Abstract
Since the role of dopamine in human emotional processing is unclear, we used a dynamic molecular imaging technique to examine whether striatal dopamine is released during processing of negative emotions in healthy volunteers. After volunteers have received an intravenous injection of a dopamine receptor ligand 11C-raclopride, they were asked to perform a task that elicited negative emotions. During task performance the ligand concentration was measured dynamically using a positron emission tomography camera. Analysis of the data indicated that the task performance is associated with dopamine release in the head of caudate and in the dorsal putamen bilaterally.
Keywords: emotion, dopamine, striatum, caudate, putamen, raclopride, molecular imaging, PET, negative emotion
We have earlier observed that dopamine is released in a number of brain areas outside the striatum during processing of negative emotions in healthy volunteers [1]. Because of methodological constraints (described later), dopamine released in the striatum could not be detected in this study. As a result, an important aspect of dopaminergic control of human emotion remains uninvestigated. Evidence from both, animal and human experiments suggest that striatal dopamine is critically involved in emotional processing. Neuroimaging studies have reported increased activation both in the dorsal [2] and ventral [3,4] striatum and in animals, changes in emotional responses are observed after activation of the striatal dopaminergic system [5].
Disrupted emotional processing in Parkinson’s [6] patients also indicate involvement of striatal dopamine. Because of this involvement activations are observed in the basal ganglia of healthy volunteers during emotional processing [2,7]. Animal experiments provide stronger evidence of striatal dopaminergic processing. Thus, it was shown that electrical stimulation of the ventral tegmental nucleus increases dopamine release and produces fear reactions in cats [8]. A neurotoxic lesion in this area causes attenuation of fear responses [9]. Further, conditioned fear responses are disrupted when dopaminergic neurons of the nucleus accumbens (NAc) are inactivated [5] and microinjection of a dopaminergic agent in the rat NAc elicits the 50 kHz ultrasonic vocalization, which is a measure of pleasure in these animals [10].
Because evidence of dopamine processing came mostly from animal experiments that used conditioned responses, validity of these data in human is controversial [11] because human emotions are dependent more on cultural and cognitive inputs [12] rather than conditioning [11]. it is possible that the human brain uses a different processing strategy than laboratory animals. Probably because of this difference, activation in the ventral striatum is not observed in healthy volunteers during processing of negative emotions [2,7], even though involvement of this area in animals has been demonstrated in a number of experiments [8]. There is therefore, a need to examine the role of striatal dopamine in the processing of human emotions.
In this experiment we used a dynamic molecular imaging technique to detect, map and measure striatal dopamine released during processing of negative emotions in healthy volunteers. Previously, this technique was used to detect dopamine released in the striatum during performance of a number of cognitive and behavioral tasks [13-16]. Using a variant of this technique, we recently detected dopamine released outside the striatum during emotional processing [1]. In the variant technique task-induced release of dopamine was detected using a high affinity dopamine receptor ligand 18F-fallypride. Because of its high affinity, this ligand is ideal for detection of dopamine released outside the striatum where receptor density is low. It however, takes several hours to bind to the receptors in high receptor-density areas (the striatum). Therefore, we could not detect striatal dopamine released during emotional processing in this experiment [1]. In the current experiment instead of 18F-fallypride, a relatively low affinity ligand 11C-raclopride was used. This ligand binds to the striatal receptors within a few minutes. However, in the low receptor density areas outside the striatum, 11C-raclopride does not bind in detectable quantity. This ligand, therefore is used for detection of striatal dopamine released in response to pharmacological and cognitive challenges [13-16]. In this experiment the ligand 11C-raclopride and a receptor kinetic model [17] was used to detect, map, and measure striatal dopamine released during processing of negative emotions in healthy volunteers.
Materials and Methods
The study was conducted on healthy right-handed native English speaking volunteers (male=4, female 4; mean age 27.7 years) who had no history of a psychiatric or neurological disorder. The study protocol and consent forms were approved by the IRB of Massachusetts General Hospital (Partner’s Healthcare), Boston.
