Abstract
Antagonist activity at the alpha3beta4 nicotinic acetylcholine receptor (nAChR) is thought to contribute to the anti-addictive properties of several compounds. However, truly selective ligands for the α3β4 nAChR have not been available. We report the discovery and SAR of a novel class of compounds that bind to the α3β4 nAChR and have no measurable affinity for the α4β2 or α7 subtypes. In functional assays the lead compound antagonized epibatidine-induced Ca2+ flux in α3β4-transfected cells in a noncompetitive manner.
Introduction
Nicotine, the addictive active ingredient in tobacco smoke acts by binding to nicotinic acetylcholine receptors (nAChRs) in the central and peripheral nervous system. nAChRs are ligand-gated ion channels in the same family as the GABA and glutamate receptors and mediate cation flux.1,2 The neuronal nAChR is a pentameric protein made up of a combination of α and β subunits,3 although fully functional homomeric proteins, particularly the prominent α7 nAChR, are also present in the brain. The α4β2 and the α7 nAChRs are by far the most prevalent in the central nervous system (CNS),4,5 whereas the α3β4 subtype is predominant in the sensory and autonomic ganglia and in a subpopulation of neurons in the medial habenula (MHN) and interpeduncular nucleus (IPN) in the CNS.6
Although nAChRs are implicated in nicotine reward, dependence and expression of withdrawal, little is known definitively about which subtypes are involved in each of these aspects of tobacco addiction. Since nicotine increases dopamine levels in the mesocorticolimbic reward circuitry, most attention has been focused on nAChR subtypes present in these pathways. Behavioral and functional studies in knockout mice have shown that the predominant α4β2 nAChR present in this circuitry is required for nicotine addiction and dependence.7–9 The α4β2 nAChR is therefore an important target for the development of smoking cessation medications and significant efforts have been directed towards the development of ligands selective for this nAChR subtype. Indeed, the α4β2 nAChR partial agonist, varenicline, which was recently approved by the FDA as a smoking cessation medication, is effective in reducing craving and preventing relapse to smoking during abstinence.10–13
Although both α4 and β2 subunits appear to be crucial for nicotine dependence, other nAChR subtypes, particularly the α3β4 nAChR, have also been implicated in addiction to nicotine and other drugs of abuse.14 The α3β4 receptor, which appears to predominate in sensory and autonomic ganglia and in the adrenal gland, is sometimes referred to as the “ganglionic nAChR”.6,15,16 In the CNS, α3β4 nAChR is present, although not in large amounts, in the mesolimbic dopamine pathway, which is known to be crucial for the rewarding effects of drugs of abuse.4 Differences in desensitization rates may, however, increase its relative influence in this brain region.4 It is abundant, however, in the medial habenula and interpeduncular nucleus—regions that receive inputs from the nucleus accumbens and send efferents to the ventral tegmental area (VTA).4,6,16,17 As discussed below, these receptors may influence drug dependence. A recent study has further indicated the importance of the α3β4 nAChR to nicotine addiction, by demonstrating that nicotine-induced hypolocomotion is reduced in β4 null mice.18 Furthermore, after chronic nicotine treatment, mecamylamine-induced withdrawal is greatly diminished in β4 null mice, but not in β2 null mice.18
Although recent studies seem to implicate a role for α3β4 nAChR in psychostimulant and drug-seeking behavior, researchers have no good tools to explore this hypothesis. Some compounds, such as epibatidine, have high affinity at this site, but virtually no available compound has sufficient selectivity for this site to allow the study of the pharmacological role of α3β4 nAChR. 18-methoxycoronaridine (18-MC), a semisynthetic iboga alkaloid congener, has been shown to block the α3β4 nAChR, and has shown intriguing effectiveness in a variety of animal models of drug dependence against morphine, methamphetamine and nicotine.19–24 Although 18-MC is an antagonist at α3β4 nAChR,25 it is not particularly selective, and binds to opioid and other receptors. Studies by Glick and colleagues, that include the synthesis and testing of more selective congeners, have further implicated α3β4 nAChR in dependence induced by nicotine and other drugs of abuse.22,26
Another non-selective α3β4 nAChR antagonist mecamylamine is an antagonist at several nAChR.27 Mecamylamine has been evaluated previously in clinical trials as a smoking cessation pharmacotherapy,28 and as an antidepressant. 29 Dextromethorphan has also been shown to have antagonist activity at α3β4 nAChR,30 however, it is also an antagonist at the NMDA receptor. Potent, selective ligands for the α3β4 nAChR have been unavailable thus far.
We recently discovered a novel compound, 5 (SR16584), which binds to the α3β4 nAChR and has no apparent affinity for α4β2 or ⟨7 nAChR. We report here the structure-activity relationships of 5 and its congeners, to understand the molecular features that afford the selectivity towards the α3β4 nAChR. We also characterized the binding of these compounds to the α3β4 nAChR and compared them to epibatidine.
