Abstract
Donor- and third-party-induced proliferation of T-helper (Th) and T-cytotoxic (Tc) cells, and their naïve and memory subsets was evaluated simultaneously in single blood samples from 77 children who received steroid-free liver transplantation (LTx) after induction with rabbit anti-human thymocyte globulin. Proliferation was measured by dilution of the intravital dye carboxy-flourescien-succinimidyl-ester (CFSE) in 3–4 day MLR co-culture. The ratio of donor: third-party-induced proliferated, (CFSElow) T-cells was reported as the immunoreactivity index (IR) for each subset. Rejectors were defined as those who experienced biopsy-proven acute cellular rejection within 60 days of the assay. IR>1 signified increased risk of rejection and IR<1 implied decreased risk.
Results
Demographics for 32 Rejectors and 45 Non-Rejectors were similar. Proliferated CFSElow T-cells and subsets were significantly higher among Rejectors, compared with Non-Rejectors. In 33 of 77 randomly selected children, logistic regression, leave-one-out cross-validation and ROC analyses showed that the IR of Tc associated best with biopsy-proven rejection (sensitivity>75%, specificity>88%). Sensitivity/specificity were replicated in the remaining 44 children, comprising the validation cohort. IR of CFSElow Tc correlated significantly with IR of pro-inflammatory, allospecific CD154+Tc (r=0.664, p=0.0005), and inversely with IR of allospecific, anti-inflammatory, CTLA4+Tc (r=−0.630, p=0.007).
Conclusions
Proliferative alloresponses of T-cytotoxic cells can identify rejection-prone children receiving LTx. (200)
Introduction
Roughly 600 children receive liver transplantation (LTx) in the United States each year (1). Over half of these children experience immunosuppressant drug failures such as organ rejection, or life-threatening infections and malignancies (2, 3). These failures may be prevented by titrating immunosuppressants precisely, based on the status of donor-specific alloimmunity at any given time. Previous work with the mixed lymphocyte response (MLR) of undifferentiated peripheral blood leukocytes (PBL) supports this view (4, 5). Enhanced donor-specific alloreactivity measured by the proliferative 3thymidine-MLR occurs in children with LTx, at the time of early rejection, and at the time of late rejection during routine clinical minimization of immunosuppressants. Because proliferation is relatively non-specific, and takes days to manifest, “bystanders” can be recruited into the proliferative response, thereby diluting donor-specific proliferation, and reducing the sensitivity of any such assay. In one adaptation of the MLR, the ELISPOT, the antigen-specificity of PBL or pre-sorted T-cells is measured by secreted IFNγ, and shows a higher sensitivity for association with rejection and non-rejection outcomes in renal transplantation (6–11). However, a large proportion of pediatric LTx recipients weigh 4–10 kg. In these children, the blood sample volume needed for the ELISPOT may be unsafe, if added to clinical sampling requirements. Another adaptation, the CFSE-MLR, which measures donor-induced proliferation by dilution of the intravital dye carboxy-flourescien-succinimidyl-ester, 5–7-day culture shows greater promise in children because of lower blood sample requirements. Because flow cytometry is used to measure dye dilution in the various T-cell subsets, each subset can be investigated separately for the strength of its association with rejection and non-rejection outcomes. One example is a suggested association between rejection-free outcomes in children who have received living donor LTx, and decreased donor-specific proliferation of the CD8+CD25+T-cell subset (12,13). However, such associations remain unconfirmed by sensitivity and specificity testing in independent replication cohorts.
In the current study, we have evaluated the sensitivity and specificity of proliferating T-helper (Th, CD4+) and T-cytotoxic (Tc, CD8+) cells and their memory (CD45RO+) and naïve (CD45RO−) subsets, for their association with rejection outcomes in 77 children with LTx. The MLR co-culture duration has been reduced to 3–4 days. All children have received lymphocyte depleting induction with rabbit anti-human thymocyte globulin (rATG, Genzyme, Cambridge, MA), and steroid-free Tacrolimus monotherapy, as described previously (4, 5). We have benchmarked the performance of each proliferating T-cell subset by seeking positive correlations with the highly sensitive allo-(antigen)-specific CD154+T-cells, which measure a pro-inflammatory alloresponse, and negative correlations with allospecific CTLA4+T-cell subsets, which represent a suppressive or anti-inflammatory T-cell phenotype. The clinical significance of allospecific CD154+T-cells and CTLA4+T-cells has been recently reported from our laboratory (14). As in these recent studies, we report the results of the CFSE-MLR as the ratio of donor- and third-party-induced proliferation, or the immunoreactivity index (IR) for each proliferating T-cell subset. We continue to hypothesize that IR>1 indicates enhanced donor-specific alloreactivity and increased risk of rejection, and IR<1 indicates decreased risk. Finally, we have randomly divided the test population into a screening and replication cohort to confirm whether associations between clinical outcomes and the alloresponsive T-cell subset are robust. Our results show that donor-specific proliferation of T-cytotoxic cells identifies rejection-prone children with LTx with good sensitivity and specificity.
