Abstract
Antigen-specific T-cells, which express CD154 rapidly, but remain untested in alloimmunity, were measured with flow cytometry in 16-hour MLR of 58 identically-immunosuppressed children with liver transplantation (LTx), to identify Rejectors (who had experienced biopsy-proven rejection within 60 days post-transplantation). Thirty one children were sampled once, cross-sectionally. Twenty seven children were sampled longitudinally, pre-LTx, and at 1–60 and 61–200 days after LTx. Results were correlated with proliferative alloresponses measured by CFSE-dye dilution (n=23), and CTLA4, a negative T-cell costimulator, which antagonizes CD154-mediated effects (n=31). In cross-sectional observations, logistic regression and leave-one-out cross-validation identified donor-specific, CD154+T-cytotoxic (Tc)-memory cells as best associated with rejection outcomes. In the longitudinal cohort, 1) the association between CD154+Tc-memory cells and rejection outcomes was replicated with sensitivity/specificity 92.3%/84.6% for observations at 1–60 days, and 2) elevated pre-LTx CD154+Tc-memory cell responses were associated with significantly increased incidence (p=0.02) and hazard (HR=7.355) of rejection in survival/proportional hazard analysis. CD154 expression correlated with proliferative alloresponses (r=0.835, p=7.1e-07), and inversely with CTLA4 expression of allospecific CD154+Tc-memory cells (r=−0.706, p=3.0e-05). Allospecific CD154+T-helper-memory cells, not CD154+Tc-memory, were inhibited by increasing Tacrolimus concentrations (p=0.026). Collectively, allospecific CD154+T-cells provide an estimate of rejection risk in children with LTx.
Introduction
Safe drug minimization is a universal goal in transplantation (1), especially among children who receive liver transplantation (LTx). Rejection is seen in 50%, while post-transplant lymphoma-like malignancies due to immunosuppressants occur in 2–8% (2–4). To avoid toxicity while preventing rejection, immunosuppressants are reduced gradually, recognizing that the risk of rejection dissipates over time (5). A significant proportion of children experience rejection during this process, likely because the risk of rejection, and its relationship to drug doses is defined by clinical judgement. Therefore, an unmet need exists to measure the risk of rejection objectively.
Prior studies with proliferative 3H-thymidine mixed leukocyte responses (MLR) show that enhanced donor specific alloreactivity persists longer among children with early LTx rejection, is associated with early and late liver rejection, and may therefore serve as one measure of the risk of rejection (6, 7). However, as is true of several biological markers, donor-specific alloresponses can show large variation, precluding reliable distinctions between rejection-prone children and those at reduced risk. The effect of biological variation can be minimized and risk assessment individualized, if the donor-specific alloresponse is expressed as a ratio with third-party alloresponse. This risk ratio, called the immunoreactivity index, if >1, indicates increased risk of rejection in proliferative MLR (7). If the index is <1, the risk of rejection is low. Even so, proliferative MLRs are unsuitable for clinical use in children, because of assay duration, and blood sample volume requirements. Also, the MLR lacks the sensitivity of antigen specificity, whose determination with intracellular cytokines entails harsh permeabilizing procedures, and prohibitive cell losses (8–12).
In this report, we have evaluated allospecific CD154+ T-helper (Th) and T-cytotoxic (Tc) cells as measures of the risk of rejection, using polychromatic flow cytometry without cell permeabilization, and 16-hour MLR. In this MLR, recipient peripheral blood mononuclear cells (PBMC) are stimulated with live, pre-labeled donor and third-party PBMCs, to better approximate in-vivo host-graft interactions between live cells. Recent reports show that intracellular CD154 is expressed rapidly, can be measured without permeabilization, and is an excellent surrogate for IFNγ+ antigen-specific Th, directed against bacterial and viral antigens (13, 14). Expressed on a variety of cells, CD154 is classically induced after T-cell activation, binds to its receptor CD40, and provides positive costimulatory signals (15). In experimental studies, CD154 blockade prevents rejection, when used alone or in combination with donor-specific transfusion, or with antibodies to Tc, or CD45 (16–18). This anti-rejection effect results from apoptosis of donor-specific Tc cells, and depends on CTLA4, an inducible T-cell inhibitory marker, because blockade of CTLA4 reverses the anti-rejection effect of CD154 blockade (16). This antagonism between CD154 and CTLA4 is also seen in experimental disease models, where CD154 overexpression characterizes the inflammatory effector phase, while resolution leads to increased CTLA4 expression, and decreased expression of CD154 (19, 20). Despite its importance in allograft rejection, CD154 has not been tested as a clinical biomarker in organ transplantation.
In this study, we first asked which allospecific CD154+ T-cell subset, memory or naive Th or Tc, best distinguishes rejecting children from those who are rejection-free, using single measurements in proximity to biopsy-proven acute cellular rejection (ACR). Next, we asked whether in serial monitoring, the most sensitive and specific subset is still associated with and predictive of biopsy-proven ACR. Finally, we asked whether allospecific CD154+T-cells are affected by concentrations of Tacrolimus, a T-cell immunosuppressant, to understand how immunosuppressants may prevent clinical rejection during periods at increased risk. Our goal has been to develop allospecific CD154+T-cells as dynamic measures of the risk of rejection, which changes over time, so that immunosuppressants maybe titrated objectively. Our purpose has not been to develop a noninvasive test of rejection, or a substitute for an allograft biopsy.
Our study finds memory CD154+Tc-memory cells as highly sensitive and specific for identifying rejection-prone children with LTx. Further, allospecific CD154+Th cells are downregulated by increasing concentrations of Tacrolimus (21). Validated by screening/replication testing, and confirmatory positive and negative correlations with proliferative alloresponses, allograft biopsies, and CTLA4 expression, allospecific CD154+T-cells may address an unmet need for viable clinical markers, which may facilitate safe drug minimization in children with LTx.
Methods
Human Subjects
Research procedures were approved by the University of Pittsburgh’s Institutional Review Board. Subjects are 58 children who received primary LTx with rabbit anti-human thymocyte globulin (rATG, Genzyme, Cambridge, MA), and steroid-free Tacrolimus (7). Allospecific CD154+ T-cells have been measured since October 2006. Single measurements were made in 31 children, who received LTx prior to October 2006, within 60 days of a biopsy, or at presentation for routine clinical follow-up. Twenty seven children, transplanted after October 2006, were sampled serially, prior to LTx, and after LTx at 1–60 days, and at 61–200 days, in accordance with an ongoing observational protocol. Clinical Tacrolimus whole blood concentration targets (FKWB) were 10–15 ng/ml at days 1–60, and 5–10 ng/ml at days 61–200. Ficoll-separated PBMC were obtained for MLR from 3–5 ml blood at each timepoint.