Results and Discussion
The lead compound 5 was discovered from screening a limited, selective library of small molecule compounds that possessed a protonatable nitrogen in an alicyclic ring, keeping in mind the 3-point pharmacophoric features of the Sheridan nicotinic pharmacophore.31 Most of the compounds screened contained a piperidine core ring, either monocyclic or bicyclic, with a lipophilic substituent on the alicyclic nitrogen and a heterocyclic aromatic substituent on the 4-position of the piperidine ring. These compounds were screened against the α3β4 and α4β2 nAChR transfected into HEK293 cells (obtained from the laboratory of Dr. Kenneth Kellar, Georgetown University). Compounds were evaluated for binding affinity, determined by inhibition of [3H]epibatidine binding to cell membranes, and for functional activity, measuring stimulation or inhibition of calcium flux using the fluorometric imaging plate reader (FLIPR). Selected compounds were also tested for binding affinity to the α7 nAChR on rat brain membranes. Table 1 provides the structures of some of the compounds screened and their binding affinities.
Table 1.
Structures and binding affinities (Ki, nM)a of selected compounds screened for nAChR affinity.
| Structure | Compound | α3β4 | α4β2 | α7 |
|---|---|---|---|---|
| Epibatidine | 0.15 ± 0.05 | 0.06 ± 0.0 | 4.16 ± 0.47 | |
| Nicotine | 480.69 ± 59.38 | 11.13 ± 1.11 | ||
| Cytisine | 202.89 ± 18.85 | 1.53 ± 0.20 | ||
| Acetylcholine | 619.63 ± 130.2 | 37.74 ± 3.73 | ||
 |
1 | >10,000 | >10,000 | |
 |
2 | >10,000 | >10,000 | |
 |
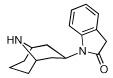
3 | >10,000 | >10,000 | |
 |
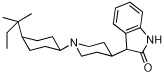
4 | 2790.3 ± 168.9 | >100,000 | |
 |
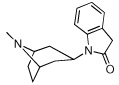
5 | 507.86 ± 162.4 | >100,000 | >100,000 |
 |
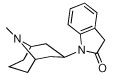
6 | 421.54 ± 53.43 | >100,000 | |
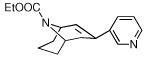 |
7 | >10,000 | >10,000 | |
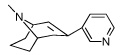 |
8 | 240.50 ± 29.95 | 10.39 ± 0.21 | |
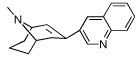 |
9 | 2614.78 ± 277 | 423.12 ± 5.90 | |
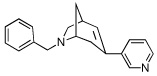 |
10 | 169.95 ± 47.91 | 1.95 ± 0.18 | 424 |
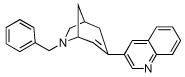 |
11 | 2477.86 ± 123 | 29.31 ± 9.26 | |
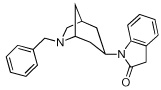 |
12 | 1836.25 ± 210 | >10,000 | |
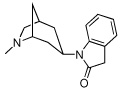 |
13 | >10,000 | >10,000 |
Binding affinities were determined by inhibition of [3H]epibatidine binding to membranes derived from HEK cells transfected with rat α3β4 and α4β2 nAChR, and rat brain membranes for α7 nAChR.
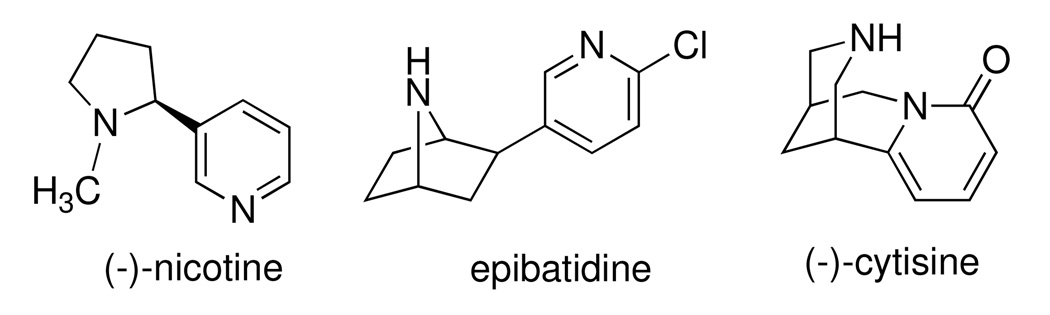
From the binding affinities in Table 1, it was apparent that compounds that contain the essential protonatable nitrogen in a monocyclic piperidine ring (1–3) have no affinity for either the α3β4 or the α4β2 nAChR, whereas those in which the nitrogen is present as part of a bicyclic core ring (4–13) possessed measurable binding affinity at one or both nAChRs. This is not surprising, and is also a trend seen in the natural product nAChR ligands such as epibatidine (Figure 1), which contains a bicyclic ring and has a 1000-fold higher affinity for nAChR than does nicotine, which contains a monocyclic pyrrolidine ring. In fact, several potent nAChR ligands contain bicyclic core rings.32–35 From the 65 compounds we tested, we identified [3.2.1]-azabicyclooctane-containing 4 and [3.3.1]-azabicyclononane-containing 5 as potential lead compounds; both have binding affinity at the α3β4 nAChR and no affinity for the α4β2 or α7 nAChR upto 100 µM concentrations. The binding affinity of 5 at α3β4 is similar to that of nicotine and cytisine at α3β4 (see Table 1); however, 5 has over 200-fold selectivity for the α3β4 nAChR and offers a good starting point for developing potent and selective α3β4 nAChR ligands. More importantly, our SAR studies suggest insights into the structural basis of subtype selectivity between the α3β4 and α4β2 nAChRs.
