Abstract
All metazoans use insulin to control energy metabolism, but they secrete it from different cells: neurons in the central nervous system in invertebrates and endocrine cells in the gut or pancreas in vertebrates. Despite their origins in different germ layers, all of these insulin-producing cells share common functional features and gene expression patterns. In this study, we tested the role in insulin-producing cells of the vertebrate homologues of Dachshund, a transcriptional regulator that marks the earliest committed progenitors of the neural insulin-producing cells in Drosophila. Both zebrafish and mice expressed a single dominant Dachshund homologue in the pancreatic endocrine lineage, and in both species loss of this homologue reduced the numbers of all islet cell types including the insulin-producing β-cells. In mice, Dach1 gene deletion left pancreatic progenitor cells unaltered, but blocked the perinatal burst of proliferation of differentiated β-cells that normally generates most of the β-cell mass. In β-cells, Dach1 bound to the promoter of the cell cycle inhibitor p27Kip1, which constrains β-cell proliferation. Taken together, these data demonstrate a conserved role for Dachshund homologues in the production of insulin-producing cells.
INTRODUCTION
The peptide hormone insulin regulates energy metabolism and growth in all metazoans (Leevers, 2001; Skorokhod et al., 1999). In mammals, the insulin-producing cells, the β-cells, reside in the pancreas, organized into the islets of Langerhans together with α-cells, δ-cells, ε-cells and PP cells that produce the peptide hormones glucagon, somatostatin, ghrelin and pancreatic polypeptide, respectively. The β-cells act as part of an integrated information network regulating energy metabolism that includes the other islet cells, the endocrine cells of the gut, and neurons, especially specific neurons in the hypothalamus.
Our understanding of how the β-cells are generated during development, and the genes involved in that process, derives largely from studies in rodents (Murtaugh, 2007; Wilson et al., 2003), and more recently in zebrafish (Field et al., 2003). The generation of β-cells during mouse development can be divided into three phases. First, during the period starting with the budding of the initial pancreatic anlage from the gut endoderm at embryonic day 9.5 (E9.5) and ending with the “secondary transition” at E13, a small number of endocrine cells differentiate from the pancreatic progenitor cells. A minority of these “primary endocrine cells” express insulin, but these insulin-expressing cells contain low levels of insulin, often co-express glucagon, and lack mature β-cell markers Nkx6.1, MafA and Pdx1 (Kim and MacDonald, 2002; Wilson et al., 2002). During the second phase, starting at E13, much larger numbers of insulin-producing cells with mature β-cell characteristics differentiate via a pathway that involves the basic helix-loop-helix transcription factor Neurogenin3 acting upstream of the transcription factors NeuroD1, Nkx2.2, Nkx6.1, MafA, and Pax4, among others. This neogenesis of β-cells via the Neurogenin3+ progenitor cells peaks around E14-15 in the mouse and has largely ceased by E18 (Jensen et al., 2000; Johansson et al., 2007; Schwitzgebel et al., 2000). The third phase, β-cell proliferation, starts shortly before the termination of β-cell neogenesis and lasts through the first few weeks of postnatal life, yielding a marked expansion of the β-cell population (Finegood et al., 1995; Sander et al., 2000).
The genes in the Neurogenin3-dependent pathway that drive β-cell neogenesis also function in neural development in the vertebrate central nervous system, especially in peptide- and monoamine-secreting neurons in the hypothalamus (Kurrasch et al., 2007) and hindbrain (Cordes, 2005). Interestingly, some cells in the vertebrate brain produce small amounts of insulin (Devaskar et al., 1994). While neuronal production of insulin is only a minor pathway in vertebrates, in invertebrates the principle insulin-producing cells (IPCs) are located in the nervous system (Rulifson et al., 2002). In Drosophila, the neural IPCs differentiate in a region analogous to the vertebrate hypothalamus, adjacent to the Corpora cardiaca cells, the fly equivalent of the vertebrate α-cells, with which they later functionally interact in regulating energy metabolism (Kim and Rulifson, 2004).
The parallels between the neural IPCs in Drosophila and the vertebrate β-cells suggest that the IPCs could be used to identify candidate genes involved in β-cell generation. A recent study of the development of the IPCs and Corpora cardiaca cells in Drosophila documented in detail the gene networks operating during the differentiation of each lineage (Wang et al., 2007). Whereas some of the genes identified -- such as eyeless, the homologue of vertebrate Pax6 -- have been implicated previously in vertebrate islet development (Sander et al., 1997; St-Onge et al., 1997), most of the vertebrate homologues of the Drosophila IPC genes have not been interrogated in pancreas development.
In particular, a single gene, dachshund, not previously implicated in islet development, uniquely identified the earliest committed progenitor of the IPCs . The dachshund gene encodes a nuclear protein required for normal eye and leg development in Drosophila (Mardon et al., 1994). A highly interactive network of genes including eyeless, eyes absent and sine oculis work with dachshund in initiating eye formation in Drosophila (Gehring, 2004). The dachshund gene family is conserved in vertebrates; three homologues have been identified previously in zebrafish (Hammond et al., 2002) and two in mice and human (Caubit et al., 1999; Davis et al., 1999; Davis et al., 2001b; Kozmik et al., 1999). The mammalian Dach genes have partially overlapping expression patterns in a variety of embryonic and adult tissues, including the eye, the hypothalamus, and the pituitary, where they also interact with mammalian members of the eyes absent (Eya) and sine oculis (Six) families, as well as other nuclear proteins (Hanson, 2001).
Dachshund family proteins lack obvious sequence similarity with other known transcription factors, but structural analysis has revealed that an N-terminal domain with sequence similarity to the ski/sno oncogenes has structural similarities to the winged-helix/forkhead DNA binding motif (Kim et al., 2002). Whether Dachshund proteins can bind DNA is unclear; but, like the Ski/Sno proteins, they can function as transcriptional repressors by linking to DNA-binding transcription factors and recruiting corepressors. For example, in regulating the proliferation of pituitary progenitor cells in mice, Dach2 binds to the Sine oculis homologue Six6, recruits the corepressors N-Cor and Sin3A/B and histone deacetylases, and suppresses the expression of the cell cycle inhibitor p27Kip1 (Li et al., 2002). In other contexts, however, such as in association with the phosphatase activity of Eya proteins (Li et al., 2003), Dachshund proteins can recruit coactivators and activate the expression of target genes. The function of Dachshund proteins, therefore, is highly dependent on their cellular and gene context.
To explore the possibility that Dachshund homologues play a role in the development of the vertebrate islet and β-cell, we determined the expression pattern and function of the genes in this family in the developing pancreas in zebrafish and mice. As in Drosophila, we found Dachshund family members expressed in the islet lineage and demonstrated their importance in expanding the endocrine cell population in both vertebrate species. These studies demonstrate a conserved role for Dachshund homologues in islet cell development, and validate the use of Drosophila to identify genes and pathways important for vertebrate islet development.
Results
Expression of dachb in the developing zebrafish pancreas
To determine if the vertebrate homologues of the Drosophila dachshund gene play a role in pancreas development, we started by examining their expression in the developing zebrafish pancreas. The zebrafish pancreas forms from two buds off the gut endoderm. The first anlage, generating only endocrine tissue, buds from the dorsal aspect of the developing gut by 24 hours post fertilization (hpf). The second anlage, located on the ventral aspect of the developing gut and anterior to the dorsal anlage, appears by 40 hpf and gives rise mainly to the pancreatic duct and exocrine cells. These two buds merge by 52 hpf to form the pancreas (Biemar et al., 2001; Field et al., 2003).
Three zebrafish dachshund homologues, dacha, dachb and dachc, have been described with distinct but overlapping expression patterns (Hammond et al., 2002); and we identified a candidate fourth homologue, dachd, in the zebrafish genome and demonstrated its expression by RT-PCR from RNA isolated from whole embryos at 24 and 48 hpf. We assessed the expression of all four dachshund homologues in the developing zebrafish pancreas by whole-mount in situ hybridization from mid-somitogenesis to 48 hpf.