Rejectors (R) were defined as children who experienced biopsy-proven ACR within 60 days after LTx, or in whom late rejection occurred in addition to an early ACR episode. The 60-day risk period for early ACR was chosen because early liver ACR occurs toward the end of the first, and beginning of the second month after rATG induction (6). The risk period for late ACR began >60 days after LTx, because late ACR is usually associated with clinical drug minimization, which was initiated 60 days after LTx. One child was assigned Rejector status because of fever and rapid elevation of liver transaminases to >400 IU/ml in the first 60 days. Bile duct dilatation or fluid collections were absent on ultrasound, and percutaneous image-guided biopsy was unavailable. Liver function tests (LFT) normalized with empiric steroid taper. Protocol biopsies are not performed in pediatric LTx recipients.
Assay optimization occurred in ten MLR co-cultures between HLA-mismatched PBMC from six adult normal control human subjects. Six-color flow cytometry simultaneously identified CD4+CD3+ (T-helper, Th), CD3+CD4− (non-T-helper cells, which were predominantly T-cytotoxic, Tc), CD45RO+ (memory) and CD45RO− (naive) cells with flourochrome labeled antibodies (BD Biosciences, San Jose, CA), after excluding dead cells with published instrumentation and protocols (Fig 1) (22). A minimum of five CD154+, CFSElow or CTLA4+ T-cells were required in each of the naive and memory Th or Tc subsets, before normalizing results per 10,000 total T-cells within that subset.
Figure 1.
Shows gating strategy used for flow cytomteric analysis. Live responder T-cells (CD3+) are identified after exclusion of donor or third-party stimulators, which are prestained with anti-CD45− allophycocyanin, and after exclusion of dead cells. T-helper (Th, CD3+CD4+) and T-cytotoxic (Tc, CD3+CD4−) families are each divided into memory (CD45RO+), and naive (CD45RO−).
Peak CD154 expression in T-cells occurred after 16-hour culture, was 2.5-fold greater among Th subsets than Tc, at (median) 50 CD154+ cells/10,000, and was inhibited by 80% after pretreatment with 20 ng/ml Tacrolimus. Tc cells were minimally inhibited. For each of 58 test subjects, 250,000–1000,000 PBMC from a single 3–5 milliliter blood sample were cultured alone and 1:1 with live donor or third-party PBMC (10). Live, non-irradiated donor and third-party PBMC were used after pre-labeling with anti-CD45-allophycocyanin. Donor cells were only available for 17 living donor LTx, and included 4 of 26 Rejectors, and 13 of 32 Non-Rejectors. For 41 recipients of cadaveric liver grafts, stimulators consisted of banked PBMC (surrogate donor, SD), with minimum one-antigen match with donor at each of the HLA-A, HLA-B and HLA-DR loci. The degree to which donor and surrogate donor PBMC stimulators were matched was similar for MLR conducted in Rejectors and Non-Rejectors (Table 1). Third-party PBMC were HLA-mismatched to recipient, donor or surrogate donor. Stimulator and responder PBMC were typed for Class I and II HLA antigens using the Dynal Reli test per manufacturer’s protocol (Dynal Biotech, Dynal RELI SSO HLA typing kits). Culture duration was 16 hours and medium included monensin and anti-CD154-phycoerythrin (13). To validate the pro-inflammatory status of CD154, proliferation, which is indicative of pro-inflammatory alloresponses, was measured with CFSE dye dilution and 3-day co-culture in parallel MLR experiments conducted with residual PBMC from 23 of 58 subjects. CTLA4, a T-cell suppressor, whose expression is negatively correlated with CD154 in animal models, was measured simultaneously with CD154 in 31 of 58 subjects using 7-and 8-color flow cytometry.
Table 1.
Summary of general demographic and the degree to which donor and surrogate donor were matched for the MLR assay in Rejectors and Non-Rejectors.
| R | NR | p-value | ||
|---|---|---|---|---|
| Age (median±SEM years) (n=58) | 5.8±1.3 | 4.7±1.3 | NS | |
| Gender (M:F) (n=58) | 11:15 | 21:11 | NS | |
| Race (Caucasian: Afro-American: Others) (n=58) | 18:1:7 | 23:4:5 | NS | |
| Surrogate Donor HLA match with Actual Donor at each loci among cadaveric Tx (Mean ± SD) (n=58) | A | 1.05 ± 0.38 | 1.21 ± 0.42 | NS |
| B | 0.62 ± 0.49 | 0.93 ± 0.47 | NS | |
| Dr | 1.05 ± 0.21 | 1.07 ± 0.27 | NS | |
| Time b/w LTx and blood sampling for Cross sectional cohort | 480±549 | 465±478 | NS | |
| FKWB at the time of the assay for Cross sectional cohort | 11.6±4.8 | 7.5±4.7 | 0.026 | |
| FKWB at the time of the assay in 6 Rejectors who were sampled within 60 days before Bx, compared with FKWB among Non-Rejectors in the Cross sectional cohort | 9.1±4.4 | 7.5±4.7 | NS | |
| FKWB at the time of the assay in 7 Rejectors who were sampled within 60 days after Bx, compared with FKWB among Non-Rejectors, in the Cross sectional cohort | 13.8±4.4 | 7.5±4.7 | 0.008 | |
| Time to Early ACR in Cross sectional cohort (mean±SD days) (n=4) | 24 ± 16 | NA | NA | |
| Time to Late ACR in Cross sectional cohort (mean±SD days) (n=9) | 670 ±553 | NA | NA | |
| Time b/w LTx and blood sampling during the 1–60-day period for Longitudinal cohort | 30±11 | 37±10 | NS | |
| Time b/w LTx and blood sampling during the 61–200-day period for Longitudinal cohort | 143±56 | 136±32 | NS | |
| FKWB at the time of assay during the 1–60 day period in the longitudinal cohort | 14±6.5 | 8.2±4.7 | 0.014 | |
| FKWB at the time of assay during the 1–60 day period in the longitudinal cohort for 9 Rejectors who were sampled before Bx. | 11.3±6.1 | 8.2±4.7 | NS | |
| FKWB at the time of assay during the 1–60 day period in the longitudinal cohort for 4 Rejectors who were sampled before Bx. | 18.4±4.8 | 8.2±4.7 | 0.004 | |
| FKWB at the time of assay during the 61–200 day period in the longitudinal cohort (n=13). | 8.5±4.5 | 5.9±2.4 | NS | |
| Time to Early ACR in the longitudinal cohort (mean±SD days) (n=13) | 29±16 | NA | NA | |
| Incidence of late ACR in the cross sectional cohort | 69% (9 of 13) | 17% (3 of 18) | 0.013 | |
| Incidence of late ACR in the Longitudinal cohort | 15% (2 of 13) | 7% (1 of 14) | - |
To develop a parameter(s) for risk of rejection, the allospecific CD154+T-cell subset best associated with biopsy-proven rejection was identified in 31 single measurements from the pre-October-2006 cohort. Logistic regression, Leave-one-out cross-validation (LOO-CV), and ROC analysis were used.