Figure 1.
Structures of known non-selective nAChR ligands with affinity for the α3β4 nAChR
Both 4 and 5 contain an azabicyclic core ring, with a dihydroindolin-2-one ring distal to the protonatable nitrogen. Although the azabicyclic rings differ by only one carbon (azabicyclooctane vs. azabicyclononane), 5 is more potent than 4 by almost a whole order of magnitude at the α3β4 nAChR. Moreover, both these bicyclic rings are larger than the azabicycloheptane ring of epibatidine, indicating that, among other factors, the α3β4 receptor may have a larger binding pocket than does α4β2, which may be distinguished by compounds containing larger rings, like 5; in contrast to epibatidine, which contains an optimally sized bicyclic ring that fits into all the nAChRs and thus lacks selectivity.
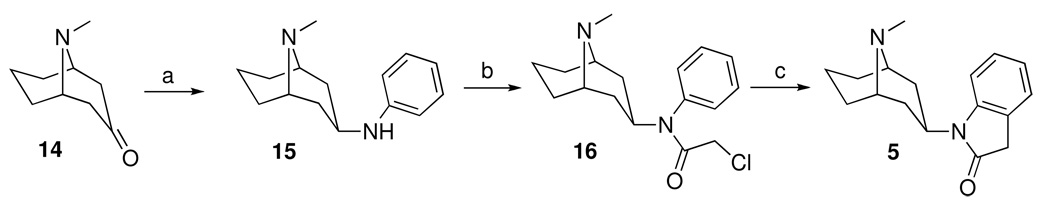
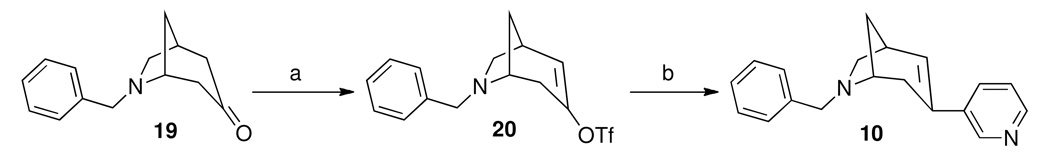
We synthesized a small series of analogs to investigate the importance of the three major pharmacophoric elements, viz. (i) the heteroaromatic ring, (ii) the bicyclic core ring and (iii) the nitrogen substituent, for selectivity to the α3β4 nAChR. The synthesis of these analogs is shown in Schemes 1–3, and followed standard methodology as shown. These analogs, shown in Table 1, were tested for their binding affinity and functional activity at the α3β4 and α4β2 nAChR and showed some interesting trends that suggested the basis for the selectivity of the lead compound 5 for the α3β4 nAChR.
Scheme 1.
Reagents and reaction conditions: a. aniline, molecular sieves, toluene, reflux, sodium cyanoborohydride, methanol; b. chloroacetyl chloride, triethylamine, methylene chloride, reflux; c. aluminum chloride, 130–160°C.
Scheme 3.
Reagents and reaction conditions: a. NaN(TMS)2, N-(5-chloro-2-pyridyl)triflimide, THF, −78°C; b. 3-pyridineboronic acid, Pd(PPh3)4, K3PO4, dioxane, 85°C.
The dihydroindolin-2-one ring plays a significant role in the selectivity of 5 for α3β4 and as discussed later, also on conferring antagonist activity at the α3β4 nAChR. When the indolinone ring of 5 was replaced with a 3-pyridyl ring, as in 8, the compound retained affinity for α3β4, but now gained significant affinity for the α4β2 receptor (10 nM) and was a potent ligand for α4β2 nAChR. This surprising result indicates that it is the dihydroindolinone ring of 5 that provides selectivity for the α3β4 receptor. In the published SAR on epibatidine, the 3-pyridyl ring is a favored heteroaromatic ring for several α4β2 ligands.32,35,36 The gain in α4β2 affinity by introducing the 3-pyridyl ring onto the [3.3.1] azabicyclo core ring of 8 agrees with published SAR trends and confirms our observation that the dihydroindolinone ring somehow precludes binding to the α4β2 receptor, thereby affording selectivity for α3β4. Replacing the heteroaromatic ring with a quinoline, as in 9, decreases binding affinity for both α4β2 and α3β4, indicating that the larger 3-quinolinyl ring is not optimal for binding at either of these receptors.
We explored the effect of substitution on the basic nitrogen and found that removal of the N-methyl group of 5 (as in compound 6) retained the affinity and selectivity for α3β4. This finding agrees with most reported observations for nAChR ligands.32,35 Interestingly, carbamate-type N-substituents, such as those present in 7 (N-ethoxycarbamate analog of 8), result in complete loss of binding affinity for both α3β4 and α4β2, confirming previous SAR that the protonatable basic nitrogen is an important requisite for binding affinity at all nAChR.
