Abstract
Aims
The adenosine A2A receptor (ADORA2A) may ameliorate deleterious physiologic effects associated with tissue injury in individuals with diabetes. We explored associations between variants of the ADORA2A gene and proliferative diabetic retinopathy (PDR) in a cohort of patients with type 1 diabetes (T1D).
Methods
The participants were from the Pittsburgh Epidemiology of Diabetes Complications prospective study of childhood-onset T1D. Stereoscopic photographs of the retinal fundus taken at baseline, then biennially, for 10 years were used to define PDR according to the modified Airlie House system. Two tagging single nucleotide polymorphisms (tSNPs; rs2236624-C/T and rs4822489-G/T) in the ADORA2A gene were selected using the HapMap (haplotype map) reference database.
Results
A significant association was observed between SNP rs2236624 and PDR in the recessive genetic model. Participants homozygous for the T allele displayed a decreased risk of developing prevalent PDR (odds ratio, OR = 0.36; p = 0.04) and incident PDR (hazard ratio = 0.156; p = 0.009), and for all cases of PDR combined (OR = 0.23; p = 0.001). The protective effect of T allele homozygosity remained after adjusting for covariates. Similarly, for SNP rs4822489, an association between PDR and T allele homozygosity was observed following covariate adjustment (OR = 0.55; 95% CI: 0.31–0.92; p = 0.04).
Conclusion
Genetic variants of ADORA2A offer statistically significant protection against PDR development in patients with T1D.
Key Words: Diabetes, Diabetic retinopathy, Single nucleotide polymorphism, Adenosine receptor
Introduction
Diabetic retinopathy (DR) is the cause of up to 24,000 incident cases of blindness in the USA each year, and is the most prevalent cause of blindness between the second and seventh decades of life [1]. Most individuals with diabetes eventually experience some form of DR during the course of their lives; however, a greater proportion of patients with type 1 diabetes (T1D) develop proliferative DR (PDR) than do patients with T2D, with reported estimates as high as 50% compared to 10%, respectively [2,3].
The presence of DR and/or severity of DR have been shown to be partially heritable [4,5,6]. The Family Investigation of Nephropathy and Diabetes-Eye study consisting of European Americans, African Americans and Mexican Americans estimated heritability for PDR to be about 25% [7]. Observed ethnic differences in susceptibility to DR also provide some support for the potential role of genetic factors in the etiology of DR [4,5,6,7,8,9,10].
Adenosine is a powerful physiologic mediator that modulates cellular damage and the resulting tissue injury caused by biologic stressors. In the presence of oxidative stress (OS), hydrogen peroxide is formed in the cytosol, rendering vascular endothelial cells more permeable [11], and adenosine appears to ameliorate this process. The effects of adenosine are directed by adenosine receptors (AR), with the adenosine A1 receptor having a proinflammatory response to tissue injury, while the adenosine A2 receptor (ADORA2A) restricts inflammation and guards tissues from further damage [12,13]. In rats, the AR exhibit variability in function and a long-term response to ischemic brain injury [14]. Other animal studies have provided evidence that ADORA2A protects the kidneys and the heart from ischemic reperfusion injury by reducing the generation of reactive oxygen species, thereby limiting mitochondrial damage and guarding against apoptosis [15,16,17].
Growing biological evidence suggests that reactive oxygen species and OS caused by long-term exposure to hyperglycemia may be responsible for some of the tissue damage associated with microvascular complications of diabetes [18,19,20]. There is evidence in bovine retinal endothelial cells that adenosine may play a role in the upregulation of expression of a gene involved in glucose transport (GLUT1) [21]. Other investigators using electroretinogram measurements were able to demonstrate that adenosine caused vasodilatation, an increase in the rod-driven b-wave, an increase in the cone-driven b-wave, followed by a decrease in the cone-driven b-wave that was mediated by ADORA2A in the cat retina [22]. Others found that adenosine or its agonist decreased the blood flow and induced apoptosis in rat or rabbit retinae [23,24,25]. Furthermore, it has been demonstrated that OS is evident in the retinae of individuals with diabetes – a phenomenon that is likely to contribute to the development of DR [26]. ADORA2A also appears to ameliorate the effects of hypoxia, inflammation, vasodilatation, intraocular hyperglycemia and the speed of glucose metabolism out of the cell [13,21,27]. Both human and animal studies provide evidence that adenosine [28] or its receptors [29] play a role in angiogenesis [30,31]. Specifically, ADORA2A[32] has been shown to increase the proliferation of human retinal endothelial cells.
Based upon the multiple roles that adenosine and the ADORA2A gene play in ameliorating the effects of biologic stressors in humans and animals, it is reasonable to consider ADORA2A as a plausible candidate gene for increased susceptibility to or protection from the development and progression of PDR. We therefore selected tag single nucleotide polymorphisms (tSNPs) from the European ancestry (CEU) HapMap (haplotype map) data that were representative of the entire ADORA2A gene, as described below in the Methods section. We evaluated the association between tSNPs and PDR in a well-characterized sample of patients with T1D.
