Abstract
Background
Cognition is affected early in Huntington's disease, and in HD animal models there is evidence that this reflects abnormal synaptic plasticity. We investigated whether there is evidence for abnormal synaptic plasticity using the human motor cortex-rTMS model, and if so, if there is any difference between premanifest HD gene carriers and very early manifest HD patients or any relationship with ratings of the severity of motor signs.
Methods
Fifteen HD gene carriers (7 premanifest, 8 very early manifest) and 14 control participants were given a continuous train of 100 bursts of theta burst stimulation (cTBS: three pulses at 50 Hz and 80% AMT repeated every 200ms). The size of the motor evoked potential was measured at regular intervals until 21 minutes after cTBS.
Results
HD gene carriers and controls responded differently to theta burst stimulation (F4.9,131.9=1.37, p=0.048) with controls having more inhibition than HD gene carriers (F1,27=13.3, p=0.001). Across all time points mean inhibition differed between the groups (F2,26=6.32, p=0.006); controls had more inhibition than either HD gene carrier subgroup (p=0.006 for premanifest and p=0.009 for early symptomatic) whereas there was no difference between premanifest and early symptomatic HD gene carriers. The measure of cortical plasticity was not associated with any clinical ratings (UHDRS motor score, estimate of age at onset).
Conclusions
Motor cortex plasticity is abnormal in HD gene carriers but is not closely linked to the development of motor signs of HD.
Keywords: repetitive transcranial magnetic stimulation, UHDRS, Huntington's disease, synaptic plasticity, theta burst stimulation
INTRODUCTION
Huntington's disease (HD) is caused by a CAG repeat expansion in the IT15 gene that leads to abnormal movements, behavioural and psychiatric problems, and declining powers of cognition [1]. Several of the symptoms of HD involve deficits in learning and memory. These encompass difficulties with attention, working and verbal episodic memory and behaviour inhibition, as well as recognition of emotion and explicit learning [2-4]. Interestingly, incidental learning seems relatively spared, at least in the premanifest and early manifest stages of the disease [4]. Given the importance of synaptic changes such as long term potentiation and depression (LTP/LTD) in these processes [5], it is perhaps not surprising that mouse models of HD show abnormal plasticity even before these animals have signs of the disease or neurone loss [6-8]. The animals have behavioural abnormalities with concomitant deficits in hippocampal LTP and LTD, and mossy fibre potentiation [9-13]. While many studies in HD mouse models examined the hippocampus and the striatum there is also evidence for altered cortical plasticity [7].
The aim of the present study was to explore whether similar abnormalities of synaptic plasticity can be observed in human carriers of the HD mutation. Although HD is often seen as a disease of the basal ganglia, there is good evidence from structural imaging and microarray analysis to suggest that cortical areas, in particular the motor cortex, are involved early in HD [14-18]. Thus we used a recently designed measure of LTD-like plasticity in the motor cortex involving repetitive transcranial magnetic stimulation (TMS) given in a theta burst pattern (theta burst stimulation, TBS) which has after-effects on the excitability of corticospinal projections that last for 30-60min after the end of stimulation [19]. Given that these disappear after pretreatment with drugs that interfere with NMDA receptor function [20], they are thought to be produced by changes in synaptic plasticity similar to those observed in animal experiments. In the present study we used this method to investigate motor cortex plasticity in a homogeneous group of premanifest and very early manifest HD patients. We were interested in two questions: is there evidence for abnormal synaptic plasticity in the motor cortex, and if so, is there any difference between premanifest HD gene carriers and very early manifest HD patients or any relationship with ratings of the severity of motor signs.
