Abstract
Two cyclopeptidic Smac mimetics, 2 and 3, were designed and synthesized. These two compounds bind to XIAP and cIAP-1/2 with low nanomolar affinities, and restore the activities of caspase-9 and caspase-3/-7 inhibited by XIAP. Compound 2 potently inhibits cancer cell growth and is 5–8 times more potent than the initial lead compound.
Keywords: Smac mimetics, IAP antagonists, Apoptosis inducers, New anticancer agents
Apoptosis, or programmed cell death, is a cell suicide process essential for normal development, host defense and suppression of oncogenesis.1 Dysfunction of apoptosis machinery is a hallmark of cancer, and targeting key apoptosis regulators has emerged as an attractive strategy for the development of new approaches to human cancer treatment.1
Inhibitors of apoptosis proteins (IAPs) are a class of key regulators of apoptosis characterized by containing one to three baculoviral IAP repeat domains (BIRs).2 Eight members of IAPs have been discovered. Of these, cIAP-1 and cIAP-2 inhibit tumor necrosis factor mediated apoptosis,3 and XIAP binds to and inhibits the activity of three caspases, an initiator caspase-9 and two effectors, caspase-3 and -7, thus blocking both the intrinsic and extrinsic apoptotic pathways.4 XIAP, cIAP-1 and cIAP-2 have three BIR domains. While the BIR3 domain of XIAP binds to initiator caspase-9, its BIR2 domain, together with the linker preceding it, binds to the effectors caspase-3 and -7.5 Although the BIR3 domain of cIAP-1 and -2 can also bind to caspase-9, biological studies indicate that they do not inhibit the activity of this caspase.
Smac (second mitochondria-derived activator of caspase) is a potent and direct endogenous inhibitor of IAPs.6 Structural and biological studies have shown that the interaction between Smac and the IAPs is mediated by the BIR domain(s) in IAPs and the four amino acid residues, AVPI, at the N-terminus of Smac.7 On one hand, Smac antagonizes cIAP-1 and cIAP-2 by direct binding, followed by induction of their rapid degradation. On the other hand, Smac blocks XIAP by concurrently binding to its BIR2 and BIR3 domains as a homodimer, thus removing the ability of XIAP to inhibit caspases.8 Using Smac AVPI peptide as a lead compound, a number of groups have designed and synthesized many peptidic and non-peptidic Smac mimetics.9 In addition, a series of bivalent Smac mimetics which mimic the interaction of dimeric Smac proteins with XIAP have been designed and synthesized.10
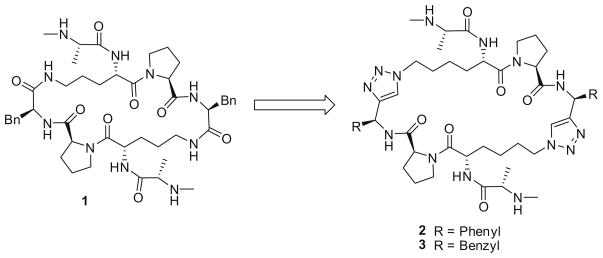
PSmac-21 (1, Fig. 1) is a cyclopeptidic Smac mimetic previously designed by our group. Previous studies indicated that (N–Me)AKPF binds to XIAP BIR3 more effectively than the Smac tetrapeptide, AVPI.11 In compound 1, two (N–Me)AKPF tetrapeptides are cyclized through two amide bonds formed between the two Lys side chain amino groups and the carboxylic acid groups in the two C-terminal Phe residues. The crystal structure of 1 in complex with recombinant XIAP BIR3 protein has been determined11 and it was found that two XIAP BIR3 protein molecules form a homodimmer with one molecule of 1. Because the dimerization of XIAP BIR3 was not observed under a ligand free condition, we proposed that it was induced by the binding of 1. Interestingly, while 1 can bind simultaneously to two recombinant XIAP BIR3 protein molecules, it can also bind to both the BIR2 and BIR3 domains of recombinant XIAP, making it a very high-affinity inhibitor against XIAP. Compound 1 is cell-permeable and effective in inhibition of cancer cell growth.11 These data indicate that 1 is a promising lead compound for further optimization.
