Abstract
In this study, a novel process of dissolving polycaprolactone (PCL) matrices in glacial acetic acid was explored in which matrices spontaneously formed upon contact with water. Scanning electron microscopy analysis showed rough architecture and holes on the self-assembled matrix relative to matrices formed after dissolving in chloroform. Immersion in the gelatin solution reduced its roughness and number of micropores. Atomic force microscopy (AFM) analysis confirmed the increased roughness of the self-assembled matrices. The roughness of the matrices decreased after incubation in 1 N NaOH for 10 min. AFM analysis also revealed that the self-assembled matrix had a net positive surface charge, whereas chloroform–cast matrix had a negative surface charge. The surface charge of self-assembled matrix after immersion in gelatin changed to negative. However, incubation in NaOH did not affect the surface charge. The tensile properties were tested in both the dry state (25 °C) and the wet state (37 °C) by immersion in phosphate-buffered saline. Self-assembled matrix had lower elastic modulus, break stress and break strain than chloroform–cast matrix in both states. The elastic modulus in the wet condition was reduced by half in self-assembled matrix but tensile strain increased. Samples were further analyzed by ramp-hold test for assessing stress relaxation behavior. Both self-assembled and chloroform–cast matrices had similar trends in stress relaxation behavior. However, stress accumulation in self-assembled matrix was half that of chloroform–cast matrix. In vitro cell cultures were conducted using human foreskin fibroblast (HFF-1) in serum-free medium. Cytoskeletal actin staining showed cell adhesion and spreading on all matrices. Cell retention was significantly increased in self-assembled matrix compared to chloroform–cast matrix. Addition of gelatin improved the retention of seeded cells on the surface. In summary, PCL matrices generated using this novel technique show significant promise in biomedical applications.
Keywords: Matrix, PCL, Stress relaxation, Roughness, Surface property
1. Introduction
Polycaprolactone (PCL) is biocompatible polyester with a low melting point (60 °C). It has been explored for forming various medical devices [1], templates for tissue regeneration [2,3] and drug delivery systems [4]. Apart its low melting point, the ability to tailor the mechanical properties and non-enzymatic degradation (by hydrolysis) rate of PCL by altering the molecular chain length are very attractive features [5]; PCL matrices formed after dissolution in chloroform show elongation up to 1000% before breaking [6]. However, the poor wettability of the prepared matrices prevents uniform distribution of proteins and cell adhesion, thus compromising their application in biomedical devices [7].
To enhance the bioreactivity of the PCL surface and to modulate cell survival, a number of studies have evaluated the effect of the solvents used during matrix generation process [8]. Typically used halogenated hydrocarbon solvents such as chloroform and dichloromethane have been shown to generate a hydrophobic surface with smooth surface characteristics. To reduce the surface hydrophobicity, grafting hydrophilic fragments of synthetic or natural polymers such as acrylates, collagen and chitosan has also been explored [9–13]. Alternatively, etching the surface at the nanoscale using sodium hydroxide is also proposed [14]. Further, melt molding techniques have used molds with different surface features [15]. Nevertheless, the effect of these modifications on the resulting polymer is not completely understood.
In this study, a novel process of dissolving PCL in acetic acid (AA) was explored which allows self-assembly of PCL in an aqueous environment. To understand the utility of these matrices in biomedical applications, their surface characteristics, effect of neutralizing in alkaline solution, tensile properties and stress relaxation properties were investigated. Matrices formed by dissolving in chloroform and air drying—an method that has been widely investigated [8,9]—were used as control. In addition, the cytocompatibility and cytoskeletal organization of well-characterized fibroblasts were tested in serum-free medium. The results showed a significant benefit of the modified process relative to casting matrices with chloroform.
2. Materials and methods
PCL of 80 kDa (in number Mn) and gelatin (type-A) were purchased from Sigma–Aldrich (St. Louis, MO). Glacial AA was purchased from Pharmco Products Inc. (Brookfield, CT). Pure ethanol was from AAPER (Shelbyville, KY). Human foreskin fibroblast cells (HFF-1, cell line) were purchased from the American Type Culture Collection (Walkersville, MD). All other chemicals were of reagent grade.
2.1. Preparing solutions and forming blends
PCL solution (10% (w/v)) was prepared in glacial AA, and the same percentage of PCL solution was prepared in chloroform. Gelatin solution (5% (w/v)) was prepared in distilled water. All solutions were prepared fresh or stored for no longer than 4 days. Long-term storage is deleterious to self-assembly of PCL matrix.
2.2. Forming matrices
PCL matrices were made in 5 cm diameter Teflon dishes for tensile testing and surface analysis using two methods:
Method 1: dispensing 2–3 ml PCL solution dropwise on top of a waterbath.
Method 2: layering 2 ml PCL solution on the bottom of the dish and dipping the entire dish into a larger container of water.
Although matrices formed spontaneously in both the modes, they were undisturbed for 5 min to allow the process of matrix formation to complete. Generated matrices were allowed to air dry. Some of those PCL matrices were incubated in gelatin solution for 30 min, and were then allowed to air dry. Both gelatin-coated and uncoated matrices were removed from the dish, and placed in an ethanol bath for 15 min. These matrices were used for tensile testing measurements and surface analysis. Chloroform–cast matrices were made in the same Teflon dishes by layering 2 ml PCL solution and air drying.
To form porous matrices, the waterbath was replaced with NaHCO3 solutions which react with AA to form CO2. To evaluate the effect of concentration of NaHCO3 solution on matrix formation, experiments were performed using 2%, 5% and 8% (w/v) NaHCO3 solutions.
The formed matrices were dipped in a 1 N sodium hydroxide solution for 10 min to test the effect of etching on surface roughness and charge.
For surface analysis of formed matrices, samples were incubated in ethanol for 10 min and allowed to dry overnight in a vacuum desiccator. Dry matrices were attached to aluminum stubs with carbon paint and sputter-coated with gold for 1 min. The surface architecture of the matrices was analyzed by scanning electron microscopy (SEM, JEOL 6360, JEOL USA Inc., Peabody, MA) at an accelerating voltage of 21 kV.