Research Design and Methods
Study Population
The participants in this study were from the Pittsburgh Epidemiology of Diabetes Complications (EDC) prospective study of childhood-onset (<17 years) T1D. All participants were listed in the Children's Hospital of Pittsburgh diabetes registry. Potential participants received a letter inviting them to have a physical examination and to complete several questionnaires. There were 979 eligible participants, 658 of whom (67.2%) participated in the entire EDC evaluation process [33,34]. The current study, Genetic Basis of Diabetic Retinopathy, consists of the 496 EDC participants for whom DNA was available (75.4%).
This study adhered to the tenets of the Declaration of Helsinki, and was approved by the institutional review board at the University of Pittsburgh. Written informed consent was obtained from the participants prior to baseline data collection. The data collection methodologies for this population have been published previously [34,35,36]. Data collection relevant to the Genetic Basis of Diabetic Retinopathy included baseline and biennial stereoscopic retinal examinations, measurement of blood pressure (systolic blood pressure, diastolic blood pressure), hypertension (HTN), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, glycosylated hemoglobin (GHb), body mass index (BMI), and documentation of ever-smoker status [34,35].
Determination of PDR
Dilated eye examinations and stereoscopic images of the retinal fundus were obtained for participants at baseline (1986–1988), then biennially, for those without PDR over the course of 10 years, and again at 18 years. ETDRS (Early Treatment Diabetic Retinopathy Study) fundus fields 1, 2 and 4 [37] were taken using the Zeiss Fundus camera (Carl Zeiss, Oberkochen, Germany), and diagnosis and severity grading were based on the assessment of these images by the Fundus Photography Reading Center at the University of Wisconsin using the Arlie House system. Three images were used instead of the standard 7 images since it has been previously validated that this gives acceptable results with good sensitivity and reliability [33,38]. PDR was defined as either grade ≥60 in one eye or grade <60 but with panretinal chorioretinal scars consistent with laser therapy, according to the modified Airlie House system. Baseline PDR was defined as the presence of PDR at the initial evaluation. Incident PDR was defined as PDR first diagnosed at a subsequent biennial follow-up time point.
Genotyping
Based on the European ancestry (CEU) HapMap (NCBI build 35) data (39), we identified 2 tSNPs for the ADORA2A gene. The 2 tSNPs selected for analyses in our study (rs2236624-C/T and rs4822489-G/T) were the only SNPs in the CEU population that met the selection criteria of minor allele frequency (MAF) of ≥0.2. The tSNPs were genotyped using TaqMan allele discrimination assays (Applied Biosystems, Foster City, Calif., USA) with the ABI Prism® 7000 Sequence Detection System, and the genotype assignments were determined by 2.0 ABI software (Applied Biosystems). The cycling conditions provided by Applied Biosystems yielded poor genotype cluster separation, and an extended protocol was used and resulted in improved separation and genotype discrimination. Conditions were 2 min of activation at 50°C, denaturing at 95°C, then 50 cycles of 15 s of denaturing at 95°C, followed by annealing and extension at 58°C for 1.5 min.
Statistical Analysis
The data were analyzed using SAS version 9.1.3 (SAS Institute Inc., Cary, N.C., USA). Selected covariates (age, BMI, diabetes duration, GHb, HDL, LDL, total cholesterol and triglycerides) found to be significant in the univariate analysis were included in the multivariate analyses. We conducted 3 separate analyses evaluating the association of selected tSNPs with incident PDR, prevalent PDR and all PDR cases combined. Using the more conservative Bonferroni adjustment, we set the levels of significance to be 0.025 and 0.05 for highly and marginally significant p values, respectively. As far as we know this is the first study investigating the potential role of ADORA2A in the pathophysiology of PDR.
Haploview was used to construct linkage disequilibrium plots. Genetic association analyses of PDR using additive, dominant and recessive genetic models were carried out using Plink version 1.06 [40]. The stepwise Cox proportional hazard backward regression method (significance level of 0.10), as implemented in SAS, was used to identify tSNPs associated with incident PDR while adjusting for significant covariates.
Results
A total of 161 participants (32%) developed incident PDR during the follow-up period (table 1a). There was no significant difference in duration of diabetes in those that developed incident PDR compared to those that did not (p = 0.77). In contrast, participants who developed incident PDR had a higher rate of HTN and higher mean levels of GHb, total cholesterol, LDL, triglycerides and BMI (table 1a). These findings suggest that those who did not develop PDR at the end of the follow-up period had better glycemic control and consequentially developed significantly fewer risk factors for cardiovascular disease than those who developed incident PDR.