Methods
All studies were approved by the University of Pittsburgh Institutional Review Board. The results of a single CFSE-MLR from 77 non-consecutive children, who received LTx, were analyzed. All children received steroid-free Tacrolimus, and 5 mg/kg rATG (15). Target Tacrolimus whole blood concentration (FKWB) were 12–15 ng/ml in the first month, and 8–10 ng/ml after the third month. By the end of month 12, target levels were 5–7 ng/ml. If acute cellular rejection (ACR) occurs, these targets are delayed by 3–6 months. Rejectors (R) were defined as children who experienced biopsy-proven ACR within 60 days after LTx, or in whom late ACR occurred in addition to an early ACR episode. The 60-day risk period for early ACR was chosen because early liver ACR occurs toward the end of the first, and beginning of the second month after rATG induction. The risk period for late ACR began >60 days after LTx, because late ACR is usually associated with clinical drug minimization, which was initiated 60 days after LTx. All Rejectors included in this study showed biopsy-proven ACR within 60 days of performing the assay (n=32). In the remaining 45 subjects who were termed Non-Rejectors, the rejection-free course was inferred from normal liver function tests (n=37), or with an allograft biopsy, if liver function tests were abnormal (n=8).
CFSE-MLR
Ficoll-separated PBL obtained from 3–4 ml of whole blood from each subject were suspended at concentration 5×105–1×106 cells/ml in RPMI with 10% fetal calf serum, and labeled with 4 μM CFSE as described previously (16). Briefly, 100,000–1,000,000 CFSE-labeled PBL from each recipient were cultured alone and 1:1 with live donor/surrogate donor or third-party PBL. Live, non-irradiated donor and third-party PBL were pre-labeled with anti-CD45-allophycocyanin prior to incubation for co-culture. This allowed separation of stimulator and responder PBL by flow cytometry, at the end of 3–4-day co-culture. Donor cells were only available for 12 living donor LTx, and included 4 of 32 Rejectors, and 8 of 45 Non-Rejectors. For 65 recipients of cadaveric liver grafts, stimulators consisted of banked PBL (surrogate donor, SD), matched with donor at a minimum of one antigen each for HLA-A, HLA-B and HLA-DR loci. All incubations were at 37°C and 5% CO2 for 3–4 days.
After incubation was completed, the cells were washed with PBS and labeled with anti-CD3-PE (phycoerythrin), anti-CD4-APC-Cy7 (allophycocyanin-cyanin-7), anti-CD45RO-Texas Red and 7-AAD (amino-actinomycin-D) prior to acquisition. Anti-CTLA-4-PE-Cy-5 (Phycoerythrin-Cyanine-5) was also added in the 17 most recent assays. Data acquisition was performed with FACS-DIVA software on the LSRII flow cytometer (Becton-Dickinson, San Jose, CA.). Both scatterplot and histogram representations were used to first separate recipient PBL from anti-CD45-APC-labeled stimulators, and dead cells (7-AAD+) from live cells. Thereafter, live proliferating recipient PBL were distinguished from non-proliferating recipient PBL on the basis of decreased fluorescence due to CFSE (CFSElow). The CFSElow daughter generations were enumerated collectively as representative of alloresponsive recipient cells. This approach differs from several previous reports in which multiple daughter generations are characterized with DNA replication software after 5–7-day co-culture. In our study, the shorter culture duration of 3–4 days, and our use of lymphocyte depleting protocols likely attenuated the proliferative response. Therefore, it was easier to visualize proliferating (CFSElow) cells as a single group, than as a collection of multiple generations of daughter cells. To reduce other sources of error, the gates enclosing proliferated CFSElow daughter generations of alloreactive cells, once identified, were kept constant while analyzing data acquired from recipient PBL incubated alone, with donor/surrogate donor, or third-party. Absolute counts of CFSElow alloreactive cells within each subset were used to calculate the IR and after normalized to per 10,000 cells.
To develop a parameter(s) for risk of rejection, the proliferated CFSElow T-cell subset best associated with biopsy-proven rejection was identified in 33 children, randomly selected from the cohort of 77 children. Logistic regression, Leave-one-out cross-validation (LOO-CV), and ROC analysis were used (17, 18).
To validate pro-inflammatory nature of the proliferative alloresponse measured by CFSElow T-cells, allo-(antigen)-specific pro-inflammatory CD154+T-cell subsets were measured in parallel MLR experiments for 23 of 77 subjects. CTLA4, a T-cell suppressor, whose expression is negatively correlated with pro-inflamatory markers in animal models, was measured simultaneously with CFSE in 17 of 77 subjects using 8-color flow cytometry.