2.3. Surface roughness and charge analysis
Surface roughness analysis of matrices was done by atomic force microscopy (AFM) using a DI Nanoscope V Multimode Scanning Probe Microscope (Digital Instruments, Veeco Metrology Group, Santa Barbara, CA) at ambient conditions, as described previously [16]. In brief, at least triplicate samples were prepared per condition. Each sample was split into four, and each of the four samples was attached to iron substrates using double-sided tape (for the roughness test) and conductive tape (for the charge test). In each sample, AFM analysis was performed by randomly choosing 10 different locations, with a field size of 5 µm × 5 µm. Topographic images were obtained in tapping mode at a scan rate of 1 Hz and 256 scanning lines using commercial silicon microcantilever probes (MikroMasch, Portland, OR) with a tip radius of 5–10 nm and a spring constant of 2–5 N m−1. Height images were captured at the probe oscillation resonance frequency of ~160 kHz. The roughness factor, Rq, is the root mean square average of the number, n, of height deviations (Zi) taken from the mean data plane, calculated using the equation:
| (1) |
in the associated software (Nanoscope, version 7).
For surface charge, topographic images were obtained in tapping mode using microcantilever probes coated with platinum–iridium with a tip radius of 20 nm. Then topographic images were rescanned in electrical force mode. Surface potential charges were recorded using the associated software (Nanoscope, version 7).
2.4. Tensile testing
Tensile testing was performed by the method previously described [17,18]. In brief, 30 mm × 10 mm rectangular strips were cut from each matrix and strained to break at a constant crosshead speed of 10 mm min−1 using and Instron 5842 (Instron Inc., Canton, MA). Tensile stress and strain were determined using the associated software Merlin (Instron Inc.). Samples were tested either in the dry state at 25 °C or in the wet state at 37 °C by immersion in phosphate-buffered saline (PBS) using a custom-built environmental chamber. The elastic modulus was calculated from the slope of the linear portion (0.1–5% strain range) of the stress–strain curve. To measure the thickness of the matrices, digital micrographs were obtained at various locations through an inverted microscope (Nikon TE2000U, Melville, NY) equipped with a CCD camera, as described previously [17]. These images were quantified for the thickness using image the analysis software Sigma Scan Pro (SPSS Science, Chicago, IL), calibrated using a micrograph of a hemocytometer at the same magnification. Four or five images were obtained per sample with at least 20 points per image. The calculated minimum thicknesses were used to determine the stress values in each sample.
2.5. Stress relaxation testing
Stress relaxation testing was performed using the previously described procedure [19]. In brief, 30 mm × 10 mm rectangular matrices were tested using the Instron 5542 mechanical testing machine. The load limits were predetermined by the linear portion of the stress–strain curves obtained previously under tensile testing. Samples were subjected to a constant step tensile strain applied at the rate of 1.0% s−1 for 50 s and the sample was allowed to relax for 100 s. Each single test had five steps of ramp-hold cycles.
2.6. Evaluating cell adhesion
HFF-1 cells were seeded onto each sample and maintained in serum-free FGM medium purchased from Lonza (Walkersville, MD). The cultures were maintained in 5% CO2/95% air at 37 °C with medium changes every 48 h. Cells were detached with 0.01% trypsin–10 µM EDTA, obtained from Invitrogen Corp. (Carlsbad, CA). Viable cell numbers were determined using the Trypan Blue dye exclusion assay. Ten thousand viable cells were seeded onto the control, and 25,000 cells were seeded uniformly onto the scaffolds. Growth medium (2 ml) was added and the cells were incubated for 48 h.
After 2 days, samples were fixed in 3.7% formaldehyde for 30 min at room temperature. Samples were washed three times with PBS, and permeabilized with −20 °C ethanol overnight at 4 °C [20]. The samples were stained with Alexa Fluor 546 phalloidin (Molecular Probes, Eugene, OR) for 3 h at −4 °C in the dark. Samples were counterstained with DAPI following the vendor’s protocol (Invitrogen) and observed under a fluorescence microscope (Nikon TE2000), and digital micrographs were collected from different locations using the attached CCD camera. Surface architecture of matrices was also analyzed by SEM to observe changes in the architecture due to incubation with cells.
2.7. Statistical analysis
All cell culture experiments were repeated three or more times with quadruplicate samples. All tensile testing and compliance testing were repeated four or more times and average and standard deviations were calculated. Significant differences between two groups were evaluated using a one way analysis of variance (ANOVA) with 99% confidence interval. When P < 0.01, the differences were considered to be statistically significant.
