Abstract
Trastuzumab mediates the lysis of HER2-expressing breast cancer cell lines by interleukin-2 (IL-2) primed natural killer (NK) cells. We hypothesized that IL-2 would augment the anti-tumor effects of trastuzumab in MBC in patients who had progressed on or within 12 months of receiving a trastuzumab-containing regimen. Secondary objectives were to measure antibody-directed cellular cytotoxicity (ADCC) against HER2 over-expressing target cells, and to measure serum cytokines. Patients received trastuzumab (4 mg/kg intravenously (IV)) every 2 weeks in combination with daily low-dose IL-2 (1 million IU/m2 subcutaneously (SC)) and pulsed intermediate-dose IL-2 (12 million IU/m2 SC). Samples were analyzed for NK cell expansion and ADCC against a HER2-positive breast cancer cell line. In addition, interferon-gamma (IFN-γ), mRNA expression in peripheral blood mononuclear cells (PBMC) and the following serum cytokines were measured: IFN-γ, monokine-induced by IFN-γ (MIG), and interferon-inducible protein ten (IP-10). The median number of treatment cycles was four (range 1–23) and the treatment was well tolerated. There were no objective responses. NK cells were not expanded and ADCC was not enhanced. Eight (62%) patients had a twofold or higher increase in mRNA transcript for IFN-γ, two (15%) patients had elevated serum levels of IFN-γ and 12 (92%) had increases angiogenic MIG and IP-10. In trastuzumab-refractory patients adding IL-2 did not produce responses and did not result in NK cell expansion. However, these patients had the ability to respond to IL-2 as evidenced by increases in IFN-γ transcripts and chemokines. The lack of NK cell expansion may explain the absence of clinical benefit.
Keywords: Trastuzumab, Her-2/neu, Interleukin-2, Breast cancer, Clinical trial
Introduction
The HER2 oncogene (also known as ErbB-2) encodes a cell surface protein with tyrosine kinase activity that confers enhanced growth characteristics when overexpressed in human cancers. In breast cancer patients, overexpression of HER2 is an independent adverse prognostic factor for disease-free and overall survival in the absence of trastuzumab, and a predictive factor for benefit from trastuzumab [1]. In 2001 Slamon et al. published the first randomized trial establishing the benefit of trastuzumab and chemotherapy in HER2 overexpressing MBC patients; this benefit extends to the adjuvant setting with improvements in disease-free and overall survival for patients with localized HER2 overexpressing breast cancers [2, 3].
One of the proposed mechanisms of trastuzumab is the generation of ADCC [4–8], and work from our laboratory demonstrates that IL-2, as well as IL-12 and IL-21, augments NK-cell-mediated ADCC against breast cancer cells coated with trastuzumab [9–11]. Phase I clinical trials of trastuzumab and IL-2 in HER2 overexpressing breast cancer patients showed acceptable side-effects, anti-tumor responses, NK cell expansion, and trastuzumab-mediated ADCC [12, 13]. The present trial was designed to estimate the response rate and side effects to trastuzumab and IL-2 in MBC patients who had previously progressed on or within 12 months of receiving a trastuzumab-containing regimen. The trial was designed to test the following hypothesis: if responses were observed and correlated with ADCC or cytokine expression in trastuzumab-refractory patients, then this would support an immune-mediated mechanism of relevance to trastuzumab in the clinic.
PTS and methods
A multi-institutional CTEP-sponsored phase II trial of trastuzumab and IL-2 was conducted at The Ohio State University (OSU) Comprehensive Cancer Center, the University of Chicago, the University of Pittsburgh, and Dartmouth University. Patients with documented HER2 overexpressing metastatic breast cancer (2+ or 3+ by the DAKO HercepTest) and the following characteristics were eligible: age ≥18 years; ≤2 prior chemotherapy regimens for metastatic disease; disease progression on or within 12 months of receiving a trastuzumab-containing regimen; measurable disease by RECIST criteria; ECOG performance status 0–2; adequate organ and bone marrow function; left ventricular ejection fraction ≥institutional lower limit of normal; total cumulative doxorubicin dose was ≤360 mg/m2; ≥3 months since treatment of any CNS metastases; and signed informed consent in accordance with federal and institutional guidelines.
Patients were excluded from enrollment for the following reasons: prior or active congestive heart failure or ischemic heart disease; concurrent use of immunosuppressive drugs; underlying immunodeficiency; concurrent active malignancy other than cervical cancer in situ; and any other medical condition deemed by the treating physician to preclude safe participation.