Results
Rejectors (R) (n=32) were similar to Non-Rejector (NR) (n=45) in general demographics (Table 1), and in the interval between LTx and assay (114±102 vs 177±863 days, p=NS). For 65 recipients of cadaveric LTx, in whom donor PBL were available, surrogate donor PBL were used to elicit donor-specific proliferation as described above. The degree to which donor and surrogate donor PBL stimulators were matched was similar for MLR conducted in Rejectors and Non-Rejectors (Table 1).
Table 1.
Summary of general demographics and the degree to which donor and surrogate donor were matched for the MLR assay in Rejectors and Non-Rejectors.
| R | NR | p-value | ||
|---|---|---|---|---|
| Age (Median±SEM years) (n=77) | 5.5±1.3 | 5.8±0.9 | NS | |
| Gender (M:F) (n=77) | 18:14 | 27:18 | NS | |
| Race (Caucasian: African-American: Others) (n=77) | 25:4:3 | 36:3:6 | NS | |
| Surrogate Donor HLA match with Actual Donor among cadaveric Tx (Mean±SD) (n=65) | A | 1.0±0.4 | 1.2±0.4 | NS |
| B | 0.6±0.5 | 0.6±0.6 | NS | |
| Dr | 1.1±0.3 | 1.1±0.3 | NS | |
| Time between LTx and blood sampling | 114±102 | 117±863 | NS | |
| Tacrolimus whole blood concentration (FKWB) at the time of the assay | 11.0±0.8 | 5.0±0.8 | 0.016 | |
| FKWB at the time of the assay in 11 Rejectors who were sampled within 60 days before biopsy, compared with FKWB among Non-Rejectors | 9.0±1.4 | 5.0±0.8 | NS | |
| FKWB at the time of the assay in 21 Rejectors who were sampled within 60 days after biopsy, compared with FKWB among Non-Rejectors | 13.0±1.1 | 5.0±0.8 | 0.0018 | |
| Time to Early acute cellular rejection (ACR) (Median±SEM days) (n=15) | 37±5 | NA | NA | |
| Time to Late ACR (Median±SEM days) (n=17) | 378±164 | NA | NA |
Summary statistics
Rejectors show significantly higher proportions of donor-induced CFSElow proliferating T cells, compared with those induced by third-party (Fig. 1). Non-Rejectors showed significantly lower proportions of donor-induced CFSElow proliferating T-cells, compared with those induced by third-party (Table 2). No differences were seen between groups in third-party induced immunoreactivity index (IR) for either subset. This resulted in significantly higher IR for all T-cell subsets except Tc-naïve subsets, among Rejectors, compared with Non-Rejectors. For allospecific CD154+T-cells and their subsets, IR was numerically higher among Rejectors for all subsets, but achieved significance only for the Tc (p=0.043), and Tc-memory (p=0.030) subsets (Table 3). An opposite trend was seen for CTLA4+T-cells and their subsets. Compared with third-party stimulation, more donor-induced CTLA4+T-cells were seen among Non-Rejectors, and less donor-induced CTLA4+T-cells were seen among Rejectors. This resulted in numerically higher IR for all CTLA4+T-cell subsets among Non-Rejectors, compared with Non-Rejectors. Significance was only achieved by IR of CTLA4+Tc (p=0.011) and Th-memory (p=0.040), which was significantly higher among Non-Rejectors (Table 4).
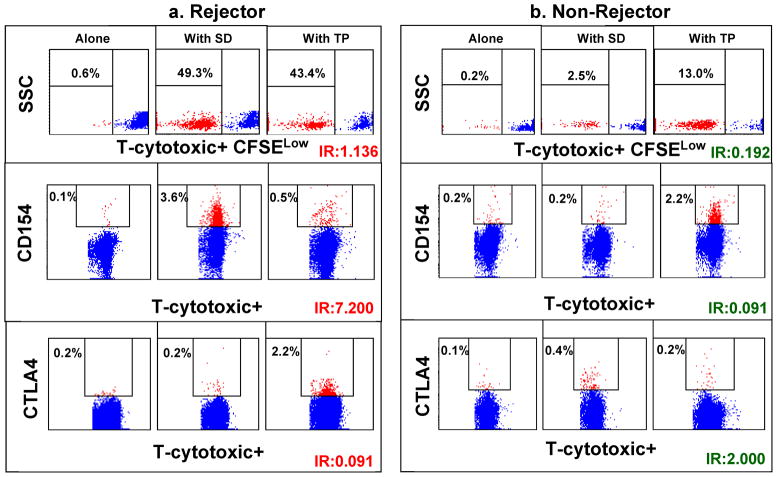
Figure 1.
Scatter plots in panel a) show significantly higher proportions of donor-specific CFSElow (uppermost) and CD154+ (middle) T-cytotoxic cells in a Rejector, compared with corresponding third-party-induced alloresponses. In the lowermost subpanel, donor-induced expression of the negative costimulator, CTLA4, is less than third-party-induced CTLA expression. For the Non-Rejector shown in panel b) the reverse features are seen in a Non-Rejector. Donor-induced markers of T-cell activation, CFSElow and CD154+ cells, are less than those induced with third-party stimulation. On the other hand, donor-induced cells expressing the negative costimulator, CTLA4, exceed third-party induced CTLA4+ cells.