The longitudinal cohort was analyzed to confirm this association, and ask whether rejection events could be predicted, and rejection risk determined. Kaplan-Meier survival analysis and Cox proportional hazard ratios were used as described previously (23).
Summary statistics were calculated after combining 31 cross-sectional observations with those obtained during the 1–60 day time period in the longitudinal cohort. Between-group comparisons for these summary observations were performed with a Mann-Whitney test or a t-test, depending upon nonviolation of statistical assumptions. Spearman tests assessed the relationship between IR for allospecific CD154+T-cells, and alloresponsive proliferated CFSElow T-cells (n=23), or allospecific CTLA4+T-cells (n=31).
To determine the effect of Tacrolimus whole blood concentrations (FKWB) on parameter(s) for rejection risk, IR CD154+ Th-memory and Tc-memory cells in blood samples obtained before, and two hours after the morning dose of Tacrolimus were compared in 7 of 58 children. The pre- and post-dose samples were also assayed respectively, for the lowest (trough) and highest (peak) interdose FKWB. This experiment was done to understand how Tacrolimus may prevent clinical rejection, during periods at increased risk of rejection.
To confirm that the risk parameter of CD154+Tc-memory measured by the CD3+CD4− phenotype, was reflective of the CD3+CD8+ phenotype, simultaneous MLR was performed using PBMC from the same blood sample from 15 of 58 children, to evaluate each phenotype. This was done, because a significant portion of our study was initiated with 6-color flow cytometry, where the Tc subset was labeled as CD3+CD4−. As experience was gained with 8-color compensation, simultaneous detection of CTLA4+ and CD154+ T-cells, and CD8+ and CD4+ T-cells became possible.
Results
Rejectors (n=26) were similar to NR (n=32) in general demographics (Table 1). In both, the cross-sectional and longitudinal cohorts, MLR were performed before and after biopsy-proven ACR. As a result, mean FKWB at the time of MLR were largely influenced by higher FKWB used to treat ACR, and were significantly higher among Rejectors (Table 1). In the cross-sectional cohort, the incidence of late ACR was significantly higher among Rejectors, compared with Non-Rejectors. Despite similar numeric trends, differences in the incidence of late rejection were not significant in the longitudinal cohort, likely because of the shorter 200-day follow-up for this group.
Summary statistics
Rejectors show significantly higher proportions of donor-induced CD154+T-cells, compared with those induced by third-party (Fig. 2). Non-Rejectors showed significantly lower proportions of donor-induced CD154+T-cells, compared with those induced by third-party (Table 2). No differences were seen between groups in third-party induced IR for either subset. This resulted in significantly higher IR for memory CD154+ Th and Tc among Rejectors, compared with NR. Differences were most significant for memory CD154+Tc. Naive Th and Tc cells were not discriminatory. Similar trends were seen for donor-induced CFSElow proliferating Tc cells for Rejectors and Non-Rejectors. An opposite trend was seen for CTLA4. Compared with third-party stimulation, more donor-induced CTLA4+T-cells were seen among Non-Rejectors, and less donor-induced CTLA4+T-cells were seen among Rejectors (Fig. 2).
Figure 2.
Scatter plots in panel a) show significantly higher proportions of donor-specific CD154+ (uppermost) and CFSElow (lowermost) T-cytotoxic cells in a Rejector, compared with corresponding third-party-induced alloresponses. In the middle subpanel, donor-induced expression of the negative costimulator, CTLA4, is less than third-party-induced CTLA expression. For the Non-Rejector shown in panel b) the reverse features are seen in a Non-Rejector. Donor-induced markers of T-cell activation, CD154 and CFSElow cells, are less than those induced with third-party stimulation. On the other hand, donor-induced cells expressing the negative costimulator, CTLA4, exceed third-party induced CTLA4+ cells.
Table 2.
Summary of naïve and memory CD154+Th and CD154+Tc in 58 LTx children. Data are summarized for 26 Rejectors and 32 Non-Rejectors. Results are expressed as Median ±SEM CD154+ cell counts/10,000 in response to donor (SD) or third-party (TP), or as the immunoreactivity index (IR) for that family or subfamily (IR=SD: TP). Alone: Recipient alone with out stimulation, SD: Stimulated with Surrogate donor, TP: Stimulated with third-party, IR: Immunoreactivity index.
| Th-Memory | Th-Naive | |||||||
|---|---|---|---|---|---|---|---|---|
| Alone | SD | TP | IR | Alone | SD | TP | IR | |
| Median ± SEM (Non-Rejector, n=32) | 17±39 | 35±42 | 68±83 | 0.394±0.082 | 3±8 | 4±9 | 14±27 | 0.099±0.130 |
| Median ± SEM (Rejector, n=26) | 36±184 | 267±119 | 132±131 | 1.730±1.029 | 4±14 | 12±33 | 8±32 | 0.835±3.188 |
| p-value | 0.250 | 0.009 | 0.439 | 0.011 | 0.833 | 0.050 | 0.949 | 0.178 |
| Tc-Memory | Tc-NAive | |||||||
| Alone | SD | TP | IR | Alone | SD | TP | IR | |
| Median ± SEM (Non-Rejector, n=32) | 16±27 | 73±52 | 200±111 | 0.501±0.106 | 5±25 | 7±39 | 22±38 | 0.348±0.216 |
| Median ± SEM (Rejector, n=26) | 17±42 | 255±166 | 112±90 | 1.912±0.437 | 7±7 | 32±38 | 32±12 | 1.238±0.601 |
| p-value | 0.754 | 0.025 | 0.528 | 8.87E-05 | 0.446 | 0.332 | 0.292 | 0.008 |
Allospecific CD154+Tc-memory cells associate best with rejection, at threshold IR≥1.13
The cross-sectional cohort consisted of 31 observations from 13 Rejectors and 18 Non-Rejectors. Among Rejectors, 6 were monitored before, and 7 after biopsies showing ACR. The most parsimonious logistic regression model was calculated using exhaustive backward and forward stepwise selection of IR for the 4 cell types as independent variables, and Rejector status as the dependent variable across 31 cross-sectional observations. The optimal model was built with CD154+Tc-memory cells alone. We then assessed five covariates, which could contribute confounding effects-age, race, gender, time from transplant, and Tacrolimus whole blood level. In additional stepwise model selection, only two covariates were tested with the original four independent variables at any given time to avoid model saturation. The optimal model was once again built with CD154+Tc-memory cells alone. Clinical status and assay results of all subjects in the screening and replication cohorts, who received biopsy, are summarized in Table 3. The biopsied cohort includes 25 of 26 Rejectors, and 13 of 32 Non-Rejectors. Biopsies representative of each group are shown in Fig. 3.