MATERIAL AND METHODS
HD gene carriers and control participants
Fifteen HD gene carriers (12 women, mean age 41.6 years, range 28-64; for CAG repeat length see table 1) and 14 control participants (9 women, mean age 42.4 years, range 28-62) were recruited. The same clinician (SJT) with long standing experience in HD examined patients clinically; motor, psychiatric and cognitive signs were scored using the Unified Huntington's Disease Rating Scale (UHDRS) [21]. Seven HD gene carriers were clinically premanifest according to the UHDRS (diagnostic confidence score of less than 4), and 8 HD gene carriers were early manifest (clinical stage 1 [22]). The predicted time to symptom onset was estimated according to Langbehn et al [23]. All HD gene carriers apart from two scored > 27 on the Mini-Mental State Examination (MMSE) [24]. Two early HD patients scored 26 on the MMSE, possibly suggesting mild cognitive impairment. Neurological examination was normal in controls, and there was no history of psychiatric disorders or substance abuse. HD gene carriers and controls were unmedicated at the time of the study. Participants gave informed written consent according to the Declaration of Helsinki, and the local ethics committee approved the study protocol.
Table 1.
Demographic, molecular genetic and clinical data from HD patients. Estimated symptom onset was calculated according to Langbehn et al (23). Since all manifest patients by definition had HD motor signs the calculated ‘onset’ is given in brackets. All premanifest patients had a diagnostic confidence score of less than 4 on the UHDRS.
| Patient | age | gender | CAG | UHDRS motor | Years to predicted onset |
|---|---|---|---|---|---|
| Premanifest 1 | 41 | F | 43 | 0 | 10.59 |
| Premanifest 2 | 39 | F | 42 | 2 | 16.10 |
| Premanifest 3 | 40 | M | 41 | 4 | 19.10 |
| Premanifest 4 | 32 | M | 40 | 0 | 33.54 |
| Premanifest 5 | 28 | F | 47 | 7 | 10.50 |
| Premanifest 6 | 38 | F | 40 | 1 | 27.68 |
| Premanifest 7 | 53 | F | 41 | 5 | 9.44 |
| Manifest 1 | 44 | F | 43 | 8 | (8.60) |
| Manifest 2 | 48 | M | 47 | 16 | (3.53) |
| Manifest 3 | 43 | F | 43 | 15 | (9.22) |
| Manifest 4 | 33 | F | 44 | 13 | (13.82) |
| Manifest 5 | 48 | F | 46 | 23 | (3.79) |
| Manifest 6 | 37 | F | 43 | 8 | (13.80) |
| Manifest 7 | 64 | F | 44 | 30 | (4.09) |
| Manifest 8 | 40 | F | 46 | 30 | (4.98) |
Electromyography recordings
Surface electromyograms (EMG) were recorded from the right first dorsal interossoeus (FDI) muscle using silver/silver-chloride disc surface electrodes (1 cm diameter) in a belly tendon montage. The EMG signal was amplified and analogue filtered (30Hz to 1kHz) with a Digitimer D150 amplifier (Digitimer Ltd., Welwyn Garden City, UK). Data (sampling rate 4kHz) was digitised for off-line analysis using Signal software (Cambridge Electronic Devices, Cambridge, UK).
Transcranial Magnetic Stimulation
HD gene carriers and controls were seated relaxed in a comfortable chair. Magnetic stimuli were given with a hand-held figure-of-eight coil (outer winding diameter 9cm) connected to a High Power Magstim 200 stimulator (Magstim Co., Whitland, Dyfed, UK). This stimulator induces a pulse with monophasic waveform. For repetitive transcranial magnetic stimulation we used a Super Rapid Magstim stimulator (Magstim Co., Whitland, Dyfed, UK). This stimulator generates a magnetic pulse with a biphasic (anterior posterior/posterior-anterior/) waveform. Both stimulators induce a current in the brain with posterior-anterior flow when the coil is placed tangentially to the scalp with the handle positioned at an angle of 45° pointing backwards. The optimal spot for right FDI stimulation was marked with a felt pen.
Motor thresholds
Resting motor threshold (RMT) was defined as the minimum intensity needed to evoke an MEP of >50μV in 5 out of 10 consecutive trials in the relaxed FDI. Active motor threshold (AMT) was defined as the minimum intensity (in % of maximum stimulator output) needed to evoke a MEP of >200μV in 5 out of 10 trials in the tonically active FDI (~10% of maximal contraction as assessed visually on an oscilloscope). Thresholds were approached from above threshold in steps of 1% stimulator output. Once no MEPs could be elicited the intensity was increased in steps of 1% stimulator output until a minimal MEP was observed. This intensity was taken as motor threshold.