Table 2.
Shows CFSElow (proliferated) Thelper and Tcytotoxic cells, and their memory and naive subsets in 3–4 day MLR.
| Th (Thelper) | Th-Memory | Th-Naïve | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALONE | SD | TP | IR | ALONE | SD | TP | IR | ALONE | SD | TP | IR | |
| NR (Median±SEM) (n=45) | 6±117 | 193±326 | 601±390 | 0.484±0.122 | 31±118 | 614±521 | 1521±721 | 0.654±0.197 | 7±86 | 160±295 | 568±343 | 0.554±0.252 |
| R (Median±SEM) (n=32) | 12±15 | 640±235 | 463±230 | 1.436±0.654 | 15±31 | 143±493 | 967±461 | 1.489±0.922 | 22±28 | 831±254 | 538±214 | 1.222±0.441 |
| p: value | NS | NS | NS | 0.003 | NS | NS | NS | 0.012 | NS | NS | NS | 0.043 |
| Tc (Tcytotoxic) | Tc-Memory | Tc-Naïve | ||||||||||
| ALONE | SD | TP | IR | ALONE | SD | TP | IR | ALONE | SD | TP | IR | |
| NR (Median±SEM) (n=45) | 25±503 | 220±384 | 561±586 | 0.536±0.105 | 44±114 | 538±562 | 848±764 | 0.558±0.324 | 29±87 | 250±302 | 409±354 | 0.660±0.242 |
| R (Median±SEM) (n=32) | 25±15 | 449±182 | 256±188 | 1.294±0.244 | 26±98 | 1256±595 | 747±615 | 1.203±0.672 | 25±38 | 465±156 | 432±161 | 1.045±0.266 |
| p: value | NS | NS | NS | 0.001 | NS | NS | NS | 0.043 | NS | NS | NS | NS |
Data are summarized for 32 Rejectors and 45 Non- Rejectors. Results are expressed as Median±SEM CFSElow cell counts/10,000 induced by donor/surrogate donor (SD) or third-party (TP), or as the immunoreactivity index (IR) for that subset (IR=SD : TP). Alone: Recipient alone with out stimulation, SD: Stimulated with Surrogate donor, TP: Stimulated with third-party, IR: Immunoreactivity index.
Table 3.
Shows allospecific CD154+ T-helper and T-cytotoxic cells, and their memory and naïve subsets in 23 children with LTx.
| Th (Thelper) | Th-Memory | Th-Naïve | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALONE | SD | TP | IR | ALONE | SD | TP | IR | ALONE | SD | TP | IR | |
| NR (Median±SEM) (n=15) | 7±7 | 11±43 | 19±36 | 0.535±0.102 | 11±50 | 24±100 | 54±119 | 0.401±0.134 | 1±13 | 4±50 | 9±56 | 0.321±0.134 |
| R (Median±SEM) (n=8) | 14±108 | 242±183 | 82±134 | 1.700±1.417 | 33±103 | 548±227 | 265±177 | 1.881±2.646 | 3±24 | 15±85 | 43±72 | 0.677±0.541 |
| p:value | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Tc (Tcytotoxic) | Tc-Memory | Tc-Naïve | ||||||||||
| ALONE | SD | TP | IR | ALONE | SD | TP | IR | ALONE | SD | TP | IR | |
| NR(Median±SEM) (n=15) | 7±32 | 18±53 | 53±80 | 0.468±0.127 | 10±53 | 92±81 | 121±174 | 0.410±0.146 | 3±53 | 7±81 | 16±61 | 0.450±0.131 |
| R(Median±SEM) (n=8) | 8±36 | 60±72 | 26±38 | 1.840±0.835 | 54±63 | 361±355 | 181±77 | 2.465±0.997 | 8±25 | 65±104 | 22±27 | 1.821±1.202 |
| p:value | NS | NS | NS | 0.043 | NS | NS | NS | 0.030 | NS | NS | NS | NS |
The subject population includes 8 Rejectors and 15 Non-Rejectors. Results are expressed as Median±SEM CD154+ cell counts/10,000 induced by donor/surrogate donor (SD) or third-party (TP), or as the immunoreactivity index (IR) for that subset (IR=SD : TP). Alone: Recipient alone with out stimulation, SD: Stimulated with Surrogate donor, TP: Stimulated with third-party, IR: Immunoreactivity index.
Table 4.