Table 3.
a and b: Summary of assay predictions expressed as IR for each T-cell subset, and corresponding biopsy result for 38 children. This biopsied cohort included 25 of 26 Rejectors (Table 3a) and 13 of 32 Non-Rejectors (Table 3b).
| Table 3a: | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| # | Bx Diagnosis | Time between sampling and Bx date | Th memory IR | Th naïve IR | Tc memory IR | Tc naive IR | Prediction based on Th memory IR | Prediction Based on Th naive IR | Prediction based on Tc memory IR | Prediction based on Tc naive IR |
| 1 | R | −6 | 0.55 | 0.00 | 1.30 | 0.00 | NR | NR | R | NR |
| 2 | R | 47 | 7.19 | 2.19 | 1.06 | 0.06 | R | R | NR | NR |
| 3 | R | 26 | 0.59 | 0.39 | 2.86 | 0.74 | NR | NR | R | NR |
| 4 | R | −6 | 0.50 | 1.10 | 1.65 | 0.62 | NR | NR | R | NR |
| 5 | R | 22 | 0.79 | 0.00 | 1.31 | 0.09 | NR | NR | R | NR |
| 6 | R | −4 | 0.94 | 0.49 | 1.53 | 1.04 | NR | NR | R | NR |
| 7 | R | 4 | 2.59 | 0.00 | 2.60 | 8.73 | R | NR | R | R |
| 8 | R | 2 | 1.82 | 0.46 | 3.71 | 0.20 | R | NR | R | NR |
| 9 | R | −9 | 16.87 | 8.43 | 2.44 | 9.66 | R | R | R | R |
| 10 | R | −2 | 2.34 | 0.00 | 1.58 | 0.00 | R | NR | R | NR |
| 11 | R | 10 | 1.94 | 0.86 | 2.33 | 0.89 | R | NR | R | NR |
| 12 | R | −1 | 2.67 | 0.66 | 1.35 | 5.45 | R | NR | R | R |
| 13 | R | 35 | 0.96 | 1.22 | 1.40 | 1.77 | NR | R | R | R |
| 14 | R | 53 | 1.14 | 0.08 | 0.39 | 5.19 | R | NR | NR | R |
| 15 | R | −37 | 3.10 | 0.16 | 3.44 | 2.25 | R | NR | R | R |
| 16 | R | 7 | 6.97 | 2.37 | 9.17 | 8.23 | R | R | R | R |
| 17 | R | 2 | 2.16 | 2.85 | 1.27 | 1.15 | R | R | R | R |
| 18 | R | −15 | 4.13 | 1.54 | 1.80 | 2.70 | R | R | R | R |
| 19 | R | −4 | 1.98 | 0.00 | 9.98 | 6.09 | R | NR | R | R |
| 20 | R | 0 | 0.47 | 2.49 | 1.20 | 2.26 | NR | R | R | R |
| 21 | R | 5 | 3.31 | 9.72 | 5.09 | 1.37 | R | R | R | R |
| 22 | R | −18 | 1.64 | 83.65 | 2.98 | 0.00 | R | R | R | NR |
| 23 | R | 0 | 1.18 | 0.81 | 2.25 | 1.50 | R | NR | R | R |
| 24 | R | −15 | 0.29 | 0.45 | 1.65 | 1.33 | NR | NR | R | R |
| 25 | R | −20 | 0.18 | 0.09 | 2.50 | 0.95 | NR | NR | R | NR |
Figure 3.
Representative liver biopsies from a Rejector and Non-Rejector. Two stains are presented for each biopsy-hematoxylin and eosin, and immunostain for T-cytotoxic cells (aminoethylcarbazol, AEC). Corresponding immunoreactivity indices (IR) of CD154+ Tc-memory cells are also shown. a) Allograft liver with no cellular infiltration of portal areas. No Acute cellular rejection (HEx200) Fig. 3b as in 3a, with few red-stained Tc (CD8+) cells (AEC) 3c. Allograft liver-central vein inflammation, hepatocyte dropout, and centrilobular extravasation–moderate centrilobular ACR (HEx200). Fig. 3d as in 3c, with a large number of red-stained Tc (AEC).
To obtain a realistic measure of model performance, leave-one-out cross validation (LOO-CV) was performed using CD154+Tc-memory cell IR as the single predictor of Rejector status on these 31 observations. Using the IR cut point from each observation drop in LOO-CV, the median was calculated for use as the threshold IR for this cell type. ROC analysis confirmed this single cutoff IR value of CD154+Tc-memory cell at 1.13, above which Rejector status is predicted (supplementary Table 1).
Allospecific CD154+Tc memory cells associate with ACR, and with risk of rejection in an independent longitudinal cohort
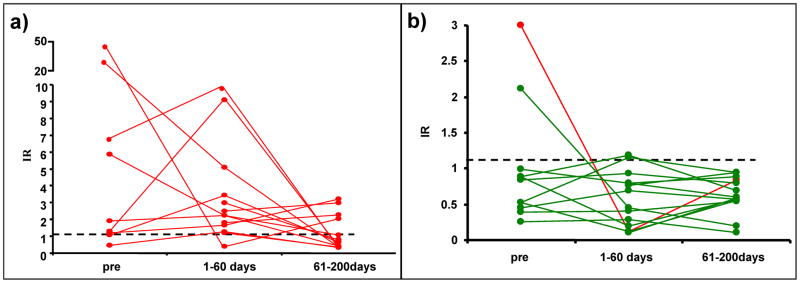
The independent longitudinal dataset consists of pre-LTx IR and two repeated measurements obtained at 1–60 and 61–200 days after LTx from 27 children (Fig. 4). Eight of 81 maximum possible measurements (≈10%) are missing-7 missing pre-LTx, and one missing at 1–60 days. Compared with Non-Rejectors, Rejectors demonstrated significantly higher IR pre-LTx (1.92±5.1 vs 0.84±0.25, p=0.01), and at 1–60 days (2.25±0.83 vs 0.68±0.11, p=0.00002), but not at 61–200 days (0.78±0.29 vs 0.6±0.07, p=NS). Specifically, IR<1.13 was achieved by all except four Rejectors at 61–200 days. Additional analyses are as follows:
Figure 4.
IR for CD154+Tc-memory cells obtained from 27 children in the longitudinal cohort are shown. The cohort consisted of 13 Rejectors and 14 Non-Rejectors. Twenty of 27 children were sampled before LTx, 26 of 27 during the 1–60-day time period, and 27 of 27 at the 61–200-day time period. Figure 4a. IR<1.13 (below dotted line) was seen in 9 of 11 Non-Rejectors, sampled before LTx, in 11 of 13 sampled during the 1–60-day period, and all 14 children sampled at the 61–200-day period. One of two Non-Rejectors showing pre-LTx IR≥1.13 (red line) went on to experience late ACR. Figure 4b. IR>1.13 (above dotted line) was seen in 7 of 9 Rejectors sampled pre-LTx, and in 12 of 13 Rejectors sampled during the 1–60-day period. All but four of 13 Rejectors achieved IR<1.13, or reduced risk of rejection at 61–200 days. Two of these four children experienced late biopsy-proven ACR.