Continuous theta burst stimulation
Theta burst stimulation (TBS) followed the protocol described in detail by Huang et al [19]. In brief, repetitive transcranial magnetic stimulation consisted of three pulses at a frequency of 50 Hz and an intensity of 80% AMT repeated with a frequency of 5 Hz (i.e. every 200ms). A continuous train of 100 bursts (300 stimuli) was given (continuous TBS, cTBS).
Data analysis
Two researchers blinded to HD gene carriers' clinical characteristics collected and analysed the TMS data. Peak to peak amplitude of MEP was measured with in-house software. To assess the effects of cTBS we examined whether there was a main effect of ‘time’ on MEP amplitude. This was tested using repeated measures analysis of variance (ANOVA). To test whether HD gene carriers, either premanifest or early manifest, differed from controls or from each other we introduced ‘group’ as between-subjects factor. In addition, we tested whether the time course of the after effects of cTBS differed between HD gene carriers and controls (interaction of ‘time’ and ‘group’ in the repeated measures ANOVAs).
A statistical difference in the ANOVAs was followed by a post-hoc paired t-test analysis. Mauchly's test tested for sphericity in the repeated measures ANOVAs, and the Greenhouse-Geisser correction was applied to the DFs if necessary. Statistical significance levels were set to p=0.05. All statistical analyses were performed using SPSS 16 for Windows software package.
RESULTS
Motor thresholds
Thresholds were similar in controls (AMT 30.8 (SD 5.5); RMT 40.6 (6.9); rapid AMT 44.8 (6.8)) and HD gene carriers (AMT 32.7 (5.7); RMT43 (7.1); rapid AMT 46.4 (8.8)).
Continuous theta burst stimulation
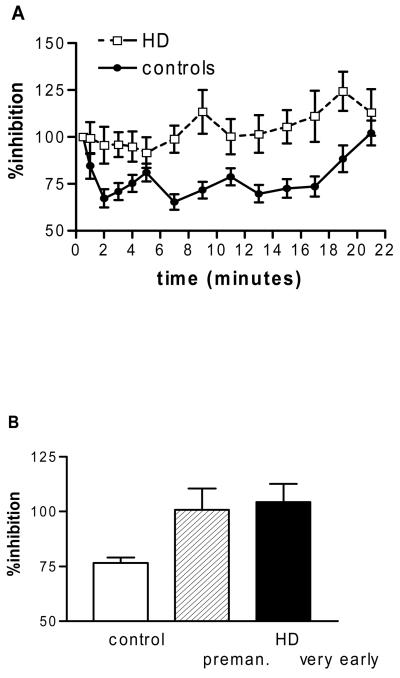
Theta burst stimulation induced inhibition in the controls that lasted for the 21 minutes observation period after the end of stimulation (repeated measures ANOVA, main effect of ‘time’, F4.3,56=4.3, p=0.003). In the group of HD gene carriers and controls together this effect was lost; both groups responded differently to theta burst stimulation (repeated measures ANOVA, interaction ‘time*group’, F4.9,131.9=1.37, p=0.048, Figure 1A) with controls having more inhibition than HD gene carriers (ANOVA on data in Figure 1A, main effect of ‘group’, F1,27=13.3, p=0.001). Looking at the time course of the effect (0-9 versus 11-21 minutes after the end of cTBS) it appeared that there was less inhibition at the later time points but this was not significant (repeated measures ANOVA, no main effect of ‘time’). We next compared all 3 groups (controls, premanifest and early manifest) using the mean inhibition across all time points. This showed a difference between the groups (ANOVA, main effect of ‘group’, F2,26=6.32, p=0.006); controls had more inhibition than either HD gene carrier subgroup (p=0.006 for premanifest and p=0.009 for early symptomatic, Figure 1B) whereas there was no difference between premanifest and early symptomatic HD gene carriers.
Figure 1.
