| Lamprologus callipterus | 2517 | Wonzye | S 8°43’, E 31°08’ | EF191085 | − |
| 4263 | Wonzye | S 8°43’, E 31°08’ | - | + | |
| 4270 | Wonzye | S 8°43’, E 31°08’ | - | + | |
| Lamprologus congoensis | 0307 | ? | AY740385 | − | |
| Lamprologus kungweensis | 2521 | Aquarium trade | EF191084 | − | |
| Lamprologus laparogramma | 0579 | Mpulungu | S 8°46’, E 31°06’ | EF191087 | − |
| Lamprologus lemairii | 0582 | Mpulungu | S 8°46’, E 31°06’ | AY740386 | − |
| Lamprologus meleagris | 1733 | Aquarium trade | DQ055027 | − | |
| Lamprologus ocellatus | 1833 | Chisansa | S 8°39’, E 31°11’ | EF191113 | − |
| Lamprologus cf. ocellatus⁎ | 2518 | Aquarium trade | EF191116 | − | |
| Lamprologus ornatipinnis | 1904 | Aquarium trade | EF191112 | − | |
| Lamprologus signatus | 0578 | Mpulungu | S 8°46’, E 31°06’ | EF191086 | − |
| Lamprologus speciosus | 1908 | Aquarium trade | EF191102 | − | |
| Lamprologus teugelsi | 2519 | Aquarium trade | HM623815 | − | |
| 5658 | Aquarium trade | - | + | ||
| 5677 | Aquarium trade | - | + | ||
| Lepidiolamprologus attenuatus | 1828 | Mpulungu | S 8°46’, E 31°06’ | DQ055036 | − |
| 3906 | Mtondwe Island | S 8°42’, E 31°07’ | - | + | |
| Lepidiolamprologus boulengeri | 1909 | Aquarium trade | DQ055040 | − | |
| Lepidiolamprologus elongatus | 3644 | Kalambo Lodge | S 8°37’, E 31°37’ | HM623829 | − |
| 3909 | Katoto | S 8°48’, E 31°01’ | - | + | |
| Lepidiolamprologus hecqui | 1913 | Aquarium trade | DQ055041 | − | |
| Lepidiolamprologus kendalli | 0355 | Aquarium trade | DQ055060 | − | |
| Lepidiolamprologus meeli | 2479 | Kigoma | S 4°52’, E 29°37’ | DQ055051 | − |
| Lepidiolamprologus nkambae | 1752 | Aquarium trade | DQ055035 | − | |
| Lepidiolamprologus profundicola | 3652 | Kasenga Rocks | S 8°43’, E 31°09’ | HM623830 | − |
| 4107 | Kasenga Rocks | S 8°43’, E 31°09’ | - | + | |
| Lepidiolamprologus sp. “meeli-boulengeri” | 1831 | Mbita Island | S 8°48’, E 31°01’ | DQ055038 | − |
| 3915 | Muzumwa | S 8°42’, E 31°12’ | - | + | |
| Lepidiolamprologus variostigma | 1738 | ? | DQ055029 | − | |
| Neolamprologus bifasciatus | 1820 | Aquarium trade | HM623809 | − | |
| Neolamprologus brevis | 1730 | Aquarium trade | EF191095 | − | |
| Neolamprologus brichardi | 1479 | ? | DQ05515 | − | |
| 5682 | Fulwe Rocks | - | + | ||
| 5683 | Fulwe Rocks | - | + | ||
| Neolamprologus buescheri | 1745 | Tembwe | S 7°14’, E 30°07’ | HM623803 | − |
| 5729 | ? | - | + | ||
| 5739 | ? | - | + | ||
| Neolamprologus calliurus | 2522 | Aquarium trade | EF191117 | − | |
| Neolamprologus caudopunctatus | 0208 | Aquarium trade | AY740388 | − | |
| Neolamprologus christyi | 3622 | Isanga | S 8°39’, E 31°12’ | HM623826 | − |
| 5617 | Isanga | S 8°39’, E 31°12’ | - | + | |
| 5664 | Isanga | S 8°39’, E 31°12’ | - | + | |
| 5676 | Isanga | S 8°39’, E 31°12’ | - | + | |
| Neolamprologus cunningtoni | 2513 | Kapata | DQ055053 | − | |
| 5695 | Wonzye | S 8°43’, E 31°08’ | - | + | |
| 5732 | Kalambo Lodge | S 8°37’, E 31°37’ | - | + | |
| Neolamprologus cylindricus | 3618 | Aquarium trade | HM623823 | − | |
| 5657 | ? | - | + | ||
| Neolamprologus devosi | 1819 | Ivagala (Malagarazi) | EF437476 | − | |
| Neolamprologus falcicula | 3436 | Aquarium trade | HM623817 | − | |
| Neolamprologus fasciatus | 2516 | Wonzye | S 8°43’, E 31°08’ | EF191120 | − |
| 4233 | Wonzye | S 8°43’, E 31°08’ | - | + | |
| 4249 | Wonzye | S 8°43’, E 31°08’ | - | + | |
| Neolamprologus furcifer | 2126 | ? | HM623812 | − | |
| 5734 | ? | - | + | ||
| Neolamprologus gracilis | 0625 | Kalo | HM623798 | − | |
| 3434 | Kigoma | S 4°52’, E 29°37’ | HM623816 | − | |
| Neolamprologus helianthus | 1477 | ? | DQ055013 | − | |
| 5668 | Aquarium trade | - | + | ||
| 5674 | Aquarium trade | - | + | ||
| Neolamprologus leleupi | 2147 | Aquarium trade | HM623814 | − | |
| 5660 | Aquarium trade | - | + | ||
| 5661 | Aquarium trade | - | + | ||
| Neolamprologus leloupi | 1906 | Aquarium trade | EF191103 | − | |
| 1907 | Aquarium trade | EF191104 | − | ||
| Neolamprologus longior | 0216 | Aquarium trade | HM623793 | − | |
| Neolamprologus marunguensis | 0618 | Kafitilila | AY740390 | − | |
| 0619 | Kafitilila | HM623797 | − | ||
| 5718 | Aquarium trade | - | + | ||
| 5719 | Aquarium trade | - | + | ||
| Neolamprologus modestus | 3457 | Kasakalawe | S 8°47’, E 31°05’ | HM623821 | − |
| 5596 | Kalambo Lodge | S 8°37’, E 31°37’ | - | + | |
| 5597 | Kalambo Lodge | S 8°37’, E 31°37’ | - | + | |
| Neolamprologus mondabu | 3433 | Mpimbwe | S 7°08’, E 30°30’ | - | + |
| Neolamprologus multifasciatus | 0310 | Aquarium trade | EF191089 | − | |
| 3nmulti | Aquarium trade | - | + | ||
| 5662 | Aquarium trade | - | + | ||
| Neolamprologus mustax | 2118 | Mtondwe Island | S 8°42’, E 31°07’ | HM623811 | − |
| 5704 | Mbita Island | S 8°48’, E 31°01’ | - | + | |
| 5659 | Kapembwa | S 8°37’, E 30°51’ | - | + | |