Dopamine released in the striatum during emotional processing was detected and mapped using a dynamic molecular imaging technique. In this technique a radio labeled ligand is administered prior to task performance. The ligand concentration is dynamically measured during the experiment using a positron emission tomography (PET) camera to estimate the rate of its displacement from dopamine receptor sites. Because the ligand competes with endogenous dopamine for occupancy of receptor sites, it is displaced at a higher rate in the areas where dopamine is released during task performance. The scan procedures used in this experiment followed the protocol described in our earlier publications [1,14-17] and outlined briefly in the following paragraphs.
After volunteers were positioned in the PET camera, their heads were fixed using an air-lock pillow. A neuroshield was placed between the head and the body to reduce detection of scattered photons. Thereafter, each volunteer received an intravenous bolus (10-15 mCi; mean 13.73 mCi) of a dopamine receptor ligand 11C-raclopride at a high specific activity (mean specific activity 1114 mCi/micromole). Immediately after the injection, an emotional task (described below) and the PET data acquisition started.
The emotional task was similar to the one we used to study extrastriatal dopamine in an earlier experiment [1]. It consisted of a Control and a Test condition. In the Control volunteers were shown a list of emotionally neutral words (e.g., PARK, PENCIL) and asked to indicate the intensity of emotion elicited by each word in a scale of 1-3 (1=no emotion; 3=intense emotional arousal). At 25 min after the ligand injection, unbeknownst to volunteers, task was changed from Control to Test condition. In the Test condition neutral words were replaced by negative emotional words (e.g., FIRE, BLOOD). In both conditions stimuli were presented for 4500 msec. It was followed by a cross-mark (500 msec). A total of 200 neutral and an equal number of emotional words were used in the experiment. During the experiment ligand concentration was measured in 30 sec epochs in the first 5 min and then at 60 sec epochs, using an ECAT EXACT HR+ PET camera operating in 3D mode.
The dynamic molecular imaging technique used in this experiment exploited the competition between the ligand 11C-raclopride and endogenous dopamine for occupancy of the same receptor binding sites. Because of this competition, dopamine released during task performance displaced the ligand from receptor sites. The rate of ligand displacement therefore increased in the brain areas where dopamine was released during task performance. We estimated the rate of displacement by dynamically measuring ligand concentration and applying the data to a receptor kinetic model developed for this purpose [17].
PET Data Analyses
Procedures used for analyses of PET data were essentially similar to those used in earlier studies [1,14-17]. Briefly, the images were reconstructed as 128×128×63 element volumes using a standard three-dimensional filtered back projection algorithm with corrections for photon attenuation, random coincidences, scatter and dead time. Thereafter, images were registered to align each frame to a common orientation, using the following procedure: First, all frames were smoothed with a 5 mm FWHM Gaussian filter, then variation in spatiotemporal distribution was corrected by registration of temporally adjacent frames and finally, using a transformation matrix all frames were aligned to a reference frame. Thereafter, a voxel-wise analysis of data was carried out on each subject using a kinetic model that was developed to detect transient changes in ligand displacement. Using this model, quantitative maps of kinetic parameters were generated for each volunteer. These data were then pooled to acquire cohort means and variances. The pooling involved elastic registration of the sum of image data of each subject to a standard template, using statistical parametric mapping software, SPM99 [18]. All images were normalized to stereotactic template. This transformation was applied to the entire dynamic sequence to allow further analysis in stereotactic space. A voxel-wise t-map was then computed to localize voxels where the rate of ligand displacement increased significantly after task initiation. Finally, time activity curves were drawn for the voxels showing maximum ligand displacement. The cerebellum was used as a reference region (because of paucity of dopamine receptors), and a time activity curve for this region was also drawn to estimate clearance rate of free and nonspecifically bound ligand.