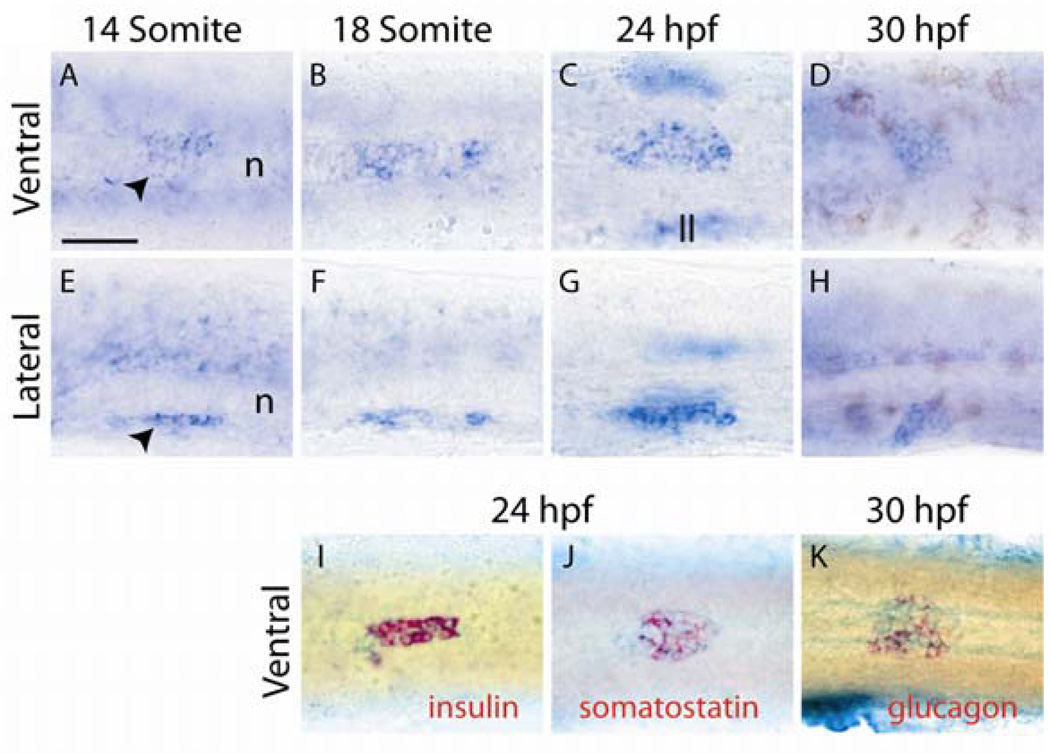
We could detect expression of dachb, but not dacha, dachc or dachd, in the region of the developing pancreas (Fig. 1 and data not shown). dachb expression was first detected at the 14-somite stage in the mid-endoderm region from which the pancreas develops (Fig. 1A, E). dachb expression peaked at 24hpf, coincident with the formation of the dorsal pancreatic bud and persisted until 30 hpf (Fig1B–D;F–H). After 30 hpf, the level of expression dramatically decreased (data not shown).
Fig. 1.
Expression of dachb in the zebrafish pancreas. Whole-mount in situ hybridization was performed for dachb (A–K, blue) and insulin (I, red), somatostatin (J, red), and glucagon (K, red) at the stages of zebrafish development shown. Ventral views (A–D;I–K), and lateral views (E–H) are shown with anterior to the left. On all the panels the yolk was manually removed from the embryos. The notochord (n) and the lateral line (ll) have been indicated in panels A, E and C. Black arrowheads: dachb-expressing cell in the pancreatic region. Scale bar, 50µm.
The localization of dachb transcripts to the dorsal pancreatic bud was confirmed by double in situ hybridization with three pancreatic endocrine markers: insulin, somatostatin and glucagon (Fig1I–K). At 24 hpf, subsets of dachb-expressing cells co-expressed insulin or somatostatin. Similarly at 30hpf, a portion of the dachb-positive cells co-expressed glucagon.
Knock-down of dachb reduces pancreatic endocrine cells
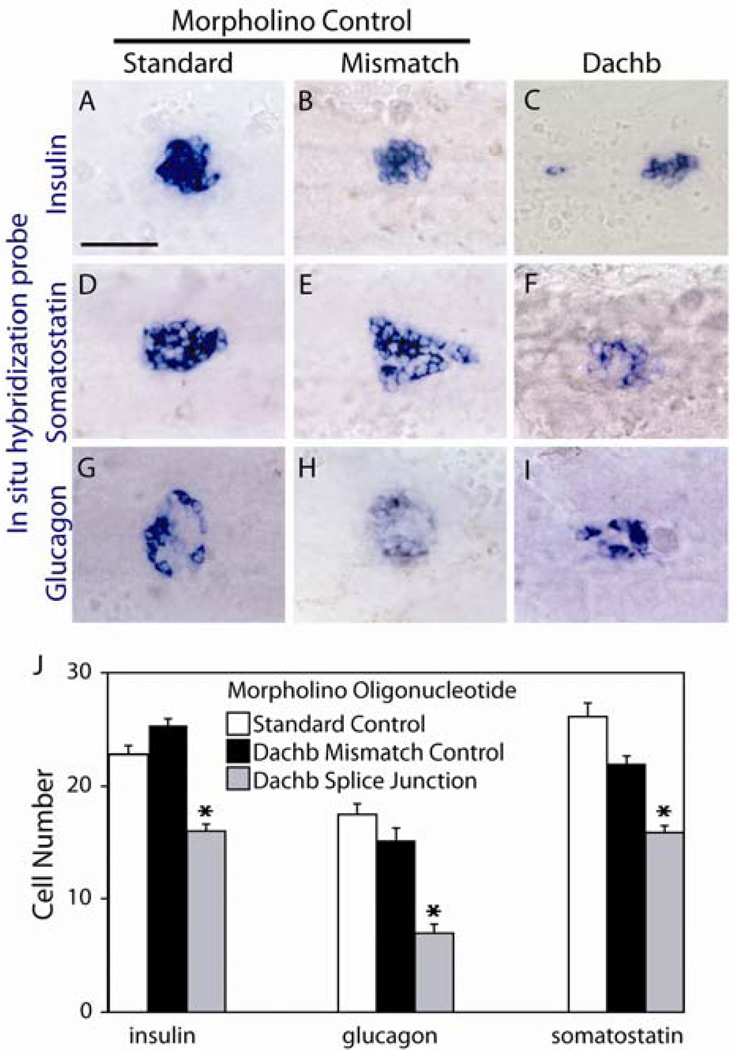
The function of Dachb in development of the zebrafish pancreas was investigated by injection of antisense morpholino oligonucleotides targeting the splicing of the dachb transcript into 1- to 2-cell stage embryos. We confirmed by RT-PCR that this morpholino oligonucleotide blocked efficiently the splicing of dachb mRNA (Supplemental Fig. 1). As negative controls, we used a morpholino oligonucleotide in which 4 nucleotides of the dachb targeting sequence were altered and an unrelated standard control morpholino oligonucleotide. Embryos were injected with 4 ng of morpholino oligonucleotide and allowed to develop until 30 hpf or 48 hpf. The expression of pancreatic markers was then analyzed quantitatively.
Injection of the dachb morpholino oligonucleotide decreased the number of hormone-expressing cells (Fig. 2C,F,I) as compared to control embryos (Fig. 2A,B,D,E,G,H). We observed a decrease of 36%, 35% and 64% in insulin-, somatostatin- and glucagon-expressing cells, respectively. In contrast, the expression of ptf1a, marking the exocrine tissue, was not affected (Supplemental Fig. 1C–E).
Fig. 2.
Inhibition of DachB expression by morpholino antisense oligonucleotides. Expression of insulin (A–C), somatostatin (D–F) and glucagon (G–I) was analyzed by whole-mount in situ hybridization at 30 hpf embryos injected with the morpholino oligonucleotides shown: a standard control (A, D, G), a mismatch control with 4 bases altered from the complimentary dachb sequence (B, E, H), and an oligonucleotide complimentary to a dachb splice junction (C, F, I). All panels present ventral views of yolk-free embryos with anterior to the left. In panel J, the numbers of cells expressing the hormones shown were assessed in morpholino-injected embryos at 30 hpf. Each data point represents the mean ± standard error of at least 40 embryos for each condition. ***p<0.001 compared with embryos injected with morpholino 4-mismatch control by Student’s t test. Scale bar, 50µm.
Taken together, these data demonstrate that one homologue of the Drosophila dachshund gene, dachb, is expressed in the developing pancreas and is involved in the formation of hormone-expressing islet cells in zebrafish.