| Neolamprologus niger | 0315 | ? | AY740391 | − | |
| Neolamprologus nigriventris | 1227 | ? | AY740392 | − | |
| Neolamprologus obscurus | 2946 | Kasakalawe | S 8°47’, E 31°05’ | HM623824 | − |
| 5741 | Kasakalawe | S 8°47’, E 31°05’ | - | + | |
| 5742 | Kasakalawe | S 8°47’, E 31°05’ | - | + | |
| Neolamprologus olivaceous | 0631 | Kyeso | S 6°30’, E 29°29’ | AY740393 | − |
| 5656 | Aquarium trade | - | + | ||
| Neolamprologus petricola | 3631 | Kapembwa | S 8°37’, E 30°51’ | HM623827 | − |
| 5675 | S of Katoto | S 8°48’, E 31°01’ | - | + | |
| Neolamprologus prochilus | 3623 | Kalambo | S 8°36’, E 31°11’ | HM623825 | − |
| 5582 | Kalambo | S 8°36’, E 31°11’ | - | + | |
| Neolamprologus pulcher | 521 | Mbita Island | S 8°48’, E 31°01’ | HM623795 | − |
| 5601 | Katukula | S 8°43’, E 30°57’ | - | + | |
| 5684 | Mbita Island | S 8°48’, E 31°01’ | - | + | |
| Neolamprologus savoryi | 613 | Kyeso | S 6°30’, E 29°29’ | HM623807 | − |
| 614 | Kimomo | HM623796 | − | ||
| 1463 | Kachese | S 8°29’, E 30°28’ | HM623800 | − | |
| 1511 | Kachese | S 8°29’, E 30°28’ | HM623810 | − | |
| 2145 | S of Loasi River | S 8°19’, E 31°04’ | HM623805 | − | |
| 2146 | Mbita Island | S 8°48’, E 31°01’ | HM623806 | − | |
| 5752 | ? | - | + | ||
| 5754 | ? | - | + | ||
| Neolamprologus sexfasciatus | 3624 | Kalambo | S 8°36’, E 31°11’ | HM623828 | − |
| 5612 | Katukula | S 8°43’, E 30°57’ | - | + | |
| 5614 | S of Katoto | S 8°48’, E 31°01’ | - | + | |
| Neolamprologus similis | 1739 | Tembwe | S 7°14’, E 20°07’ | DQ055030 | − |
| 1902 | ? | EF191100 | − | ||
| Neolamprologus sp. “eseki” | 0347 | ? | HM623794 | − | |
| Neolamprologus splendens | 628 | Kasu | HM623799 | − | |
| Neolamprologus tetracanthus | 3458 | Mpulungu | S 8°46’, E 31°06’ | HM623822 | − |
| 5611 | Kalambo Lodge | S 8°37’, E 31°37’ | - | + | |
| 5697 | Kalambo Lodge | S 8°37’, E 31°37’ | - | + | |
| Neolamprologus toae | 0217 | Aquarium trade | AY682543 | − | |
| 2148 | Aquarium trade | HM623813 | − | ||
| 5693 | Aquarium trade | - | + | ||
| 5694 | Aquarium trade | - | + | ||
| Neolamprologus tretocephalus | 1731 | Aquarium trade | DQ055026 | − | |
| 5649 | Aquarium trade | - | + | ||
| 5650 | Aquarium trade | - | + | ||
| Neolamprologus walteri | 1816 | near Kigoma (hilltop) | S 4°53’, E 29°36’ | HM623808 | − |
| Telmatochromis bifrenatus | 0106 | Aquarium trade | DQ055009 | − | |
| Telmatochromis dhonti | 1829 | Mbita Island | S 8°48’, E 31°01’ | HM623787 | − |
| 1830 | Mtondwe Island | S 8°42’, E 31°07’ | HM623804 | − | |
| 5651 | ? | - | + | ||
| 5652 | ? | - | + | ||
| Telmatochromis temporalis | 1475 | Tongwa | S 8°40’, E 30°53’ | HM623789 | − |
| 2117 | Mbita Island | S 8°48’, E 31°01’ | HM623788 | − | |
| 5717 | Funda | S 8°46’, E 30°59’ | - | + | |
| 5735 | ? | - | + | ||
| Telmatochromis vittatus | 2119 | Mtondwe Island | S 8°42’, E 31°07’ | AY682545 | − |
| 5594 | Isanga | S 8°39’, E 31°12’ | - | + | |
| Variabilichromis moorii | 1508 | Kachese | S 8°29’, E 30°28’ | DQ055016 | − |
| 5751 | ? | - | + | ||
| 5753 | ? | - | + | ||
| Outgroups | |||||
| Boulengerochromis microlepis | AF317229 | ||||
| Bathybates leo | 2226 | AY663731 | − | ||
| Hemibates stenosoma | 2234 | AY663719 | − | ||
| Telotrematocara macrostoma | 2483 | AY663715 | − | ||
| Ophthalmotilapia ventralis | U07257 | − | |||
| Callochromis macrops | 1854 | AY337795 | − | ||
| 5653 | - | + | |||
| 5654 | - | + | |||
| Cyphotilapia frontosa | U07247 | − | |||
| Triglachromis otostigma | 0103 | AY337769 | − | ||
| Limnochromis auritus | 0059 | AY337766 | − | ||
| Paracyprichromis brieni | AF398223 | − | |||
| Perissodus microlepis | AF317265 | − | |||
| 3240 | EF437483 | + | |||
| 3470 | EF437484 | + | |||
| 3597 | EF437485 | + | |||
| Benthochromis tricoti | 1480 | EU753962 | − | ||
| Ctenochromis horei | 2524 | EU753935 | − | ||
| Petrochromis sp. “macrognathus rainbow” | 8259 | GQ995772 | − | ||
| Tropheus moorii | AB018975 | − | |||
| 5598 | - | + | |||
| 5600 | - | + | |||
| Astatotilapia calliptera | EU753934 | − | |||
| Aulonocara sp. | 6662 | GQ995712 | − | ||
| Labidochromis caeruleus | 3055 | AY740383 | − | ||
| Melanochromis auratus | AY930063 | − | |||
| Nimbochromis livingstoni | 3057 | EU753948 | − | ||
| Nimbochromis venustus | 3056 | EU753947 | − | ||
| Pseudotropheus tropheops | 3058 | AY740384 | − | ||
| Eretmodus cyanostictus | 1102 | DQ055010 | − | ||
| 5678 | - | + | |||
| Spathodus erythrodon | 0092 | DQ055008 | − | ||
| 5673 | - | + | |||
| Spathodus marlieri | 0058 | HM623786 | − | ||
| Tanganicodus irsacae | 0066 | DQ055007 | − |
Sequences not generated in the framework of this study were obtained from GenBank from following publications: Kocher et al. (1995); Klett and Meyer (2002); Salzburger et al. (2002a, 2005); Koblmüller et al. (2004, 2005, 2007a,b, 2008, 2010); Duftner et al. (2005); Schelly et al. (2006).