Figure 1.
Design of cyclic Smac mimetics 2 and 3.
In this Letter, we report the design, synthesis and evaluation of two new cyclopeptidic Smac mimetics, 2 (SM-162) and 3 (SM-163), in which two amide bonds in 1 were replaced with two triazole structures, in an effort to reduce its peptidic characteristics.
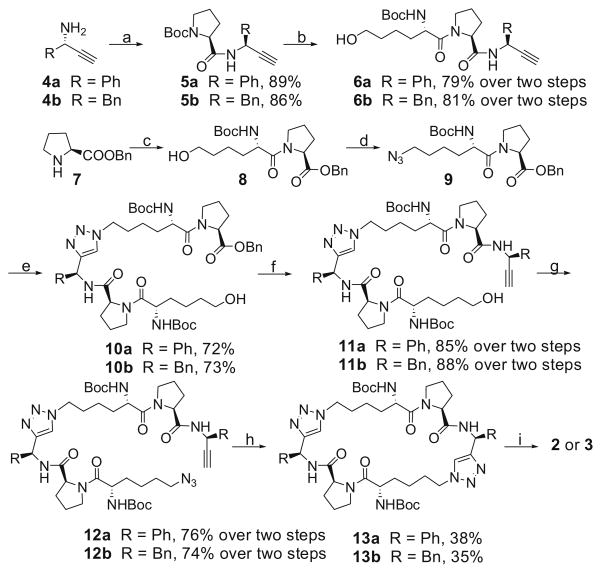
The synthesis of compounds 2 and 3 is shown in Scheme 1. We use a ‘click reaction’ as the key cyclization step instead of the macro cyclo-amidation reaction, which is used more frequently for large cyclopeptides.12 The chiral amines 4a and 4b were prepared by reported methods.13 Condensation of N-Boc-L-proline with 4a or 4b gave two amides 5a and 5b. Removal of the Boc protecting group from these two amides followed by condensation of the resulting ammonium salts with N-Boc-L-6-hydroxynorleucine yielded compounds 6a and 6b. Condensation of L-proline benzyl ester hydrochloride (7) with N-Boc-L-6-hydroxynorleucine afforded amide 8. Mesylation of the hydroxyl group in 8 followed by substitution of the mesylate with sodium azide furnished compound 9. Cycloaddition of 6a or 6b with azide 9 yielded 10a and 10b, respectively. Hydrolysis of the benzyl esters group in 10a and 10b followed by condensation of the resulting acids with 4a and 4b, respectively, afforded compounds 11a and 11b. Mesylation of the hydroxyl group in 11a and 11b and subsequent substitution of the mesylates with sodium azide furnished 12a and 12b. Macro-cyclization of 12a and 12b using ‘click reaction’ furnished the cyclopeptides 13a and 13b, removal of whose Boc protecting groups followed by condensation of the ammonium salts with N-methyl- N-Boc-L-alanine provided two amides. Finally, removal of the Boc protecting groups gave compounds 2 and 3.14
Scheme 1.