Allospecific CD154+Tc memory cells associate with ACR
Of 27 maximum measurements, 26 were available at 1–60 days. These 26 observations were compared with the cutoff IR of 1.13, which was derived from the cross-sectional cohort. Twelve of 13 Rejectors show IR≥1.13 (sensitivity 92.3%), while 11 of 13 Non-Rejectors show IR<1.13 (specificity 84.6%, (supplementary table 2). All but four Rejectors were sampled prior to biopsies showing ACR. Observations at 1–60 days were made in proximity to biopsy-proven early rejection, and most closely approximate cross-sectional data. Therefore, the association between rejection and the Tc-memory cell is replicated.
Pre-LTx IR≥1.13 associates with increased incidence and hazard of ACR
Kaplan-Meier survival analysis and Cox proportional hazard ratio were used to ask whether pre-LTx IR≥1.13 (n=9) and <1.13 (n=11) indicated respectively, increased and decreased risk of rejection. Increased pre-LTx risk (IR>1.13) was associated with significantly greater rejection incidence, (77.8% vs. 18.2%, p=0.02, Fisher exact test), delayed reduction of risk to IR<1.13, (166±22 vs 56±19 days, p=0.016), and increased hazard of rejection (Cox proportional hazard ratio, HR=7.355) (Supplementary table 3). Specifically, 7 of 9 children with pre-LTx IR≥1.13 experienced rejection, and 9 of 11 children with pre-LTx IR<1.13 did not experience rejection during the first 60 days. (Fig 5a).
Figure 5.
A) Rejection-free survival during the first 200 days after LTx for children whose CD154+Tc-memory cell showed high pre-LTx rejection risk, or IR≥1.13 is significantly lower, compared with children with low pre-LTx risk or IR<1.13. (B) Time in days required to achieve low rejection risk, or IR<1.13 after LTx was significantly longer in Rejectors, compared with Non-Rejectors (median 167 vs 56 days, p=0.016).
Rejectors show delayed resolution of risk to IR<1.13
When survival and hazard analysis is applied to compare subjects grouped by Rejector (n=13) and Non-Rejector status (n=14), Rejectors demonstrated significantly higher pre-LTx risk, IR=9.57±5.09 vs 0.98±0.25, p=0.01 (Mann-Whitney test), delayed reduction of risk to IR<1.13 (167±2 vs 27±24 days, p<0.001), and a significantly lower hazard of achieving IR<1.13 in 200-day follow-up (HR=0.062), compared with Non-Rejectors (n=14) (Fig. 5b).
Correlation between IR for allospecific CD154+T-cells and CFSElowT-cells for 23 of 58 summary observations was most significant for memory Tc (Spearman coefficient r=0.835, p=7.1e-07, 95% CI 0.645 to 0.927, Fig. 6a) and less so for memory Th (Spearman coefficient r=0.524, p=0.010, 95% CI 0.143 to 0.769). Correlations were not significant for naive subsets. Subjects included eight Rejectors and fifteen Non-Rejectors (Table 4).
Figure 6.
a) Highly significant correlations exist between IR for CFSElow Tc-memory, and allospecific CD154+ Tc-memory cells in 23 children (Spearman rho=0.835, p=7.1e-07). b) The negative correlation between IR for allospecific CD154 Tc-memory and IR for allospecific CTLA4+Tc-memory cells is highly significant in 31 of 58 recipients. Ranked placement shows Non-Rejectors clustered in the lower right hand side of the graph, while Rejectors are clustered in the upper left hand side (Spearman rho −0.706, p=3.0e-05).
Table 4.
Shows CFSElow (proliferated) cells in the T-helper and T-cytotoxic memory and naive subfamilies in 3 day co-culture experiment. Data are summarized for 8 Rejectors and 15 Non- Rejectors. Results are expressed as Median ±SEM CFSElow cell counts/10,000 in response to donor (SD) or third-party (TP), or as the immunoreactivity index (IR) for that family or subfamily (IR=SD: TP).
| Th-Memory | Th-Naïve | |||||||
|---|---|---|---|---|---|---|---|---|
| Alone | SD | TP | IR | Alone | SD | TP | IR | |
| Median ± SEM (Non-Rejector, n=15) | 40 ±191 | 328 ±1228 | 1000 ±1735 | 0.556 ±0.141 | 3 ±45 | 160 ±444 | 568 ±610 | 0.559 ±0.276 |
| Median ± SEM (Rejector, n=8) | 3 ±57 | 1490 ±1086 | 748 ±456 | 2.499 ±1.110 | 18 ±67 | 893 ±615 | 827 ±185 | 1.675 ±0.457 |
| p-value | NS | NS | NS | 0.0007 | NS | NS | NS | 0.010 |
| Tc-Memory | Tc-Naïve | |||||||
| Alone | SD | TP | IR | Alone | SD | TP | IR | |
| Median ± SEM (Non-Rejector, n=15) | 36 ±282 | 538 ±1143 | 801 ±1782 | 0.545 ±0.068 | 51 ±114 | 204 ±504 | 306 ±653 | 0.677 ±0.649 |
| Median ± SEM (Rejector, n=8) | 14 ±354 | 3641 ±1889 | 747 ±1396 | 1.481 ±0.804 | 111 ±137 | 661 ±265 | 495 ±249 | 1.213 ±0.465 |
| p-value | NS | NS | NS | 0.0001 | NS | NS | NS | 0.039 |
Negative correlations were significant between IR for CD154+Tc-memory and CTLA4+Tc-memory in 31 of 58 summary observations (Spearman rho= −0.706, 95% CI −0.85 to −0.47, p=3.0e-05, Fig. 6b), but not for memory Th and naive Th/Tc (not shown). Subjects included eight Rejectors, and twelve Non-Rejectors (Table 5).
Table 5.