In a previous publication (Koblmüller et al., 2007a), this sample has been erroneously identified as Neolamprologus wauthioni (see Büscher, 2007).
Appendix B. Supplementary material
A chronogram of the diversification of the Lamprologini based on complete ND2 sequences and applying following calibration points (as uniform priors): (i) the onset of the diversification of the C-lineage in the course of the so-called “primary radiation”, which was proposed to have coincided with the formation of tropical clearwater habitat with deep-water conditions 5–6 MYA (Salzburger et al., 2002a); (ii) a maximum age of 0.57–1 MY for the Lake Malawi cichlid species flock, based on the age of the refilling of Lake Malawi (Delvaux, 1995; Sturmbauer et al., 2001); (iii) an age of 1.1–3.5 MY for the split of the Congo-lamprologines, based on the time window for a Lukuga-connection between Lake Tanganyika and the Congo system (Lezzar et al., 1996; Cohen et al., 1997). Divergence time estimates are represented as the mean node height of the 95% highest posterior density (HPD) interval from a BEAST maximum-clade-credibility tree. Gray bars span the 95% HPD for each well supported node. No bars are assigned to nodes with low posterior probability. Calibration points are marked by arrows. Riverine lamprologine taxa are shaded in gray.
A chronogram of the diversification of the Lamprologini based on complete ND2 sequences and applying following calibration points (as uniform priors): (i) the onset of the diversification of the C-lineage in the course of the so-called “primary radiation”, which was proposed to have coincided with the formation of tropical clearwater habitat with deep-water conditions 5–6 MYA (Salzburger et al., 2002a); (ii) a maximum age of 0.57–1 MY for the Lake Malawi cichlid species flock, based on the age of the refilling of Lake Malawi (Delvaux, 1995; Sturmbauer et al., 2001). Divergence time estimates are represented as the mean node height of the 95% highest posterior density (HPD) interval from a BEAST maximum-clade-credibility tree. Gray bars span the 95% HPD for each well supported node. No bars are assigned to nodes with low posterior probability. Calibration points are marked by arrows. Riverine lamprologine taxa are shaded in gray.
A chronogram of the diversification of the Lamprologini based on complete ND2 sequences and applying following calibration points (as uniform priors): (i) the onset of the diversification of the C-lineage at 9–12 MYA, assuming that the primary Tanganyika radiation had taken place at the onset of the Lake formation 9–12 MYA as a consequence of allopatric diversification in a series of small shallow lakes in the area currently occupied by the Congo River system and Lake Tanganyika (Tiercelin and Mondeguer, 1991; Cohen et al., 1993), implying that the secondary radiation observed in most Lake Tanganyika cichlid tribe (Koblmüller et al., 2004, 2005, 2007a,b, 2010; Duftner et al. 2005) coincided with the formation of a real lacustrine habitat with deep-water conditions about 5–6 MYA, compatible with the fossil calibrated diversification scenario discussed by Genner et al. (2007); (ii) an age of 1.1–3.5 MY for the split of the Congo-lamprologines, based on the time window for a Lukuga-connection between Lake Tanganyika and the Congo system (Lezzar et al., 1996; Cohen et al., 1997). Divergence time estimates are represented as the mean node height of the 95% highest posterior density (HPD) interval from a BEAST maximum-clade-credibility tree. Gray bars span the 95% HPD for each well supported node. No bars are assigned to nodes with low posterior probability. Calibration points are marked by arrows. Riverine lamprologine taxa are shaded in gray.
A chronogram of the diversification of the Lamprologini based on complete ND2 sequences and assuming an age of 30 MYA (normally distributed prior with a mean of 30 MYA; S.D, 3.0 MY) for the MRCA of the C-lineage, as was proposed by Genner et al. (2007) based on a presumed Gondwanan origin of the family Cichlidae. Divergence time estimates are represented as the mean node height of the 95% highest posterior density (HPD) interval from a BEAST maximum-clade-credibility tree. Gray bars span the 95% HPD for each well supported node. No bars are assigned to nodes with low posterior probability. Calibration points are marked by arrows. Riverine lamprologine taxa are shaded in gray.