Table 1.
Patient characteristics
| a Patients who developed incident PDR compared to patients who were PDR free at the end of follow-up | |||
|---|---|---|---|
| Incident PDR (n = 161) | No PDR at end of follow-up (n = 183) | p | |
| Age, years | 25.8 ± 6.9 | 24.8 ± 8.1 | 0.1992 |
| Duration, years | 17.0 ± 6.3 | 16.3 ± 7.1 | 0.7675 |
| Male/female, n | 79/82 | 92/91 | 0.9132 |
| GHb, % | 10.7 ± 1.9 | 9.9 ± 1.7 | <0.0001 |
| Prevalence of HTN, n | 15 (9.3) | 7 (3.8) | 0.0435 |
| Total cholesterol, mg/dl | 190 ± 40.0 | 173.3 ± 32.5 | <0.0001 |
| HDL cholesterol, mg/dl | 54.7 ± 12.1 | 55.3 ± 12.0 | 0.6155 |
| LDL cholesterol, mg/dl | 114.3 ± 31.4 | 102.2 ± 26.6 | 0.0004 |
| Triglycerides, mg/dl | 106.6 ± 87.0 | 81.1 ± 50.1 | 0.0012 |
| BMI | 23.7 ± 3.0 | 22.9 ± 3.4 | 0.0466 |
| Ever smoker, n | 46 (28.8) | 61 (34.7) | 0.1602 |
| b Patients who had PDR at baseline compared to patients who were PDR free at baseline | |||
|---|---|---|---|
| Prevalent PDR (n = 142) | No PDR at baseline (n = 183) | p | |
| Age, years | 37.2 ± 5.8 | 24.8 ± 8.1 | <0.0001 |
| Duration, years | 25.1 ± 6.1 | 16.3 ± 7.1 | <0.0001 |
| Male/female, n | 79/63 | 92/91 | 0.4562 |
| GHb, % | 10.3 ± 1.8 | 9.9 ± 1.7 | 0.0020 |
| Prevalence of HTN, % | 35.9 | 3.8 | <0.0001 |
| Total cholesterol, mg/dl | 204.9 ± 45.3 | 173.3 ± 32.5 | 0.0001 |
| HDL cholesterol, mg/dl | 51.7 ± 11.3 | 55.3 ± 12.0 | 0.0058 |
| LDL cholesterol, mg/dl | 128.9 ± 38.0 | 102.2 ± 26.6 | <0.0001 |
| Triglycerides, mg/dl | 120.8 ± 71.2 | 81.1 ± 50.1 | <0.0001 |
| BMI | 24.2 ± 3.3 | 22.9 ± 3.4 | 0.0051 |
| Ever smoker, n | 66 (47.5) | 61 (34.7) | 0.9456 |
| c All patients who developed PDR (incident and prevalent) compared to patients who were PDR free at the end of follow-up | |||
|---|---|---|---|
| Any PDR (n = 306) | No PDR (n = 185) | p | |
| Age, years | 29.0 ± 7.3 | 24.7 ± 7.9 | <0.375 |
| Duration, years | 20.8 ± 7.8 | 16.2 ± 7.0 | <0.009 |
| Male/female, n | 159/147 | 94/91 | 0.456 |
| GHb, % | 10.5 ± 1.8 | 9.9 ± 1.7 | <0.001 |
| Prevalence of HTN, n | 68 (90.7) | 7 (9.3) | <0.001 |
| Total cholesterol, mg/dl | 204.9 ± 45.3 | 173.6 ± 32.5 | <0.001 |
| HDL cholesterol, mg/dl | 53.4 ± 12.1 | 55.2 ± 11.9 | 0.145 |
| LDL cholesterol, mg/dl | 120.9 ± 35.2 | 102.5 ± 26.7 | <0.001 |
| Triglycerides, mg/dl | 113.2 ± 79.5 | 81.7 ± 50.0 | <0.001 |
| BMI | 23.9 ± 3.1 | 22.9 ± 3.4 | 0.016 |
| Ever smoker, n | 113 (64.6) | 62 (35.4) | 0.434 |
Values denote means ± SD unless otherwise specified. Figures in parentheses are percentages.
There were significant differences between participants with prevalent PDR and those who had not developed PDR at the time of their baseline evaluation (table 1b). Those with prevalent PDR tended to be older, had a longer duration of T1D and higher levels of GHb; they also had higher levels of other cardiovascular disease risk factors including total cholesterol, LDL, triglycerides and lower levels of HDL. In addition, they were heavier and had a higher prevalence of HTN. The characteristics of those who developed incident or prevalent PDR during the follow-up period versus those who did not develop PDR during the follow-up period are displayed in table 1c and are consistent with the finding reported above.