Expression of Dach1 in the mouse pancreas
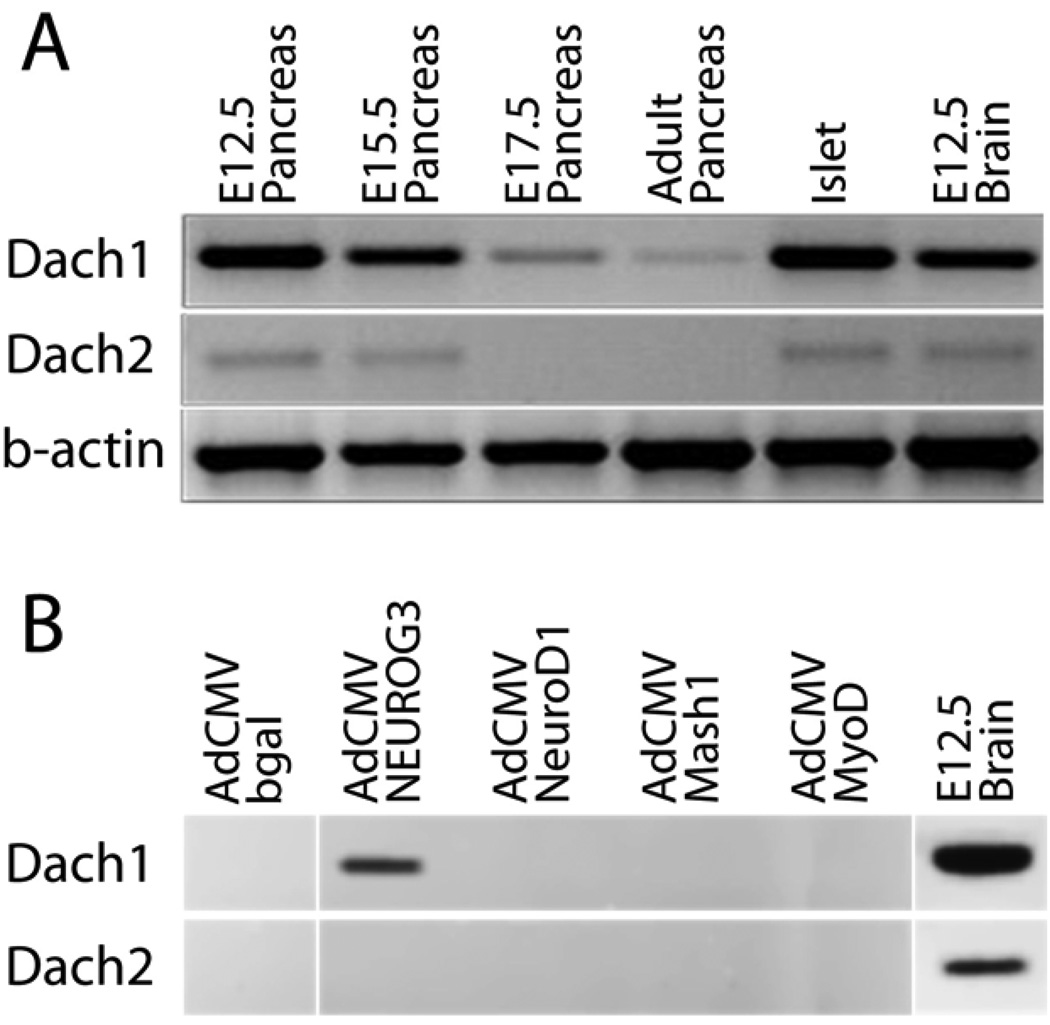
The mouse genome contains 2 dachshund homologues, Dach1 and Dach2 (Davis et al., 2001b; Hammond et al., 1998). To assess the expression of the two Dach genes in the developing and adult mouse pancreas, we first performed RT-PCR on RNA from E12.5, E15.5, E17.5, adult total pancreases and adult islets using gene specific primers (Fig. 3A). Dach1 mRNA was detected at all embryonic stages examined and also in RNA from adult mouse islets. Dach2 mRNA was detected at lower levels throughout pancreas development and in mature tissue. Similar results were obtained by real-time RT-PCR (data not shown). Parallel RT-PCR analysis identified members of the sine oculis and eyes absent gene families (Supplementary Fig. 2).
Fig.3.
Expression of Dach mRNA in the mouse. RT-PCR was performed with gene specific primers with RNA purified from isolated mouse tissues at the indicated ages in A, and from the mouse pancreatic ductal cell line mPAC L20 infected with adenovirus shown in B. Products were not amplified in the absence of RT (data not shown).
The proendocrine bHLH transcription factor Neurogenin3 can induce endocrine differentiation in pancreatic duct cells in vitro (Gasa et al., 2004; Heremans et al., 2002). We found that adenovirus expressing Neurogenin3, but not NeuroD1, could induce Dach1 expression in pancreatic duct cells (Fig. 3B), placing Dach1 in a small group of genes induced by Neurogenin3, but not NeuroD1 (Gasa et al., 2008). Dach2 expression, in contrast, was unaffected by Neurogenin3 or NeuroD1.
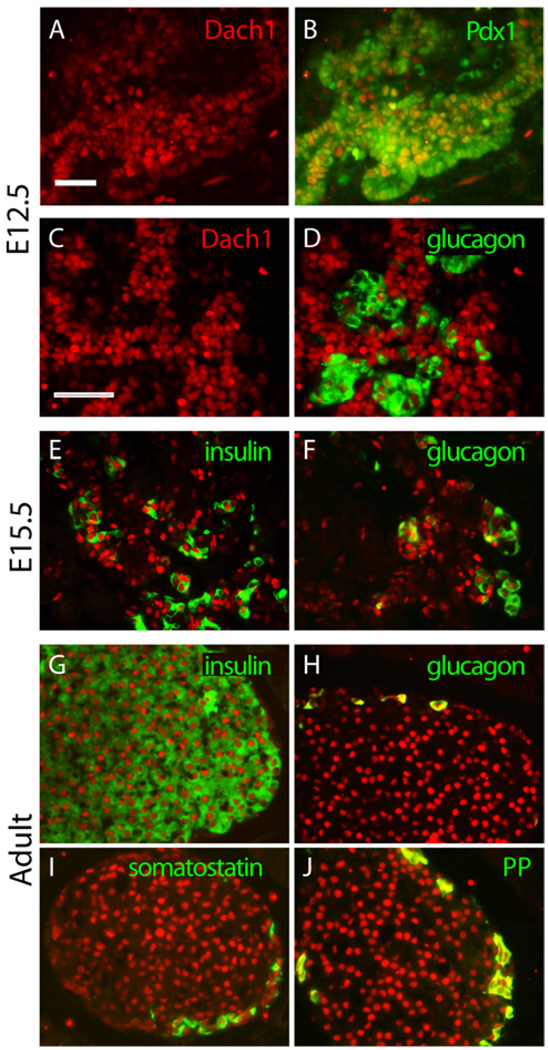
Next, we defined the pattern of Dach1 expression in the developing mouse pancreas by immunohistochemistry. The pancreas expressed detectable levels of Dach1 protein as early as E10.5 (data not shown); and at E12.5, Dach1 protein was detected throughout the pancreatic epithelium (Fig. 4A, C), where it co-localized with the pancreatic-duodenal homeodomain transcription factor Pdx1 in most cells (Fig. 4B). Double immunofluorescence staining also detected Dach1 in a subset of the glucagon-positive cells at E12.5 (Fig. 4D).
Fig.4.
Expression of Dach1 in the mouse pancreas. Immunofluorescence staining for Dach1 is shown in red at E12.5 (A–D), E15.5 (E, F), and adult (G – J). Double immunofluorescence staining for transcription factor Pdx1 or islet hormones (green) was performed for Pdx1 (B) glucagon (D, F and H), insulin (E and G), somatostatin (I), and pancreatic polypeptide (PP, in J). Scale bar, 100µm.