Response to continuous theta burst (cTBS) stimulation. A. In controls cTBS inhibits motor cortex excitability up to 20 minutes whereas there is no effect in HD patients (ANOVA, main effect of ‘group’, F1,27=13.3, p=0.001). B. The mean inhibition differed between the groups (ANOVA, main effect of ‘group’, F2,26=6.32, p=0.006); controls had more inhibition than either patient subgroup (p=0.006 for premanifest and p=0.009 for early symptomatic), whereas there was no difference between premanifest and early symptomatic patients. Error bars are standard error of the mean.
For subsequent analyses with clinical parameters we used the mean inhibition across all time points.
Correlation of cortical plasticity with clinical measures
We first tested whether the response to cTBS was related to UHDRS motor score. To this end we used backward logistic regression analysis with UHDRS motor score as the dependent variable and age, CAG repeat length, and the overall average of inhibition induced by cTBS as the dependent variables. Only age and CAG repeat length predicted UHDRS motor score (F2,12=10.64, p=0.002, adjusted R2=0.58). We next examined if the response to theta burst stimulation changed when HD gene carriers approach the onset of motor signs. To this end we assessed how the estimate of predicted age at onset was predicted using age, CAG repeat length, and cTBS data. The cTBS data did not improve prediction over age and CAG repeat length (F2,12=29.6, p<0.001, adjusted R2=0.8). We then examined in the same way how the response to cTBS was predicted. There was no association with any of the variables.
DISCUSSION
We used theta burst stimulation, a novel form of repetitive transcranial magnetic stimulation, to investigate motor cortical plasticity. In controls, we were able to reproduce the inhibitory effects shown previously [19]. However, cTBS did not induce any changes in HD expansion mutation carriers, both premanifest or very early motor manifest. Thus our data in human HD are in agreement with evidence for abnormal plasticity in HD mouse models [9-13].
Since HD gene carriers differed from controls we next distinguished between clearly premanifest HD gene carriers and those that had very early motor signs. Reduced motor cortex plasticity was not associated with the stage of HD – there was no difference between premanifest and early symptomatic patients – or with clinical ratings of motor signs. This is similar to a previous study that found that MEP recruitment curves at rest were also reduced to the same extent in premanifest and manifest HD gene carriers suggesting that both are likely to be an intrinsic consequence of the abnormal genotype rather than a consequence of the symptoms [26]. In the context of life-long expression of mutant huntingtin it is interesting to note that mutant huntingtin decreases expression of brain-derived neurotrophic factor (BDNF) and its tropomyosin-related kinase B receptor in neocortex and hippocampus of humans [27, 28] and mice [28-32]. In the healthy animal, BDNF is almost exclusively produced by the cortex from where it is transported in cortico-striatal axons to the striatum, which does not express BDNF itself [33]. Transgenic mice with reduced cortical BDNF expression develop striatal degeneration and expression profiles very similar to R6/2 transgenics and human HD. This suggeststhat the cortical generation of BDNF and its axonal transport to the striatum provides important trophic support for the striatum even though so far BDNF has not been shown to be a causative factor in humans [18]. BDNF is also a potent, positive modulator of LTP induced in slice preparations by naturalistic TBS [34]. Reduced levels of BDNF in cortex could therefore be one factor that contributes to loss of response to the cTBS protocol in the present data even though we acknowledge that hippocampal neurones and motor cortical neurones are likely to differ electrophysiologically. Consistent with this, in a recent study we found that a common BDNF polymorphism (BDNF Val66Met) in healthy subjects was also predictive of a loss of response to cTBS [35]. Indeed, BDNF has been shown to rescue synaptic plasticity in hippocampal slices of a knock-in mouse model of HD [36] .
In summary we demonstrate that motor cortex plasticity is abnormal in HD expansion mutation carriers.Premanifest and early motor manifest HD gene carriers were similar, and the amount of plasticity was not associated with age, CAG repeat length or UHDRS motor score. This suggests that abnormal motor cortex plasticity may not be closely linked to the development of motor signs of HD. However, one of the limitations of our study is the small numbers of participants in the premanifest and very early manifest group. What determines the development of motor signs, and the possible contribution of abnormal motor cortex function in the premanifest phase of HD needs to be the subject of further research.