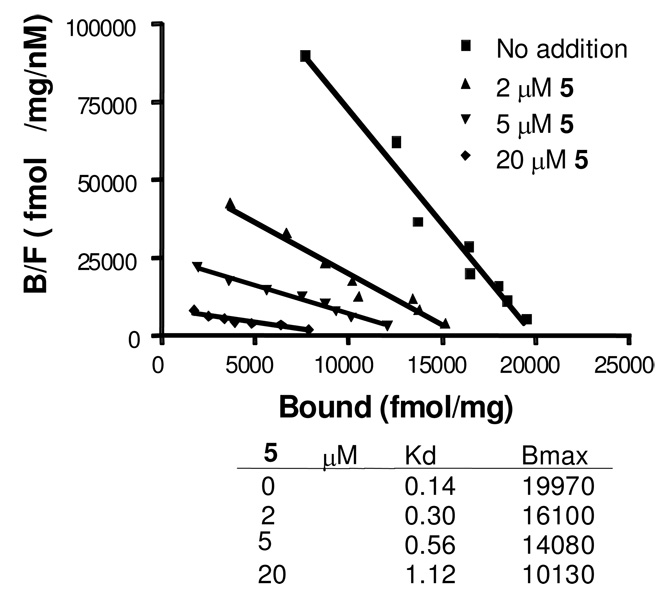
We determined the mode of binding and functional activity of 5 at α3β4 nAChR. Saturation binding experiments at α3β4 using [3H]epibatidine indicated that 5 and cytisine bind noncompetitively with epibatidine, as indicated by a change in both Kd and Bmax when using increasing concentrations of 5 (Figure 2). However, the moderate Ki of both compounds in displacing [3H]epibatidine in binding experiments appears to indicate that both compounds may bind at sites partially overlapping the epibatidine binding.
Figure 2.
Scatchard analysis of [3H]epibatidine binding at α3β4 nAChR alone and in the presence of various concentrations of the inhibitor 5.
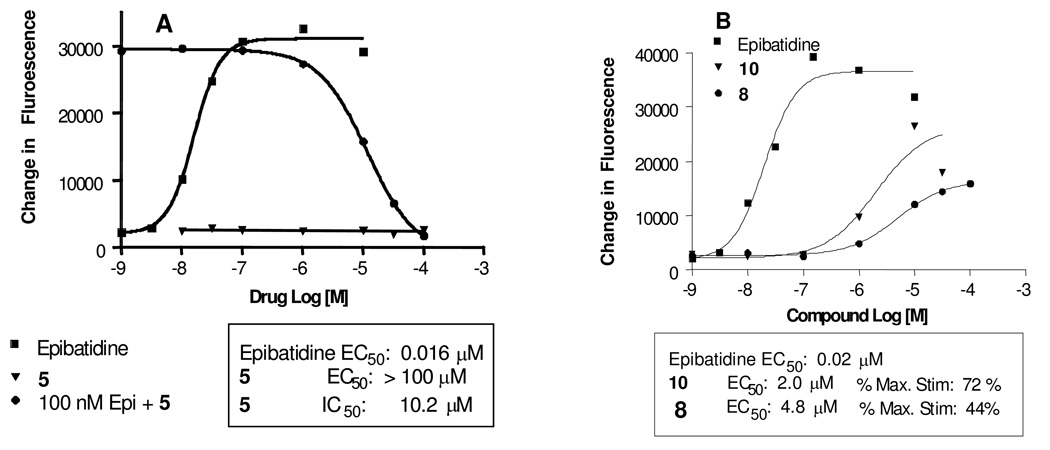
We used the high-throughput FLIPR and calcium flux, to evaluate the functional activity of these compounds. As seen in Figure 3, addition of the nAChR agonist epibatidine leads to a 1000-fold increase in intracellular Ca2+-induced fluorescence. The potencies of epibatidine (0.030 ±0.004 µM) and nicotine (8.7 ± 0.9 µM, data not shown) are similar to those reported by Fitch et al.37 Both 5 and its desmethyl analog 6 were antagonists in the FLIPR assay. They showed no agonist activity alone and fully reversed the epibatidine-induced Ca2+ fluorescence, with potencies in the micromolar range (as shown for 5 in Figure 3). The 3-pyridyl containing analog 8, on the other hand, showed partial agonist activity in the FLIPR assay. This result supports our hypothesis that the larger bicyclic benzo-fused dihydroindolinone ring plays a role not only in the selectivity but also confers antagonistic activity to this class of azabicyclononane-type nAChR ligands. To the best of our knowledge, the dihydroindolinone ring is a new heterocyclic ring, not present in any nAChR ligands reported to date. Notably, 5 has no affinity at the α7 nAChR (Table 1). Compound 5 therefore represents a novel class of α3β4-selective nAChR ligands that may be valuable for studying the pharmacological role of this receptor in drug abuse and reward pathways.
Figure 3.