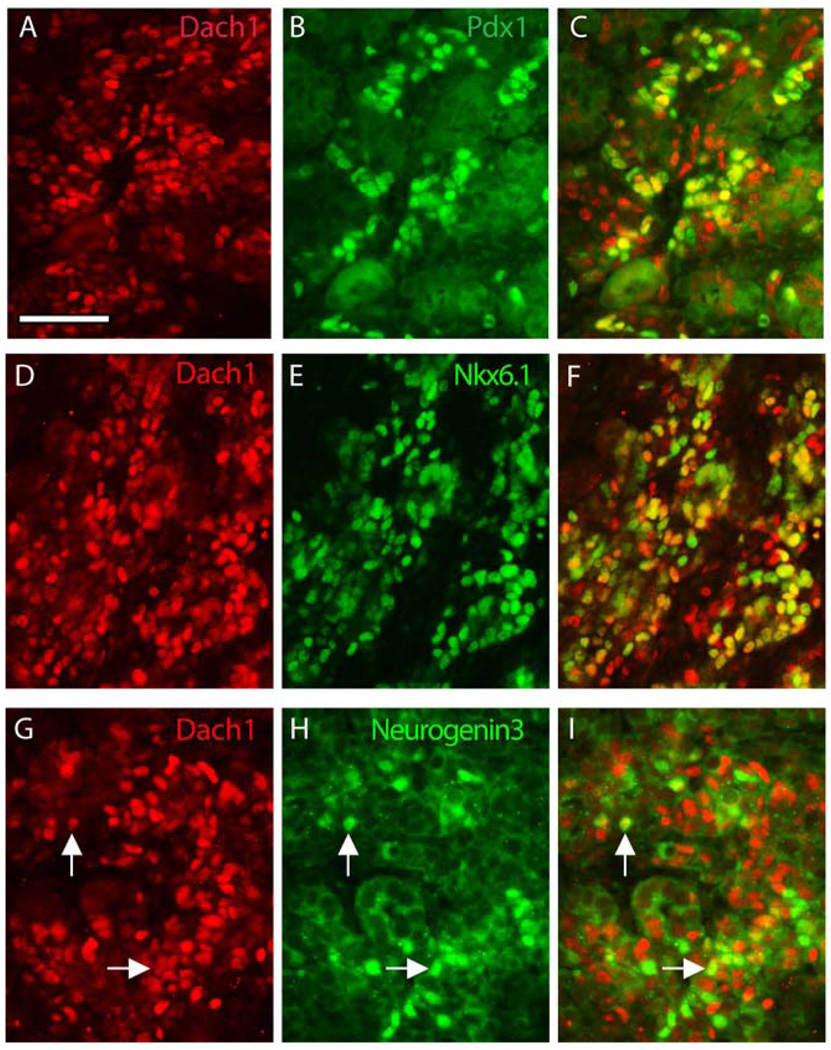
After the secondary transition at E13, when the undifferentiated pancreatic progenitor cells in the epithelium rapidly begin to differentiate into endocrine and exocrine cells, Dach1 expression was detected in a more restricted pattern. At E15.5, the majority of the insulin-producing and glucagon-producing endocrine cells expressed Dach1 protein (Fig. 4E, F), but Dach1 was absent from terminally differentiated exocrine cells. At this stage, the expression of Dach1 overlapped with other pancreatic endocrine transcription factors, including the homeodomain factors Pdx1 (Fig. 5A–C), which is expressed at high levels in mature β-cells, and Nkx6.1 (Fig. 5D–F), which is expressed at high levels in differentiating and mature β-cells, and to a lesser degree the bHLH factor Neurogenin3 (Fig. 5G–I), which marks islet cell progenitors (Schwitzgebel et al., 2000).
Fig.5.
Expression of Dach1with islet transcriptional regulators in the mouse pancreas at E15.5. Immunofluorescence staining for Dach1 is shown in red (A, C, D, F, G, I). Double immunofluorescence staining for islet transcription factors (green) was performed for Pdx1 (B, C), Nkx6.1 (E, F) and Neurogenin3 (H, I). Nuclei expressing both proteins appear yellow (C, F, I). Examples of co-staining nuclei in panels G–I are indicated with white arrows. Scale bar, 50µm.
By E18.5 (data not shown) and in the adult pancreas (Fig. 4G–J), Dach1 expression was fully restricted to the islets of Langerhans, where it was detected in α-, β-, δ- and PP-cells.
Pancreas development in mice lacking Dach1
To test the role of Dach1 in pancreatic development in the mouse, we examined mice with a targeted disruption of the Dach1 gene. Dach1−/− mice survive to birth, but die shortly thereafter (Backman et al., 2003; Davis et al., 2001a). At E18.5, one day prior to birth, the pancreases of Dach1−/− embryos were normal in size and gross appearance (data not shown). Hematoxylin-eosin staining of the E18.5 pancreases revealed no obvious morphological discrepancy between wild-type and Dach1−/− mice (Fig. 6A, B). Immunofluorescent staining for acinar (Amylase), ductal (Mucin-1) and mesenchymal (Vimentin) markers at E18.5 also detected no abnormalities (Supplemental Fig 3, data not shown).
Fig.6.
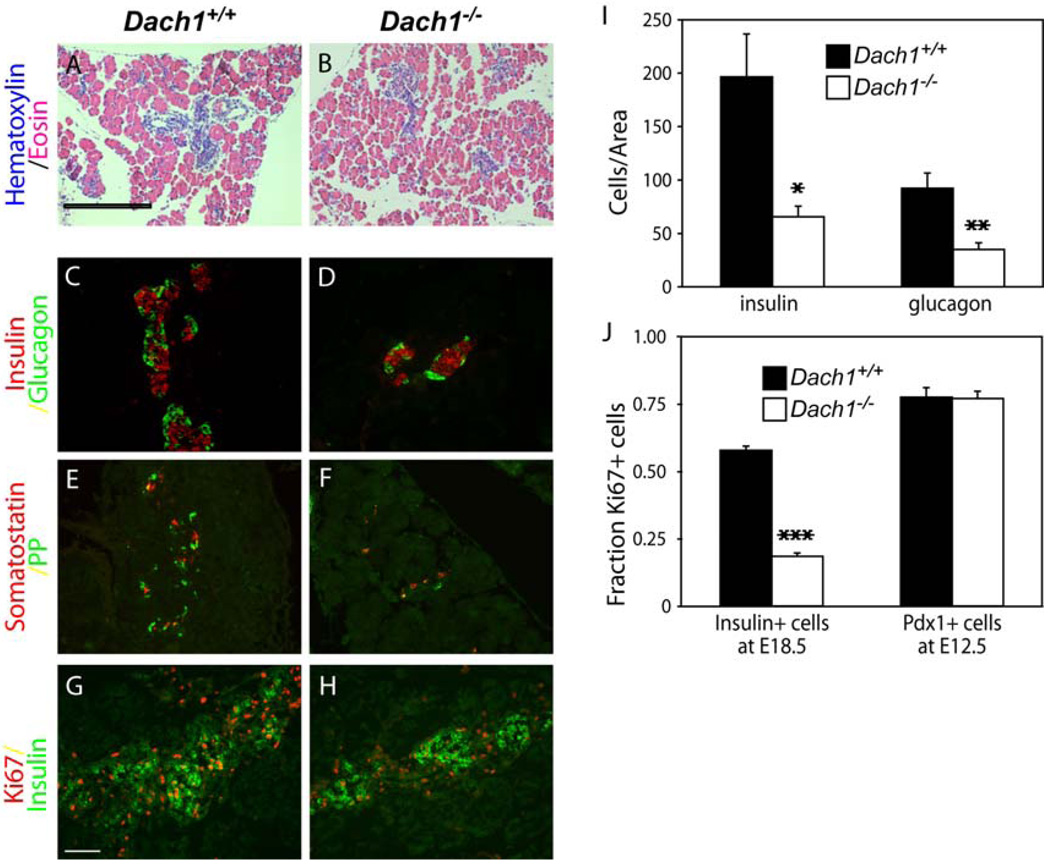
Pancreas development in the absence of Dach1 at E18.5. Hematoxylin-eosin staining demonstrates the morphology of the pancreas in Dach1+/+ (A) and Dach1−/− (B) mouse embryos. Scale bar, 500 µm. Immunofluorescent co-staining for insulin (C, D; red), glucagon (C, D; green), somatostatin (E, F; green) and pancreatic polypeptide (PP; E, F; red) was performed on the pancreas in Dach1+/+ (C, E) and Dach1−/− (D, F) mouse embryos at E18.5. Scale bar, 100 µm. Immunofluorescent co-staining for insulin (green) and Ki67 (red) was performed on the pancreas in Dach1+/+ (G) and Dach1−/− (H) mouse embryos at E18.5. Scale bar: 100 µm. I. Insulin- and glucagon-positive cells from Dach1+/+ (black bars) and Dach1−/− (white bars) embryos were counted and expressed as the total number of cells per total pancreatic area. J. Percentage of β-cells replicating in Dach1+/+ (black bars) and Dach1−/− (white bars) embryos at E18.5 was assessed by counting the number of cells co-staining for insulin and Ki67 and dividing by the total number of cells staining for insulin; and the percent of pancreatic progenitor cells replicating in Dach1+/+ (black bars) and Dach1−/− (white bars) embryos at E12.5 was assessed by counting the number of cells co-staining for Pdx1 and Ki67 and dividing by the total number of cells staining for Pdx1. Each data point represents the mean of 4 embryos ± standard error of the mean.*p < 0.02, **p < 0.01, and ***p < 0.001 compared with Dach1+/+ embryos by Student’s t test.