The rs2236624 tSNP is in Hardy-Weinberg equilibrium (p > 0.05), while the rs4822489 tSNP is not (p = 0.05) and shows some signs of heterozygote advantage: GG = 195, GT = 212 and TT = 84. The minor allele frequency for rs2236624 is 0.21, and for rs4822489 it is 0.39, and the r2 between the SNPs is 0.36. In the recessive genetic model, comparing all participants with PDR (i.e. prevalent plus incident cases), carriers homozygous for any T allele of SNP rs2236624 had a significant protective odds ratio (OR) of 0.23 compared to carriers of any C allele (table 2). Interestingly, the protective effect of the homozygote T allele improved (OR of 0.23 vs. 0.18) following adjustment for important covariates (i.e. duration of diabetes, GHb, HTN status and LDL cholesterol (table 2). Age, gender, BMI, ever-smoker status, HDL cholesterol and triglycerides dropped out of the model. Similarly, carriers homozygous for the T allele of SNP rs4822489 had a protective OR of 0.65 compared to carriers of any G allele, and the observed protective effect of the T allele improved after adjusting for covariates (OR = 0.55) (table 2).
Table 2.
Association of ADORA SNPs with all cases of PDR under 3 genetic models
| SNP (reference allele) | OR | 95% CI | p |
|---|---|---|---|
| rs2236624 (T) | |||
| Recessive adjusted | 0.18 | 0.06–0.59 | 0.004 |
| Dominant adjusted | 1.24 | 0.78–1.95 | 0.363 |
| Additive adjusted | 0.93 | 0.64–1.34 | 0.690 |
| rs4822489 (T) | |||
| Recessive adjusted | 0.55 | 0.31–0.97 | 0.040 |
| Dominant adjusted | 0.97 | 0.62–1.49 | 0.873 |
| Additive adjusted | 0.84 | 0.62–1.13 | 0.240 |
A djusted for duration of T1D, LDL, hypertension and GHb.
Modeling Prevalent and Incident PDR and Variants of the ADORA2A Separately
Among prevalent cases and in a recessive model, carriers homozygous for the T allele (rs2236624) had a protective OR of 0.36 compared to carriers of any C allele (p = 0.04; 95% CI: 0.128–0.991). The observed protective effect was enhanced with covariate adjustment. The additive and dominant models did not reveal any association with prevalent PDR in the univariate or multivariate models for PDR (data not shown). SNP rs4822489 was not associated with prevalent PDR in the additive, dominant or recessive models (data not shown).
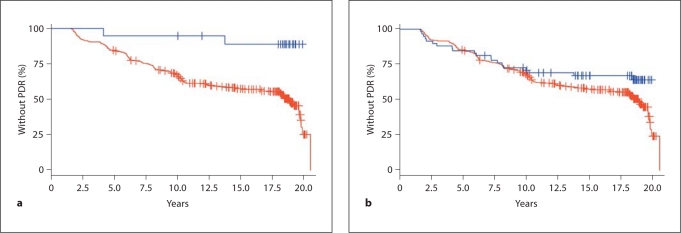
There were 162 incident cases of PDR during the 20-year follow-up period. The Cox proportional hazards regression showed that carriers homozygous for the T allele of SNP rs2236624 were less likely to progress to PDR compared to carriers homozygous for the C allele, resulting in a protective hazard ratio (HR) of 0.156 (95% CI: 0.040–0.63; p = 0.009); covariate adjustment significantly improved the observed protective effect from a HR of 0.156 to 0.090 (95% CI: 0.01–0.64; p = 0.016). Kaplan-Meier analysis showed that only 11% of those homozygous for the T allele progressed to PDR during follow-up compared to 48.9% of carriers of any copy of the C allele (fig. 1a). The two-group log rank χ2 test showed that the observed difference in time free of PDR (i.e. time to event) was significantly different (p = 0.0027) between carriers homozygous for the T allele and carriers of any copy of the C allele.
Fig. 1.
a Kaplan-Meier curve showing follow-up time for development of PDR in 2.5-year increments by rs2236624 genotype. Blue line: T allele. Red line: C allele. b Kaplan-Meier curve showing follow-up time for development of PDR in 2.5-year increments by rs4822489 genotype. Blue line: T allele. Red line: G allele.
Similar, though not as dramatic, results were observed for rs4822489: carriers homozygous for the T allele of SNP rs4822489 were less likely than carriers of any G allele to progress to PDR during follow-up (HR = 0.67; 95% CI: 0.42–1.07; p = 0.093). Thirty-four percent of those homozygous for the T allele progressed to PDR compared to 49% among carriers of any G allele. The two-group log rank χ2 test revealed marginal significance (p = 0.091) (fig. 1b).
Haplotypes were generated from the 2 SNPs with the following frequencies: TC 594 (61.36%), GC 172 (17.77%), TG 1 (0.10%) and GG 201 (20.86%). The TG haplotype was excluded from the analysis as there was only 1 individual with this haplotype. None of the haplotypes were associated with PDR (data not shown).