Reagents and conditions: (a) N-Boc-L-proline, EDC, HOBt, N,N-diisopropylethylamine, CH2Cl2; (b) (i) 4 N HCl in 1,4-dioxane, MeOH; (ii) N-Boc-L-6-hydroxynorleucine, EDC, HOBt, N,N-diisopropylethylamine, CH2Cl2; (c) N-Boc-L-6-hydroxynorleucine, EDC, HOBt, N,N-diisopropylethylamine, CH2Cl2, 87%; (d) (i) MsCl, N,N-diisopropylethylamine, CH2Cl2; (ii) NaN3, DMF, 82% over two steps; (e) 6a or 6b, CuSO4, (+)-sodium L-ascorbate, t-BuOH/H2O 1:1, 74%; (f) (i) H2, 10% Pd–C, MeOH; (ii) 4a or 4b, EDC, HOBt, N,N-diisopropylethylamine, CH2Cl2; (g) (i) MsCl, N,N-diisopropylethylamine, CH2Cl2; (ii) NaN3, DMF, 82% over two steps; (h) CuSO4, (+)-sodium L-ascorbate, t-BuOH/H2O 1:1; (i) (i) 4 N HCl in 1,4-dioxane, MeOH; (ii) N-methyl-N-Boc-L-alanine, EDC, HOBt, N,N-diisopropylethylamine, CH2Cl2; (iii) 4 N HCl in 1,4-dioxane, MeOH; compound 2: 79% over three steps, compound 3: 72% over three steps.
Compounds 2 and 3 were tested for their binding affinities to recombinant XIAP and cIAP-1/2 proteins in an FP-based binding assay using Smac-1F as a tracer.11 The results are summarized in Table 1.
Table 1.
Binding affinities of compounds 1, 2 and 3 to XIAP L-BIR2–BIR3, L-BIR2, cIAP-1 BIR3 and cIAP-2 BIR3 proteins
| Compounds | XIAP L-BIR2–BIR3
IC50 (nM) |
XIAP L-BIR2 IC50 (μM) | cIAP-1 BIR3 Ki (nM) | cIAP-2 BIR3 Ki (nM) | |
|---|---|---|---|---|---|
| Site 1 | Site 2 | ||||
| 1 | 1.0 | 481.0 | 53.9 ± 20.9 | 84 ± 9 | 292 ± 41 |
| 2 | 0.43 | 28.0 | 3.2 ± 1.6 | 11 ± 2 | 24 ± 5 |
| 3 | 1.3 | 1106.0 | 40.5 ± 9.2 | 161 ± 20 | 393 ± 69 |
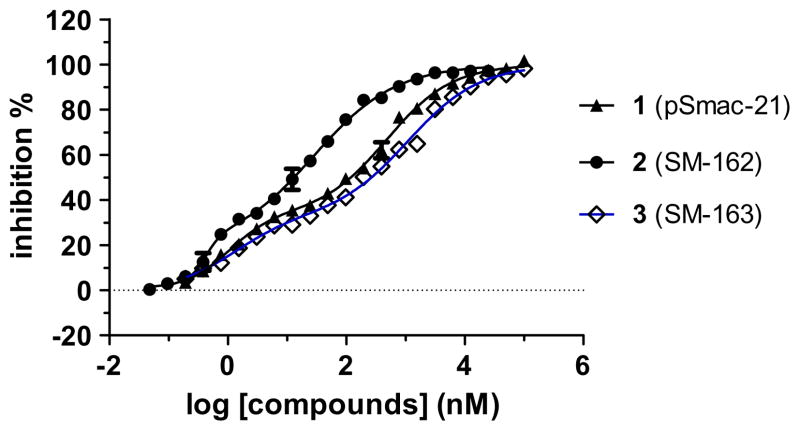
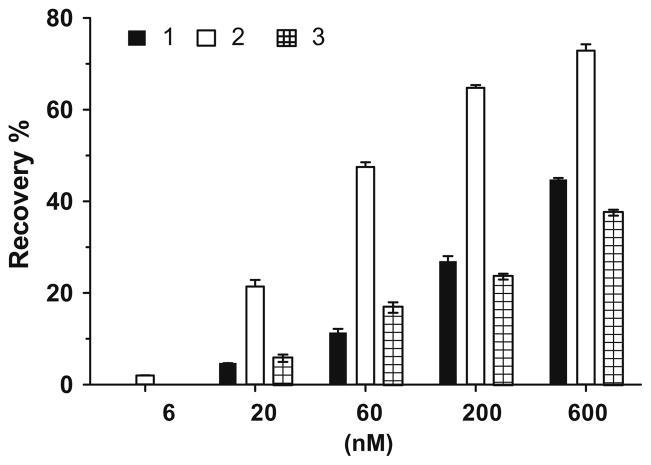
The binding curves of 1, 2 and 3 to XIAP protein containing linker-BIR2–BIR3 (L-BIR2–BIR3) indicate that these compounds have two binding sites or modes (Fig. 2). Compound 2 has IC50 values of 0.43 and 28 nM, respectively, for the two sites, whereas compound 3 has IC50 values of 1.3 and 1106 nM, respectively. In comparison, compound 1 has IC50 values of 1.0 and 481 nM, respectively, for the two sites.