3. Results
3.1. Macroscopic properties of matrices
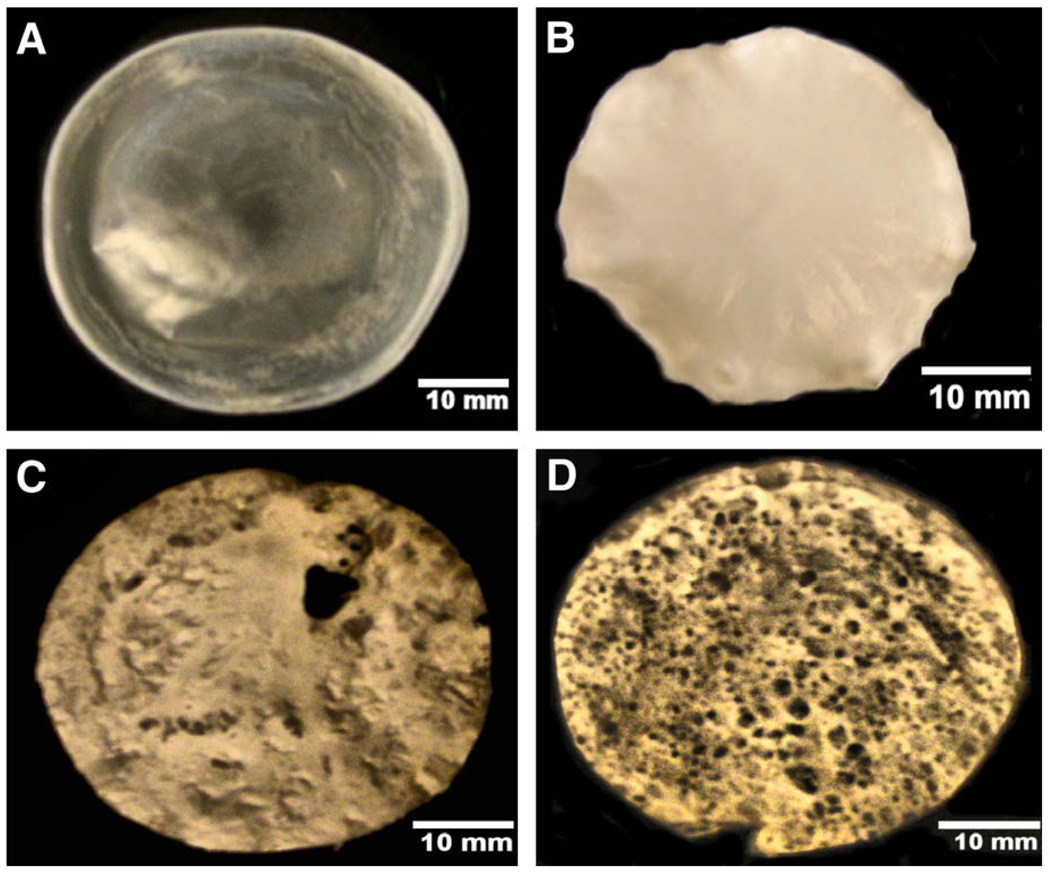
Dispersion of PCL solution in a waterbath formed matrices quickly in both methods. However, dropwise dispersion (method 1) of PCL solutions formed a uniform matrix (Fig. 1B) unlike spreading PCL solution (method 2) (Fig. 1C); matrices generated using method 1 had more uniform thickness, and these matrices were used for the subsequent analyses described below. When PCL solution stored for more than 4 days was used, no spontaneous matrix formation was observed. This could probably be due to the acid hydrolysis decreasing the molecular weight of the polymer and the inability to form an interpenetrating network. All solutions for subsequent testing were freshly prepared for forming matrices.
Fig. 1.
Macroscopic view of the PCL matrices. (A) Matrix formed in chloroform; (B) matrix formed in AA by method 1; (C) matrix formed in AA by method 2; (D) matrix formed using 8% (w/v) NaHCO3.
To test the possibility of incorporating porous architecture into the matrices, solutions were dispersed in 8% (w/v) sodium bicarbonate solution. Matrices formed much faster in these baths than in a waterbath and formation of bubbles due to the release of CO2 was also observed. These bubbles created pores in the formed matrices (Fig. 1D). When self-assembled matrices were immersed in gelatin solution, macroscopically their characteristics were identical to Fig. 1B (data not shown).
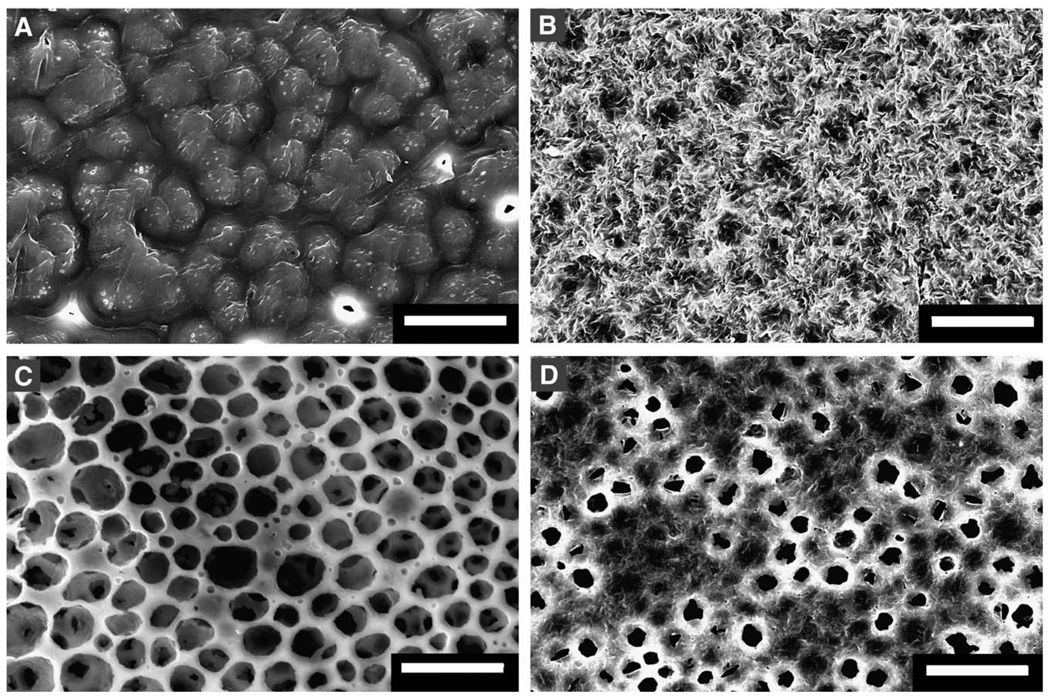
Next, formed matrices were analyzed using SEM to better understand the microarchitecture. Chloroform–cast matrices had a smooth surface and appeared like a fused network of crystals (Fig. 2A) with no porosity on both sides of the matrix. However, self-assembled PCL matrices had a rougher surface architecture and the surface facing the air had a number of micropores (Fig. 2B). Matrices formed in NaHCO3 bath showed a number of micropores although the walls appeared smooth. Upon immersion in gelatin solution, self-assembled matrices (Fig. 2D) had a reduction in pore size and surface roughness, probably due to the presence of gelatin. However, no significant change was observed in chloroform–cast PCL samples after dipping in gelatin solution.
Fig. 2.
Comparison of surface characteristics of PCL matrices. Micrographs of matrices formed by dissolving (A) PCL in chloroform; (B) PCL in AA; (C) PCL in AA and reaction with NaHCO3; (D) PCL in AA and incubation in gelatin solution. Scale bar = 50 µm.