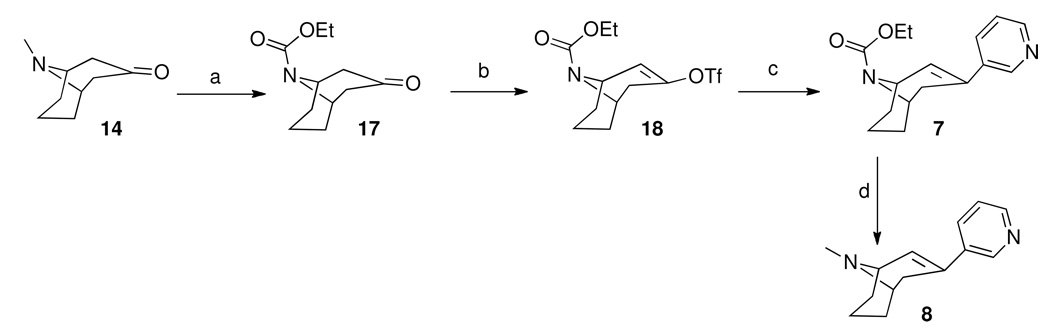
Ligand-induced Ca2+ fluorescence in α3β4 nAChR containing HEK cells using FLIPR. (A) agonist activity of epibatidine and antagonist activity of 5. (B) agonist activity of epibatidine and the partial agonist activity of 10 and 8.
From our screening efforts, we also identified a new bicyclic core ring, the 2-azabicyclo[3.2.1]octane (an isomer of tropane) that provided potent binding affinity for α4β2 nAChR. The compound containing this isotropane core scaffold and the α4β2-favored 3-pyridyl ring, 10 (Table 1), has a 2 nM binding affinity for α4β2 and about 100-fold selectivity over α3β4 receptors and greater than 200-fold selectivity over α7 nAChR. Interestingly, 10 contains an N-benzyl substituent on the basic nitrogen. Furthermore, in functional assays, 10 is a partial agonist at α4β2 nAChR. This is unusual, because most SAR on α4β2 show that for agonist activity, only small alkyl substituents are tolerated at the basic nitrogen.32,35 Our results show that in this isotropane class, the larger N-benzyl substituent can still impart agonist activity at the α4β2 receptor. At α3β4 nAChR, the 3-pyridyl containing 10 is also a partial agonist, similar to the 3-pyridyl containing azabicyclononane 8 (Figure 3B). The 3-quinolinyl analog 11 in the isotropane series has decreased activity at both receptors, similar to the trend also seen with the in the azabicyclononane series (compound 9). However, when the 3-pyridyl ring in the isotropane series is replaced with the dihydroindolinone ring (compound 12), it completely abolished affinity at the α4β2 nAChR, although it did bind to the α3β4 nAChR, albeit with lower affinity than the isotropane 10 or the azabicyclononane compound 5. This further confirms our SAR that the dihydroindolinone ring somehow prevents binding to the α4β2 nAChR, a trend also seen with the azabicyclononane series of ligands.
The SAR on the two pharmacophoric features, the heteroaromatic ring and the core bicyclic ring, provides approaches to design α3β4-selective nAChR ligands. The heteroaromatic dihydroindolinone ring precludes binding to the α4β2 nAChR when present on any of the core bicyclic rings (see 4, 5 and 12), thus conferring selectivity for the α3β4 nAChR. Among the azabicyclic core rings, the larger azabicyclononane affords good binding affinity for the α3β4 nAChR and the possibility of preferential binding to the α3β4 nAChR, with an appropriate heteroaromatic pharmacophore, as in 5. We are currently optimizing the pharmacophoric elements of the azabicyclononane class of α3β4 ligands to improve binding affinity, and these results will be reported in due course.
In summary, we have discovered a new class of nAChR ligands that do not appear to bind other major nAChR subtypes at concentrations greater than 100 µM and therefore, show excellent selectivity (>200-fold) for the α3β4 nAChR. Our SAR studies show that the azabicyclo[3.3.1]nonane core ring as well as the dihydroindolinone heteroaromatic ring play a role in conferring the selectivity for the α3β4 nAChR, whereas only the dihydroindolinone-containing ligands possess antagonist activity at α3β4 nAChR. These SAR may lead to a better understanding of structural features for selectivity and functional activity at the nAChR. Such selective and potent α3β4 nAChR antagonists will be useful for testing the hypothesis that α3β4 receptor antagonists can attenuate rewarding properties of nicotine and other drugs of abuse.14,38
EXPERIMENTAL DETAILS
General
1H and 13C NMR spectra were recorded on a Varian Gemini 300 MHz spectrometer (300 MHz and 75 MHz, respectively) and are internally referenced to chloroform at δ 7.27. Data for 13C are reported in terms of chemical shift. Mass spectra were obtained using a ThermoFinnigan LCQ Duo LC/MS/MS instrument and an electrospray ionization probe. Thin-layer chromoatgraphy was run on Analtech Uniplate silica gel TLC plates. Flash chromatography was carried out using silica gel, Merck grade 9385, 230–400 mesh. The purity of the final compounds reported was confirmed by HPLC and mass spectra, using the following conditions: column: Phenomenex Synergi 4m Fusion RP, 250×4.60 mm; mobile phase: (A) MeCN (0.1% TFA):(B) H2O (0.1% TFA) (gradient 20%A to 100%A, 15 mins); flow rate: 1 mL/min; detection: PDA 254 nm. The purity of all final compounds was greater than 95%. Experimental details for intermediates from Schemes 1–3 are reported in the Supporting Information.