Results
Emotional words presented in the Test condition elicited significantly (p<0.001) stronger emotion than the words shown in the Control. Mean emotional ratings (1=no emotion; 3=intense emotional arousal) in the Control and Test conditions were 1.29±0.19 and 2.15±0.52 respectively. There was no significant difference in the mean response time in the Control condition (1654±207 msec) and Test (1581±190 msec). Questionnaires filled in the debriefing confirmed that the words in the Test condition elicited negative emotions and that there was a perceptible difference in state of emotional arousal in the Control and Test condition.
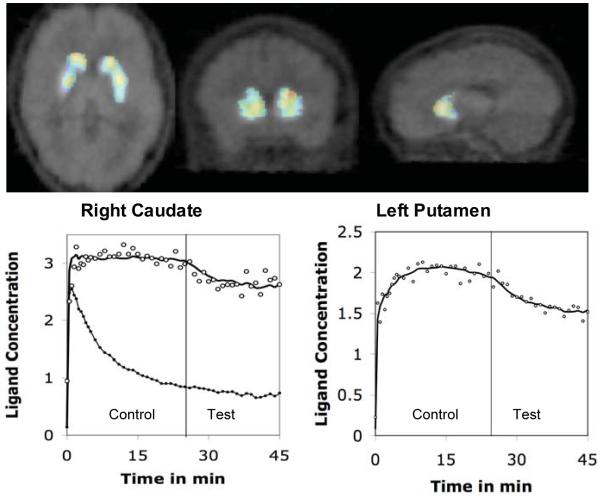
The PET data were analyzed using linear extension of simplified reference region model [17]. Using this model we computed t-values of the difference in the rate before and after task initiation. The rate increased significantly in a number of striatal areas. The group mean of t-values pooled across volunteers was significant (t>3.0) bilaterally in the head of caudate and in the middle of dorsal putamen, indicating dopamine release in these areas during task performance. The maximum t-values measured in a voxel in the ‘activated’ areas were 3.43 and 4.03 in the left and right caudate; and 4.03 and 3.6 in the left and right putamen respectively. Approximate Talairach coordinates of the voxels that had maximum t-values were (x,y,z) 10, 16, 6 and −12, 15, 10 for the caudate and 17, 8, −4 and −22, −4, 6 for the putamen activation (Figure 1). We also estimated the ligand binding potential (BP) during the experiment at each of the activated voxels. The mean BP estimated in the right and left caudate were 2.3 and 2.2 respectively. It was 1.8 in the right putamen and 1.9 in the left putamen. We did not find significant task-induced changes in the rate of ligand displacement in the reference region – cerebellum. This indicates that the changes observed in the striatum were due to the ligand displacement elicited by task-induces release of striatal dopamine.
FIGURE 1.
t-map showing striatal areas where the rate of ligand (11C-raclopride) displacement increased significantly (t>3) after initiation of a task that elicited negative emotion (vertical line). The time-activity curves show the concentration history and least square fits for the ligand in activated regions of the right caudate and left putamen. The lower curve in the left panel represents concentration history in the reference region (cerebellum) where the rate of ligand displacement did not change significantly after task initiation.
Discussion
The results of this experiment complement and extend our earlier observation of increased dopamine release in the amygdala, prefrontal cortex and medial temporal lobe during processing of negative emotions in healthy volunteers [1]. The findings of dopamine release in the dorsal striatum (caudate and putamen) is consistent with the observation of increased regional cerebral blood flow in this areas during processing of self generated negative emotion of sadness [2] and transiently elicited sadness [7]. It appears that the increased blood flow observed in these experiments was due to increased activity of the striatal dopamine system.
Since dopamine release in the mid-dorsal putamen is associated primarily with motor planning [14-16], its activation in the current experiment could be related to the planning and execution of emotional motor response (e.g., facial, autonomic). Dopamine of the caudate is associated with processing of motor memory [14,15] and reward [13] in human and with encoding of fear related stimuli [19] in animals. Since memories of past experiences and reward potentials of stimuli determine their affective values, the caudate activation observed in this experiment could be associated with the assignment of these values. The dopamine system of the caudate is uniquely suited for this role because it receives input from the orbitofrontal cortex where the valence of emotional stimuli are processed [20].