3.2. Analysis of surface roughness
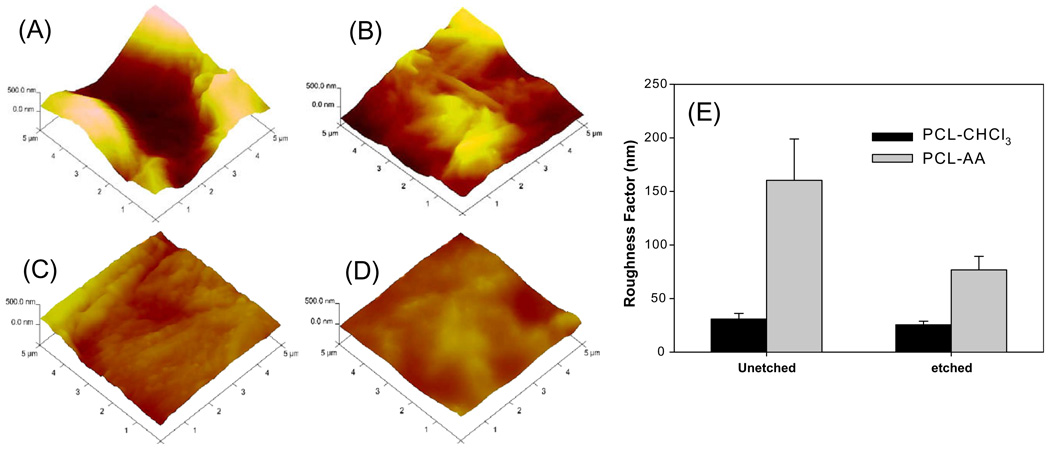
Next, the effectiveness of generating matrices on the surface roughness was compared with the chloroform–cast PCL matrices. AFM analysis of formed matrices in AA (Fig. 3A) showed a statistically significant increase in roughness relative to chloroform–cast matrices (Fig. 3C). While average roughness factor of PCL matrices formed in AA was ~150 nm, employing chloroform resulted in an average roughness factor of 30 nm (Fig. 3E).
Fig. 3.
Surface roughness characteristics. (A) PCL in AA; (B) PCL in AA after etching; (C) PCL in chloroform; (D) PCL in chloroform after etching; (E) variation in roughness values. Average and standard deviations from at least four samples for each condition are shown. Micrographs correspond to 5 µm × 5 µm area and the roughness coordinate is 500 nm.
To assess the effect of incubation in an alkaline solution for neutralization of AA bound to self-assembled matrix, PCL matrices were immersed in 1 N NaOH for 10 min. These results showed a significant reduction in the roughness (referred as etching in Fig. 3) of self-assembled PCL matrices. After 10 min of incubation in NaOH at room temperature, the roughness factor decreased to ~75 nm. There was only a marginal reduction in chloroform–cast PCL matrices (Fig. 3D). Thus while neutralizing the remaining AA in self-assembled PCL matrices, one has to consider the effect of alkaline solution concentration and the incubation time.
3.3. Alteration in surface charge
Next, we investigated whether the self-assembled PCL matrix shows a different surface charge relative to chloroform–cast matrices. For this purpose, formed matrices were assessed for surface charge using AFM. The analyzed surface in contact with the probe was that in contact with the waterbath. These results showed that self-assembled matrices had a net positive surface charge (~+9.988), whereas chloroform–cast matrices had a negative surface charge (~−9.998). Since gelatin has a net negative charge in acidic conditions, immersion in gelatin solution resulted in ionic binding of gelatin to the PCL surface. The surface charge of gelatin-coated self-assembled PCL matrix changed to negative. This suggested the possibility of immobilizing gelatin via ionic interactions. Interestingly, the alkaline etching did not alter the surface charge characteristics in both processes.
3.4. Effect on tensile properties
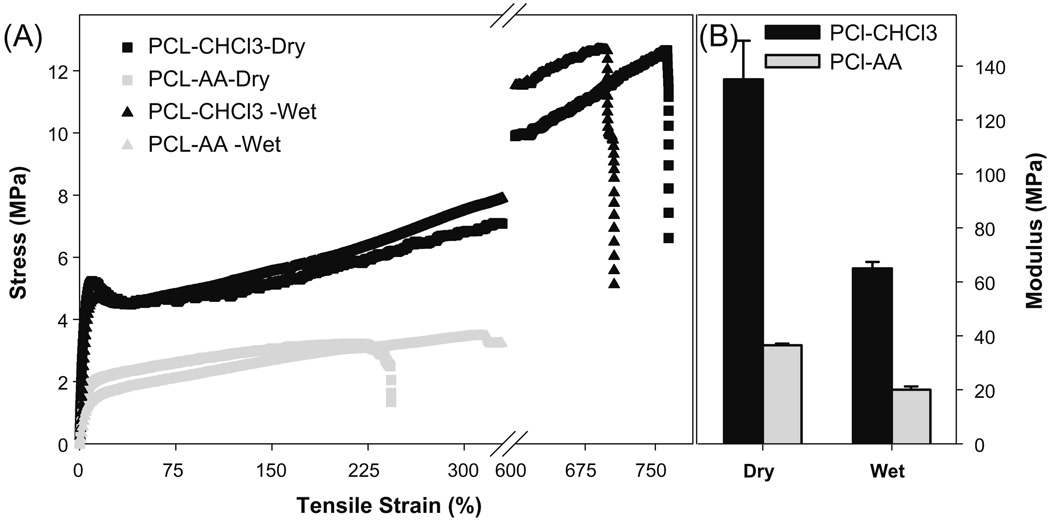
Next, alterations in the tensile strength of matrices were evaluated. These results (Fig. 4A) showed that chloroform–cast matrix had a higher tensile stress and strain than self-assembled matrix. The break strain of chloroform–cast matrix was more than 700%, and the break stress was ~12 MPa. The break strain of self-assembled matrix was ~230%, and its break stress was between 3.0 and 3.5 MPa. Elastic modulus was estimated using the initial linear range (0.1–5%) of the stress–strain curve. These results showed that self-assembled matrices were more elastic than chloroform–cast matrices (Fig. 4B). The elastic modulus of the self-assembled matrix was 40 MPa, relative to 140 MPa in chloroform–cast matrices. Both scaffolds had a similar thickness (~100 µm). However, this does not mean that the packing density of the two conditions is the same. This observed difference in elastic modulus could be due to reduced packing of polymer chains with an interpenetrated network formation during self-assembly.