Immunohistochemical staining with antibodies against islet hormones insulin, glucagon, somatostatin and PP at E18.5 revealed that endocrine cells still clustered into islet structures, but the size of the aggregates was reduced in Dach1−/− embryos (Fig. 6D, F) compared to their wild-type littermates (Fig. 6C, E). Staining for Pax6, a marker for endocrine cells, confirmed the reduction in total endocrine cells in the Dach1−/− embryos (Supplemental Fig. S3). Cell counting normalized to pancreatic area at E18.5 demonstrated a 67% reduction in the number of insulin-producing β-cells in Dach1−/− pancreases relative to their wild-type littermates, and a 63% reduction in glucagon-producing α-cells (Fig. 6I). Although reduced in number, the remaining β-cells in the Dach1−/− embryos appeared to be fully differentiated, as judged by their expression of Glut2, a characteristic marker of mature β-cells (Supplemental Fig. S3). This defect in the pancreas was not apparent earlier at E15.5 (Supplemental Fig. S4).
Role of Dach1 in islet cell proliferation
The reduction in islet cells observed in the Dach1−/− embryos could result from decreased generation of new islet cells from progenitor cells, or decreased proliferation or increased apoptosis of differentiated islet cells. Islet cells differentiate from pancreatic progenitor cells via islet progenitor cells that transiently express the bHLH transcription factor Neurogenin3. Staining for Neurogenin3 during the peak of islet cell genesis at E15.5, we did not detect a decrease in Neurogenin3-expressing islet cell progenitors in the Dach1−/− pancreases (data not shown). Similarly, the frequency of apoptotic β-cells as judged by co-staining of insulin with cleaved caspase-3 was very low at E18.5 in wild type embryos (0.38%) and was not increased in the Dach1−/− pancreases (0.2%) (data not shown).
Staining for Ki-67, a marker of actively proliferating cells, revealed approximately a three-fold decrease in the proliferation of insulin-positive cells in Dach1−/− embryos at E18.5 compared to their wild-type littermates (Fig. 6G,H, J). In contrast, the proliferation of Pdx1-positive pancreatic progenitors at E12.5 was unaffected in the Dach1−/− embryos (Fig. 6J), a result that is consistent with the observation that the overall size of the pancreas was unchanged in the Dach1−/− embryos. Taken together, these results suggest that Dach1 plays a role specifically in the proliferation of in the terminally differentiated islet cells, but not in their differentiation or in the proliferation of the pancreatic progenitor cells.
Dach1 binds to the p27Kip1 promoter
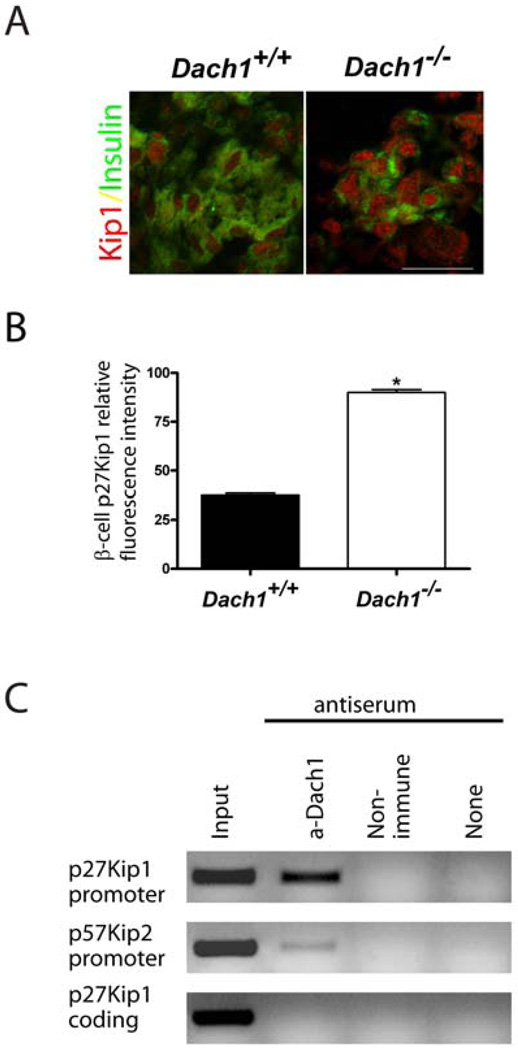
During the perinatal expansion of the islet cell population, the cyclin-dependent kinase inhibitor p27Kip1 plays a crucial role in limiting the proliferation of terminally differentiated β-cells (Georgia and Bhushan, 2006). We could readily detect a two-fold increase in p27Kip1 in the endocrine pancreas at E18.5 (Fig.7A, B and Supplemental Fig. 5). Dach2, in association with the homeodomain factor Six6, can regulate the proliferation of retinal and pituitary progenitors by directly repressing the promoter for the gene encoding p27Kip1 (Li et al., 2002). In the β-cell line βTC3, which expresses Dach1 (data not shown), chromatin immunoprecipitation demonstrated that Dach1 binds to the promoter of the Cdkn1b gene that encodes p27Kip1, but not to a site 2 KB downstream in the coding region (Fig.7C). Similarly, but to a lesser degree, the Dach1 antiserum also pulled down the promoter of the Cdkn1c gene that encodes p57Kip2.
Fig. 7.
Cell cycle regulation by Dach1. A. Immunofluorescent co-staining was performed for cyclin-dependent kinase inhibitor p27Kip1 (red) and insulin (green) on sectioned pancreas from Dach1+/+ and Dach1−/− mouse embryos at E18.5. Scale bar, 25µm. B. The intensity of p27Kip1 protein expression in insulin-positive cells in E18.5 pancreases from Dach1+/+ (black bar) and Dach1−/− (white bar) embryos was quantified and expressed as the intensity normalized to cell area.*p<0.0001. C. Chromatin IP studies were performed by immunoprecipitating cross-linked chromatin with antiserum against Dach1, with control IgG or without antiserum. Fragments of the mouse genes shown were amplified by PCR from the precipitates or the input DNA. Each data point represents the mean of quantification in 4 embryos ± standard error of the mean.
Discussion
Given the unique position of the dachshund gene in identifying the earliest committed progenitors of the insulin-producing cells in the Drosophila central nervous system (Wang et al., 2007), we explored whether dachshund homologues are similarly involved in the differentiation of vertebrate β-cells in the pancreatic islets of Langerhans. We found that all islet lineages expressed a dachshund homologue: dachb in zebrafish and Dach1 in mice. Furthermore, we found that all islet lineages required these genes for generating a full complement of cells. Finally, for the β-cell lineage, we demonstrated in mice that the decrease in cell number resulted from a decrease in proliferation. These data demonstrate the conservation of a role for members of the Dachshund family of transcriptional regulators in the development of insulin-producing cells in both invertebrates and vertebrates.
The expression of Dach1 in the mouse pancreas followed a pattern common to a number of islet transcription factors including Sox4, Mnx1 (Hb9), Pdx1, Nkx2.2, and Nkx6.1: early expression in all pancreatic progenitor cells, followed by deactivation prior to the secondary transition and reactivation in the endocrine lineage (Murtaugh, 2007). Interestingly, these factors often have different functions during their different phases of expression. For example, despite their early broad expression, the loss of neither Nkx2.2 nor Nkx6.1 has any apparent effect on the growth and differentiation of the early pancreatic anlage; but during their second phase of expression their loss dramatically impairs the differentiation of the β-cells (Sander et al., 2000; Sussel et al., 1998). Similarly, in the Dach1−/− mouse embryos, the development of the early pancreatic buds proceeded normally, and it was only during the second expression phase that a phenotype became evident, and then only in the endocrine lineage.