In animals dopamine of the dorsal striatum has limited role in emotional processing. Most animal studies have implicated the ventral striatum. Thus, stimulation of the ventral tegmental nucleus elicits fear response in cats and a neurotoxic lesion in this area attenuates fear [9]. Similalry, inactivation of the nucleus accumbens impairs conditioned fear response [5]. In animals nucleus accumbens is involved in the processing the positive emotions also. Thus, Enhanced dopamine efflux from this nucleus is observed when animals receive food reward [21] and intranuclear injection of a dopaminergic agent in the rat elicits the 50 kHz ultrasonic vocalization, which is a measure of pleasure in these animals [10].
In the current experiment dopamine was released in the dorsal striatum, not in the ventral striatum. It suggests that dopamine processing of human and animal emotions are not identical. While in animals both positive and negative emotions are processed in the ventral striatum, human striatum appears to make a distinction between the two types of emotion. The ventral striatum processes only positive emotions and negative emotions are processed in the dorsal striatum. This distinction is consistent with the observations of neuroimaging experiments. These experiments have reported ventral striatal activation during presentation of pleasant music [3], positive words [4], and financial reward [22]. In contrast, negative emotional stimuli do not activate the ventral striatum. Most of these stimuli activate dorsal striatal structures (mostly the caudate). Thus, dorsal striatum is activated during self generated sadness [2], transiently elicited sadness [7] and financial penalties [22].
It is therefore not surprising that negative emotional stimuli did not release dopamine in the ventral striatum in this experiment. This finding is consistent with clinical observation in early stage Parkinson’s patients who have difficulty processing the negative but not positive emotions [6]. Since dopamine is depleted only in the dorsal striatum in early stages it suggests that negative emotions are processed in the dorsal striatum. This discussion on differences in the activity patterns in the human and animals brains assumes that the reliability of human data acquired using neuroimaging and molecular imaging techniques is comparable to the animal data obtained using more invasive procedures.
The findings of this experiment extend and complement our earlier observation of increased dopamine release in the amygdala, prefrontal and medial temporal areas during processing of negative emotions [1]. Taken together, it appears that the dopamine system of these extrastriatal structures communicate with the striatal system during emotional processing. Anatomically, these structures are connected not only by the dopaminergic pathways that project to the striatum (nigrostriatal), amygdala, hippocampus (mesolimbic), and prefrontal cortex (mesocortical); but also by direct neural connections. Thus, amygdala is connected to the dorsal striatum by amygdalo-striatal pathway that originates in the central nucleus of amygdala and via synaptic relay in the substantia nigra pars compacta, terminates in the striatum [23]. The striatum and prefrontal cortex are connected via dopaminergic striatofrontal projections. Further, the evidence suggests that the hippocampus, striatum and amygdala are interconnected via dopamine dependent neural connections [24].
Conclusion
The results of this experiment indicate that the dorsal striatal dopamine system is involved in the processing of negative human emotions. This is in contrast to the findings in animals in which the ventral striatal dopamine system processes both, the negative and positive emotions. Human and animal emotions are therefore, processed differently by the dopamine system. These findings suggest that impaired emotional processing observed in psychiatric (e.g., PTSD, schizophrenia) and neuropsychiatric (e.g. Parkinson’s disease) conditions could be due to dysregulated dopamine neurotransmission.