Fig. 4.
Effect of different solvents on stress–strain behavior of PCL matrices. (A) Stress–strain behavior; (B) elastic modulus of matrices calculated from the linear region of the stress–strain curve.
When matrices were tested in the hydrated condition (Fig. 4A), break stress was similar to dry conditions but break strain increased in self-assembled matrices. However, in chloroform–cast matrices both break stress and break strain decreased slightly. Elastic modulus (Fig. 4B) decreased in both cases by nearly 50%.
3.5. Effect on stress relaxation properties
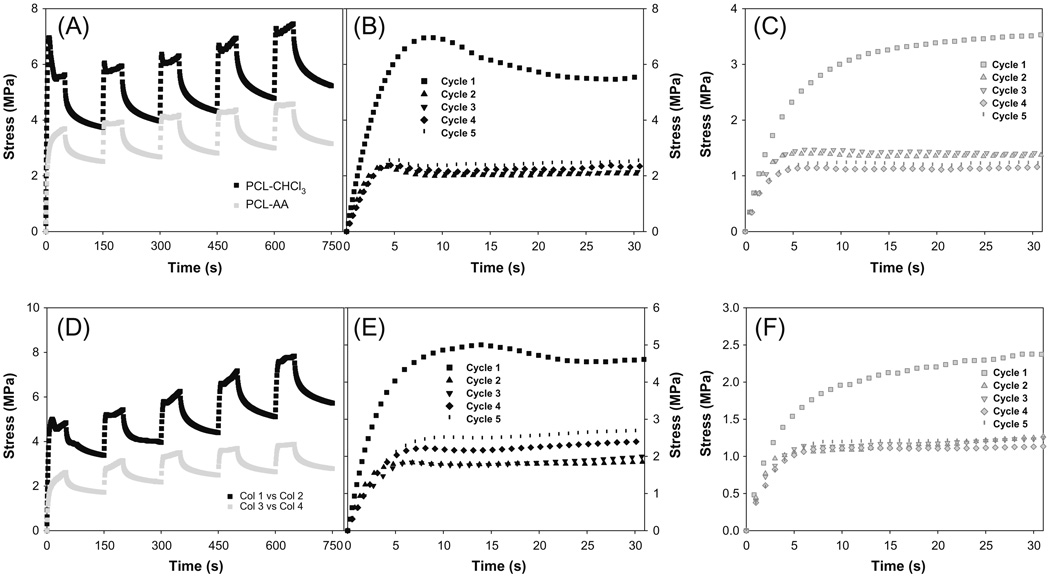
PCL is known to behave like a viscoelastic material which stores and dissipates energy within the complex molecular structure, producing hysteresis and allowing creep and stress relaxation to occur [21]. Hence, a full description of the mechanical response of materials requires nonlinear viscoelastic behavior. To understand the effect of self-assembly on the polymeric matrix, stress relaxation was tested. These results (Fig. 5) showed that both self-assembled and chloroform–cast matrices showed similar behavior in ramp and hold cycles. In the first ramp and hold cycle, both matrices showed high stress values for the same amount of strain. However, in the successive ramp-hold conditions, there were no significant differences. After the first ramp-hold, successive ramp-hold tests showed similar stress levels, suggesting that the hysteresis loss could be negligible and hence that it might be possible to use these matrices in applications where tissues will be subjected to cyclical loading conditions. Electrospun PCL fibers have been shown to have similar behavior [21]. Thus, one could refer to the first cycle as the preconditioning cycle. Nevertheless, self-assembled matrices experienced nearly half the stress relative to chloroform–cast matrices for the same strain range in all the cycles. This supported the fact that chloroform–cast matrices were harder than self-assembled matrices.
Fig. 5.
Stress relaxation behavior of different materials. (A) Effect of casting process; (B) loading behavior of chloroform–cast matrix in different cycles at the rate of 1.0% s−1 for 30 s; (C) loading behavior of self-assembled matrix in different cycles at the rate of 1.0% s−1 for 30 s; (D) effect of casting process at wet condition (37 °C); (E) loading behavior of chloroform–cast matrix in different cycles at the rate of 1.0% s−1 for 30 s at wet condition (37 °C); (F) loading behavior of self-assembled matrix in different cycles at the rate of 1.0% s−1 for 30 s at wet condition (37 °C).
One interesting difference between the two types of matrices was the level of stress experienced during the loading part of each cycle. Chloroform–cast matrices showed an initial surge in stress which reduced to a lower level despite further application of strain. This behavior was not observed in self-assembled matrices. One could attribute this difference in behavior to the alteration in the interpenetrating network architecture which accumulates and disperses the stress during loading. Importantly, preconditioning and relaxation allowed the self-assembled matrices to go beyond the break strain range observed in linear tensile loading, particularly in self-assembled PCL matrices.