Treatment regimen and toxicity assessment
Cycle 1 was 21 days in length and subsequent cycles were 14 days in length. In cycle 1 trastuzumab 4 mg/kg IV was given over 90 min on day 1 and over 30 min on day 8. In subsequent cycles, trastuzumab was administered at 4 mg/kg IV on day 1. Low-dose IL-2 at 1 million IU/m2 (for NK cell expansion) was administered SC on days 2–7 and days 12–21 of cycle 1. In subsequent cycles, low-dose IL-2 was administered on days 4–14. Intermediate-dose IL-2 at 12 million IU/m2 (for NK cell activation) was administered SC on days 9–11 of cycle 1, and in subsequent cycles on days 1–3 (Fig. 1). Patients were issued pre-filled syringes containing IL-2 for self-administration. Patients continued on therapy until disease progression. Toxicities were assessed by the NCI Common Toxicity Criteria version 2.0 prior to study registration, on days 1 and 8 of cycle 1, and on day 1 of subsequent cycles. Low and intermediate dose IL-2 were held for the development of grade 3, 4 toxicity until it resolved to grade 1 and subsequent doses were reduced by 25%.
Fig. 1.
Treatment schema
Assessment of disease response
Patients underwent imaging studies pre-study and every four treatment cycles. Response to treatment was assessed using RECIST criteria [14].
Procurement of patient serum and peripheral blood mononuclear cells
Blood for correlative studies was drawn at baseline and on the first and third day cycles 2 and 4. Serum and peripheral blood mononuclear cells (PBMCs) were procured by Ficoll density gradient centrifugation (Sigma–Aldrich, St. Louis, MO) within 6 h of phlebotomy. Serum was snap-frozen and stored at −80°C. Half of the PBMCs obtained from each sample were cryopreserved and stored at −134°C in liquid nitrogen. The remaining half of the PBMCs was processed for total RNA. Cell viability after thawing was routinely greater than 90%. Identification of NK cell expansion and activation was by previously established methods [12].
Reverse transcriptase real-time PCR
Real-time reverse transcriptase PCR (RT-PCR) was used to determine the relative increase in IFN-γ transcript following administration of IL-2. Total cellular RNA was isolated from patient PBMCs that had been processed with RNA STAT-60 solution (Tel-Test Inc., Friendswood, TX). The RNA was quantitated, and cDNA was generated from 3 μg of RNA with random hexamers and MMLV-RT according to the manufacturer’s recommendations (Gibco Life Technologies, Rockville, MD). Using the cDNA as template, RT-PCR for IFN-γ transcript was performed with primer and probe sets specific for the cytokine transcript and a β-actin internal control (PE Applied Biosystems, Foster City, CA). Data were analyzed according to the comparative CT method and normalized against the β-actintranscript as previously described [10]. Results are semi-quantitative and represent the fold difference in transcript levels in a particular sample as compared to the levels in that patient’s pre-treatment (or baseline) sample.
Cytokine enzyme-linked immunosorbent assays
Serum samples were thawed on ice and analyzed in triplicate for levels of cytokine IFN-γ and the anti-angiogenic chemokines IP-10 and MIG by enzyme-linked immunosorbent assay (ELISA) using commercially available monoclonal antibody pairs (Endogen Inc., Woburn, MA). On the basis of the manufacturer’s guidelines, a standard sandwich ELISA for each human cytokine was developed, and cytokine concentrations were determined by the use of standard curve regression analysis. The lower limit of detection for all ELISAs was 10 pg/ml.
Antibody-dependent cellular cytotoxicity (ADCC) assays
Frozen patient PBMCs were thawed, enumerated, and plated in 96-well V-bottom plates in culture medium supplemented with 0.1 nM recombinant human IL-2 and incubated overnight at 37°C. Trastuzumab-labeled HER2 overexpressing SKBR3 cells (or polyclonal IgG as a negative control) were added to the PBMCs at various effector:target ratios for 4 h incubation at 37°C. Supernatants were harvested for quantification of chromium release. Maximum and minimum releases were determined in 5% SDS, respectively. Percent specific lysis was calculated as previously described [9].