Shows CTLA4+ cells in the T-helper and T-cytotoxic memory and naïve subsets. These measurements were done simultaneously with CD154 measurements in the same sample after 16-hour co-culture. Data are summarized for 31 of 58 recipients, in whom these measurements were performed (13 Rejectors and 18 Non- Rejectors). Results are expressed as Median±SEM CTLA+ cells/10,000 in response to donor (SD) or third-party (TP), or as the immunoreactivity index (IR) for that subset (IR=SD: TP).
| Th-Memory | Th-Naive | |||||||
|---|---|---|---|---|---|---|---|---|
| Alone | SD | TP | IR | Alone | SD | TP | IR | |
| Median ± SEM (Non-Rejector, n=18) | 151±42 | 210±185 | 214±88 | 1.317±0.163 | 10±3 | 57±14 | 27±8 | 1.523±1.865 |
| Median ± SEM (Rejector, n=13) | 86±32 | 141±25 | 226±63 | 0.799±0.187 | 4±7 | 13±12 | 28±5 | 0.368±0.322 |
| p-value | 0.300 | 0.139 | 0.750 | 0.086 | 0.523 | 0.021 | 0.369 | 0.207 |
| Tc-Memory | Tc-Naive | |||||||
| Alone | SD | TP | IR | Alone | SD | TP | IR | |
| Median ± SEM (Non-Rejector, n=18) | 203±126 | 389±201 | 291±54 | 2.449±0.349 | 19±15 | 49±16 | 29±24 | 1.360±1.414 |
| Median ± SEM (Rejector, n=13) | 134±89 | 238±97 | 387±285 | 0.682±0.087 | 5±9 | 11±8 | 28±7 | 0.760±0.487 |
| p-value | 0.545 | 0.036 | 0.077 | 5.41E-05 | 0.489 | 0.024 | 0.192 | 0.196 |
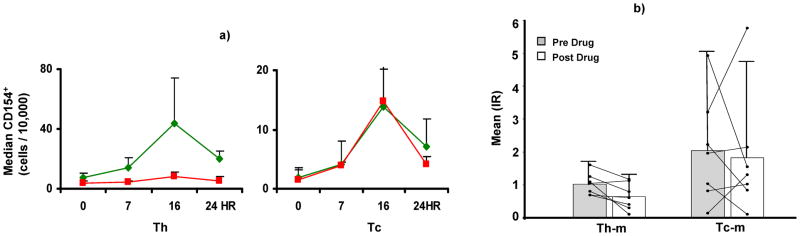
IR of CD154+Th-memory (Pre-dose vs. post-dose 1.0±0.3 vs. 0.6±0.4, p=0.026), not CD154+Tc-memory (Pre-dose vs. post-dose 2.0±1.6 vs. 1.8±1.9, p= NS, Fig. 7), decreased significantly as FKWB rose in the post-dose blood sample, compared with pre-dose FKWB (post-dose vs. pre-dose FKWB 18.8±10.4 vs. 7.6±3.6 ng/ml, p=0.014).
Figure 7.
This figure summarizes experiments demonstrating properties of allospecific CD154+ Tc-memory cells as markers of rejection-prone recipients. a) Summary data for six allostimulated normal control MLR show that CD154 expression peaks at 16 hours, is 2.5 fold greater in Th cells, compared with Tc, and is inhibited by 80% in Th cells, pretreated with Tacrolimus. Tc cells are minimally inhibited by Tacrolimus. B) Post-dose IR (Mean±SD) for Th-memory cells obtained from 7 children with LTx who are Rejectors are lower than pre-dose IR. Post-dose IR for Tc-memory decreases numerically compared with pre-dose IR, but the change is not statistically significant. Changes in pre- and post-dose IR for each subject are connected by lines.
Discussion
Our studies suggest that allospecific CD154+T-cells in MLR are as informative as proliferative T-cell alloresponses, but more suited for clinical monitoring of pediatric recipients weighing 3–5 kilograms, because they can be detected in <24 hours, in 3 ml blood samples. Unlike less successful previous efforts involving 6–8 hour mitogen stimulation, detection is facilitated by longer 16-hour allostimulation (24).
Compared with Non-Rejectors, Rejectors show significantly more donor-specific CD154+T-cells distributed among memory Th and Tc, providing part corroboration that graft-specific effectors can originate from either subset (25, 26). However, IR for CD154+Tc-memory, rather than absolute cell count, is most associated with biopsy-proven rejection. Derived from a cross-sectional cohort of 31 children, threshold IR of 1.13 for CD154+Tc-memory correctly separates Rejector from Non-Rejectors with sensitivity and specificity of 92.3% and 84.6%, respectively, during the 1–60-day risk period for early ACR in an independent, longitudinally monitored cohort. We believe that the risk parameter, IR, contributes to the high sensitivity and specificity, by reducing the effect of biological variation. The IR expresses increased or decreased donor-specific alloreactivity with respect to a reference, the third-party alloresponse, making the risk parameter unique to each recipient, but comparable between cohorts. Additional findings further support a potential role for CD154, and Tc-memory cells as clinical markers of ACR. An inverse relationship between CD154, which is overexpressed during risk periods for rejection, and CTLA4, which is overexpressed during periods of reduced risk for rejection, is best seen in Tc-memory cells. Also, in previous studies, CD154 is only expressed in biopsy tissue from liver allografts with rejection (27).
To qualify as a dynamic risk parameter, a “baseline” threshold IR of CD154+Tc-memory cell must predict future rejection events in longitudinal follow-up. Criteria for “baseline” are met by pre-LTx IR, which was available for 20 of 27 longitudinally monitored children. The threshold IR≥1.13 derived from cross-sectional monitoring in proximity to rejection events qualifies as a dynamic measure indicative of increased risk of rejection. In survival analysis and Cox proportional hazard modeling, children with increased pre-LTx risk, or IR≥1.13, showed a 78% incidence of ACR, and 7.355 greater hazard of ACR, compared with 18% ACR incidence if pre-LTx risk was low (IR <1.13). Additional analyses of two repeated post-LTx measurements from each of 27 children further show the dynamic nature of this risk parameter. In all but four children, the IR of CD154+Tc-memory cells declined to IR<1.13 within the 200-day follow-up period. The time to achieve low risk (IR<1.13) was significantly longer if pre-LTx risk was high (IR≥1.13). Further, the hazard of achieving a low risk of rejection, or IR<1.13, during the 200-day monitoring period was greatly reduced among Rejectors, to 6.2% of that seen among Non-Rejectors. These findings lead to the inference that IR≥1.13 for CD154+Tc-memory cells may be indicative of future rejection events. Supporting this inference, two of 4 children with IR≥1.13 at 200-day follow-up went on to experience ACR after 200 days. Also, one individual with pre-LTxIR≥1.13, who was classified as a Non-Rejector because of the absence of “early” ACR at 1–60 days, went on to experience “late” ACR 99 days after LTx. Pre-transplant ELISPOT monitoring of adult renal transplant recipients also supports a predictive role for allorective T-cells. Half of all recipients with >25 IFNγ+ donor-responsive PBMC went on to develop early renal allograft ACR (28). Additional validation of allospecific CD154+Tc-memory cells as predictive markers will require an independent longitudinal cohort.