References
- Awata S., Munehara H., Kohda M. Social system and reproduction of helpers in a cooperatively breeding cichlid fish (Julidochromis ornatus) in Lake Tanganyika: field observations and parentage analyses. Behav. Ecol. Sociobiol. 2005;58:506–516. [Google Scholar]
- Awata S., Takeuchi H., Kohda M. The effect of body size on mating system and parental roles in a biparental cichlid fish (Julidochromis transcriptus): a preliminary laboratory experiment. J. Ethol. 2006;24:125–132. [Google Scholar]
- Balshine-Earn S., Lotem A. Individual recognition in a cooperatively breeding cichlid: evidence from video playback experiments. Behaviour. 1998;135:369–386. [Google Scholar]
- Barluenga M., Meyer A. The Midas cichlid species complex: incipient sympatric speciation in Nicaraguan cichlid fishes? Mol. Ecol. 2004;13:2061–2076. doi: 10.1111/j.1365-294X.2004.02211.x. [DOI] [PubMed] [Google Scholar]
- Boulenger G.A. Report on the collection of fishes made by Mr. J.E.S. Moore in Lake Tanganyika during his expedition, 1895–1996. Trans. Zool. Soc. Lond. 1898;15:87–96. [Google Scholar]
- Brandstätter A., Salzburger W., Sturmbauer C. Mitochondrial phylogeny of the Cyprichromini, a lineage of open-water cichlid fishes endemic to Lake Tanganyika, East Africa. Mol. Phylogenet. Evol. 2005;34:382–391. doi: 10.1016/j.ympev.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Büscher H.H. Neolamprologus wauthioni – ein verschollener Buntbarsch aus dem Tanganjikasee. DATZ. 2007;60:58–61. [Google Scholar]
- Clabaut C., Salzburger W., Meyer A. Comparative phylogenetic analyses of the adaptive radiation in Lake Tanganyika cichlid fishes: nuclear sequences are less homoplasious but also less informative than mitochondrial DNA. J. Mol. Evol. 2005;31:666–681. doi: 10.1007/s00239-004-0217-2. [DOI] [PubMed] [Google Scholar]
- Cohen A.S., Soreghan M.J., Scholz C.A. Estimating the age of ancient lakes: an example from Lake Tanganyika, East African rift system. Geology. 1993;21:511–514. [Google Scholar]
- Cohen A.S., Lezzar K.E., Tiercelin J.J., Soreghan M. New palaeographic and lake-level reconstructions of Lake Tanganyika: implications for tectonic, climatic and biological evolution in a rift lake. Basin Res. 1997;9:107–132. [Google Scholar]
- Colombe J., Allgayer R. Description de Variabilichromis, Neolamprologus, et Paleolamprologus genres nouveaux du Lac Tanganika, avec redescription des genres Lamprologus Schilthuis, 1891 et Lepidiolamprologus Pellegrin, 1904 (Pisces: Teleostei: Cichlidae) Rev. Fr. Cichlidophile. 1985;49(9–16):21–28. [Google Scholar]
- Day J.J., Santini S., Garcia-Moreno J. Phylogenetic relationships of the Lake Tanganyika cichlid tribe Lamprologini: the story from mitochondrial DNA. Mol. Phylogenet. Evol. 2007;46:629–642. doi: 10.1016/j.ympev.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Day J.J., Cotton J.A., Barraclough T.G. Tempo and mode of diversification of Lake Tanganyika cichlid fishes. PLoS ONE. 2008;3:2. doi: 10.1371/journal.pone.0001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvaux D. Age of Lake Malawi (Nyasa) and water level fluctuations. Mus. R. Afr. Centr. Tervuren (Belg.) Dept. Geol. Min. Rapp. Ann. 1995;1995–1996:99–108. [Google Scholar]
- De Vos L., Seegers L., Taverne L., Thys van den Audenaerde D. L’ichtyofaune du bassin de la Malagarasi (Système du Lac Tanganyika): une synthèse de la connaissance actuelle. Ann. Mus. R. Afr. Centr. Sci. Zool. 2001;285:117–135. [Google Scholar]
- Dierkes P., Taborsky M., Kohler U. Reproductive parasitism of broodcare helpers in a cooperatively breeding fish. Behav. Ecol. 1999;10:510–515. [Google Scholar]
- Dierkes P., Heg D., Taborsky M., Skubic E., Achmann R. Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol. Lett. 2005;8:968–975. doi: 10.1111/j.1461-0248.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- Dierkes P., Taborsky M., Achmann R. Multiple paternity in the cooperatively breeding fish Neolamprologus pulcher. Behav. Ecol. Sociobiol. 2008;62:1581–1589. [Google Scholar]
- Dominey W. Effects of sexual selection and life history on speciation: species flocks in African cichlids and Hawaiian Drosophila. In: Echelle A.A., Kornfield I., editors. Evolution of Fish Species Flocks. University of Maine at Orono Press; Orono: 1984. pp. 231–249. [Google Scholar]
- Drummond A.J., Ho S.Y.W., Phillips M.J., Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond, A.J., Ho, S.Y.W., Rawlence, N., Rambaut, A., 2007. A rough guide to BEAST 1.4. Available from: <http://beast.bio.ed.ac.uk/>.
- Drummond A.J., Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duftner N., Koblmüller S., Sturmbauer C. Evolutionary relationships of the Limnochromini, a tribe of benthic deep water cichlid fishes endemic to Lake Tanganyika, East Africa. J. Mol. Evol. 2005;60:277–289. doi: 10.1007/s00239-004-0017-8. [DOI] [PubMed] [Google Scholar]
- Duftner N., Sefc K.M., Koblmüller S., Nevado B., Verheyen E., Phiri H., Sturmbauer C. Distinct population structure in a phenotypically homogeneous rock-dwelling cichlid fish from Lake Tanganyika. Mol. Ecol. 2006;15:2381–2396. doi: 10.1111/j.1365-294X.2006.02949.x. [DOI] [PubMed] [Google Scholar]
- Duftner N., Sefc K.M., Koblmüller S., Salzburger W., Taborsky M., Sturmbauer C. Parallel evolution of facial stripe patterns in the Neolamprologus brichardi/pulcher species complex endemic to Lake Tanganyika. Mol. Phylogenet. Evol. 2007;45:706–715. doi: 10.1016/j.ympev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Egger B., Koblmüller S., Sturmbauer C., Sefc K.M. Nuclear and mitochondrial data reveal different evolutionary processes in the Lake Tanganyika cichlid genus Tropheus. BMC Evol. Biol. 2007;7:137. doi: 10.1186/1471-2148-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B., Mattersdorfer K., Sefc K.M. Variable discrimination and asymmetric preferences in laboratory tests of reproductive isolation between cichlid colour morphs. J. Evol. Biol. 2010;23:433–439. doi: 10.1111/j.1420-9101.2009.01906.x. [DOI] [PubMed] [Google Scholar]