Statistical methods
Response rate was the primary endpoint. The trial design was a Simon mini–max two-stage design with parameters p0 = 0.20, p1 = 0.40, α = 0.10, and β = 0.10 [15]. If four out the first 17 patients responded, then an additional 20 patients would be enrolled, for a total of 37 patients. Secondary endpoints were to evaluate the ability of PBMC to conduct ADCC against HER2 overexpressing target cells, and measure serum cytokines and chemokines including IFN-γ, MIP-1α, TGF-β, MIG and IP-10.
Results
Patient characteristics
Patient characteristics are described in Table 1. The median age was 52 years (range 30–71 years); the median number of prior chemotherapy regimens was two (range 1–4); 11 (85%) had 3+ tumors by HerceptTest; and 7 (54%) had more than one site of metastatic disease. Thirteen patients were enrolled before the trial was closed to due to the lack of objective responses by RECIST and no expansion or activation of the NK cell population.
Table 1.
Patient characteristics
| N (%) | |
|---|---|
| Median age (years) | 52 (range 30–71 years) |
| Race | |
| Caucasian | 10 (77) |
| African American | 3 (23) |
| ECOG performance status | |
| 0 | 7 (54) |
| 1 | 6 (46) |
| Prior chemotherapy for metastases | |
| 1 | 2 (15) |
| 2 | 5 (39) |
| 3 | 6 (46) |
| Number of metastatic sites | |
| 1 | 6 (46) |
| 2 | 4 (31) |
| ≥3 | 3 (23) |
Toxicities
Overall, the majority of toxicity was grade 2 or less (Table 2). Grade 3 toxicities included 1 (8%) diarrhea, 1 (8%) nausea, 1 (8%) vomiting, and 1 (8%) pleural effusion. IL-2 dose reductions were required in 2 (15%) patients who experienced grade 4 toxicities: 1 (8%) with hypercalcemia and dizziness; and 1 (8%) with fever and pain.
Table 2.
Adverse events N (%)
| Grade 0,1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Hematologic | ||||
| Anemia | 13 (100) | 0 | 0 | 0 |
| Neutropenia | 12 (92) | 1 (8) | 0 | 0 |
| Constitutional | ||||
| Fever | 10 (77) | 2 (15) | 0 | 1 (8) |
| Fatigue | 11 (85) | 2 (15) | 0 | 0 |
| Weight loss | 13 (100) | 0 | 0 | 0 |
| Anorexia | 13 (100) | 0 | 0 | 0 |
| Cardiac | ||||
| Hypotension | 13 (100) | 0 | 0 | 0 |
| Pericardial effusion | 13 (100) | 0 | 0 | 0 |
| Pulmonary | ||||
| Dyspnea | 11 (85) | 2 (15) | 0 | 0 |
| Pleural effusion | 12 (92) | 0 | 1 (8) | |
| Gastrointestinal | ||||
| Stomatitis | 12 (92) | 1 (8) | 0 | 0 |
| Nausea | 9 (69) | 3 (23) | 1 (8) | 0 |
| Vomiting | 11 (85) | 1 (8) | 1 (8) | 0 |
| Diarrhea | 12 (92) | 0 | 1 (8) | 0 |
| Musculoskeletal | ||||
| Myalgias/arthralgia | 12 (92) | 1 (8) | 0 | 0 |
| Neurology | ||||
| Dizziness | 12 (92) | 0 | 0 | 1 (8) |
| Mood alteration | 12 (92) | 1 (8) | 0 | 0 |
| Pain | 12 (92) | 0 | 0 | 1 (8) |
| Injection site reaction | 10 (77) | 3 (23) | 0 | 0 |
| Hypercalcemia | 12 (92) | 0 | 0 | 1 (8) |
Treatment summary
The median number of days on study was 58 (range 29–326 days) and the median number of cycles was four (range 1–23 cycles). Twelve patients had PD and one voluntarily withdrew consent from the trial. The median time to progression (TTP) was 51 days (range 29–326 days).