1-(9-methyl-9-azabicyclo[3.3.1]non-3-yl)-1,3-dihydro-2H-indol-2-one (5)
A mixture of 16 (576 mg, 1.88 mmol) and aluminum chloride (1.00 g, 7.51 mmol) under argon was placed in a 160°C oil bath and stirred for 15 min and then, allowed to cool to 130°C and kept at this temperature 1.75 h. A solution of 1N sodium hydroxide (10 mL) was added and the resulting mass was sonicated to homogeneity and extracted three times with methylene chloride, dried (sodium sulfate) and evaporated to an oil. The oil was purified by flash chromatography eluting with 0–6% methanol containing 5% of 28% ammonium hydroxide/methylene chloride to give 5 as a colorless oil (free base, 219 mg, 43%). This was treated HCl in ether and evaporated to dryness to an off-white solid, mp 268–269°C. 1H NMR (HCl salt; 300 MHz, CD3OD) δ 1.63 (br d, J = 14.4 Hz, 2H, H-6ax, H-8ax), 1.75 (br d, J = 14.4 Hz, 1H, H-7ax), 2.22 (m, 2H, 2-H, H-4), 2.35-2.48 (m, 3H, H-6eq, H-7eq, H-8eq), 2.79 (ddd, J = 10.4, 10.1, 2.3 Hz, 2H, H-2, H-4), 2.98 (s, 3H, N-CH3), 3.55 (s, 2H, indolinyl CH2), 3.74 and 3.77 (2 br s, J = 11.2 Hz, 2H, H-1, H-5), 4.90–5.05 (m, 1H, H-3), 7.05 (t, J = 7.6 Hz, 1H, Ar-H8), 7.25–7.32 (m, 3H, Ar-H5, Ar-H6, Ar-H7). MS (ESI) m/z: 271.2 (M+H)+.
9-methyl-3-(pyridin-3-yl)-9-azabicyclo[3.3.1]non-2-ene (8)
To a solution of compound 19 (67 mg, 0.25 mmol) in THF (10 mL), was added lithium aluminum hydride (108 mg, 4.74 mmol). The resultant mixture was stirred at room temperature for 30 minutes, and the excess of lithium aluminum hydride destroyed with ethyl acetate till no gas was released, then water (0.5 mL) was added. The mixture was filtered and the solid washed with ethyl acetate (10 mL). The filtrate and washings were combined, dried over sodium sulfate, and evaporated to dryness. The residue was subjected to chromatography on silica gel, eluting with a solvent mixture of dichloromethane and methanol (20%), to afford 14 mg of desired product 8 (35%) as a light yellow oil. 1H NMR (300 MHz, CDCl3) δ 1.36-1.62 (m, 4H, H-6, H-7, H-8), 1.78-1.96 (m, 2H, H-6, H-8), 2.03 (dd, J = 18.3, 1.5 Hz, 1H, H-4), 2.37 (s, 3H, N-CH3), 2.74 (ddd, J = 18.4, 6.9 Hz, 1.0 Hz, 1H, H-4), 3.14 (br, 1H, H-5), 3.37 (br, 1H, H-1), 6.06 (dt, J = 4.8, 2.1 Hz, 1H, H-2), 7.19 (ddd, J = 8.1, 4.8, 0.9 Hz, 1H, Ar-H5), 7.63 (ddd, J = 8.8, 2.4, 1.5 Hz, 1H, Ar-H4), 8.43 (dd, J = 4.8, 1.5 Hz, 1H, Ar-H6), 8.63 (d, J = 2.1 Hz, 1H, Ar-H2). 13C NMR (300 MHz, CDCl3) δ 15.4 (C-7), 26.5 (C-6), 28.1 (C-8), 32.9 (C-4), 41.8 (C-9, N-CH3), 52.7 (C-1), 55.4 (C-5), 123.2 (Ar-C5), 125.5 (C-2), 132.0 (Ar-C4), 133.9 (Ar-C3), 135.6 (C-3), 146.5 (Ar-C2), 148.3 (Ar-C6). MS (APCI) m/z: 215.1 (M+H)+.