This endocrine phenotype, a decrease in cell number late in fetal development, can be attributed to a decrease in proliferation as we observed in the example of the β-cells in the Dach1−/− embryos, since no decrease in Neurogenin3-expressing endocrine progenitors or increase in apoptosis was observed. In mice, the newly differentiated β-cells start to replicate late in embryonic development, in a wave of expansion that extends into the early postnatal period (Finegood et al., 1995; Georgia and Bhushan, 2004; Sander et al., 2000). This perinatal proliferation depends on the activity of cyclin D2 (Georgia and Bhushan, 2004; Kushner et al., 2005), which associates with cyclin-dependent kinase Cdk4 (Rane et al., 1999) to move mouse β-cells from the G0 to the G1 phase of the cell cycle, and is restricted by the activity of the cyclin-dependent kinase inhibitor p27Kip1 (Georgia and Bhushan, 2006), which inhibits cyclin-Cdk complexes (for a review see (Cozar-Castellano et al., 2006)). We found that Dach1 was linked in β-cells to the promoter of p27Kip1, thus potentially keeping cell cycle inhibition in check and allowing β-cell proliferation.
The balance of Dach transcriptional repression and p27Kip1 inhibition of cell cycle progression also controls the expansion of key cell populations during the development of the pituitary and the retina in mice (Li et al., 2002). p27Kip1 controls the proliferation of retinal and pituitary precursors, and in its absence mice develop pituitary tumors and hyperplastic retina (Nakayama et al., 1996). In association with the Sine oculis homologue Six6, Dach2 binds to the p27Kip1 promoter in retinal and pituitary progenitor cells and regulates their proliferation by repressing p27Kip1 expression (Li et al., 2002). In an analogous manner in pancreatic endocrine cells, products of one or more of the sine oculis gene homologues that we detected in the pancreas may recruit Dach1 to the p27Kip1 promoter.
Unlike in the differentiated endocrine cells, loss of Dach1 from the pancreatic progenitor cells that normally express it prior to E13 had no impact on the proliferation of these cells in the Dach1−/− embryos. In place of p27Kip1, the related cyclin-dependent kinase inhibitor p57Kip2 plays a dominant role in regulating cell cycle progression of the early progenitors during pancreas formation in mice. In the absence of p57Kip2, increased numbers of these progenitors enter the cell cycle; while increasing p57Kip2 expression by removal of the transcriptional inhibitor Hes1 inhibits progenitor cell proliferation (Georgia et al., 2006). Interestingly, although we could detect the presence of Dach1 protein on the p57Kip2 promoter by chromatin immunoprecipitation in pancreatic cells, this binding did not translate into substantial effects on the proliferation of these cells.
The absence of a phenotype in the pancreatic progenitor cells in the Dach1−/− mouse pancreas underscores the context dependence of Dach function. The activity of Dach proteins on a particular promoter depends on the set of interacting proteins expressed in that cell and present in the nucleus and the subset of those proteins recruited to the promoter (Heanue et al., 1999; Ikeda et al., 2002; Li et al., 2003; Li et al., 2002). Neurogenin3 initiates the second phase of Dach1 expression in the pancreas by activating its expression in the endocrine lineage; and in parallel, it initiates the transcription of a unique set of genes involved in the differentiation, maintenance and function of the endocrine cells, including other transcription factors, signaling molecules, and regulators of the cell cycle (Gasa et al., 2004; Juhl et al., 2008; Petri et al., 2006; Treff et al., 2006; White et al., 2008). The proteins expressed by this set of genes then collaborate with Dach1 in regulating the proliferation of the differentiated endocrine cells and permit the remarkable proliferative plasticity that underlies the ability of β-cells to adjust to changing energy balance.
We propose that the Dachshund family of transcriptional regulators represents part of an evolutionarily conserved genetic network governing the formation of cells producing insulin and interrelated metabolic hormones. With the relocation of these cells during evolution from the ectoderm-derived central nervous system to the endoderm derived gut, much of this network moved intact. The example of the Dachshund family demonstrates that evolutionary conservation can be exploited by using invertebrate model organisms to identify genes and pathways relevant to the development of islet cells in fish, mice and humans. Understanding the determinants of β-cell production and expansion will provide new insights into the impairment of these processes in diabetes and new strategies for therapeutic β-cell replacement for people with diabetes.
Materials and methods
Animals
All animal studies were approved by the UCSF Institutional Animal Care and Use Committee. The Dach1 mutant mouse line and the mouse insulin I gene promoter (MIP)-GFP line have been described previously (Backman et al., 2003; Hara et al., 2003). Mice were housed on a 12-hr light-dark cycle in a controlled climate. Timed matings were carried out with embryonic day 0.5 being set as midday of the day of discovery of a vaginal plug. Zebrafish (Danio rerio) were raised and cared for according to standard protocols (Westerfield, 1995). Wild-type embryos from the AB and TL strains and ptf1∷GFP embryos (Godinho et al., 2007) were used and staged according to Kimmel (Kimmel et al., 1995).
Cell culture and infection of recombinant adenoviruses
Mouse mPAC L20 (Yoshida and Hanahan, 1994) and βTC3 (Efrat et al., 1988) were cultured in DMEM supplemented with 10% FCS and antibiotics. Viral infections were performed as previously described (Gasa et al., 2004).
In situ hybridization
Using the N and C box conserved regions of the three known zebrafish dachshund homologues as well as translated full length reading frames, we searched the UCSC genome browser (www.genome.ucsc.edu) for other zebrafish dachshund homologs. We detected another candidate dachshund gene on zebrafish chromosome 9, which has been subsequently identified in silico and listed in the NCBI database as hypothetical protein LOC560080. Using primers unique to this sequence, we amplified from a mixture of RNA from 24 and 48 hpf embryos a single cDNA whose sequence matched this putative dachshund homologue.
Single and double hybridizations and detections were carried out as previously described (Hauptmann and Gerster, 1994) on whole-mount wild-type embryos. Anti-sense RNA probes were prepared by transcribing linearized cDNA clones with SP6, T7, or T3 polymerase using digoxigenin or fluorescein labeling mix (Roche, Basel, Switzerland). Experimental and control embryos were developed for the same amount of time in the final colorimetric reaction for each probe tested. The dachb (Hammond et al., 2002), preproinsulin (Milewski et al., 1998), glucagon (Argenton et al., 1999), and somatostatin2 (Devos et al., 2002) probes were described previously.
Cell counting was performed directly after in situ hybridization under the microscope by focusing successively on each layer of stained cells in whole-mount embryos. The NBT/BCIP staining was monitored in order to avoid an overstaining which would have prevented the visualization of the individual cell boundaries. For mounting, the yolk was removed manually and the embryos where then mounted with the ventral side on top. Imaging was then performed using a Zeiss AxioImager Brightfield Microscope. Captured imaged were further processed with Adobe Illustrator CS2 for figure mounting.
Morpholino oligonucleotide injection
The morpholino oligonucleotides (Gene Tools, Eugene, OR) used were a dachb antisense oligonucleotide that targets the exon2-intron2 splice boundary, CTCAATGAGGGTTTACCTGTGGGTG; its 4-mismatch version (mismatch bases are underlined),CTCAAAGAGCGATTAGCTGTCGGTG; and the standard control morpholino provided by Gene Tools. 4 ng of morpholino nucleotides diluted in water were injected into the yolk of each embryo. Rhodamine dextran was added at 0.5% to the samples to visualize injection efficiency.
To assess the effect of the dachb morpholino, we extracted RNA from 24hpf morpholino-injected embryos using Trizol according to manufacturer’s instructions (Invitrogen, Carlsbad, CA). cDNA was prepared by in vitro transcription with 1µg of total RNA using SuperscriptII reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo(dT) primers (Invitrogen). 2 µl of cDNA product was used for each PCR reaction under the following conditions: 1 cycle of 94°C for 2’, 60°C for 1’, 72°C for 1’, followed by 35 cycles of 94°C for 1’, 60°C for 1’, 72°C for 1’. The primers used for the PCR amplification on the obtained cDNA were as follows: A: 5’-ACGACTGCACCAACGCAAGC-3’, B: 5’-TGTACCGGCGTTAGAGTTCA-3’, C: 5’-CTGCCTAAAACCAGAATATTACTGT-3’, D: 5’-GAGGAAATTGAGGCTCATCT-3’.