3.6. Effect on cellular activity
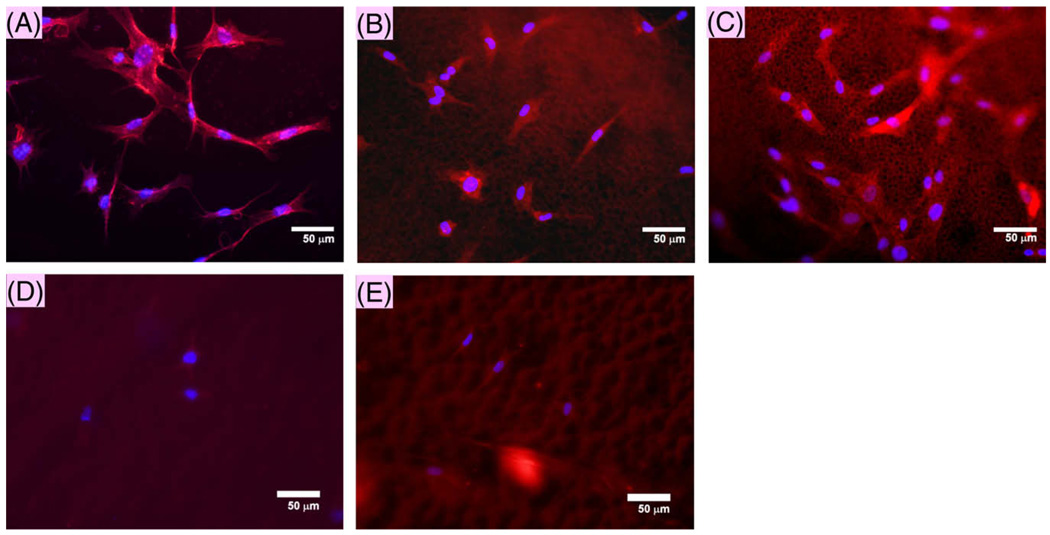
Microarchitecture analysis along with surface charge showed the possibility of immobilizing gelatin directly onto PCL. To understand the functional implications of these changes, the cell morphologies of HFF-1 were evaluated in serum-free conditions. These results (Fig. 6) showed increased presence of HFF-1 cells on self-assembled PCL matrices, with well spread spindle shape. Self-assembled matrices immersed in gelatin showed a qualitative improvement in the retention of seeded cells on the surface. Further, HFFs showed peripheral distribution of actin filaments, similar to cells on tissue culture plastic (TCP) surface. On the other hand, chloroform–cast matrices showed very few cells with and without gelatin coating. This significant difference suggests that forming self-assembled matrices in aqueous medium helps improve cell adhesion to PCL even in the absence of serum proteins. Counterstaining with DAPI confirmed the presence of nuclei, along with the actin fiber, suggesting that the PCL structure promotes cell attachment.
Fig. 6.
Cell spreading of fibroblasts. Fluorescence micrographs of cells stained for actin and nucleus after 48 h of incubation. (A) Tissue culture plastic, (B) PCL–AA, (C) PCL–AA + gelatin, (D) PCL–chloroform, (E) PCL–chloroform + gelatin.
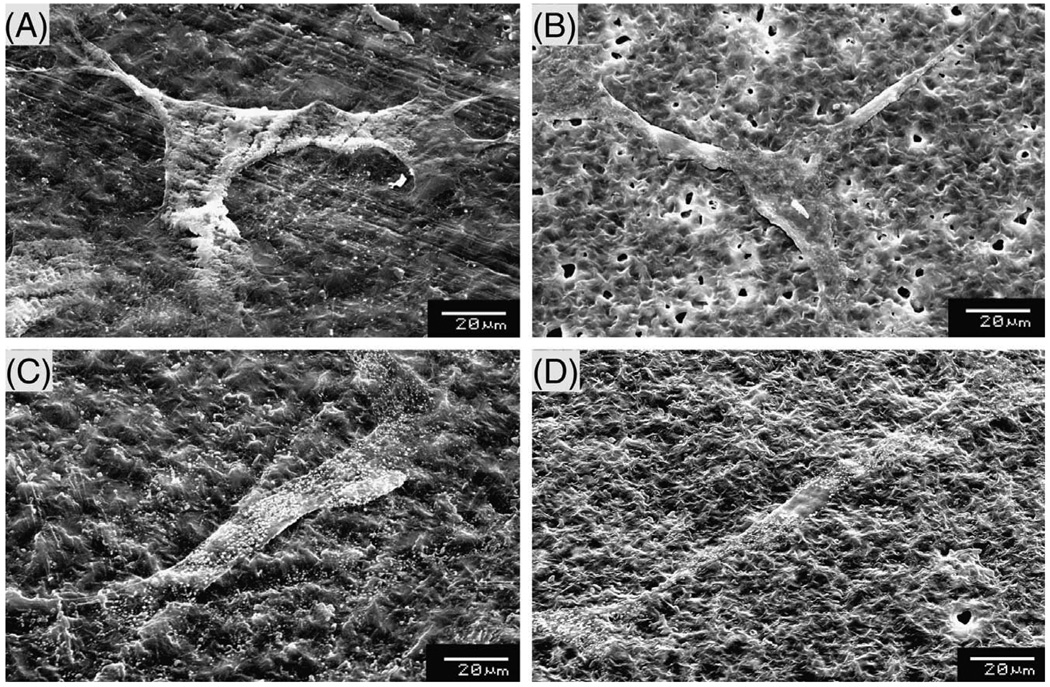
To understand the cellular interactions better, samples were evaluated using SEM (Fig. 7). The morphology of self-assembled matrices was similar to those observed prior to cell seeding; PCL-only matrices showed increased surface roughness and the presence of gelatin reduced the roughness. Cells attached and spread throughout matrix despite the presence of micropores in the matrix on self-assembled matrices. On chloroform–cast matrices, not many cells were detected, probably due to loss of weakly adherent cells during the sample preparation.
Fig. 7.
Cell spreading of fibroblasts analyzed by SEM: (A) chloroform–cast matrix; (B) PCL–AA; (C) chloroform–cast matrix and incubation in gelatin solution; (D) PCL– AA + gelatin.
4. Discussion and conclusions
In this study, a novel method of generating PCL-based matrices in an aqueous environment was evaluated. The self-assembly of PCL into matrices was observed in concentrated (>5%) solutions of PCL upon contact with water. This precipitation into stable matrices did not occur with PCL of 10 kDa Mn, probably due to the lack of stable interpenetrating network formation. The matrices that formed had increased roughness relative to chloroform–cast matrices. Other researchers have measured the surface roughness of chloroform–cast PCL matrices and reported a roughness of less than 100 nm [9].