Correlative assays
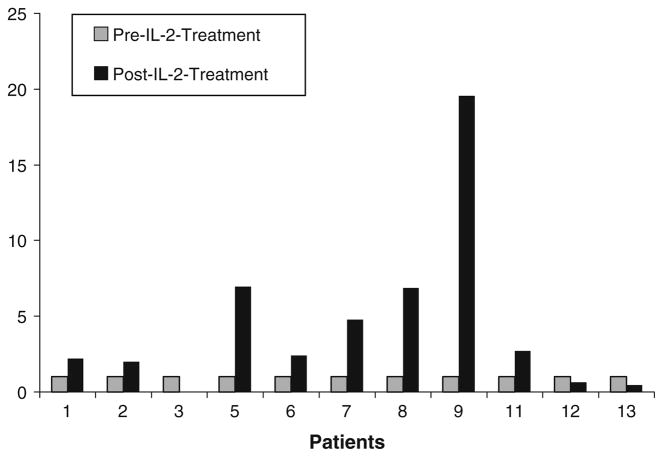
Correlative studies were performed to determine the immune effects of IL-2 when given with trastuzumab. The NK cell population was neither expanded nor activated (data not shown); ADCC was measured in six patients and did not increase (Fig. 2). PTS 2, 9, and 12 had increases in serum IFN-γ during treatment (Fig. 3). RT-PCR was used to quantitate serum IFN-γ transcript levels pre- and post-IL-2 treatment. Eight (62%) patients exhibited at least a twofold increase in IFN-γ transcript levels, with patients nine having more than a 19-fold increase (Fig. 4). The antiangiogenic chemokines MIG and IP-10 rose significantly over baseline in all 12 (92%) patients for whom post-treatment samples were available (Fig. 5a, b).
Fig. 2.
ADCC by patient PBMCs against trastuzumab-coated SKBR3 tumor cells was measured pre- and post-IL-2 treatment. ADCC values were calculated as the percent lysis of trastuzumab-coated SKBR3 tumor cells after subtraction of lysis of these cells in the absence of trastuzumab. ADCC was not significant in any patient
Fig. 3.
Serum IFN-γ was measured at the indicated time points (C cycle; D day). Represented here are the only three patients in whom there was detectable IFN-γ. Results were normalized against a β-actin internal control
Fig. 4.
IFN-γ transcripts were measured by RT-PCR both pre- and post-IL-2 treatment, and are displayed here as fold increase. Transcripts increased in eight patients after IL-2 treatment
Fig. 5.
a Serum levels of MIG and b IP-10 were measured at various time points. Shown here are the baseline and peak levels
Discussion
The IL-2 and trastuzumab regimen was tolerable with the majority of toxicities grades 1 or 2. However, there were neither observed anti-tumor responses nor evidence of NK cell expansion or increase in ADCC. In contrast, two previous trials of trastuzumab and low-dose IL-2, using similar doses to this trial, demonstrated increases in NK cells, trastuzumab-mediated ADCC, and anti-tumor responses [12, 13]. The patients enrolled in the prior trials were similar to those in the current trial in that the majority had prior chemotherapy; however, few of them had prior trastuzumab and there was no information about the response to prior trastuzumab. In the current trial all patients were trastuzumab-refractory defined as either relapsing on or within 12 months of receiving a trastuzumab-containing regimen.
Among the limitations of the current trial is the small number of patients enrolled from four institutions. PBMC collections were stored frozen at their respective institutions and subsequently shipped to OSU. Technical or methodological problems with the PMBC acquisition or the assays cannot be excluded as a possible reason for the observed null result. Another possibility is the trial closed after 13 patients were enrolled without any responses. Using the trial assumptions and parameters outlined in the statistical methods (p0 = 0.20, p1 = 0.40, α = 0.10, and β = 0.10), four or more responses were required in the first 17 patients to proceed to second stage. Given that 0/13 patients responded and true response rate (or p1) is 40%, the probability of the next four patients responding is only 2.6%. Likewise, the 95% upper confidence bound (1-sided) for 0/13 responses is 21%. If the true response rate is 21%, then the probability of the next four patients responding is even lower at 0.2%. Despite early closure of this trial, it is very unlikely that clinically meaningful response or benefit was missed.
The patients in this trial were specifically selected as being refractory to trastuzumab. Possibly, the lack of IL-2-induced expansion and activation of NK cells is related to trastuzumab resistance implying that ADCC is an important mechanism of trastuzumab activity in vivo. While there is no direct evidence to support or refute this hypothesis, there is an expanding literature that suggests the immune system may be a potential mechanism of relevance to trastuzumab and other therapeutic monoclonal antibodies. Several recent neoadjuvant trastuzumab trials serve as examples. In one report, subsequent responders to neoadjuvant trastuzumab had more tumor-associated lymphoid infiltrates containing CD56-positve (NK) cells at baseline than non-responders [16]; in another trial after trastuzumab treatment there was a statistically significant correlation between extent of anti-tumor response and ADCC [17]. In a non-randomized trial of neoadjuvant docetaxel with trastuzumab, docetaxel alone, or anthracycline-based non-taxane chemotherapy, after treatment with trastuzumab there were increases in NK cells and the cytoplasmic markers of cytolytic activity granzymes B and TiA1 both within and surrounding residual tumor foci relative to the non-trastuzumab treated groups [18].