6-benzyl-3-(pyridin-3-yl)-6-azabicyclo[3.2.1]oct-2-ene (10)
A mixture of 21 (140 mg, 0.40 mmol), 3-pyridineboronic acid (55 mg, 0.45 mmol), Pd(PPh3)4 (23 mg, 0.02 mmol), K3PO4 (128 mg, 0.60 mmol), and dioxane (4 mL) was stirred at 85°C overnight (17 h). The mixture was treated with NaOH (2M) to strong basic (pH > 12) and extracted with ethyl acetate (3 X 10 mL). The extract was washed with brine, dried over sodium sulfate, and evaporated to dryness. The residue was subjected to chromatography on silica gel, eluting with a mixture solvent of ethyl acetate/hexanes/methanol (5:5:1) to afford 36 mg of 10 (32%) as a light yellow oil. 1H NMR (300 MHz, CDCl3) δ 1.71 (d, J = 10.5 Hz, 1H, H-8), 1.96-1.86 (m, 1H, H-8), 2.43 (d, J = 17.1 Hz, 1H, H-4), 2.55 (d, J = 17.1 Hz, 1H, H-4), 2.66-2.71 (m, 1H, H-5), 2.83 (dd, J = 8.7, 4.8 Hz, 1H, H-7eq), 2.97 (d, J = 9.0 Hz, 1H, H-7ax), 3.46 (m, 1H, H-1), 3.74 (d, J = 10.5 Hz, 1H, CH2-Ph), 3.87 (d, J = 10.5 Hz, 1H, CH2-Ph), 6.48 (d, J = 6.1, 1H, H-2), 7.10-7.36 (m, 6H, Ar-H, pyridyl H-5), 7.58 (ddd, J = 8.8, 2.3, 1.6 Hz, 1H, pyridyl H-4), 8.38 (dd, J = 5.0, 1.6 Hz, 1H, pyridyl H-6), 8.58 (dd, J = 2.4, 0.6 Hz, 1H, pyridyl H-2). 13C NMR (300 MHz, CDCl3) δ 32.8 (C-1), 35.7 (C-4), 35.8 (C-8), 57.6 (C-5), 59.6 (C-7), 62.4 (Ar-CH2-N), 123.0 (pyridyl C-5), 126.8 (Ar C-4), 128.2 (Ar C-3, Ar C-5,), 128.5 (Ar C-2), 131.6 (C-2), 131.7 (C-3), 131.9 (pyridyl C-4), 135.9 (pyridyl C-3), 146.5 (pyridyl C-2), 148.0 (pyridyl C-6). MS (APCI) m/z: 277.0 (M+H)+.
Supplementary Material
Scheme 2.
Reagents and reaction conditions: a. ethyl chloroformate, K2CO3, toluene, 90–100°C; b. NaN(TMS)2, N-(5-chloro-2-pyridyl)triflimide, THF, −78°C; c. 3-pyridineboronic acid, Pd(PPh3)4, K3PO4, dioxane, 85°C; d. LAH, THF.
Acknowledgment
This work was supported by a NIH-NIDA grant R01-DA020811.
ABBREVIATIONS
- nAChR
nicotinic acetylcholine receptors
- CNS
central nervous system
- FDA
Food and Drug Administration
- MHN
medial habenula
- IPN
interpeduncular nucleus
- VTA
ventral tegmental area
- 18-MC
18-methoxycoronaridine
- FLIPR
fluorometric imaging plate reader
Footnotes
Supporting Information Available: Experimental details and analytical data for all intermediates from Schemes 1–3, biological evaluation and in vitro assay protocols. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog. Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 2.McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 3.McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 4.Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J. Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- 5.Xiao Y, Kellar KJ. The comparative pharmacology and up-regulation of rat neuronal nicotinic receptor subtype binding sites stably expressed in transfected mammalian cells. J. Pharmacol. Exp. Ther. 2004;310:98–107. doi: 10.1124/jpet.104.066787. [DOI] [PubMed] [Google Scholar]
- 6.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- 8.Picciotto MR. Common aspects of the action of nicotine and other drugs of abuse. Drug Alcohol Depend. 1998;51:165–172. doi: 10.1016/s0376-8716(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 9.Epping-Jordan MP, Picciotto MR, Changeux JP, Pich EM. Assessment of nicotinic acetylcholine receptor subunit contributions to nicotine self-administration in mutant mice. Psychopharmacology (Berl) 1999;147:25–26. doi: 10.1007/s002130051135. [DOI] [PubMed] [Google Scholar]
- 10.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. J.A.M.A. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. J.A.M.A. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 12.West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales D, Rennard SI, Jorenby DE, Reeves KR. Comment: Oral varenicline for smoking cessation. Ann. Pharmacother. 2007;41:720–721. doi: 10.1345/aph.1H310a. [DOI] [PubMed] [Google Scholar]
- 14.Glick SD, Maisonneuve IM, Kitchen BA, Fleck MW. Antagonism of alpha 3 beta 4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur. J. Pharmacol. 2002;438:99–105. doi: 10.1016/s0014-2999(02)01284-0. [DOI] [PubMed] [Google Scholar]
- 15.Poth K, Nutter TJ, Cuevas J, Parker MJ, Adams DJ, Luetje CW. Heterogeneity of nicotinic receptor class and subunit mRNA expression among individual parasympathetic neurons from rat intracardiac ganglia. J. Neurosci. 1997 17;:586–596. doi: 10.1523/JNEUROSCI.17-02-00586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci. 2009;29:2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. Alpha3beta4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 18.Salas R, Cook KD, Bassetto L, De Biasi M. The alpha3 and beta4 nicotinic acetylcholine receptor subunits are necessary for nicotine-induced seizures and hypolocomotion in mice. Neuropharmacology. 2004;47:401–407. doi: 10.1016/j.neuropharm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- 20.Glick SD, Maisonneuve IM, Visker KE, Fritz KA, Bandarage UK, Kuehne ME. 18-Methoxycoronardine attenuates nicotine-induced dopamine release and nicotine preferences in rats. Psychopharmacology (Berl) 1998;139:274–280. doi: 10.1007/s002130050716. [DOI] [PubMed] [Google Scholar]