Shows allospecific CTLA4+ T-helper and T-cytotoxic cells, and their memory and naïve subsets measured simultaneously with CFSElow T-cells and their subsets in 3–4 day MLR.
| Th (Thelper) | Th-Memory | Th-Naïve | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alone | SD | TP | IR | Alone | SD | TP | IR | Alone | SD | TP | IR | |
| NR (Median±SEM) (n=10) | 13±51 | 72±71 | 44±55 | 1.383±0.160 | 29±371 | 326±1047 | 162±999 | 1.490±0.258 | 20±107 | 40±119 | 20±270 | 0.889±0.350 |
| R (Median±SEM) (n=7) | 4±19 | 22±30 | 37±97 | 1.074±0.228 | 78±23 | 190±42 | 251±104 | 0.852±0.206 | 3±3 | 7±8 | 14±16 | 0.845±0.232 |
| p-value | NS | NS | NS | NS | NS | NS | NS | 0.040 | NS | NS | NS | NS |
| Tc (Tcytotoxic) | Tc-Memory | Tc-Naïve | ||||||||||
| Alone | SD | TP | IR | Alone | SD | TP | IR | Alone | SD | TP | IR | |
| NR (Median±SEM) (n=10) | 17±24 | 41±50 | 32±28 | 1.315±0.148 | 128±256 | 327±250 | 259±112 | 1.153±0.533 | 16±207 | 36±86 | 23±54 | 1.558±0.254 |
| R (Median±SEM) (n=7) | 5±8 | 17±13 | 41±14 | 0.838±0.158 | 189±91 | 168±146 | 775±147 | 0.274±0.169 | 3±8 | 10±6 | 12±11 | 0.844±0.520 |
| p-value | NS | NS | NS | 0.011 | NS | NS | NS | NS | NS | NS | NS | NS |
The subject population of 17 children, in whom both measurements were conducted simultaneously included 7 Rejectors and 10 Non- Rejectors. Results are expressed as Median±SEM CTLA4+ cell counts/10,000 induced by donor/surrogate donor (SD) or third-party (TP), or as the immunoreactivity index (IR) for that subset (IR=SD : TP). Alone: Recipient alone with out stimulation, SD: Stimulated with Surrogate donor, TP: Stimulated with third-party, IR: Immunoreactivity index.
CFSElow proliferating T-cytotoxic cells associate best with rejection, at threshold IR>0.914
To determine associations, 33 observations from 16 Rejectors and 17 Non-Rejectors were analyzed. Among Rejectors, 4 were monitored before, and 12 after biopsies showing ACR. The most parsimonious logistic regression model was calculated using exhaustive backward and forward stepwise selection of IR for Th and Tc, and their 4 subsets (Th-naïve, Th-memory, Tc-naïve, and Tc-memory) as independent variables, and Rejector status as the dependent variable. The optimal model was built with CFSElow proliferating Tc alone. We then assessed five covariates, which could contribute confounding effects-age, race, gender, time from transplant, and Tacrolimus whole blood concentration (FKWB). In additional stepwise model selection, only two covariates were tested with the original six independent variables at any given time to avoid model saturation. The optimal model was once again built with CFSElow proliferating Tc alone.
To obtain a realistic measure of model performance, leave-one-out cross validation (LOO-CV) was performed using the IR of CFSElow proliferating Tc as the single predictor of Rejector status on these 33 observations. Using the IR cut point from each observation drop in LOO-CV, the median was calculated for use as the threshold IR for this cell type. ROC analysis confirmed this single cutoff IR value of CFSElow proliferating Tc at 0.914, at or above which Rejector status is predicted. Based on this cutoff, 12 of 16 Rejectors (sensitivity 75%) and 15 of 17 Non-Rejectors (specificity 88%) were identified correctly in the 33 subjects who made up the screening cohort.
Sensitivity and specificity of CFSElow Tc for predicting clinical status is replicated in the independent replication cohort
In the remaining 44 of 77 children, an IR ≥ 0.914 for CFSElow Tc was seen in 12 of 16 Rejectors (sensitivity 75%), while an IR<0.914 was seen in 23 of 28 Non-rejectors (specificity 82%). Clinical status and assay results of 32 Rejectors and 8 of 45 Non-Rejectors who received biopsy, are summarized in Table 5. Supplementary Table 1 is also included to show that allospecific CFSElow Th are not discriminatory.
Table 5.
Summary of assay predictions expressed as IR for Tcytotoxic (Tc) cells and Tc subsets, and corresponding biopsy result for 40 children. This biopsied cohort included all 32 Rejectors and 8 of 45 Non-Rejectors.