RT-PCR analysis
Mouse whole pancreata were dissolved in RLT-buffer (Qiagen, Valencia, CA) and total RNA was prepared according to the manufacturer’s protocol RNAeasy (Qiagen). Total RNA was treated with DNase (Qiagen). cDNA was prepared by in vitro transcription using SuperscriptII reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo(dT) primers (Invitrogen). MIP-GFP cell sorting, RNA preparation, reverse transcription and amplification were performed as described previously (Miyatsuka et al., 2009) from E17.5 pancreas and adult islets. cDNA product from 20 ng of original total RNA or 0.2 µl of the MIP-GFP amplified cDNA was used for each PCR reaction under the following conditions: 1 cycle of 94°C for 2’, 60°C for 1’, 72°C for 1’, followed by 35 cycles of 94°C for 1’, 60°C for 1’, 72°C for 1’. Mouse β-actin was used as the internal control. Sequences of primers are available on request.
Immunohistochemistry
The whole mouse embryos at E12.5 or E15.5 or isolated pancreases from E18.5 embryos or adults were fixed in 4% (w/v) paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 1 hour to overnight at 4°C and either paraffin embedded or frozen in OCT and then sectioned. Hematoxylin/eosin and immunofluorescence analysis were performed as described previously (Sander et al., 1997) . The primary antibodies used in these assays were the following: rabbit anti-Dach1 diluted 1:100 (Proteintech Group Inc., Chicago, IL), guinea-pig anti-insulin diluted 1:2000 (Linco, Billerica, MA), guinea-pig anti-glucagon diluted 1:2000 (Linco), mouse anti-glucagon 1:2000 (Sigma, St Louis, MO), rat anti-somatostatin diluted 1:500 (Chemicon, Billerica, MA), guinea-pig anti-pancreatic polypeptide diluted 1:100 (Linco), guinea-pig anti-PDX1 diluted 1:2000 (Sander et al., 2000), mouse monoclonal anti-Nkx6.1 diluted 1:50 (Developmental Studies Hybridoma Bank), guinea-pig anti-Ngn3 diluted 1:1000 (Schwitzgebel et al., 2000), mouse anti-Ki-67 diluted 1: 100 (BD Pharmingen, San Jose, CA), rabbit anti-p27Kip1 (C-19) diluted 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human caspase 3 diluted 1:400 (Cell signaling technology, Danvers, MA), rabbit anti-Pax6 diluted 1:500 (Chemicon, Billerica, MA), rabbit anti-Amylase diluted 1:750 (Sigma, St Louis, MO), hamster anti-Mucin diluted 1:200 (Thermo Scientific, Fremont, CA) and rabbit anti-Glut2 diluted 1:500 (Chemicon, Billerica, MA). The following secondary antibodies were used for immunofluorescence: Cy3-conjugated anti-rabbit 1:800, Cy3-conjugated anti-guinea pig 1:800, Cy3-conjugated anti-rat 1:800, FITC-conjugated anti-guinea pig 1:200, FITC-conjugated anti-rat 1:200, and FITC-conjugated anti-mouse 1:200 (Jackson Laboratories, Bar Harbor, Main). Fluorescence was visualized and photographed with a Zeiss Axiophoto2 plus microscope.
Cell counting and protein quantifications in mouse
To obtain a representative average of the number of hormone-positive cells, entire pancreases were used for quantification. Immunofluorescence staining was performed on 6 µm sections and positive cells were counted on every fifth section throughout the pancreas at E18.5 from a minimum of four embryos per genotype. The average cell number was determined from all sections counted. After immunohistochemistry, pancreatic epithelial areas were outlined and measured with the OpenLab software. The values of cell counting were normalized to tissue area. Quantification of proliferating β-cells was performed on paraffin wax-embedded sections by counting of insulin and insulin/Ki-67 double-positive cells. The cells from every tenth section were counted throughout the pancreas at E18.5 from three embryos per genotype.
For the quantification of insulin cells at E15.5, five sections of pancreatic tissue from two Dach1+/+ and from two Dach1−/− animals were stained for insulin. The insulin-positive cells were counted and the obtained numbers were normalized to the pancreatic tissue area. The tissue area was determined using ImageJ software (NIH).
For the quantification of p27Kip1, eight sections of pancreatic tissue from four Dach1+/+ and from four Dach1−/− animals (E18.5) were co-stained for p27Kip1 and insulin. The staining was visualized using a Zeiss confocal microscope (LSM 510Meta). The intensity of p27Kip1 staining in insulin-positive cells was quantified using ImageJ software. p27Kip1 was quantified in 50 insulin-positive cells per animal (a total number of 200 cells was quantified per condition). The intensity (pixels) of p27Kip1 staining in insulin-positive cells was normalized to the nuclear area.
Chromatin immunoprecipitation assays
Mouse βTC3 cells were grown to 50–60% confluence; and cross-linking, chromatin preparation, immunoprecipitation and PCR were performed as previously described (Lynn et al., 2007) using 100 µg of chromatin and 2.5 µg normal rabbit IgG (sc-2027; Santa Cruz Biotechnology) or rabbit anti-Dach1 antibody (Proteintech Group Inc., Chicago, IL). The PCR primers for the Cdkn1b gene promoter (p27Kip1) located at −1.6 to −1.3 kb and coding sequence at +2.0 to +2.3 kb have been described previously (Li et al., 2002). The PCR primers for the Cdkn1c gene promoter (p57Kip2) were located at −1.5 kb (5’-ACACAGGGACAGAACAAAGC-3’) to −1.3 kb (5’-TCAAGTCAAACCCTGAAGCC-3’).
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the German and Stainier laboratories and Gerold Grodsky for helpful advice and criticism, Takeshi Miyatsuka and Greg Ku for the preparation of the MIP-GFP cDNA. This work was supported by NIH grants R01 DK21344 (M.S.G.), R01 DK075032 (D.Y.S.), U19 DK61245 (M.S.G. and D.Y.S.), and cores from P30 DK063720, Larry L. Hillblom Foundation grants 2002/1E and 2007/1B, American Diabetes Association Grant 7-07-MN-21, a Kraft Family Stem Cell Fellowship (AM) and Juvenile Diabetes Research Foundation Postdoctoral Fellowship 3-2005-902 (BAA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTIONS
A.K. and M.S.G. initiated the project; A.K., A.M., B.A.A. and M.S.G. designed the experiments; A.K., A.M., B.A.A. and N.N. performed the research; A.K., A.M., B.A.A., N.N. and M.S.G. analyzed the data; and A.K., A.M., B.A.A. and M.S.G. wrote the paper.