Figure 2.
Binding curves of compound 1, 2 and 3 to XIAP protein containing linker-BIR2–BIR3.
The weaker binding site may correspond to the BIR2 domain in XIAP, so we also determined the binding affinities of 1, 2 and 3 to XIAP protein containing only linker-BIR2 (Table 1). In this assay, compounds 1, 2 and 3 have IC50 values of 53.9, 3.2, and 40.5 μM, respectively. Hence, these data showed that the weaker binding site for these compounds is not simply due to their interaction to XIAP BIR2. Further investigations are needed to define precisely how these compounds interact with XIAP linker-BIR2–BIR3 protein.
These three compounds also potently bind to cIAP-1 and cIAP-2 proteins (Table 1). Compounds 1, 2 and 3 bind to cIAP-1 BIR3 protein with Ki values of 84, 11 and 161 nM, respectively, and to cIAP-2 with Ki values of 292, 24 and 393 nM, respectively.
Taken together, our binding data thus showed that compound 2 in which R is a phenyl group is much more potent than compound 3, in which R is a benzyl group. In fact, compound 2 also binds more potently than compound 1 to all the three IAP proteins.
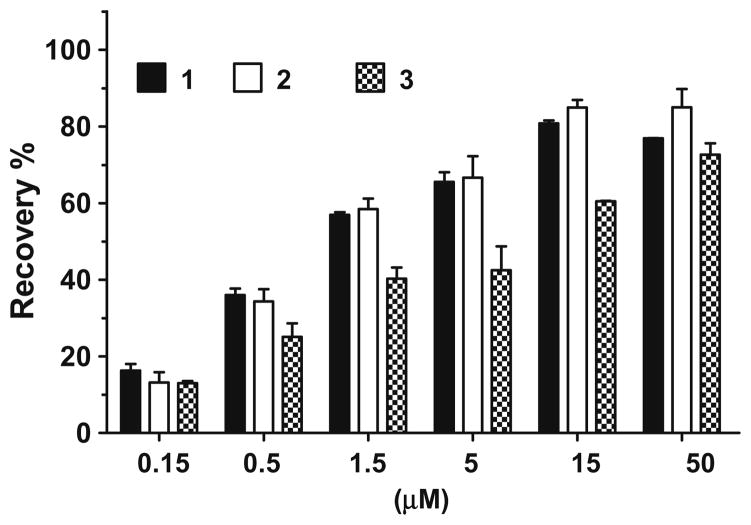
We evaluated these three compounds for their ability to restore the activity of caspase-9 and -3 inhibited by XIAP in cell-free caspase functional assays. A Caspase-Glo 9 assay kit (Promega) was used in the caspase-9 assay and caspase-3 Fluorescent Assay kit (BD Bioscience) in the caspase-3 assay. In the caspase-9 functional assay, XIAP protein containing linker-BIR2–BIR3 dose dependently inhibits the activity of caspase-9, achieving 80% inhibition at concentrations of 500 nM. Each of the three compounds can dose dependently restore the activity of caspase-9 (Fig. 3). While compounds 1 and 2 are equally potent, compound 3 is less potent. At 500 nM and 1.5 μM, compounds 1 and 2 can restore the caspase-9 activity by 40% and 60%, respectively. In a caspase-3 functional assay, 20 nM of XIAP protein containing linker-BIR2–BIR3 can inhibit 90% of the enzymatic activity of caspase-3 (Fig. 4). Compound 2 is the most potent compound in antagonizing XIAP and restoring the activity of caspase-3. Compound 2 at 60 nM restores the activity of caspase-3 by 50% (Fig. 4). These functional data show that compound 2 is a potent XIAP antagonist.