The above results, in addition to several other studies of trastuzumab and interleukin-2 and 12 in breast cancer [5, 9, 10, 19, 20] as well as of other monoclonal antibodies in other tumor types [21–24] appear to support an immune-mediated mechanism of possible relevance to the therapeutic effects of therapeutic monoclonal antibodies. However, all the relevant cell populations and cytokines, as well as the characteristics of the breast cancer patients most or least likely to benefit from this approach have yet to be elucidated. Ultimately, a randomized clinical trial will be required to evaluate this type of immune-mediated mechanism.
In a prior phase I trial of trastuzumab and interleukin-12 in patients with metastatic HER2-overexpressing solid tumors, the majority of them breast cancers, increased serum IFN-γ, MIG, and IP-10 were found in patients with objective responses or stable disease [25]. Transcripts for IFN-γ and the antiangiogenic chemokines MIG and IP-10 were increased in the majority of patients in the current trial despite the lack of NK cell expansion or activation. These results suggest that trial patients were able to respond to IL-2 and other interleukins in ways consistent with prior studies [9, 25]. The observed rise in IFN-γ transcript after IL-2 may be attributed to other immune cell populations such as CD4+ helper T cells or CD8+ cytotoxic T cells increased expression of IFN-γ leading to increases in MIG and IP-10 [26].
In summary, there was no significant expansion of the NK cells nor was there enhanced ADCC against trastuzumab-coated tumor cells in this trial leading to the hypothesis that expansion and activation of NK cells may be required for or serve as a marker associated with clinical benefit from the combination of trastuzumab and interleukins. Future trials of this approach should not include breast cancer patients who are not trastuzumab refractory.
Acknowledgments
The authors wish to thank Amy Stark MS for biostatistical support, and all the patients and their families who participated in this trial. This work was supported by an unrestricted grant from Genentech and the NIH/NCI grants P30 (CA16058-29), K24 (CA93670-02), P01 CA95426, U01 (CA76576-07) and N01 (CM-62201). AM is a NRSA T32 fellow (5T32CA009338-28).
Footnotes
Presented in part at the American Society of Clinical Oncology Meetings June 2007.
Contributor Information
Aruna Mani, Division of Hematology and Oncology, Department of Internal Medicine, Ohio State University Medical Center, Starling Loving Hall Rm B405, 320 W. 10th Avenue, Columbus, OH 43210, USA.
Julie Roda, Integrated Biomedical Sciences Graduate Program, Department of Molecular Virology, Ohio State University, Columbus, OH, USA.
Donn Young, Department of Biostatistics, Ohio State University, Columbus, OH, USA.
Michael A. Caligiuri, Division of Hematology and Oncology, Department of Internal Medicine, Ohio State University Medical Center, Starling Loving Hall Rm B405, 320 W. 10th Avenue, Columbus, OH 43210, USA
Gini F. Fleming, University of Chicago, Chicago, IL, USA
Peter Kaufman, Dartmouth University, Hanover, NH, USA.
Adam Brufsky, University of Pittsburgh, Pittsburgh, PA, USA.
Susan Ottman, Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
William E. Carson, III, Division of Surgical Oncology, Department of Surgery, Ohio State University, Columbus, OH 43210, USA.