- 21.Glick SD, Maisonneuve IM, Dickinson HA. 18-MC reduces methamphetamine and nicotine self-administration in rats. Neuroreport. 2000;11:2013–2015. doi: 10.1097/00001756-200006260-00041. [DOI] [PubMed] [Google Scholar]
- 22.Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, Fleck MW. Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration. Eur. J. Pharmacol. 2004;492:159–167. doi: 10.1016/j.ejphar.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 23.Glick SD, Ramirez RL, Livi JM, Maisonneuve IM. 18-Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in rats. Eur. J. Pharmacol. 2006;537:94–98. doi: 10.1016/j.ejphar.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur. J. Pharmacol. 2002;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- 25.Glick SD, Maisonneuve IM, Szumlinski KK. 18-Methoxycoronaridine (18-MC) and ibogaine: comparison of antiaddictive efficacy, toxicity, and mechanisms of action. Ann. N. Y. Acad. Sci. 2000;914:369–386. doi: 10.1111/j.1749-6632.2000.tb05211.x. [DOI] [PubMed] [Google Scholar]
- 26.Kuehne ME, He L, Jokiel PA, Pace CJ, Fleck MW, Maisonneuve IM, Glick SD, Bidlack JM. Synthesis and biological evaluation of 18-methoxycoronaridine congeners. Potential antiaddiction agents. J. Med. Chem. 2003;46:2716–2730. doi: 10.1021/jm020562o. [DOI] [PubMed] [Google Scholar]
- 27.Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J. Pharmacol. Exp. Ther. 2001;297:646–656. [PubMed] [Google Scholar]
- 28.Buchhalter AR, Fant RV, Henningfield JE. Novel pharmacological approaches for treating tobacco dependence and withdrawal: current status. Drugs. 2008;68:1067–1088. doi: 10.2165/00003495-200868080-00005. [DOI] [PubMed] [Google Scholar]
- 29.Lippiello PM, Beaver JS, Gatto GJ, James JW, Jordan KG, Traina VM, Xie J, Bencherif M. TC-5214 (S-(+)-mecamylamine): a neuronal nicotinic receptor modulator with antidepressant activity. CNS Neurosci. Ther. 2008;14:266–277. doi: 10.1111/j.1755-5949.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez SC, Bertolino M, Xiao Y, Pringle KE, Caruso FS, Kellar KJ. Dextromethorphan and its metabolite dextrorphan block alpha3beta4 neuronal nicotinic receptors. J. Pharmacol. Exp. Ther. 2000;293:962–967. [PubMed] [Google Scholar]
- 31.Sheridan RP, Nilakantan R, Dixon JS, Venkataraghavan R. The ensemble approach to distance geometry: application to the nicotinic pharmacophore. J. Med. Chem. 1986;29:899–906. doi: 10.1021/jm00156a005. [DOI] [PubMed] [Google Scholar]
- 32.Romanelli MN, Gratteri P, Guandalini L, Martini E, Bonaccini C, Gualtieri F. Central nicotinic receptors: structure, function, ligands, and therapeutic potential. ChemMedChem. 2007;2:746–767. doi: 10.1002/cmdc.200600207. [DOI] [PubMed] [Google Scholar]
- 33.Cassels BK, Bermúdez I, Dajas F, Abin-Carriquiry JA, Wonnacott S. From ligand design to therapeutic efficacy: the challenge for nicotinic receptor research. Drug Discovery Today. 2005;10:1657–1665. doi: 10.1016/S1359-6446(05)03665-2. [DOI] [PubMed] [Google Scholar]
- 34.Astles PC, Baker SR, Boot JR, Broad LM, Dell CP, Keenan M. Recent progress in the development of subtype selective nicotinic acetylcholine receptor ligands. Curr. Drug Targets CNS Neurol. Disord. 2002;1:337–348. doi: 10.2174/1568007023339256. [DOI] [PubMed] [Google Scholar]
- 35.Breining SR. Recent developments in the synthesis of nicotinic acetylcholine receptor ligands. Curr. Top. Med. Chem. 2004;4:609–629. doi: 10.2174/1568026043451131. [DOI] [PubMed] [Google Scholar]
- 36.Carroll FI. Epibatidine structure-activity relationships. Bioorg. Med. Chem. Lett. 2004;14:1889–1896. doi: 10.1016/j.bmcl.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Fitch RW, Xiao Y, Kellar KJ, Daly JW. Membrane potential fluorescence: a rapid and highly sensitive assay for nicotinic receptor channel function. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4909–4914. doi: 10.1073/pnas.0630641100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Struthers AM, Wilkinson JL, Dwoskin LP, Crooks PA, Bevins RA. Mecamylamine, dihydro-beta-erythroidine, and dextromethorphan block conditioned responding evoked by the conditional stimulus effects of nicotine. Pharmacol. Biochem. Behav. 2009;94:319–328. doi: 10.1016/j.pbb.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartlett PA, McQuaid LA. Total synthesis of (.+-.)-methyl shikimate and (.+-.)-3-phosphoshikimic acid. J. Amer. Chem. Soc. 1984;106:7854–7860. [Google Scholar]
- 40.Pitner JBP, Abraham P, Joo YJ, Triggle DJ, Carroll FI. Synthesis and stereoselective reduction of (±)–, (+)– and (−)-6-substituted-6-azabicyclo[3.2.1]octan-3-one. J. Chem. Soc. Perkin Trans. 1. 1991:1375–1381. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.