Figure 3.
Functional antagonism of Smac mimetics to XIAP-LB2B3 inhibition of caspase-9 activity. 500 nM of XIAP protein was used in the assay.
Figure 4.
Functional antagonism of Smac mimetics to inhibition of caspase-3 activity by XIAP-linker-BIR2–BIR3 protein. 20 nM of XIAP protein was used in the assay.
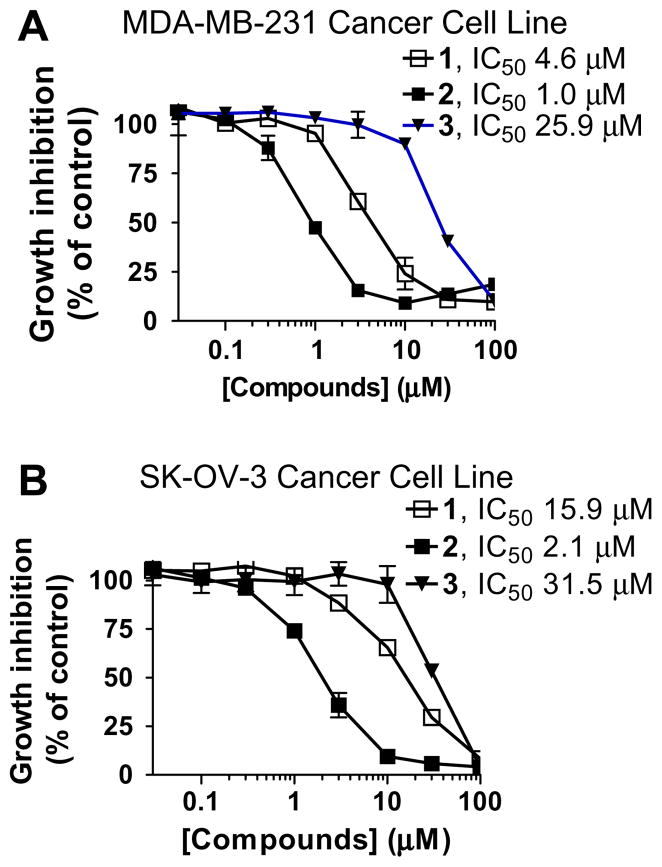
One major goal in the design of compounds 2 and 3 is to reduce the peptidic nature of compound 1 and to improve the cellular activity. These compounds were evaluated together for their activity in inhibition of cell growth in the MDA-MB-231 breast cancer and SK-OV-3 ovarian cancer cell lines, which are sensitive to Smac mimetics as single agents. Although 3 is weaker than 1, compound 2 is 5–8 times more potent than 1 in inhibition of cell growth in both cancer cell lines (Fig. 5). Compound 2 has IC50 values of 1.0 and 2.1 μM, respectively, in the MDA-MB-231 and SK-OV-3 cancer cell lines.
Figure 5.
Cell growth inhibition of compounds 1, 2 and 3 in (A) MDA-MB-231 and (B) SK-OV-3 cancer cell lines. Cells were treated for 4 days and cell growth was determined using a standard WST-8 assay.