Our identification of the Tc-memory as potential “precursor” cells, which may herald LTx rejection in pre-LTx monitoring, may be explained by pre-existing donor-specific memory within this subset, possibly due to contact with cross-reactive, non-allogeneic antigens (29). However, IR≥1.13 was also seen in 9 of 26 Rejectors in our study, in whom monitoring was only possible after a biopsy had been performed, and the treatment of ACR had been initiated. In these children, normal liver biochemistry suggested resolution of ACR, despite IR≥1.13. Pre- and post-dose measurements, in which the IR for Th-memory decreases with increasing FKWB, but the Tc is unaffected, offer some explanations. It can be speculated that after treatment of rejection, donor-specific “effector” subpopulations within Th and Tc subsets decline to thresholds, which are amenable to additional reductions at peak FKWB. It can also be speculated that the resolution of ACR allows protective Th residing within allografts, e.g. T-regulatory cells, to better neutralize the effect of circulating donor-specific CD154+ Tc-memory cells, which remain selectively more donor-reactive, even at peak FKWB. Another hypothesis worthy of investigation is whether Tacrolimus-resistant subpopulations predominate among donor-specific CD154+ Tc-memory cells at the time of rejection. Increased resistance to inhibition by Tacrolimus has been observed in mitogen-stimulated Tc from Rejectors in our previous studies (6). Further, although unproven in humans, durable tolerance requires memory Tc ablation in non-human primates (17).
Our preliminary effort to define the risk of rejection with allospecific CD154+Tc-memory has obvious limitations. Initially, we defined Tc by the phenotype, CD3+CD4− with 6-color flow cytometry, because CD3+CD4−CD8− cells constitute <5% of this phenotype. Six flourochromes helped us localize donor-specificity to memory subsets in living recipient T-cells, without a confounding admixture with stimulator cells, or dead cells arising from co-culture conditions. With implementation of 8-color cytometry, simultaneous experiments in 15 children showed that the CD3+CD4− phonotype indeed represented its dominant constituent, the CD3+CD8+Tc. The IR for memory CD154+Tc were similar, whether the Tc subset was defined as CD3+CD4− or CD3+CD8+ (Table 6). Another limitation is the small numbers of children with LTx available at any center, the small size of the average recipient, ranging from 4–15 kilograms, the conditions for phlebotomy, whereby the volume of blood needed for pre-biopsy and diagnostic tests during suspected rejection often precluded safe research sampling simultaneously, and the safe limit for combined clinical and research blood sampling of 3 ml/kg in any 8-week period. As a result, parallel measurement of proliferative alloresponses, and research blood sampling at the time of diagnosis of rejection, could not be performed in all recipients. Third, we have used “surrogate donor PBMC” to unmask donor-specific CD154+T-cells in nearly 2/3rd or 41 of 58 children. Donor PBMC or splenocytes from cadaveric donors, which remain the dominant source of liver allografts, are usually consumed in the tissue-typing process. However, surrogate donor PBMC were matched with corresponding donors at a minimum of three antigenic loci. Also, the degree of matching between “surrogate donors” and corresponding donors was similar, when Rejectors and Non-Rejectors were compared. Therefore, outcome-specific differences in allospecific CD154+Tc could not be attributed to more immunogenic “surrogate-donor” stimulators among Rejectors. Banking of donor splenocytes has been initiated, where possible, to eliminate future concerns.
Table 6.
Comparison of immunoreactivity index (IR) calculated with memory CD154+CD3+CD4− and memory CD154+CD3+CD8+ staining in 15 LTx Recipients. Data are summarized for 10 Rejectors and 5 Non-Rejectors. No significant differences are seen.
| Tc phenotype | CD154+Tc-MEMORY IR |
|---|---|
| CD3+CD4− (Rejectors) (n=10) | 1.833 ± 0.40 |
| CD3+CD8+ (Rejectors) (n=10) | 1.835 ± 0.42 |
| P(T<=t) two-tail | NS |
| CD3+CD4− (Non-Rejectors) (n=5) | 0.439 ± 0.10 |
| CD3+CD8+ (Non-Rejectors) (n=5) | 0.536 ± 0.15 |
| P(T<=t) two-tail | NS |
Finally, we make no claims about CD154+Tc-memory cells as effectors of rejection. Our primary intent was to preserve the low yield of cells from lymphocyte-depleted children by avoiding additional assay-related losses, and develop a generic risk parameter. Therefore, allospecific CD154+ alloresponses were benchmarked to proliferative alloresponses, and not to intracellular effector cytotoxins such as perforin and granzyme. After 16-hour co-culture, up to 94% of cells from Non-Rejectors, and 86% cells from Rejectors were viable, and our risk parameter achieved a high sensitivity for detecting Rejectors in screening/replication testing.
Notwithstanding these obvious limitations, and experiments performed to address them within the clinical constraints of our rare test population, our study shows for the first time, that the longitudinal risk of liver ACR in children can be estimated with allospecific CD154+T-cells. Further, memory CD154+Tc may be important constituents of donor-specific “precursor” T-cells.
Supplementary Material
Table 3b.
| # | Bx Diagnosis | Time between sampling and Bx date | Th memory IR | Th naïve IR | Tc memory IR | Tc naive IR | Prediction based on Th memory IR | Prediction Based on Th naive IR | Prediction based on Tc memory IR | Prediction based on Tc naive IR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NR | 24 | 0.45 | 0.00 | 0.53 | 0.65 | NR | NR | NR | NR |
| 2 | NR | 1 | 0.09 | 0.02 | 0.09 | 0.01 | NR | NR | NR | NR |
| 3 | NR | 1 | 0.84 | 0.32 | 0.39 | 0.23 | NR | NR | NR | NR |
| 4 | NR | −8 | 1.16 | 1.09 | 1.60 | 1.33 | R | NR | R | R |
| 5 | NR | 55 | 0.36 | 0.28 | 0.38 | 0.00 | NR | NR | NR | NR |
| 6 | NR | 4 | 0.55 | 0.00 | 0.44 | 0.74 | NR | NR | NR | NR |
| 7 | NR | 38 | 0.39 | 0.09 | 0.79 | 0.08 | NR | NR | NR | NR |
| 8 | NR | 13 | 0.03 | 0.03 | 0.56 | 0.72 | NR | NR | NR | NR |
| 9 | NR | 10 | 0.33 | 0.10 | 0.79 | 0.44 | NR | NR | NR | NR |
| 10 | NR | −5 | 0.23 | 0.02 | 1.18 | 0.00 | NR | NR | R | NR |
| 11 | NR | 0 | 0.38 | 0.97 | 0.12 | 1.69 | NR | NR | NR | R |
| 12 | NR | 1 | 1.09 | 0.00 | 1.15 | 0.62 | NR | NR | R | NR |
| 13 | NR | −28 | 0.29 | 0.19 | 0.76 | 0.22 | NR | NR | NR | NR |
Acknowledgments
5RO1AI073895-02, 5-RO1AI49156-05, Children’s Hospital of Pittsburgh Research Foundation, and Hillman Foundation of Pittsburgh.