Acknowledgements
Grant support: NIH (1R21MH073624 and 1R21MH079435), Dana Foundation, and Shriners Hospital for Children.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Badgaiyan R, Fischman A, Alpert N. Dopamine release during human emotional processing. Neuroimage. 2009;47:2041–2045. doi: 10.1016/j.neuroimage.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- [3].Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13:15–19. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- [5].Haralambous T, Westbrook RF. An infusion of bupivacaine into the nucleus accumbens disrupts the acquisition but not the expression of contextual fear conditioning. Behav Neurosci. 1999;113:925–940. doi: 10.1037//0735-7044.113.5.925. [DOI] [PubMed] [Google Scholar]
- [6].Dara C, Monetta L, Pell MD. Vocal emotion processing in Parkinson’s disease: reduced sensitivity to negative emotions. Brain Res. 2008;1188:100–111. doi: 10.1016/j.brainres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- [7].George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- [8].Levita L, Dalley JW, Robbins TW. Nucleus accumbens dopamine and learned fear revisited: a review and some new findings. Behav Brain Res. 2002;137:115–127. doi: 10.1016/s0166-4328(02)00287-5. [DOI] [PubMed] [Google Scholar]
- [9].Levita L, Dalley JW, Robbins TW. Disruption of Pavlovian contextual conditioning by excitotoxic lesions of the nucleus accumbens core. Behav Neurosci. 2002;116:539–552. doi: 10.1037//0735-7044.116.4.539. [DOI] [PubMed] [Google Scholar]
- [10].Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- [11].Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neurosci Biobehav Rev. 2006;30:173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- [12].Paul ES, Harding EJ, Mendl M. Measuring emotional processes in animals: the utility of a cognitive approach. Neurosci Biobehav Rev. 2005;29:469–491. doi: 10.1016/j.neubiorev.2005.01.002. [DOI] [PubMed] [Google Scholar]
- [13].Pappata S, Dehaene S, Poline JB, Gregoire MC, Jobert A, Delforge J, et al. In vivo detection of striatal dopamine release during reward: a PET study with [(11)C]raclopride and a single dynamic scan approach. NeuroImage. 2002;16:1015–1027. doi: 10.1006/nimg.2002.1121. [DOI] [PubMed] [Google Scholar]
- [14].Badgaiyan RD, Fischman AJ, Alpert NM. Explicit Motor Memory Activates the Striatal Dopamine System. NeuroReport. 2008;19:409–412. doi: 10.1097/WNR.0b013e3282f6435f. [DOI] [PubMed] [Google Scholar]
- [15].Badgaiyan RD, Fischman AJ, Alpert NM. Striatal dopamine release in sequential learning. NeuroImage. 2007;38:549–556. doi: 10.1016/j.neuroimage.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Badgaiyan RD, Fischman AJ, Alpert NM. Striatal dopamine release during unrewarded motor task in human volunteers. Neuroreport. 2003;14:1421–1424. doi: 10.1097/00001756-200308060-00003. [DOI] [PubMed] [Google Scholar]
- [17].Alpert NM, Badgaiyan RD, Livini E, Fischman AJ. A novel method for noninvasive detection of neuromodulatory changes in specific neurotransmitter systems. NeuroImage. 2003;19:1049–1060. doi: 10.1016/s1053-8119(03)00186-1. [DOI] [PubMed] [Google Scholar]
- [18].Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- [19].White NM. Effect of nigrostriatal dopamine depletion on the post-training, memory-improving action of amphetamine. Life Sci. 1988;43:7–12. doi: 10.1016/0024-3205(88)90230-5. [DOI] [PubMed] [Google Scholar]
- [20].Smith AP, Henson RN, Dolan RJ, Rugg MD. fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage. 2004;22:868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- [21].Ahn S, Phillips AG. Dopamine efflux in the nucleus accumbens during within-session extinction, outcome-dependent, and habit-based instrumental responding for food reward. Psychopharmacology (Berl) 2007;191:641–651. doi: 10.1007/s00213-006-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferreira TL, Shammah-Lagnado SJ, Bueno OF, Moreira KM, Fornari RV, Oliveira MG. The indirect amygdala-dorsal striatum pathway mediates conditioned freezing: insights on emotional memory networks. Neuroscience. 2008;153:84–94. doi: 10.1016/j.neuroscience.2008.02.013. [DOI] [PubMed] [Google Scholar]
- [24].Okubo Y, Olsson H, Ito H, Lofti M, Suhara T, Halldin C, et al. PET mapping of extrastriatal D2-like dopamine receptors in the human brain using an anatomic standardization technique and [11C]FLB 457. Neuroimage. 1999;10:666–674. doi: 10.1006/nimg.1999.0502. [DOI] [PubMed] [Google Scholar]