- Frostman P., Sherman P.T. Behavioral response to familiar and unfamiliar neighbors in a territorial cichlid, Neolamprologus pulcher. Ichthyol. Res. 2004;51:283–285. [Google Scholar]
- Genner M.J., Seehausen O., Lunt D.H., Joyce D.A., Shaw P.W., Carvalho G.R., Turner G.F. Age of cichlids: new dates for ancient lake fish radiations. Mol. Biol. Evol. 2007;24:1269–1282. doi: 10.1093/molbev/msm050. [DOI] [PubMed] [Google Scholar]
- Gray S.M., McKinnon J.S. Linking color polymorphism maintenance and speciation. Trends. Ecol. Evol. 2007;22:71–79. doi: 10.1016/j.tree.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Heg D., Bachar Z., Taborsky M. Cooperative breeding and group structure in the Lake Tanganyika cichlid Neolamprologus savoryi. Ethology. 2005;111:1017–1043. [Google Scholar]
- Heg D., Bachar Z. Cooperative breeding in the Lake Tanganyika cichlid Julidochromis ornatus. Environ. Biol. Fish. 2007;76:265–281. [Google Scholar]
- Hendy M.D., Penny D. A framework for the quantitative study of evolutionary trees. Syst. Zool. 1989;38:197–309. [Google Scholar]
- Hert E. Individual recognition of helpers by the breeders in the cichlid fish Lamprologus brichardi (Poll, 1974) Zeitschr. Tierpsych. 1985;68:313–325. [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MrBayes: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Johnson T.C., Scholz C.A., Talbot M.R., Kelts K., Ricketts R.D., Ngobi G., Beuning K., Ssemmanda I., McGill J.W. Late Pleistocene desiccation of Lake Victoria and rapid evolution of cichlid fishes. Science. 1996;273:1091–1093. doi: 10.1126/science.273.5278.1091. [DOI] [PubMed] [Google Scholar]
- Katoh R., Munehara H., Kohda M. Alternative male mating tactics of the substrate brooding cichlid Telmatochromis temporalis in Lake Tanganyika. Zool. Sci. 2005;22:555–561. doi: 10.2108/zsj.22.555. [DOI] [PubMed] [Google Scholar]
- Klett V., Meyer A. What, if anything, is a Tilapia? Mitochondrial ND2 phylogeny of Tilapiines and evolution of parental care systems in the African cichlid fishes. Mol. Biol. Evol. 2002;19:865–883. doi: 10.1093/oxfordjournals.molbev.a004144. [DOI] [PubMed] [Google Scholar]
- Koblmüller S., Salzburger W., Sturmbauer C. Evolutionary relationships in the sand-dwelling cichlid lineage of Lake Tanganyika suggest multiple colonization of rocky habitats and convergent origin of biparental mouthbrooding. J. Mol. Evol. 2004;58:79–96. doi: 10.1007/s00239-003-2527-1. [DOI] [PubMed] [Google Scholar]
- Koblmüller S., Duftner N., Katongo C., Phiri H., Sturmbauer C. Ancient divergence in bathypelagic Lake Tanganyika deepwater cichlids: mitochondrial phylogeny of the tribe Bathybatini. J. Mol. Evol. 2005;60:297–314. doi: 10.1007/s00239-004-0033-8. [DOI] [PubMed] [Google Scholar]
- Koblmüller S., Duftner N., Sefc K.M., Aibara M., Stipacek M., Blanc M., Egger B., Sturmbauer C. Reticulate phylogeny of gastropod-shell-breeding cichlids from Lake Tanganyika – the result of repeated introgressive hybridization. BMC Evol. Biol. 2007;7:7. doi: 10.1186/1471-2148-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblmüller S., Egger B., Sturmbauer C., Sefc K.M. Evolutionary history of Lake Tanganyika’s scale-eating cichlid fishes. Mol. Phylogenet. Evol. 2007;44:1295–1305. doi: 10.1016/j.ympev.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Koblmüller S., Sefc K.M., Duftner N., Warum M., Sturmbauer C. Genetic population structure as indirect measure of dispersal ability in a Lake Tanganyika cichlid fish. Genetica. 2007;130:121–131. doi: 10.1007/s10709-006-0027-0. [DOI] [PubMed] [Google Scholar]
- Koblmüller S., Sefc K.M., Sturmbauer C. The Lake Tanganyika cichlid species assemblage: recent advances in molecular phylogenetics. Hydrobiologia. 2008;615:5–20. [Google Scholar]
- Koblmüller S., Schliewen U.K., Duftner N., Sefc K.M., Katongo C., Sturmbauer C. Age and spread of the haplochromine cichlid fishes in Africa. Mol. Phylogenet. Evol. 2008;49:153–169. doi: 10.1016/j.ympev.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Koblmüller S., Duftner N., Sefc K.M., Aigner U., Rogetzer M., Sturmbauer C. Phylogeographic structure and gene flow in the scale-eating cichlid Perissodus microlepis (Teleostei, Perciformes, Cichlidae) in southern Lake Tanganyika. Zool. Scripta. 2009;38:257–268. [Google Scholar]
- Koblmüller S., Egger B., Sturmbauer C., Sefc K.M. Rapid radiation, ancient incomplete lineage sorting and ancient hybridization in the endemic Lake Tanganyika cichlid tribe Tropheini. Mol. Phylogenet. Evol. 2010;55:318–334. doi: 10.1016/j.ympev.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Koch M., Koblmüller S., Sefc K.M., Duftner N., Katongo C., Sturmbauer C. Evolutionary history of the endemic Lake Tanganyika cichlid fish Tylochromis polylepis: a recent intruder to a mature adaptive radiation. J. Zool. Syst. Evol. Res. 2007;45:64–71. [Google Scholar]
- Kocher T.D., Thomas W.K., Meyer A., Edwards S.V., Pääbo S., Villablanca F.X., Wilson A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA. 1989;86:6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher T.D., Conroy J.A., McKaye K.R., Stauffer J.R., Lockwood S.F. Evolution of NADH dehydrogenase subunit 2 in East African cichlid fish. Mol. Phylogenet. Evol. 1995;4:420–432. doi: 10.1006/mpev.1995.1039. [DOI] [PubMed] [Google Scholar]
- Kuhner M.K. Coalescent genealogy samplers: windows into population history. Trends. Ecol. Evol. 2009;24:86–93. doi: 10.1016/j.tree.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwamura T. The evolution of parental care and mating systems among Tanganyikan cichlids. In: Kawanabe H., Hori M., Nagoshi M., editors. Fish Communities in Lake Tanganyika. Kyoto University Press; Kyoto: 1997. pp. 57–86. [Google Scholar]
- Lezzar K.E., Tiercelin J.J., De Batist M., Cohen A.S., Bandora R., van Rensbergen C., Le Turdu C., Mifundu W., Klerkx J. New seismic stratigraphy and Late Tertiary history of the North Tanganyika basin, East African rift system deduced from multichannel and high-piston core evidence. Basin Res. 1996;8:1–28. [Google Scholar]