Discussion
The analyses of this prospective study of persons with T1D demonstrate that variants of the ADORA2A gene confer significant protection against the development of PDR in persons with T1D. At baseline, the OR of developing PDR was only 0.23 for carriers of 2 copies of the T allele compared to carriers of any copy of the C allele. Remarkably, only 10% of the carriers of 2 copies of the T allele developed incident PDR compared to 50% for carriers of any copy of the C allele. As far as we know, this is the first study to implicate the ADORA2A gene in PDR in individuals with childhood-onset T1D.
The reason for this decreased association for carriers homozygous for the T allele with PDR is not clear and warrants further investigation. While they appear to be protected against development of PDR, we note that the number of participants homozygous for the T allele was small (n = 24 for rs2236624), and only 2 of the 24 developed PDR during follow-up. For rs4822489, 84 were homozygotes for the protective allele, of which 20 progressed to PDR during follow-up. However, the biologic functions of the ADORA2A gene, specifically its role in angiogenesis and protection against biologic stressors associated with diabetes, suggest that the OR and HR associated with these data are unlikely to be due solely to small sample sizes of participants homozygous for the T allele.
The ADORA2A gene is part of the G-protein-coupled receptor super family. It is expressed in the basal ganglia, blood vessels, platelets and other tissues in the body. It encompasses 1 intron and 2 exons [41,42,43,44]. SNP rs2236624 is in linkage disequilibrium with 15 currently known SNPs. Four of these SNPs are within exons but are synonymous coding SNPs, 3 are intronic, 6 lie in the 3′>untranslated region, 1 is a nonsynonymous SNP and another is a frame shift polymorphism [45].
At this stage of the investigation, the reasons for the differences in the phenotypes associated with the 2 tSNPs in our study are not clear and require further exploration. Investigators have reported that ADORA2A receptor activation may decrease vascular endothelial growth factor (VEGF) and endothelial cell production in rodent pheochromocytoma cells [46]. Similar modulation of VEGF may occur in humans and, thus, the possibility of influencing the development and/or the progression of PDR.
Myriad physiological processes may be affected by adenosine and its receptors in vivo since it is found in the vasculature of all organs and is a byproduct of adenosine triphosphate metabolism. Variation in ADORA2A expression and the ability of the receptor to bind with adenosine may have an impact on its ability to attenuate the negative effects of OS and other biologic homeostatic processes in ways that have yet to be elucidated. To date adenosine has been linked to retinal VEGF production [32,47] and regulation of angiogenesis via VEGF production [32,48]. It is also suspected to play a role in interleukin-8, mast cell and insulin-like-growth-factor-induced angiogenesis [49].
ADORA2A stimulation has been linked to psychomotor depression, sleep induction, immune suppression and vasodilatation, while its suppression has been linked to HTN, aggression and inflammation, alleviation of symptoms of ethanol withdrawal and amelioration of neurotoxicity [44]. There is evidence that ADORA2A activation decreases the expression of VEGF, a primary mitogen associated with the development of DR [46]. Experimental models have also shown that the ADORA2A receptor plays a role in glucose transport, vasodilatation, hypoxia, and inflammation, resolution of inflammation and prevention of apoptosis [13,16,21,50,51].
This study evaluated only the ADORA2A gene, which is one of 4 members of the adenosine receptor family. ADORA1 plays a significant role in the proinflammatory responses of the cell, while ADORA2A and ADORA2B play significant roles in limiting the cell response to inflammation, thereby producing an antiinflammatory response. ADORA3 plays a role in the cell's response to ischemia [13]. The potential role of the remaining 3 genes of the adenosine receptor family in PDR susceptibility deserves further investigation.
The majority (98%) of the participants enrolled in this study of T1D were of European ancestry. As a result, the generalizability of these findings awaits confirmation in other ancestral population groups. The fact that the mean diabetes duration was 19 years at the time of entry into the study limited our ability to explore the natural history of DR in reference to specific genotypes. However, because 85% of the cohort had some form of DR at the time of entry into the study, we were able to explore the effect of genetic variation on the progression to PDR. It would be beneficial to explore the pathophysiologic role of the ADORA2A gene in patients with T2D.
Replication of our observed associations in other cohorts, fully evaluating this segment of the gene for underlying variability, as well as functional characterization of this variability regarding receptor function or mRNA stability are potential areas of further investigation. Based on the HapMap populations, there is a high frequency of the T allele in populations of Asian ancestry (29.8% in Han Chinese from Beijing China, CHB, and 22.1% in Japanese from Tokyo, JPT) and Western and Northern European ancestry (CEU; 23.5%). In contrast, it is far less common in the HapMap Yoruba sample from Ibadan, Nigeria (YRI; 0.9%). These observations suggest that a more comprehensive documentation of the global distribution of the genetic variation of the ADORA2A gene may contribute to the understanding of how this gene influences differential susceptibility to visual complications of diabetes, particularly the growing evidence that DR is less prevalent in West African individuals with diabetes than in Caucasians [52].