Assessed tensile properties showed a reduction in self-assembled matrix relative to chloroform–cast matrix. However, the self-assembled matrices had a break stress that is comparable to that of small intestinal submucosa (3 MPa) [22], a natural matrix utilized in tissue regeneration. Further, the calculated elastic modulus was comparable to that of small intestinal submucosa. Hence, the reduction in elastic modulus is advantageous in tissue regeneration strategies as it reduces the mismatch in mechanical properties with the surrounding tissues. Measured stress relaxation characteristics showed less accumulation of stress in self-assembled matrices relative to chloroform–cast materials. A significant difference between self-assembled matrix and chloroform–cast matrix was relaxation during the loading phase of chloroform–cast matrix. This was not observed in self-assembled matrices, which could be attributed to alteration in the packing of the polymeric chains. However, one has to perform experiments with varying loading and relaxation times to better understand the viscoelastic behavior as viscoelastic behavior significantly depends on the loading and relaxation times. Further, one has to test the effect of temperature on the viscoelastic behavior.
Previously, our laboratory has shown the possibility of blending PCL with chitosan uniformly at low concentrations (<2%) using 70% AA solution [16,18]. When crystalline properties were evaluated (using wide-angle X-ray diffraction, melting temperature and glass transition temperature), no significant changes were observed in PCL crystalline properties. The increased roughness could be attributed to the different mode of assembly of PCL, similar to results reports by others using different solvents [8]. One possibility for different assembly is that the dissolution of PCL in acidic environment could introduce a charge in the PCL backbone. This could create an affinity for water on one side while the nonfunctionalized regions still retain hydrophobicity. Thus the hydrophobic regions could randomly aggregate, forming a rough architecture. Interestingly, surface formed in contact with water was hydrophilic as water uniformly distributed when added dropwise onto the dry matrix. However, there could be a change in the surface characteristics on the side exposed to air. One has to explore the differences in the characteristics of the matrix on both sides and understand the mechanism of self-assembly.
When cellular activity was measured, increased cell adhesion was observed on self-assembled matrices. Further, cell spreading was similar to TCP, unlike other reports in which cell spreading was shown to be minimal on chloroform–cast PCL matrices [23]. In the absence of serum, adhesion to chloroform–cast PCL was minimal. Thus the new simple technique shows a significant improvement in cellular interactions. Since matrix topography affects cell spreading characteristics [24], the observed variation in cellular activity could be attributed to the significant increase roughness. A number of previous studies have investigated systematically the effect of surface porosity and roughness on cell proliferation rates [25,26]. Further, this increase may be required to observe the marked effect as nanoscale (~100 nm) variation in surface roughness showed no significant difference in cellular activity [27]. Alternatively, the observed differences could also be due to the changes in the surface energy along with the changes in the adhesion of proteins present in the medium. However, detailed cell colonization studies, such as growth characteristics and alteration in secretion of matrix elements, have to be analyzed. In addition, analysis of cellular activity needs to be extended to include other types of cells. These findings open a new set of possibilities to understand the influence of charge and roughness separately on macromolecular interactions and cell colonization, which has not been well understood in tissue regeneration.
This study assessed only the formation of matrices in an aqueous environment. To utilize this technique, one has to explore the possibility of easy layering onto various devices in an aqueous environment. The utility of this technique in forming three-dimensional porous structures needs to be explored and compared to other techniques [28]. In addition, the three-dimensional matrices formed via a reaction to generate CO2 had pores with a wide distribution in size. Further studies are necessary to improve the method of forming porous structures with better control of pore size. Nevertheless, there are many approaches to incorporating the porous architecture. For example, one could use the self-assembled PCL matrix to support porous structures formed using natural polymers [29].
In summary, the results show that PCL matrices can be generated using the novel technique in aqueous media. Self-assembled matrices showed an increase in roughness, which was decreased after immersion in NaOH. Measured tensile properties showed a reduction in elastic modulus but with similar stress relaxation behavior relative to chloroform–cast matrices. Cell adhesion and spreading were significantly improved on the self-assembled matrix. Thus, this technique offers a significant opportunity to enhance the use of PCL in biomedical applications.
Acknowledgments
Financial support was provided by the National Institutes of Health (1R21DK074858). Authors would like to thank Ms. Pooja Iyer for help on tissue culture and Mr. Rahul Mirani on stress relaxation experiments.
Appendix A: Figures with essential colour discrimination
Certain figures in this article, particularly Figures 1, 3 and 6, are difficult to interpret in black and white. The full colour images can be found in the on-line version, at doi: 10.1016/j.actbio.2009.08.002.