Charles L. Shapiro, Email: charles.shapiro@osumc.edu, Division of Hematology and Oncology, Department of Internal Medicine, Ohio State University Medical Center, Starling Loving Hall Rm B405, 320 W. 10th Avenue, Columbus, OH 43210, USA
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra 043186. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM 200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Barok M, Isola J, Palyi-Krekk Z, Nagy P, Juhasz I, Vereb G, Kauraniemi P, Kapanen A, Tanner M, Vereb G, Szollosi J. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6:2065–2072. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Hironaka K, Okawaki M, Okita R, Matsuura K, Ohshita A, Toge T. HER2-specific cytotoxic activity of lymphokine-activated killer cells in the presence of trastuzumab. Anticancer Res. 2005;25:827–832. [PubMed] [Google Scholar]
- 6.Mellstedt H. Monoclonal antibodies in human cancer. Drugs Today (Barc) 2003;39(suppl C):1–16. [PubMed] [Google Scholar]
- 7.Cooley S, Burns LJ, Repka T, Miller JS. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp Hematol. 1999;27:1533–1541. doi: 10.1016/S0301-472X(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 8.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 9.Carson WE, Parihar R, Lindemann MJ, Personeni N, Dierksheide J, Meropol NJ, Baselga J, Caligiuri MA. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31:3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::AID-IMMU3016 >3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE., III Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol. 2006;177:120–129. doi: 10.4049/jimmunol.177.1.120. [DOI] [PubMed] [Google Scholar]
- 12.Fleming GF, Meropol NJ, Rosner GL, Hollis DR, Carson WE, III, Caligiuri M, Mortimer J, Tkaczuk K, Parihar R, Schilsky RL, Ratain MJ. A phase I trial of escalating doses of trastuzumab combined with daily subcutaneous interleukin 2: report of cancer and leukemia group B 9661. Clin Cancer Res. 2002;8:3718–3727. [PubMed] [Google Scholar]
- 13.Repka T, Chiorean EG, Gay J, Herwig KE, Kohl VK, Yee D, Miller JS. Trastuzumab and interleukin-2 in HER2-positive metastatic breast cancer: a pilot study. Clin Cancer Res. 2003;9:2440–2446. [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89) 90015-9. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.V97.10.3146. [DOI] [PubMed] [Google Scholar]
- 17.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, Oliviero B, Ballardini B, Da Prada G, Zambelli A, Costa A. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 18.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubo M, Morisaki T, Kuroki H, Tasaki A, Yamanaka N, Matsumoto K, Nakamura K, Onishi H, Baba E, Katano M. Combination of adoptive immunotherapy with Herceptin for patients with HER2-expressing breast cancer. Anticancer Res. 2003;23:4443–4449. [PubMed] [Google Scholar]
- 20.Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE. III natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006;66:517–526. doi: 10.1158/0008-5472.CAN-05-2429. [DOI] [PubMed] [Google Scholar]
- 21.Gluck WL, Hurst D, Yuen A, Levine AM, Dayton MA, Gockerman JP, Lucas J, Denis-Mize K, Tong B, Navis D, Difrancesco A, Milan S, Wilson SE, Wolin M. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-hodgkin’s lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin Cancer Res. 2004;10:2253–2264. doi: 10.1158/1078-0432.CCR-1087-3. [DOI] [PubMed] [Google Scholar]
- 22.Eisenbeis CF, Grainger A, Fischer B, Baiocchi RA, Carrodeguas L, Roychowdhury S, Chen L, Banks AL, Davis T, Young D, Kelbick N, Stephens J, Byrd JC, Grever MR, Caligiuri MA, Porcu P. Combination immunotherapy of B-cell non-Hodgkin’s lymphoma with rituximab and interleukin-2: a preclinical and phase I study. Clin Cancer Res. 2004;10:6101–6110. doi: 10.1158/1078-0432.CCR-04-0525. [DOI] [PubMed] [Google Scholar]
- 23.van Herpen CM, van der Laak JA, de Vries IJ, van Krieken JH, de Wilde PC, Balvers MG, Adema GJ, De Mulder PH. Intratumoral recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies loco-regional lymph node architecture and induces natural killer cell infiltration in the primary tumor. Clin Cancer Res. 2005;11:1899–1909. doi: 10.1158/1078-0432.CCR-04-1524. [DOI] [PubMed] [Google Scholar]
- 24.Roda JM, Joshi T, Butchar JP, McAlees JW, Lehman A, Tridandapani S, Carson WE., 3rd The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res. 2007;13:6419–6428. doi: 10.1158/1078-0432.CCR-07-0865. [DOI] [PubMed] [Google Scholar]
- 25.Parihar R, Nadella P, Lewis A, Jensen R, De Hoff C, Dierksheide JE, VanBuskirk AM, Magro CM, Young DC, Shapiro CL, Carson WE., 3rd A phase I study of interleukin-12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon gamma production in a subset of patients. Clin Cancer Res. 2004;10:5027–5037. doi: 10.1158/1078-0432.CCR-04-0265. [DOI] [PubMed] [Google Scholar]
- 26.Vremec D, O’Keeffe M, Hochrein H, Fuchsberger M, Caminschi I, Lahoud M, Shortman K. Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood. 2007;109:1165–1173. doi: 10.1182/blood-2006-05-015354. [DOI] [PubMed] [Google Scholar]