In summary, findings from this study should spark interest in all of the AR as potential modifiers in diabetes complications. If replicated, this association of ADORA2A with PDR could lead to additional studies of ADORA2A and its role in humans with DR and other microvascular complications of diabetes. Illustratively, the potential therapeutic effects of adenosine in ischemic retinal diseases have been suggested [22].
Acknowledgements
We would like to thank/acknowledge Dr. Adebowale Adeyemo, Deputy Director, of the Center for Research on Genomics and Global Health, National Human Genome Research Institute, National Institutes of Health (NIH), for his contributions to the manuscript. This research was conducted at the University of Pittsburgh and supported by grants NIH/National Institute of Nursing Research 1F31NR008970-01A2 ‘Genetic Basis of Diabetic Retinopathy’, Charles B. (PI), American Nurses Foundation ‘Genes Implicated in Time to Onset and Severity of Diabetic Retinopathy’, Charles B. (PI), and NIH/National Institute of Diabetes and Digestive and Kidney Diseases R01 DK034818-21 ‘Epidemiology of Diabetes Complications, Phase II’, Orchard (PI). This research was also supported in part by the Intramural Research Program of the National Human Genome Research Institute, NIH, in the Center for Research in Genomics and Global Health (Z01HG200362).
References
- 1.American Diabetes Association . All about diabetes. In: American Diabetes Association, editor. All About Diabetes. American Diabetes Association; 2008. [Google Scholar]
- 2.Frank R. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. 3. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 4.Hallman M, Huber C, Gonzalez V, Klein BE, Klein R, Hanis C. Familial aggregation of severity of diabetic retinopathy in Mexican Americans from Starr County, Texas. Diabetes Care. 2005;28:1163–1168. doi: 10.2337/diacare.28.5.1163. [DOI] [PubMed] [Google Scholar]
- 5.Rema M, Saravanan R, Deepa R, Mohan V. Familial clustering of diabetic retinopathy in South Indian type 2 diabetic patients. Diabet Med. 2002;19:910–916. doi: 10.1046/j.1464-5491.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arar NH, Freedman BI, Adler SG, Iyengar SK, Chew EY, Davis MD, Satko SG, Bowden DW. Heritability of severity of diabetic retinopathy: the FIND-Eye study. Invest Ophthalmol Vis Sci. 2008;49:3839–3845. doi: 10.1167/iovs.07-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes Control and Complications Trial Research Group Clustering of long-term complications in families with diabetes in the Diabetes Control and Complications Trial. Diabetes. 1997;46:1829–1839. [PubMed] [Google Scholar]
- 9.Harris M, Klein R, Cowie C, Rowland M, Byrd-Holt D. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A US population study. Diabetes Care. 1998;21:1230–1235. doi: 10.2337/diacare.21.8.1230. [DOI] [PubMed] [Google Scholar]
- 10.Tudor S, Hamman R, Baron A, Johnson D, Shetterly S. Incidence and progression of diabetic retinopathy in Hispanics and non-Hispanic whites with type 2 diabetes. San Luis Valley Diabetes Study, Colorado. Diabetes Care. 1998;21:53–61. doi: 10.2337/diacare.21.1.53. [DOI] [PubMed] [Google Scholar]
- 11.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 12.Salmon JE, Brogle N, Brownlie C, Edberg JC, Kimberly RP, Chen B-X, Erlanger BF. Human mononuclear phagocytes express adenosine a1-receptors. J Immunol. 1993;151:2775–2785. [PubMed] [Google Scholar]
- 13.McKusick VA. Mendelian Inheritance in Man. A Catalog of Human Genes and Genetic Disorders. Baltimore: Johns Hopkins University Press; 2006. [Google Scholar]
- 14.Trincavelli ML, Melani A, Guidi S, Cuboni S, Cipriani S, Pedata F, Mattini C. Regulation of A2A adenosine receptor expression and functioning following permanent focal ischemia in rat brain. J Neurochem. 2008;104:479–490. doi: 10.1111/j.1471-4159.2007.04990.x. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Park S-S, Mueller RA, Bagnell RC, Patterson C, Boysen PG. Adenosine produces nitric oxide and prevents mitochondrial oxidant damage in rat cardiomyocytes. Cardiovasc Res. 2005;65:803–812. doi: 10.1016/j.cardiores.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Mueller RA, Park S-S, Boysen PG, Cohen MV, Downey JM. Cardioprotection with adenosine A2 receptor activation at reperfusion. J Cardiovasc Pharmacol. 2005;46:794–802. doi: 10.1097/01.fjc.0000188161.57018.29. [DOI] [PubMed] [Google Scholar]