References
- 1.van der Giessen WJ, Lincoff AM, Schwartz RS, van Beusekom HM, Serruys PW, Holmes DR, Jr, et al. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94(7):1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- 2.Li WJ, Cooper JA, Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2(4):377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Htay AS, Teoh SH, Hutmacher DW. Development of perforated microthin poly(epsilon-caprolactone) films as matrices for membrane tissue engineering. J Biomater Sci Polym Ed. 2004;15(5):683–700. doi: 10.1163/156856204323046933. [DOI] [PubMed] [Google Scholar]
- 4.Aliabadi HM, Mahmud A, Sharifabadi AD, Lavasanifar A. Micelles of methoxy poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization and controlled delivery of cyclosporine A. J Control Release. 2005;104(2):301–311. doi: 10.1016/j.jconrel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Engelberg I, Kohn J. Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials. 1991;12(3):292–304. doi: 10.1016/0142-9612(91)90037-b. [DOI] [PubMed] [Google Scholar]
- 6.Averous L, Moro L, Dole P, Fringant C. Properties of thermoplastic blends: starch-polycaprolactone. Polymer. 2000;41(11):4157–4167. [Google Scholar]
- 7.Lee JW, Kim YH, Park KD, Jee KS, Shin JW, Hahn SB. Importance of integrin beta1-mediated cell adhesion on biodegradable polymers under serum depletion in mesenchymal stem cells and chondrocytes. Biomaterials. 2004;25(10):1901–1909. doi: 10.1016/j.biomaterials.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Tang ZG, Black RA, Curran JM, Hunt JA, Rhodes NP, Williams DF. Surface properties and biocompatibility of solvent-cast poly[ε-caprolactone] films. Biomaterials. 2004;25(19):4741–4748. doi: 10.1016/j.biomaterials.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Z, Teoh SH. Surface modification of ultra thin poly(epsilon-caprolactone) films using acrylic acid and collagen. Biomaterials. 2004;25(11):1991–2001. doi: 10.1016/j.biomaterials.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Jones DS, Djokic J, McCoy CP, Gorman SP. Poly(epsilon-caprolactone) and poly(epsilon-caprolactone)-polyvinylpyrrolidone-iodine blends as ureteral biomaterials: characterisation of mechanical and surface properties, degradation and resistance to encrustation in vitro. Biomaterials. 2002;23(23):4449–4458. doi: 10.1016/s0142-9612(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 11.Jones DS, McLaughlin DW, McCoy CP, Gorman SP. Physicochemical characterisation and biological evaluation of hydrogel-poly(epsilon-caprolactone) interpenetrating polymer networks as novel urinary biomaterials. Biomaterials. 2005;26(14):1761–1770. doi: 10.1016/j.biomaterials.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Williamson MR, Black R, Kielty C. PCL-PU composite vascular scaffold production for vascular tissue engineering: attachment, proliferation and bioactivity of human vascular endothelial cells. Biomaterials. 2006;27(19):3608–3616. doi: 10.1016/j.biomaterials.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Chung TW, Wang YZ, Huang YY, Pan CI, Wang SS. Poly(epsilon-caprolactone) grafted with nano-structured chitosan enhances growth of human dermal fibroblasts. Artif Organs. 2006;30(1):35–41. doi: 10.1111/j.1525-1594.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 14.Serrano MC, Portoles MT, Vallet-Regi M, Izquierdo I, Galletti L, Comas JV, et al. Vascular endothelial and smooth muscle cell culture on NaOH-treated poly(epsilon-caprolactone) films: a preliminary study for vascular graft development. Macromol Biosci. 2005;5(5):415–423. doi: 10.1002/mabi.200400214. [DOI] [PubMed] [Google Scholar]
- 15.Hanson SJ, Jamshidi K, Eberhart RC. Mechanical evaluation of resorbable copolymers for end use as vascular grafts. ASAIO Trans. 1988;34(3):789–793. [PubMed] [Google Scholar]
- 16.Sarasam AR, Krishnaswamy RK, Madihally SV. Blending chitosan with polycaprolactone: effects on physicochemical and antibacterial properties. Biomacromolecules. 2006;7(4):1131–1138. doi: 10.1021/bm050935d. [DOI] [PubMed] [Google Scholar]
- 17.Raghavan D, Kropp BP, Lin HK, Zhang Y, Cowan R, Madihally SV. Physical characteristics of small intestinal submucosa scaffolds are location-dependent. J Biomed Mater Res A. 2005;73A(1):90–96. doi: 10.1002/jbm.a.30268. [DOI] [PubMed] [Google Scholar]
- 18.Sarasam A, Madihally SV. Characterization of chitosan-polycaprolactone blends for tissue engineering applications. Biomaterials. 2005;26(27):5500–5508. doi: 10.1016/j.biomaterials.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 19.Mirani RD, Pratt J, Iyer P, Madihally SV. The stress relaxation characteristics of composite matrices etched to produce nanoscale surface features. Biomaterials. 2009;30(5):703–710. doi: 10.1016/j.biomaterials.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials. 2005;26(36):7616–7627. doi: 10.1016/j.biomaterials.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Duling RR, Dupaix RB, Katsube N, Lannutti J. Mechanical characterization of electrospun polycaprolactone (PCL): a potential scaffold for tissue engineering. J Biomech Eng. 2008;130(1):011006. doi: 10.1115/1.2838033. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence BJ, Maase EL, Lin HK, Madihally SV. Multilayer composite scaffolds with mechanical properties similar to small intestinal submucosa. J Biomed Mater Res A. 2009;88(3):634–643. doi: 10.1002/jbm.a.31903. [DOI] [PubMed] [Google Scholar]
- 23.Serrano MC, Pagani R, Vallet-Reg M, Pe J, Rámila A, Izquierdo I, et al. In vitro biocompatibility assessment of poly([varepsilon]-caprolactone) films using L929 mouse fibroblasts. Biomaterials. 2004;25(25):5603–5611. doi: 10.1016/j.biomaterials.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Siewe M, Madihally SV. Effect of spatial architecture on cellular colonization. Biotechnol Bioeng. 2006;93(1):64–75. doi: 10.1002/bit.20703. [DOI] [PubMed] [Google Scholar]
- 25.Walboomers XF, Croes HJ, Ginsel LA, Jansen JA. Growth behavior of fibroblasts on microgrooved polystyrene. Biomaterials. 1998;19(20):1861–1868. doi: 10.1016/s0142-9612(98)00093-3. [DOI] [PubMed] [Google Scholar]
- 26.Degirmenbasi N, Ozkan S, Kalyon DM, Yu X. Surface patterning of poly(l-lactide) upon melt processing: in vitro culturing of fibroblasts and osteoblasts on surfaces ranging from highly crystalline with spherulitic protrusions to amorphous with nanoscale indentations. J Biomed Mater Res A. 2009;88(1):94–104. doi: 10.1002/jbm.a.31874. [DOI] [PubMed] [Google Scholar]
- 27.Serrano MC, Pagani R, Vallet-Regi M, Pena J, Ramila A, Izquierdo I, et al. In vitro biocompatibility assessment of poly(epsilon-caprolactone) films using L929 mouse fibroblasts. Biomaterials. 2004;25(25):5603–5611. doi: 10.1016/j.biomaterials.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Coombes AG, Rizzi SC, Williamson M, Barralet JE, Downes S, Wallace WA. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials. 2004;25(2):315–325. doi: 10.1016/s0142-9612(03)00535-0. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence BJ, Maase EL, Lin HK, Madihally SV. Multilayer composite scaffolds with mechanical properties similar to small intestinal submucosa. J Biomed Mater Res A. 2009;88(3):634–643. doi: 10.1002/jbm.a.31903. [DOI] [PubMed] [Google Scholar]