In summary, we designed two cyclopeptidic Smac mimetics, 2 and 3, from our previous lead compound 1 by replacing two amide bonds with two triazole structures and efficiently synthesized these using ‘click reaction’ as a key step for macrocyclization. Compound 2 is more potent than compound 1 in binding to XIAP, cIAP-1 and cIAP-2 and in antagonizing XIAP in cell-free caspase functional assays. Importantly, compound 2 is 5–8 times more potent than compound 1 in inhibition of cell growth in two cancer cell lines. Taken together, our data indicate that compound 2 represents a promising new lead compound for further optimization toward the development of a new class of anticancer agents by targeting multiple IAP proteins.
Acknowledgments
The financial supportfrom the National Cancer Institute, National Institutes of Health, USA (R01CA127551 and R01CA109025), Susan G. Komen Foundation and the Breast Cancer Research Foundation is greatly appreciated.
References and notes
- 1.(a) Thompson CB. Science. 1995;267:1456. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]; (b) Nicholson DW. Nature. 2000;407:810. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]; (c) Reed JC. Nat Rev Drug Disc. 2002;1:111. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 2.(a) Deveraux QL, Reed JC. Genes Dev. 1999;1:239. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]; (b) Salvesen GS, Duckett CS. Nat Rev Mol Cell Biol. 2002;3:401. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 3.(a) Fotin-Mleczek M, Henkler F, Samel D, Reichwein M, Hausser A, Parmryd I, Scheurich P, Schmid JA, Wajant H. J Cell Sci. 2002;115:2757. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]; (b) Deng Y, Ren X, Yang L, Lin Y, Wu X. Cell. 2003;115:61. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 4.Holcik M, Gibson H, Korneluk RG. Apoptosis. 2001;6:253. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 5.(a) Riedl SJ, Shi Y. Nat Rev Mol Cell Biol. 2004;5:897. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]; (b) LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. Oncogene. 1998;17:3247. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]; (c) Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H. Cell. 2001;104:781. [PubMed] [Google Scholar]
- 6.(a) Du C, Fang M, Li Y, Li L, Wang X. Cell. 2000;102:33. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]; (b) Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Cell. 2000;102:43. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 7.(a) Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y. Nature. 2000;408:1008. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]; (b) Liu Z, Sun C, Olejniczak ET, Meadows R, Betz SF, Oost T, Herrmann J, Wu JC, Fesik SW. Nature. 2000;408:1004. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]; (c) Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. Nature. 2001;410:112. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]; (d) Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mol Cell. 2003;11:519. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 8.(a) Samuel T, Welsh K, Lober T, Togo SH, Zapata JM, Reed JC. J Biol Chem. 2006;281:1080. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]; (b) Yang QH, Du C. J Biol Chem. 2004;279:16963. doi: 10.1074/jbc.M401253200. [DOI] [PubMed] [Google Scholar]; (c) Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. Cell. 2007;131:669. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]; (d) Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. Cell. 2007;131:682. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]; (e) Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Cancer Cell. 2007;12:445. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Liu M, Tomita Y, Pan H, Yoshioka Y, Krajewski K, Roller PP, Wang S. J Am Chem Soc. 2004;126:16686. doi: 10.1021/ja047438+. [DOI] [PubMed] [Google Scholar]; (b) Oost TK, Sun C, Armstrong RC, Al-Assaad AS, Betz SF, Deckwerth TL, Ding H, Elmore SW, Meadows RP, Olejniczak ET, Oleksijew A, Oltersdorf T, Rosenberg SH, Shoemaker AR, Tomaselli KJ, Zou H, Fesik SW. J Med Chem. 2004;47:4417. doi: 10.1021/jm040037k. [DOI] [PubMed] [Google Scholar]; (c) Sun H, Nikolovska-Coleska Z, Lu J, Qiu S, Yang CY, Gao W, Meagher J, Stuckey J, Wang S. J Med Chem. 2006;49:7916. doi: 10.1021/jm061108d. [DOI] [PubMed] [Google Scholar]; (d) Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJ, Okawa DC, Flygare JA, Vucic D, Fairbrother WJ, Deshayes K. ACS Chem Biol. 2006;1:525. doi: 10.1021/cb600276q. [DOI] [PubMed] [Google Scholar]; (e) Sun H, Nikolovska-Coleska Z, Lu J, Meagher JL, Yang CY, Qiu S, Tomita Y, Ueda Y, Jiang S, Krajewski K, Roller PP, Stuckey JA. J Am Chem Soc. 2007;129:15279. doi: 10.1021/ja074725f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Sun H, Stuckey JA, Nikolovska-Coleska Z, Qin D, Meagher JL, Qiu S, Lu J, Yang CY, Saito NG, Wang S. J Med Chem. 2008;51:7169. doi: 10.1021/jm8006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PGA. Science. 2004;305:1471. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]; (b) Sun H, Nikolovska-Coleska Z, Lu J, Meagher JL, Yang CY, Qiu S, Tomita Y, Ueda Y, Jiang S, Krajewski K, Roller PP, Stuckey JA, Wang S. J Am Chem Soc. 2007;129:15279. doi: 10.1021/ja074725f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolovska-Coleska Z, Meagher JL, Jiang S, Yang CY, Qiu S, Roller PP, Stuckey JA, Wang S. Biochemistry. 2008;47:9811. doi: 10.1021/bi800785y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem, Int Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Messina F, Botta M, Corelli F, Schneider MP, Fazio F. J Org Chem. 1999;64:3767. doi: 10.1021/jo982513i. [DOI] [PubMed] [Google Scholar]
- 14.(a) Chemical data for compound 2: 1H NMR (300 M Hz, D2O) δ 7.75 (s, 2H), 7.39–7.25 (m, 6H), 7.21–7.10 (m, 4H), 6.22 (s, 2H), 4.79 (m, 2H), 4.58 (m, 2H), 4.45–4.19 (m, 6H), 3.82 (m, 2H), 3.78 (m, 2H), 3.49 (m, 2H), 2.62 (s, 6H), 2.31 (m, 2H), 2.05–1.85 (m, 6H), 1.84–1.73 (m, 4H), 1.68–1.50 (m, 4H), 1.41 (d, J = 7.0 Hz, 6H), 1.32–1.13 (m, 4H); 13C NMR (75 MHz, D2O) δ 172.83×2, 172.07×2, 170.02×2, 148.31×2, 139.78×2, 129.49×2, 128.73×2, 127.68×2×2, 124.67×2, 61.61×2, 57.45×2, 52.18×2, 50.98×2, 49.89×2, 48.75×2, 31.59×2, 30.65×2, 30.05×2, 29.51×2, 25.40×2, 21.94×2, 15.96×2; ESI MS: m/z 957.5 (M+Na)+. (b) Selected chemical data for compound 3: 1H NMR (300 M Hz, D2O) δ 1H NMR (300 M Hz, D2O) δ 7.70 (s, 2H), 7.30–7.08 (m, 10H), 5.33 (t, J = 7.8 Hz, 2H), 4.62 (m, 2H), 4.40–4.31 (m, 4H), 4.07 (m, 2H), 3.81 (m, 2H), 3.68–3.52 (m, 4H), 3.25–3.12 (m, 4H), 2.57 (s, 6H), 2.06–1.65 (m, 14H), 1.48–1.27 (m, 4H), 1.26 (d, J = 7.2 Hz, 6H), 1.05 (m, 2H); 13C NMR (75 MHz, D2O) δ 173.50×2, 172.40×2, 170.09×2, 148.25×2, 137.81×2, 129.88×2, 129.17×2, 127.52×2, 123.88×2, 62.11×2, 57.45×2, 51.35×2, 50.32×2, 48.60×2, 47.25×2, 39.72×2, 31.70×2, 30.72×2, 29.81×2, 29.63×2, 25.01×2, 21.43×2, 16.31×2; ESI MS: m/z 985.5 (M+Na)+.