- 17.Yonehana T, Gemba M. Ameliorative effect of adenosine on hypoxia-reoxygenation injury in LLC-PK1, a porcine kidney cell line. Jpn J Pharmacol. 1999;80:163–167. doi: 10.1254/jjp.80.163. [DOI] [PubMed] [Google Scholar]
- 18.Kostolanska J, Jakus V, Barak L. HbA1c and serum levels of advanced glycation and oxidation protein products in poorly and well-controlled children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2009;22:433–442. doi: 10.1515/jpem.2009.22.5.433. [DOI] [PubMed] [Google Scholar]
- 19.Kostolanska J, Jakus V, Barak L. Monitoring of early and advanced glycation in relation to the occurrence of microvascular complications in children and adolescents with type 1 diabetes mellitus. Physiol Res. 2009;58:553–561. doi: 10.33549/physiolres.931612. [DOI] [PubMed] [Google Scholar]
- 20.Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11:3071–3109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 21.Takagi H, Kinh GL, Aiello LP. Hypoxia upregulates glucose transport activity through and adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes. 1998;47:1480–1488. doi: 10.2337/diabetes.47.9.1480. [DOI] [PubMed] [Google Scholar]
- 22.Macaluso C, Frishman LJ, Frueh B, Kaelin-Lang A, Onoe S, Niemeyer G. Multiple effects of adenosine in the arterially perfused mammalian eye: possible mechanisms for the neuroprotective function of adenosine in the retina. Doc Ophthalmol. 2003;106:51–59. doi: 10.1023/a:1022456615715. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama T, Oku H, Komori A, Ikeda T. Effect of P2X7 receptor activation on the retinal blood velocity of diabetic rabbits. Arch Ophthalmol. 2006;124:1143–1149. doi: 10.1001/archopht.124.8.1143. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama T, Kobayashi M, Kawamura H, Li Q, Puro DG. Enhancement of P2X7-induced pore formation and apoptosis: an early effect of diabetes on the retinal microvasculature. Invest Ophthalmol Vis Sci. 2004;45:1026–1032. doi: 10.1167/iovs.03-1062. [DOI] [PubMed] [Google Scholar]
- 25.Liao SD, Puro DG. NAD+-induced vasotoxicity in the pericyte-containing microvasculature of the rat retina: effect of diabetes. Invest Ophthalmol Vis Sci. 2006;47:5032–5038. doi: 10.1167/iovs.06-0422. [DOI] [PubMed] [Google Scholar]
- 26.Pan H-Z, Zhang H, Chang D, Li H, Sui H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br J Ophthalmol. 2008;92:548–551. doi: 10.1136/bjo.2007.130542. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto T, Umenmura S, Toya Y, Uchibori T, Kogi K, Takgi N, Ishii M. Identification of adenosine A2 receptor-cAMP system in human aortic endothelial cells. Biochem Biophys Res Commun. 1994;199:905–910. doi: 10.1006/bbrc.1994.1314. [DOI] [PubMed] [Google Scholar]
- 28.Adair TH, Montani JP, Strick DM, Guyton AC. Vascular development in chick embryos: a possible role for adenosine. Am J Physiol. 1989;256:H240–H246. doi: 10.1152/ajpheart.1989.256.1.H240. [DOI] [PubMed] [Google Scholar]
- 29.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90:531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- 30.Sapieha P, Hamel D, Shao Z, Rivera JC, Zaniolo K, Joyal JS, Chemtob S. Proliferative retinopathies: angiogenesis that blinds. Int J Biochem Cell Biol. 2010;42:5–12. doi: 10.1016/j.biocel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Sapieha P, Sirinyan M, Hamel D, Zaniolo K, Joyal J-S, Cho J-H, Honoré J-C, Kermorvant-Duchemin E, Varma DR, Tremblay S, Leduc M, Rihakova L, Hardy P, Klein WH, Mu X, Mamer O, Lachapelle P, di Polo A, Beauséjour C, Andelfinger G, Mitchell G, Sennlaub F, Chemtob S. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- 32.Grant MB, Tarnuzzer RW, Caballero S, Ozeck MJ, Davis MI, Spoerri PE, Feoktistov I, Biaggioni I, Shryock JC, Belardinelli L. Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res. 1999;85:699–706. doi: 10.1161/01.res.85.8.699. [DOI] [PubMed] [Google Scholar]
- 33.Kostraba JN, Klein R, Dorman JS, Becker DJ, Drash AL, Maser RE, Orchard TJ. The epidemiology of diabetes complications study. Am J Epidemiol. 1991;133:381–391. doi: 10.1093/oxfordjournals.aje.a115892. [DOI] [PubMed] [Google Scholar]