- Maan M.E., Taborsky M. Sexual conflict over breeding substrate causes female expulsion and offspring loss in a cichlid fish. Behav. Ecol. 2008;19:302–308. [Google Scholar]
- Matsumoto K., Kohda M. Male foraging avoidance in female feeding territories in a harem polygynous cichlid in Lake Tanganyika. J. Ethol. 2007;25:21–27. [Google Scholar]
- McCracken K.G., Sorensen M.D. Is homoplasy or lineage sorting the source of incongruent mtDNA and nuclear gene tree in stiff-tailed ducks (Nomonyx-Oxyura)? Syst. Biol. 2005;54:35–55. doi: 10.1080/10635150590910249. [DOI] [PubMed] [Google Scholar]
- Nakano S., Nagoshi M. Brood defence and parental roles in abiparental cichlid fish Laprologus toae in Lake Tanganyika. Jpn. J. Ichthyol. 1990;36:468–476. [Google Scholar]
- Nei M., Li W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevado B., Koblmüller S., Sturmbauer C., Snoeks J., Usano-Alemany J., Verheyen E. Complete mtDNA replacement in a Lake Tanganyika cichlid fish. Mol. Ecol. 2009;18:4240–4255. doi: 10.1111/j.1365-294X.2009.04348.x. [DOI] [PubMed] [Google Scholar]
- Ota K., Kohda M. Description of alternative male reproductive tactics in a shell-brooding cichlid, Telmatochromis vittatus, in Lake Tanganyika. J. Ethol. 2006;24:9–15. [Google Scholar]
- Pellegrin J. Contribution a l’étude anatomique, biologique, et taxonomique des poissons de la famille des Cichlides. Mém. Soc. Zool. France. 1904;16:41–402. [Google Scholar]
- Poll M. Poissons Cichlidae. Résultats scientifiques de l’exploration hydrobiologique du Lac Tanganika (1946–1947) Inst. R. Sci. Nat. Belg. 1956;3(Fasc. 5B):1–619. [Google Scholar]
- Poll M. Contribution à la fauna ichthyologique du lac Tanganika, d’après les récoltes de P. Brichard. Rev. Zool. Afr. 1974;88:99–100. [Google Scholar]
- Poll M. Classification des Cichlidae du lac Tanganika. Tribus, genres et espèces. Acad. R. Belg. Mem. Cl. Sci. 1986;45(2 sér.):1–163. [Google Scholar]
- Posada D., Crandall K.A. ModelTest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Pulquério M.F., Nichols R.A. Dates from the molecular clock: how wrong can we be? Trends. Ecol. Evol. 2007;22:180–184. doi: 10.1016/j.tree.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Rambaut, A., Drummond, A.J., 2008. Tracer v1.4: MCMC Trace Analysis Tool. Available from: <http://tree.bio.ed.ac.uk/software/tracer/>.
- Rambaut, A., 2009. FigTree v1.2.2. Available from: <http://tree.bio.ed.ac.uk/software/figtree/>.
- Risch S., Snoeks J. Geographic variation in Neolamprologus niger (Poll, 1956) (Perciformes: Cichlidae) from Lake Tanganyika (Africa) Zootaxa. 2008;1857:21–32. [Google Scholar]
- Salzburger W., Meyer A., Baric S., Verheyen E., Sturmbauer C. Phylogeny of the Lake Tanganyika cichlid species flock and its relationship to the Central and East African haplochromine cichlid fish faunas. Syst. Biol. 2002;51:113–135. doi: 10.1080/106351502753475907. [DOI] [PubMed] [Google Scholar]
- Salzburger W., Baric S., Sturmbauer C. Speciation via introgressive hybridization in East African cichlids? Mol. Ecol. 2002;11:619–625. doi: 10.1046/j.0962-1083.2001.01438.x. [DOI] [PubMed] [Google Scholar]
- Salzburger W., Mack T., Verheyen E., Meyer A. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of haplochromine cichlid fishes. BMC Evol. Biol. 2005;5:17. doi: 10.1186/1471-2148-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzburger W. The interaction of sexually and naturally selected traits in the adaptive radiation of cichlid fishes. Mol. Ecol. 2009;18:169–185. doi: 10.1111/j.1365-294X.2008.03981.x. [DOI] [PubMed] [Google Scholar]
- Sanders K.L., Lee M.S.Y. Evaluating molecular clock calibrations using Bayesian analyses with soft and hard bounds. Biol. Lett. 2007;3:275–279. doi: 10.1098/rsbl.2007.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Gashagaza M.M. Shell-brooding cichlid fishes of Lake Tanganyika: their habitats and mating systems. In: Kawanabe H., Hori M., Nagoshi M., editors. Fish Communities in Lake Tanganyika. Kyoto University Press; Kyoto: 1997. pp. 219–240. [Google Scholar]
- Sato T., Hirose M., Taborsky M., Kimura S. Size-dependent male alternative reproductive tactics in the shell-brooding cichlid fish Lamprologus callipterus in Lake Tanganyika. Ethology. 2004;110:49–62. [Google Scholar]
- Schelly R., Stiassny M.L.J., Seegers L. Neolamprologus devosi sp. n., a new riverine lamprologine cichlid (Teleostei, Cichlidae) from the lower Malagarasi River, Tanzania. Zootaxa. 2003;373:1–11. [Google Scholar]
- Schelly R.C., Stiassny M.L.J. Revision of the Congo River Lamprologus Schilthuis, 1891 (Teleostei: Cichlidae), with descriptions of two new species. Am. Mus. Nov. 2004;3451:1–40. [Google Scholar]
- Schelly R., Salzburger W., Koblmüller S., Duftner N., Sturmbauer C. Phylogenetic relationships of the lamprologine cichlid genus Lepidiolamprologus (Teleostei: Perciformes) based on mitochondrial and nuclear sequences, suggesting introgressive hybridization. Mol. Phylogenet. Evol. 2006;38:426–438. doi: 10.1016/j.ympev.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Schelly R., Takahashi T., Bills R., Hori M. The first case of aggressive mimicry among lamprologines in a new species of Lepidiolamprologus (Perciformes: Cichlidae) from Lake Tanganyika. Zootaxa. 2007;1638:39–49. [Google Scholar]
- Schilthuis L. On a collection of fishes from the Congo; with description of some new species. Tijd. Nederl. Dierk. 1891;3:83–91. [Google Scholar]
- Schluter D., Price T. Honesty, perception and population divergence in sexually selected traits. Proc. R. Soc. Lond. B. 1993;253:117–122. doi: 10.1098/rspb.1993.0089. [DOI] [PubMed] [Google Scholar]
- Schmidt H.A., Strimmer K., Vingron M., von Haeseler A. TREE–PUZZLE: a maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18:502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- Schütz D., Parker G.A., Taborsky M., Sato T. An optimality approach to male and female body sizes in an extremely size-dimorphic cichlid fish. Evol. Ecol. Res. 2006;8:1393–1408. [Google Scholar]