REFERENCES
- Argenton F, Zecchin E, Bortolussi M. Early appearance of pancreatic hormone-expressing cells in the zebrafish embryo. Mech Dev. 1999;87:217–221. doi: 10.1016/s0925-4773(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Backman M, Machon O, Van Den Bout CJ, Krauss S. Targeted disruption of mouse Dach1 results in postnatal lethality. Dev Dyn. 2003;226:139–144. doi: 10.1002/dvdy.10210. [DOI] [PubMed] [Google Scholar]
- Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Developmental Biology. 2001;230:189–203. doi: 10.1006/dbio.2000.0103. [DOI] [PubMed] [Google Scholar]
- Caubit X, Thangarajah R, Theil T, Wirth J, Nothwang HG, Ruther U, Krauss S. Mouse Dac, a novel nuclear factor with homology to Drosophila dachshund shows a dynamic expression in the neural crest, the eye, the neocortex, and the limb bud. Dev Dyn. 1999;214:66–80. doi: 10.1002/(SICI)1097-0177(199901)214:1<66::AID-DVDY7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cordes SP. Molecular genetics of the early development of hindbrain serotonergic neurons. Clin Genet. 2005;68:487–494. doi: 10.1111/j.1399-0004.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF. Molecular control of cell cycle progression in the pancreatic β-cell. Endocr Rev. 2006;27:356–370. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Shen W, Heanue TA, Mardon G. Mouse Dach, a homologue of Drosophila dachshund, is expressed in the developing retina, brain and limbs. Dev Genes Evol. 1999;209:526–536. doi: 10.1007/s004270050285. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Shen W, Sandler YI, Amoui M, Purcell P, Maas R, Ou CN, Vogel H, Beaudet AL, Mardon G. Dach1 mutant mice bear no gross abnormalities in eye, limb, and brain development and exhibit postnatal lethality. Mol Cell Biol. 2001a;21:1484–1490. doi: 10.1128/MCB.21.5.1484-1490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ, Shen W, Sandler YI, Heanue TA, Mardon G. Characterization of mouse Dach2, a homologue of Drosophila dachshund. Mech Dev. 2001b;102:169–179. doi: 10.1016/s0925-4773(01)00307-0. [DOI] [PubMed] [Google Scholar]
- Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994;269:8445–8454. [PubMed] [Google Scholar]
- Devos N, Deflorian G, Biemar F, Bortolussi M, Martial JA, Peers B, Argenton F. Differential expression of two somatostatin genes during zebrafish embryonic development. Mech Dev. 2002;115:133–137. doi: 10.1016/s0925-4773(02)00082-5. [DOI] [PubMed] [Google Scholar]
- Efrat S, Linde S, Kofod H, Spector D, Delannoy M, Grant S, Hanahan D, Baekkeskov S. β-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc. Natl. Acad. Sci USA. 1988;85:9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol. 2003;261:197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of β-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44:249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- Gasa R, Mrejen C, Leachman N, Otten M, Barnes M, Wang J, Chakrabarti S, Mirmira R, German M. Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. Proc Natl Acad Sci U S A. 2004;101:13245–13250. doi: 10.1073/pnas.0405301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasa R, Mrejen C, Lynn FC, Skewes-Cox P, Sanchez L, Yang KY, Lin CH, Gomis R, German MS. Induction of pancreatic islet cell differentiation by the neurogenin-neuroD cascade. Differentiation. 2008;76:381–391. doi: 10.1111/j.1432-0436.2007.00228.x. [DOI] [PubMed] [Google Scholar]
- Gehring WJ. Historical perspective on the development and evolution of eyes and photoreceptors. Int J Dev Biol. 2004;48:707–717. doi: 10.1387/ijdb.041900wg. [DOI] [PubMed] [Google Scholar]
- Georgia S, Bhushan A. b cell replication is the primary mechanism for maintaining postnatal b cell mass. J Clin Invest. 2004;114:963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgia S, Bhushan A. p27 Regulates the transition of β-cells from quiescence to proliferation. Diabetes. 2006;55:2950–2956. doi: 10.2337/db06-0249. [DOI] [PubMed] [Google Scholar]
- Georgia S, Soliz R, Li M, Zhang P, Bhushan A. p57 and Hes1 coordinate cell cycle exit with self-renewal of pancreatic progenitors. Dev Biol. 2006;298:22–31. doi: 10.1016/j.ydbio.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Hammond KL, Hanson IM, Brown AG, Lettice LA, Hill RE. Mammalian and Drosophila dachshund genes are related to the Ski proto-oncogene and are expressed in eye and limb. Mech Dev. 1998;74:121–131. doi: 10.1016/s0925-4773(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Hammond KL, Hill RE, Whitfield TT, Currie PD. Isolation of three zebrafish dachshund homologues and their expression in sensory organs, the central nervous system and pectoral fin buds. Mech Dev. 2002;112:183–189. doi: 10.1016/s0925-4773(01)00637-2. [DOI] [PubMed] [Google Scholar]
- Hanson IM. Mammalian homologues of the Drosophila eye specification genes. Semin Cell Dev Biol. 2001;12:475–484. doi: 10.1006/scdb.2001.0271. [DOI] [PubMed] [Google Scholar]
- Hauptmann G, Gerster T. Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 1994;10:266. doi: 10.1016/0168-9525(90)90008-t. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Reshef R, Davis RJ, Mardon G, Oliver G, Tomarev S, Lassar AB, Tabin CJ. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 1999;13:3231–3243. doi: 10.1101/gad.13.24.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans Y, Van De Casteele M, in't Veld P, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol. 2002;159:303–312. doi: 10.1083/jcb.200203074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Watanabe Y, Ohto H, Kawakami K. Molecular interaction and synergistic activation of a promoter by Six, Eya, and Dach proteins mediated through CREB binding protein. Mol Cell Biol. 2002;22:6759–6766. doi: 10.1128/MCB.22.19.6759-6766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and β-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Juhl K, Sarkar SA, Wong R, Jensen J, Hutton JC. The mouse pancreatic endocrine cell transcriptome defined in the embryonic Ngn3 null mouse. Diabetes. 2008 doi: 10.2337/db07-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev. 2002;12:540–547. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kim SS, Zhang RG, Braunstein SE, Joachimiak A, Cvekl A, Hegde RS. Structure of the retinal determination protein Dachshund reveals a DNA binding motif. Structure. 2002;10:787–795. doi: 10.1016/s0969-2126(02)00769-4. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kozmik Z, Pfeffer P, Kralova J, Paces J, Paces V, Kalousova A, Cvekl A. Molecular cloning and expression of the human and mouse homologues of the Drosophila dachshund gene. Dev Genes Evol. 1999;209:537–545. doi: 10.1007/s004270050286. [DOI] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci. 2007;27:13624–13634. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF. Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol Cell Biol. 2005;25:3752–3762. doi: 10.1128/MCB.25.9.3752-3762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers SJ. Growth control: invertebrate insulin surprises! Curr Biol. 2001;11:R209–R212. doi: 10.1016/s0960-9822(01)00107-5. [DOI] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–1183. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci U S A. 2007;104:10500–10505. doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- Milewski WM, Duguay SJ, Chan SJ, Steiner DF. Conservation of PDX1 structure, function, and expression in zebrafish. Endocrinology. 1998;139:1440–1449. doi: 10.1210/endo.139.3.5768. [DOI] [PubMed] [Google Scholar]
- Miyatsuka TM, Li Z, German MS. Chronology of islet differentiation revealed by temporal cell labeling. Diabetes. 2009;58:1863–1868. doi: 10.2337/db09-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh LC. Pancreas and β-cell development: from the actual to the possible. Development. 2007;134:427–438. doi: 10.1242/dev.02770. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Petri A, Ahnfelt-Ronne J, Frederiksen KS, Edwards DG, Madsen D, Serup P, Fleckner J, Heller RS. The effect of neurogenin3 deficiency on pancreatic gene expression in embryonic mice. J Mol Endocrinol. 2006;37:301–316. doi: 10.1677/jme.1.02096. [DOI] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in b-islet cell hyperplasia. Nat Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of β-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Skorokhod A, Gamulin V, Gundacker D, Kavsan V, Muller IM, Muller WE. Origin of insulin receptor-like tyrosine kinases in marine sponges. Biol Bull. 1999;197:198–206. doi: 10.2307/1542615. [DOI] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic b cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Treff NR, Vincent RK, Budde ML, Browning VL, Magliocca JF, Kapur V, Odorico JS. Differentiation of embryonic stem cells conditionally expressing neurogenin 3. Stem Cells. 2006;24:2529–2537. doi: 10.1634/stemcells.2006-0082. [DOI] [PubMed] [Google Scholar]
- Wang S, Tulina N, Carlin DL, Rulifson EJ. The origin of islet-like cells in Drosophila identifies parallels to the vertebrate endocrine axis. Proc Natl Acad Sci U S A. 2007;104:19873–19878. doi: 10.1073/pnas.0707465104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: Guide for the Laboratory Use of Zebrafish (Danio Rerio) third ed 1995. [Google Scholar]
- White P, May CL, Lamounier RN, Brestelli JE, Kaestner KH. Defining pancreatic endocrine precursors and their descendants. Diabetes. 2008;57:654–668. doi: 10.2337/db07-1362. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Kalamaras JA, German MS. Expression pattern of IAPP and prohormone convertase 1/3 reveals a distinctive set of endocrine cells in the embryonic pancreas. Mech Dev. 2002;115:171–176. doi: 10.1016/s0925-4773(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mech Dev. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hanahan D. Murine pancreatic ductal adenocarcinoma produced by in vitro transduction of polyoma middle T oncogene into the islets of Langerhans. American Journal of Pathology. 1994;145:671–684. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.