- 34.Costacou T, Chang Y, Ferrell RE, Orchard TJ. Identifying genetic susceptibilities to diabetes-related complications among individuals at low risk of complications: an application of tree-structured survival analysis. Am J Epidemiol. 2006;164:862–872. doi: 10.1093/aje/kwj287. [DOI] [PubMed] [Google Scholar]
- 35.Lloyd CE, Klein R, Maser RE, Kuller LH, Becker DJ, Orchard TJ. The progression of retinopathy over 2 years: the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study. J Diabetes Complications. 1995;9:140–148. doi: 10.1016/1056-8727(94)00039-q. [DOI] [PubMed] [Google Scholar]
- 36.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 37.Diabetic Retinopathy Study Research Group A modification of the Arlie House classification of diabetic retinopathy: Diabetic Retinopathy Study report No. 7. Invest Ophthalmol Vis Sci. 1981;21:210–226. [PubMed] [Google Scholar]
- 38.Moss SE, Meuer S, Klein R, et al. Are seven standard photographic fields necessary for classification of diabetic retinopathy? Invest Ophthalmol Vis Sci. 1989;30:823–828. [PubMed] [Google Scholar]
- 39.International HapMap Consortium The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 40.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira M, Bender D, Maller J, Sklar P, de Bakker P, Daly M, Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maglott D, Ostell J, Pruitt KD, Tatusova T. Entrez Gene: gene-centered information at NCBI. Nucleic Acids Res. 2007;35:D26–D31. doi: 10.1093/nar/gkl993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hubbard TJP, Aken BL, Beal K, Ballester B, Caccamo M, Chen Y, Clarke L, Coates G, Cunningham F, Cutts T, Down T, Dyer SC, et al. Ensembl 2007. Nucleic Acids Res. 2007;35:D610–D617. doi: 10.1093/nar/gkl996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le F, Townsend-Nicholson A, Baker E, Sutherland GR, Schofield PR. Characterization and chromosomal localization of the human A2A adenosine receptor gene: ADORA2A. Biochem Biophys Res Commun. 1996;223:461–467. doi: 10.1006/bbrc.1996.0916. [DOI] [PubMed] [Google Scholar]
- 44.Yu L, Firth MC, Suzuki Y, Peterfreund RA, Gearan T, Sugano S, Schwarzschild MA, Weng Z, Fink SJ, Chen J-F. Characterization of genomic organization of the adenosine A2A receptor gene by molecular and bioinformatics analysis. Brain Res. 2004;1000:156–173. doi: 10.1016/j.brainres.2003.11.072. [DOI] [PubMed] [Google Scholar]
- 45.Sherry S, Ward M, Kholodov M, Baker J, Phan L, Smigielski E, Sirotkin K. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olah ME, Roudabush FL. Down-regulation of vascular endothelial growth factor expression after A2A adenosine receptor activation in PC12 pheochromocytoma cells. J Pharmacol Exp Ther. 2000;293:779–787. [PubMed] [Google Scholar]
- 47.Lutty G, Matthews M, Merges C, McLeod D. Adenosine stimulates canine retinal microvascular endothelial cell migration and tube formation. Curr Eye Res. 1998;17:594–607. [PubMed] [Google Scholar]
- 48.Leibovich S, Chen J, Pinhal-Enfield G, Belem P, Elson G, Rosania A, et al. Synergistic upregulation of vascular endothelial growth factor expression in murine macrophages by adenosine A2 receptor agonists and endotoxin. Am J Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valls MD, Cronstein BN, Montesinos MC. Adenosine receptor agonists for promotion of dermal wound healing. Biochem Pharmacol. 2009;77:1117–1124. doi: 10.1016/j.bcp.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frobert O, Hanink G, Simonsen U, Gravholt CH, Levin M, Deussen A. Adenosine concentration in the porcine coronary artery wall and A2A receptor involvement in hypoxia-induced vasodilation. J Physiol. 2006;570:375–384. doi: 10.1113/jphysiol.2005.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H, Emala C. Systemic adenosine given after ischemia protects renal function via A2A adenosine receptor activation. Am J Kidney Dis. 2001;38:610–618. doi: 10.1053/ajkd.2001.26888. [DOI] [PubMed] [Google Scholar]
- 52.Rotimi C, Danile H, Zhou J, Obisesan A, Chen G, Chen Y, Amoah A, Opoku V, Acheampong J, Agyenim-Boateng K, Eghan BA, Jr, Oli J, Okafor G, Ofoegbu E, Osotimehin B, Abbiyesuku F, Johnson T, Fasanmade O, Doumatey A, Aje T, Collins F, Dunston G. Prevalence and determinants of diabetic retinopathy and cataract in West African type 2 diabetes patients. Ethn Dis. 2003;13:S100–S107. [PubMed] [Google Scholar]