| Immunoreactivity Index (IR) | Assay prediction | |||||||
|---|---|---|---|---|---|---|---|---|
| # | Days between biopsy and assay | Biopsy Diagnosis | Tc | Tc- memory | Tc-naïve | Tc | Tc- memory | Tc- naive |
| 1 | 54 | NR | 0.292 | 0.000 | 0.432 | NR | NR | NR |
| 2 | −36 | NR | 0.820 | 0.613 | 10.320 | NR | NR | R |
| 3 | 60 | NR | 1.463 | 0.257 | 1.180 | R | NR | R |
| 4 | 0 | NR | 0.722 | 0.158 | 0.771 | NR | NR | NR |
| 5 | −55 | NR | 0.208 | 0.000 | 0.289 | NR | NR | NR |
| 6 | 7 | NR | 0.000 | 0.148 | 0.638 | NR | NR | NR |
| 7 | 0 | NR | 1.113 | 0.929 | 1.236 | R | R | R |
| 8 | 12 | NR | 1.413 | 0.000 | 1.000 | R | NR | R |
| 1 | −49 | R | 0.345 | 0.306 | 0.376 | NR | NR | NR |
| 2 | −49 | R | 1.772 | 0.415 | 1.542 | R | NR | R |
| 3 | −36 | R | 0.124 | 0.269 | 0.143 | NR | NR | NR |
| 4 | −33 | R | 0.997 | 0.000 | 1.004 | R | NR | R |
| 5 | −33 | R | 0.799 | 0.400 | 1.037 | NR | NR | R |
| 6 | −11 | R | 0.324 | 0.669 | 0.354 | NR | NR | NR |
| 7 | −9 | R | 1.004 | 1.037 | 0.999 | R | R | R |
| 8 | −6 | R | 0.729 | 3.622 | 1.143 | NR | R | R |
| 9 | −3 | R | 1.735 | 1.932 | 1.645 | R | R | R |
| 10 | −1 | R | 1.349 | 3.037 | 1.232 | R | R | R |
| 11 | −1 | R | 3.480 | 7.863 | 4.672 | R | R | R |
| 12 | 3 | R | 5.806 | 21.151 | 3.197 | R | R | R |
| 13 | 3 | R | 0.548 | 0.344 | 0.617 | NR | NR | NR |
| 14 | 4 | R | 2.009 | 1.483 | 0.896 | R | R | NR |
| 15 | 5 | R | 1.187 | 0.879 | 1.130 | R | NR | R |
| 16 | 5 | R | 1.328 | 1.497 | 0.951 | R | R | R |
| 17 | 7 | R | 1.317 | 1.204 | 0.640 | R | R | NR |
| 18 | 7 | R | 1.053 | 1.074 | 1.053 | R | R | R |
| 19 | 7 | R | 1.455 | 1.784 | 0.972 | R | R | R |
| 20 | 11 | R | 0.963 | 0.554 | 0.565 | R | NR | NR |
| 21 | 14 | R | 1.855 | 1.476 | 0.993 | R | R | R |
| 22 | 14 | R | 1.528 | 1.118 | 2.462 | R | R | R |
| 23 | 15 | R | 1.857 | 3.078 | 2.944 | R | R | R |
| 24 | 17 | R | 2.066 | 1.486 | 1.696 | R | R | R |
| 25 | 21 | R | 0.441 | 0.777 | 0.353 | NR | NR | NR |
| 26 | 23 | R | 1.831 | 2.448 | 1.152 | R | R | R |
| 27 | 26 | R | 0.947 | 0.833 | 0.953 | R | NR | R |
| 28 | 27 | R | 2.213 | 5.203 | 1.613 | R | R | R |
| 29 | 32 | R | 1.270 | 1.201 | 1.273 | R | R | R |
| 30 | 40 | R | 0.692 | 0.653 | 0.603 | NR | NR | NR |
| 31 | 51 | R | 6.464 | 1.513 | 7.930 | R | R | R |
| 32 | 53 | R | 1.188 | 1.459 | 2.496 | R | R | R |
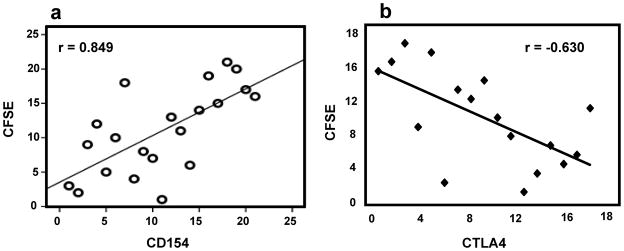
The IR of CFSElow Tc correlated positively with IR of allospecific CD154+T-cytotoxic cells and its subsets. In order of decreasing significance, this correlation was best for Tc-memory (r=0.849, p=3.04E-07, Figure 2a), intermediate for Tc (r=0.664, p=0.0005), and least for Tc-naïve (r=0.580, p=0.004). The 23 children in whom both tests were performed in parallel experiments included 8 Rejectors and 15 Non-Rejectors. Correlations were not significant among Th and Th subsets (not shown).
Figure 2.
a) Highly significant correlations exist between IR for CFSElow T-cytotoxic cells, and allospecific CD154+ T-c-memory cells in 23 children (Spearman rho=0.849, p=3.04E-07). b) The negative correlation between IR for allospecific CFSElow T-cytotoxic and IR for allospecific CTLA4+Tcytotoxic cells is highly significant in 17 of 77 recipients. (Spearman rho −0.630, p=0.007).
The IR of CFSElow Tc correlated negatively with IR of allospecific CTLA4+Tc (Spearman r=−0.630, p=0.007, Fig. 2b). The 17 children in whom CFSElow and CTLA4+ T-cells were measured simultaneously in the same assay included 7 Rejectors and 10 Non-Rejectors. Correlations were not significant among Th and the subsets (not shown).