Mario Roederer, PhD, and Steve Perfetto, MS, NIH-NIAID for Technical advice for polychromatic flow cytometry.
Special thanks: The Puleo, Giventer-Braff and Herridge families.
References
- 1.Newell KA, Larsen CP. Tolerance assays: measuring the unknown. Transplantation. 2006;81:1503–09. doi: 10.1097/01.tp.0000222912.69532.1e. [DOI] [PubMed] [Google Scholar]
- 2.Jain A, Mazariegos G, Kashyap R, Kosmach-Park B, Starzl TE, Fung J, Reyes J. Pediatric liver transplantation in 808 consecutive children: A single center experience spanning 20 years. Transplantation. 2002;73:941–47. doi: 10.1097/00007890-200203270-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin SR, Atkison P, Anand R, Lindblad AS SPLIT Research Group. Studies of pediatric liver transplantation 2002: patient and graft survival and rejection in pediatric recipients of a first liver transplant in the United States and Canada. Pediatr Transplant. 2004;8:273–83. doi: 10.1111/j.1399-3046.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Elmagd KM, Zak M, Stamos JM, Bond GJ, Jain A, Ezzelarab M, Mazariegos GV, Sindhi R, Marcos A, Demetris AJ, Fung JJ, Reyes JD. De novo malignancies after intestinal and multivisceral transplantation. Transplantation. 2004;77:1719–25. doi: 10.1097/01.tp.0000131164.43015.4b. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Lakkis FG. The unfinished legacy of liver transplantation: emphasis on immunology. Hepatology. 2006;43(Suppl S):151–63. doi: 10.1002/hep.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sindhi R, Magill A, Abdullah A, Seward J, Tresgaskes M, Bentlejewski C, Zeevi A. Enhanced donor-specific alloreactivity occurs independent of immunosuppression in children with early liver allograft rejection. Am J Transplant. 2005;5:96–102. doi: 10.1111/j.1600-6143.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 7.Khera N, Janosky J, Zeevi A, Mazariegos G, Marcos A, Sindhi R. Persistent donor-specific alloreactivity may portend delayed liver rejection during drug minimization in children. FBS. 2007;12:660–63. doi: 10.2741/2090. [DOI] [PubMed] [Google Scholar]
- 8.Kerman RH, Susskind B, Katz SM, Van Buren CT, Kahan BD. Postrenal transplant MLR hypo-responders have fewer rejections and better graft survival than MLR hyper-responders. Transplant Proc. 1997;29:1410–11. doi: 10.1016/s0041-1345(96)00613-6. [DOI] [PubMed] [Google Scholar]
- 9.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–37. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 10.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–81. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 11.De Haan A, Van Der Gun I, Van Der Bij W, De Leij LF, Prop J. Detection of alloreactive T cells by flow cytometry: a new test compared with limiting dilution assay. Transplantation. 2002;74:562–70. doi: 10.1097/00007890-200208270-00023. [DOI] [PubMed] [Google Scholar]
- 12.Martins S, St John LS, Champlin RE, Wieder ED, McMannis J, Molldrem JJ, Komanduri KV. Functional assessment and specific depletion of alloreactive human T cells using flow cytometry. Blood. 2004;104:3429–36. doi: 10.1182/blood-2004-05-1918. [DOI] [PubMed] [Google Scholar]
- 13.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–17. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 14.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–24. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 15.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2000;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000;164:512–21. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 17.Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, Sogawa H, Murakami T, Strom TB, Colvin RB, Sachs DH, Benichou G, Cosimi AB, Kawai T. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;5:1055–61. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim EY, Lee EN, Lee J, Park HJ, Chang CY, Jung da Y, Choi SY, Lee SK, Joh JW, Kim SJ. Two-signal blockade with anti-CD45RB and anti-CD154 monoclonal antibodies inhibits graft rejection via CD4-dependent mechanisms in allogeneic skin transplantation. Exp Mol Med. 2006;38:284–94. doi: 10.1038/emm.2006.34. [DOI] [PubMed] [Google Scholar]
- 19.Issazadeh S, Navikas V, Schaub M, Sayegh M, Khoury S. Kinetics of expression of costimulatory molecules and their ligands in murine relapsing experimental autoimmune encephalomyelitis in vivo. J Immunol. 1998;161:1104–12. [PubMed] [Google Scholar]
- 20.Im SH, Barchan D, Maiti PK, Fuchs S, Souroujon MC. Blockade of CD40 ligand suppresses chronic experimental myasthenia gravis by down-regulation of Th1 differentiation and up-regulation of CTLA-4. J Immunol. 2001;166:6893–98. doi: 10.4049/jimmunol.166.11.6893. [DOI] [PubMed] [Google Scholar]
- 21.Fung JJ. Tacrolimus and transplantation: a decade in review. Transplantation. 2004;77:41–43. doi: 10.1097/01.tp.0000126926.61434.a5. [DOI] [PubMed] [Google Scholar]
- 22.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, Roederer M. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods. 2006;313:199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Kopycka-Kedzierawski DT, Billings RJ. A longitudinal study of caries onset in initially caries-free children and baseline salivary mutans streptococci levels: a Kaplan-Meier survival analysis. Community Dent Oral Epidemiol. 2004 Jun;32(3):201–9. doi: 10.1111/j.1600-0528.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett A, McCall J, Ameratunga R, Munn S. The kinetics of CD154 (CD40L) expression in peripheral blood mononuclear cells of healthy subjects in liver allograft recipients and X-linked hyper-IgM syndrome. Clin Transplant. 2000 Dec;14(6):520–8. doi: 10.1034/j.1399-0012.2000.140602.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Fujisawa H, Zhuang L, et al. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol. 2000;165:6783–90. doi: 10.4049/jimmunol.165.12.6783. [DOI] [PubMed] [Google Scholar]
- 26.Dearman RJ, Moussavi A, Kemeny DM, Kimber I. Contribution of CD4+ and CD8+ T lymphocyte subsets to the cytokine secretion patterns induced in mice during sensitization to contact and respiratory chemical allergens. Immunol. 1996;89:502–10. doi: 10.1046/j.1365-2567.1996.d01-778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartlett AS, McCall JL, Ameratunga R, Yeong ML, Gane E, Munn SR. Analysis of intragraft gene and protein expression of the costimulatory molecules, CD80, CD86 and CD154, in orthotopic liver transplant recipients. Am J Transplant. 2003 Nov;3(11):1363–8. doi: 10.1046/j.1600-6135.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 28.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-Transplant IFN-gamma ELISPOTs are associated with post-Transplant renal function in African American renal transplant recipients. Am J Transplant. 2005 Aug;5(8):1971–5. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 29.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999 Aug 15;163(4):2267–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.