- Schwarzer J., Misof B., Tautz D., Schliewen U.K. The root of the East African cichlid radiations. BMC Evol Biol. 2009;9:181. doi: 10.1186/1471-2148-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O. Explosive speciation rates and unusual species richness in haplochromine cichlid fishes: effects of sexual selection. Adv. Ecol. Res. 2000;31:237–274. [Google Scholar]
- Seehausen O., Terai Y., Magalhaes I., Carleton K.L., Mrosso H.D.J., Miyagi R., van der Sluijs I., Schneider M.V., Maan M.E., Tachida H., Imai H., Okada N. Speciation through sensory drive in cichlid fish. Nature. 2008;455:620–626. doi: 10.1038/nature07285. [DOI] [PubMed] [Google Scholar]
- Sefc K.M., Baric S., Salzburger W., Sturmbauer C. Species-specific population structure in rock-specialized sympatric cichlid species in Lake Tanganyika, East Africa. J. Mol. Evol. 2007;64:33–49. doi: 10.1007/s00239-006-0011-4. [DOI] [PubMed] [Google Scholar]
- Sefc K.M., Mattersdorfer K., Sturmbauer C., Koblmüller S. High frequency of multiple paternity in broods of a socially monogamous cichlid fish with biparental nest defence. Mol. Ecol. 2008;17:2531–2543. doi: 10.1111/j.1365-294X.2008.03763.x. [DOI] [PubMed] [Google Scholar]
- Shapiro B., Rambaut A., Drummond A.J. Choosing appropriate substitution models for the phylogenetic analysis of protein-coding sequences. Mol. Biol. Evol. 2006;23:7–9. doi: 10.1093/molbev/msj021. [DOI] [PubMed] [Google Scholar]
- Shimodaira H., Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 1999;16:1114–1116. [Google Scholar]
- Soltis P.S., Soltis D.E., Savolainen V., Crane P.R., Barraclough T.G. Rate heterogeneity among lineages of tracheophytes: integration of molecular and fossil data and evidence for molecular living fossils. Proc. Natl. Acad. Sci. USA. 2002;99:4430–4435. doi: 10.1073/pnas.032087199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stager J.C., Johnson T.C. The late Pleistocene desiccation of Lake Victoria and the origin of its endemic biota. Hydrobiologia. 2008;596:5–16. [Google Scholar]
- Stiassny M.L.J. Phylogenetic interrelationships of the family Cichlidae: an overview. In: Keenleyside M.H.A., editor. Cichlid Fishes, Behaviour, Ecology and Evolution. Chapman and Hall; London: 1991. pp. 1–35. [Google Scholar]
- Stiassny M.L.J. Atavisms, phylogenetic character reversals, and the origin of evolutionary novelties. Neth. J. Zool. 1992;42:260–276. [Google Scholar]
- Stiassny M.L.J. A phylogenetic overview of the lamprologine cichlids of Africa (Teleostei, Cichlidae): a morphological perspective. S. Afr. J. Sci. 1997;93:513–523. [Google Scholar]
- Stiver K.A., Dierkes P., Taborsky M., Balshine S. Dispersal patterns and status change in a co-operatively breeding cichlid Neolamprologus pulcher: evidence from microsatellite analyses and behavioural observations. J. Fish Biol. 2004;65:91–105. [Google Scholar]
- Strimmer K., von Haeseler A. Likelihood-mapping: a simple method to visualize phylogenetic content of a sequence alignment. Proc. Natl. Acad. Sci. USA. 1997;94:6815–6819. doi: 10.1073/pnas.94.13.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmbauer C., Meyer A. Mitochondrial phylogeny of the endemic mouthbrooding lineages of cichlid fishes from Lake Tanganyika in Eastern Africa. Mol. Biol. Evol. 1993;10:751–768. doi: 10.1093/oxfordjournals.molbev.a040042. [DOI] [PubMed] [Google Scholar]
- Sturmbauer C., Verheyen E., Meyer A. Mitochondrial phylogeny of the Lamprologini, the major substrate spawning lineage of cichlid fishes from Lake Tanganyika in Eastern Africa. Mol. Biol. Evol. 1994;11:691–703. doi: 10.1093/oxfordjournals.molbev.a040148. [DOI] [PubMed] [Google Scholar]
- Sturmbauer C., Baric S., Salzburger W., Rüber L., Verheyen E. Lake level fluctuations synchronize genetic divergence of cichlid fishes in African lakes. Mol. Biol. Evol. 2001;18:144–154. doi: 10.1093/oxfordjournals.molbev.a003788. [DOI] [PubMed] [Google Scholar]
- Sturmbauer C., Hainz U., Baric S., Verheyen E., Salzburger W. Evolution of the tribe Tropheini from Lake Tanganyika: synchronized explosive speciation producing multiple evolutionary parallelism. Hydrobiologia. 2003;500:51–64. [Google Scholar]
- Sturmbauer C., Hahn C., Koblmüller S., Postl L., Sinyinza D., Sefc K.M. Variation of territory size and defense behavior in breeding pairs of the endemic Lake Tanganyika cichlid fish Variabilichromis moorii. Hydrobiologia. 2008;615:49–56. [Google Scholar]
- Sunobe T., Munehara H. Mating system and kin relationship between adults and young in the shell-brooding cichlid fish Neolamprologus meeli in Lake Tanganyika. J. Ethol. 2003;21:87–92. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2002. PAUP∗. Phylogenetic Analysis Using Parsimony (∗and Other Methods). Version 4.0b10. [Google Scholar]
- Taborsky M., Limberger D. Helpers in fish. Behav. Ecol. Sociobiol. 1981;8:143–145. [Google Scholar]
- Taborsky M. Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim. Behav. 1984;32:1236–1252. [Google Scholar]
- Takahashi K., Terai Y., Nishida M., Okada N. A novel family of short interspersed repetitive elements (SINEs) from cichlids: the patterns of insertion of SINEs at orthologous loci support the proposed monophyly of four major groups of cichlid fishes in Lake Tanganyika. Mol. Biol. Evol. 1998;15:391–407. doi: 10.1093/oxfordjournals.molbev.a025936. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Terai Y., Nishida M., Okada N. Phylogenetic relationships and ancient incomplete lineage sorting among cichlid fishes in Lake Tanganyika as revealed by analysis of the insertion of retroposons. Mol. Biol. Evol. 2001;18:2057–2066. doi: 10.1093/oxfordjournals.molbev.a003747. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Systematics of Tanganyikan cichlid fishes (Teleostei: Perciformes) Ichthyol. Res. 2003;2003:367–382. [Google Scholar]
- Takahashi T., Watanabe K., Munehara H., Rüber L., Hori M. Evidence for divergent natural selection of a Lake Tanganyika cichlid inferred from repeated radiations in body size. Mol. Ecol. 2009;18:3110–3119. doi: 10.1111/j.1365-294X.2009.04248.x. [DOI] [PubMed] [Google Scholar]
- Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Taylor M.I., Rüber L., Verheyen E. Microsatellites reveal high levels of population substructuring in the species-poor Eretmodine cichlid lineage from Lake Tanganyika. Proc. R. Soc. Lond. B. Biol. Sci. 2001;268:803–808. doi: 10.1098/rspb.2000.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai Y., Seehausen O., Sasaki T., Takahashi K., Mizoiri S., Sugawara T., Sato T., Watanabe M., Konijnendijk N., Mrosso H.D.J., Tachida H., Imai H., Shichida Y., Okada N. Divergent selection on opsins drives incipient speciation in Lake Victoria cichlids. PLoS Biol. 2006;4:2244–2251. doi: 10.1371/journal.pbio.0040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiercelin J.J., Mondeguer A. The geology of the Tanganyika trough. In: Coulter G.W., editor. Lake Tanganyika and its Life. Oxford University Press; New York: 1991. pp. 7–48. [Google Scholar]
- Verburg P., Bills R. Two new cichlid species Neolamprologus (Teleostei: Cichlidae) from Lake Tanganyika, East Africa. Zootaxa. 2007;1612:25–44. [Google Scholar]
- Wagner C.E., McCune A.R. Contrasting patterns of spatial genetic structure in sympatric rock-dwelling cichlid fishes. Evolution. 2009;63:1312–1326. doi: 10.1111/j.1558-5646.2009.00612.x. [DOI] [PubMed] [Google Scholar]
- Walter B., Trillmich F. Female aggression and male peace-keeping in a cichlid fish harem: conflict between and within sexes in Lamprologus ocellatus. Behav. Ecol. Sociobiol. 1994;34:105–112. [Google Scholar]
- Yamagishi S., Kohda M. Is the cichlid fish Julidochromis marlieri polyandrous? Ichthyol. Res. 1996;43:469–471. [Google Scholar]
- Yanagisawa Y. Social organization oa a polygynous cichlid Lamprologus furcifer in Lake Tanganyika. Jpn. J. Ichthyol. 1987;34:82–90. [Google Scholar]
- Yang Z. Estimating the pattern of nucleotide substitution. J. Mol. Evol. 1994;39:306–314. doi: 10.1007/BF00178256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A chronogram of the diversification of the Lamprologini based on complete ND2 sequences and applying following calibration points (as uniform priors): (i) the onset of the diversification of the C-lineage in the course of the so-called “primary radiation”, which was proposed to have coincided with the formation of tropical clearwater habitat with deep-water conditions 5–6 MYA (Salzburger et al., 2002a); (ii) a maximum age of 0.57–1 MY for the Lake Malawi cichlid species flock, based on the age of the refilling of Lake Malawi (Delvaux, 1995; Sturmbauer et al., 2001); (iii) an age of 1.1–3.5 MY for the split of the Congo-lamprologines, based on the time window for a Lukuga-connection between Lake Tanganyika and the Congo system (Lezzar et al., 1996; Cohen et al., 1997). Divergence time estimates are represented as the mean node height of the 95% highest posterior density (HPD) interval from a BEAST maximum-clade-credibility tree. Gray bars span the 95% HPD for each well supported node. No bars are assigned to nodes with low posterior probability. Calibration points are marked by arrows. Riverine lamprologine taxa are shaded in gray.
A chronogram of the diversification of the Lamprologini based on complete ND2 sequences and applying following calibration points (as uniform priors): (i) the onset of the diversification of the C-lineage in the course of the so-called “primary radiation”, which was proposed to have coincided with the formation of tropical clearwater habitat with deep-water conditions 5–6 MYA (Salzburger et al., 2002a); (ii) a maximum age of 0.57–1 MY for the Lake Malawi cichlid species flock, based on the age of the refilling of Lake Malawi (Delvaux, 1995; Sturmbauer et al., 2001). Divergence time estimates are represented as the mean node height of the 95% highest posterior density (HPD) interval from a BEAST maximum-clade-credibility tree. Gray bars span the 95% HPD for each well supported node. No bars are assigned to nodes with low posterior probability. Calibration points are marked by arrows. Riverine lamprologine taxa are shaded in gray.
A chronogram of the diversification of the Lamprologini based on complete ND2 sequences and applying following calibration points (as uniform priors): (i) the onset of the diversification of the C-lineage at 9–12 MYA, assuming that the primary Tanganyika radiation had taken place at the onset of the Lake formation 9–12 MYA as a consequence of allopatric diversification in a series of small shallow lakes in the area currently occupied by the Congo River system and Lake Tanganyika (Tiercelin and Mondeguer, 1991; Cohen et al., 1993), implying that the secondary radiation observed in most Lake Tanganyika cichlid tribe (Koblmüller et al., 2004, 2005, 2007a,b, 2010; Duftner et al. 2005) coincided with the formation of a real lacustrine habitat with deep-water conditions about 5–6 MYA, compatible with the fossil calibrated diversification scenario discussed by Genner et al. (2007); (ii) an age of 1.1–3.5 MY for the split of the Congo-lamprologines, based on the time window for a Lukuga-connection between Lake Tanganyika and the Congo system (Lezzar et al., 1996; Cohen et al., 1997). Divergence time estimates are represented as the mean node height of the 95% highest posterior density (HPD) interval from a BEAST maximum-clade-credibility tree. Gray bars span the 95% HPD for each well supported node. No bars are assigned to nodes with low posterior probability. Calibration points are marked by arrows. Riverine lamprologine taxa are shaded in gray.
A chronogram of the diversification of the Lamprologini based on complete ND2 sequences and assuming an age of 30 MYA (normally distributed prior with a mean of 30 MYA; S.D, 3.0 MY) for the MRCA of the C-lineage, as was proposed by Genner et al. (2007) based on a presumed Gondwanan origin of the family Cichlidae. Divergence time estimates are represented as the mean node height of the 95% highest posterior density (HPD) interval from a BEAST maximum-clade-credibility tree. Gray bars span the 95% HPD for each well supported node. No bars are assigned to nodes with low posterior probability. Calibration points are marked by arrows. Riverine lamprologine taxa are shaded in gray.