Discussion
Our study shows that allospecific CFSElow Tc can identify rejection-prone children with LTx, when assayed in a flow cytometric CFSE-MLR. Proliferating Tc show very good sensitivity and specificity of 75 and 80% respectively, for association with rejection outcomes, when a threshold IR of CFSElow Tc ≥0.914 derived from a screening cohort of 33 children was applied to an independent replication cohort of 44 children. This association compares favorably with sensitivity ranging from 65–75% and specificity ranging from 80–90% for the ELISPOT reported from other laboratories, and with 92% sensitivity and 84% specificity for the allospecific CD154+Tc-memory cell reported from our laboratory (6, 7, 14). The proliferating cell type best associated with rejection outcomes is the parent Tc subpopulation, rather than its memory or naïve subsets, likely because the proliferative alloresponse of all Tc subsets is necessary to achieve a good association with outcomes. The validity of CFSElow Tc as a pro-inflammatory marker, and as the cell type best associated with rejection outcomes is also confirmed by positive and negative correlations with other assays and markers. The IR of allostimulated CFSElow Tc correlates significantly with the IR of either allospecific CD154+Tc, or its CD154+Tc-memory or CD154+Tc-naïve subsets. All allospecific CD154+Tc subsets represent the pro-inflammatory, allo-(antigen)-specific response of recipient T-cells. Significant negative correlations are seen between the IR of proliferating Tc, and IR of CTLA4+Tc. Because, CTLA4 expression was measured in the same MLR experiment as CFSElow Tc, it represents that anti-inflammatory or suppressive polarity of alloresponsive Tc.
It is reasonable to ask why the Tc is better than the Th, or the combined Th+Tc populations, in its association with rejection outcomes. We believe that the relatively drug-resistant Tc can better reflect the host-graft interaction at any given time, because this interaction is less confounded by immunosuppressant drugs. In support, we cite the relative resistance of mitogen-stimulated Tc, and of allospecific CD154+Tc to inhibition by Tacrolimus in our studies of children with LTx (4, 14). The importance of alloresponsive Tc as a sensitive and specific marker of rejection-prone recipients suggested in our ex-vivo assays does not necessarily imply an in vivo role for this cell type in the rejection response. However, durable tolerance in a primate model receiving anti-CD154 costimulation blockade treatment was only achieved with added Tc ablation (19).
The limitations of our study include the cross-sectional nature of the observations, which may have yielded a false-positive association. For this reason, we tested the robustness of our association using screening/validation cohorts, and by including only those measurements from rejectors, which were obtained within 60 days of a liver allograft biopsy. Because of the risk of life-threatening hemorrhage from liver biopsies, protocol biopsies are not the standard of care. Therefore, biopsy data is only available from 8 Non-Rejectors, in whom elevated liver function tests required that rejection be ruled out. Another limitation is our use of “surrogate donor” PBL as stimulators, a fact necessitated by the predominant use of cadaveric liver donors, from whom spleen tissue is consumed during the tissue typing process for organ allocation. To simulate the donor cell to the extent possible, “surrogate” donor PBL were matched at a minimum one antigen each at the HLA-A, -B and -DR loci. Finally, our intent to develop a more rapid proliferative MLR, which could permit more responsive clinical drug management, led us to use 3–4-day co-culture instead of the standard 5–7-day co-culture. The resulting lower yield of proliferating daughter generations precluded additional analyses, e.g. estimates of “precursor frequencies”, etc.
With the availability of a 16-hour MLR, which measures allo-(antigen)-specific CD154+Tc-memory cells, the role of the proliferative CFSE-MLR has reduced in our laboratory. However, when significant lymphocyte depletion lowers the total yield of PBL to <500,000 cells for the entire MLR set-up, we find that the CFSE-MLR is more suitable. The 3–5 day co-culture conditions of the CFSE-MLR expand the numbers of evaluable alloresponsive PBL, and facilitate a reliable assessment of donor-specific T-cell subsets. Experience with allospecific CD154+ T-cytotoxic memory cells suggests that enhanced donor-specific alloreactivity manifests as an IR > 1 before LTx in nearly 80% rejection-prone children with LTx, and declines subsequently at highly variable rates, especially among rejectors (14, 5). Therefore, a clinically relevant strategy would be to establish a baseline proliferative alloresponse prior to LTx with the CFSE-MLR. Subsequent monitoring may be timed to coincide with periods when the risk of rejection is greatest, for e.g. during the first and second months after LTx, and whenever major reductions in immunosuppression are being contemplated, especially among rejection-prone children. Clinical practice standards mandate that such a strategy must be first tested under IRB/FDA-approved research protocols prior to clinical implementation. These efforts remain ongoing at our center.
Supplementary Material
Acknowledgments
5RO1AI073895-02, 5-RO1AI49156-05, Children’s Hospital of Pittsburgh Research Foundation, and Hillman Foundation of Pittsburgh.
The Rosner family for their generous support and encouragement.
References
- 1.UNOS OPTN Annual report. 2007. [Google Scholar]
- 2.Martin SR, Atkison P, Anand R, Lindblad AS SPLIT Research Group. Studies of pediatric liver transplantation 2002: patient and graft survival and rejection in pediatric recipients of a first liver transplant in the United States and Canada. Pediatr Transplant. 2004;8:273–83. doi: 10.1111/j.1399-3046.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 3.Jain A, Mazariegos G, Kashyap R, Kosmach-Park B, Starzl TE, Fung J, Reyes J. Pediatric liver transplantation in 808 consecutive children: A single center experience spanning 20 years. Transplantation. 2002;73:941–47. doi: 10.1097/00007890-200203270-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sindhi R, Magill A, Abdullah A, Seward J, Tresgaskes M, Bentlejewski C, Zeevi A. Enhanced donor-specific alloreactivity occurs independent of immunosuppression in children with early liver allograft rejection. Am J Transplant. 2005;5:96–102. doi: 10.1111/j.1600-6143.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 5.Khera N, Janosky J, Zeevi A, Mazariegos G, Marcos A, Sindhi R. Persistent donor-specific alloreactivity may portend delayed liver rejection during drug minimization in children. FBS. 2007;12:660–63. doi: 10.2741/2090. [DOI] [PubMed] [Google Scholar]
- 6.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-Transplant IFN-gamma ELISPOTs are associated with post-Transplant renal function in African American renal transplant recipients. Am J Transplant. 2005 Aug;5(8):1971–5. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 7.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999 Aug 15;163(4):2267–75. [PubMed] [Google Scholar]
- 8.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 9.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: New answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 10.De Haan A, Van Der Gun I, Van Der Bij W, De Leij LF, Prop J. Detection of alloreactive T cells by flow cytometry: A new test compared with limiting dilution assay. Transplantation. 2002;74:562–570. doi: 10.1097/00007890-200208270-00023. [DOI] [PubMed] [Google Scholar]
- 11.Martins S, St John LS, Champlin RE, et al. Functional assessment and specific depletion of alloreactive human T cells using flow cytometry. Blood. 2004;104:3429–3436.LR. doi: 10.1182/blood-2004-05-1918. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, Ohdan H, Onoe T, Asahara T. Multiparameter flow cytometric approach for simultaneous evaluation of proliferation and cytokine-secreting activity in T-cells responding to allo-stimulation. Immunol Invest. 2004 Aug;33(3):309–24. doi: 10.1081/imm-120038079. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Ohdan H, Onoe T, Mitsuta H, Tashiro H, Itamoto T, Asahara T. Low incidence of acute rejection after living-donor liver transplantation: immunologic analyses by mixed lymphocyte reaction using a carboxyfluorescein diacetate succinimidyl ester labeling technique. Transplantation. 2005 May 15;79(9):1262–7. doi: 10.1097/01.tp.0000161667.99145.20. [DOI] [PubMed] [Google Scholar]
- 14.Ashokkumar C, Talukdar A, Sun Q, Higgs BW, Janosky J, Wilson P, Mazariegos G, Jaffe R, Demetris A, Dobberstein J, Soltys K, Bond G, Thomson AW, Zeevi A, Sindhi R. llospecific CD154+ T Cells Associate with Rejection Risk After Pediatric Liver Transplantation. Am J Transplant. 2008 Oct 31; doi: 10.1111/j.1600-6143.2008.02459.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes J, Mazariegos GV, Abu-Elmagd K, Macedo C, Bond GJ, Murase N, Peters J, Sindhi R, Starzl TE. Intestinal transplantation under tacrolimus monotherapy after perioperative lymphoid depletion with rabbit anti-thymocyte globulin (thymoglobulin) Am J Transplant. 2005;5(6):1430–6. doi: 10.1111/j.1600-6143.2005.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AshokKumar C, Abdullah A, Logar A, Wilson P, Talukdar A, Chien N, Singh M, Sindhi R. Handbook of Human Immunology. 2. Chapter 20. Functional Assessment of Immunosuppression: Monitoring post-transplant alloreactivity with flow cytometric mixed lymphocyte co-cultures; pp. 589–597. [Google Scholar]
- 17.Bautista D, Arana E, Marti-Bonmati, Luis, Paredes R. Validation of logistic regression methods in small samples: application to calvarial lesions diagnosis. J Clin Epidemiol. 1999;52:237–241. doi: 10.1016/s0895-4356(98)00165-6. [DOI] [PubMed] [Google Scholar]
- 18.Fawcett T. An introduction to ROC analysis. Pattern Recognition Letters. 2006;27:861–874. [Google Scholar]
- 19.Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, Sogawa H, Murakami T, Strom TB, Colvin RB, Sachs DH, Benichou G, Cosimi AB, Kawai T. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;5:1055–61. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.