ACKNOWLEDGEMENTS
We thank all our patients, and the controls, for participating in this study. We are grateful to Dr Ed Wild and Ms Susie Henley for helping with patient recruitment. SJT is funded by the Medical Research Council, the Wellcome Trust, the Brain Research Trust, the UK Huntington's disease association and the High Q Foundation.
Footnotes
Disclosure: The authors report no conflict of interest
COMPETING INTERESTS
None declared.
REFERENCES
- 1.Walker FO. Huntington's disease. Lancet. 2007;369:218–28. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 2.Wolf RC, Vasic N, Schonfeldt-Lecuona C, Landwehrmeyer GB, Ecker D. Dorsolateral prefrontal cortex dysfunction in presymptomatic Huntington's disease: evidence from event-related fMRI. Brain. 2007;130:2845–57. doi: 10.1093/brain/awm210. Epub 007 Sep 13. [DOI] [PubMed] [Google Scholar]
- 3.Henley SM, Wild EJ, Hobbs NZ, et al. Defective emotion recognition in early HD is neuropsychologically and anatomically generic. Neuropsychologia. 2008;46:2152–60. doi: 10.1016/j.neuropsychologia.2008.02.025. Epub 008 Mar 6. [DOI] [PubMed] [Google Scholar]
- 4.Feigin A, Ghilardi MF, Huang C, et al. Preclinical Huntington's disease: compensatory brain responses during learning. Ann Neurol. 2006;59:53–9. doi: 10.1002/ana.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 6.Lione LA, Carter RJ, Hunt MJ, Bates GP, Morton AJ, Dunnett SB. Selective discrimination learning impairments in mice expressing the human Huntington's disease mutation. J Neurosci. 1999;19:10428–37. doi: 10.1523/JNEUROSCI.19-23-10428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazarakis NK, Cybulska-Klosowicz A, Grote H, et al. Deficits in experience-dependent cortical plasticity and sensory-discrimination learning in presymptomatic Huntington's disease mice. J Neurosci. 2005;25:3059–66. doi: 10.1523/JNEUROSCI.4320-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Raamsdonk JM, Pearson J, Slow EJ, Hossain SM, Leavitt BR, Hayden MR. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J Neurosci. 2005;25:4169–80. doi: 10.1523/JNEUROSCI.0590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodgson JG, Agopyan N, Gutekunst CA, et al. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–92. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- 10.Usdin MT, Shelbourne PF, Myers RM, Madison DV. Impaired synaptic plasticity in mice carrying the Huntington's disease mutation. Hum Mol Genet. 1999;8:839–46. doi: 10.1093/hmg/8.5.839. [DOI] [PubMed] [Google Scholar]
- 11.Murphy KP, Carter RJ, Lione LA, et al. Abnormal synaptic plasticity and impaired spatial cognition in mice transgenic for exon 1 of the human Huntington's disease mutation. J Neurosci. 2000;20:5115–23. doi: 10.1523/JNEUROSCI.20-13-05115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson HE, Reim K, Brose N, Morton AJ, Jones S. A similar impairment in CA3 mossy fibre LTP in the R6/2 mouse model of Huntington's disease and in the complexin II knockout mouse. Eur J Neurosci. 2005;22:1701–12. doi: 10.1111/j.1460-9568.2005.04349.x. [DOI] [PubMed] [Google Scholar]
- 13.Milnerwood AJ, Cummings DM, Dallerac GM, et al. Early development of aberrant synaptic plasticity in a mouse model of Huntington's disease. Hum Mol Genet. 2006;15:1690–703. doi: 10.1093/hmg/ddl092. Epub 2006 Apr 6. [DOI] [PubMed] [Google Scholar]
- 14.Henley SM, Frost C, MacManus DG, Warner TT, Fox NC, Tabrizi SJ. Increased rate of whole-brain atrophy over 6 months in early Huntington disease. Neurology. 2006;67:694–6. doi: 10.1212/01.wnl.0000230149.36635.c8. [DOI] [PubMed] [Google Scholar]
- 15.Hodges A, Strand AD, Aragaki AK, et al. Regional and cellular gene expression changes in human Huntington's disease brain. Hum Mol Genet. 2006;15:965–77. doi: 10.1093/hmg/ddl013. Epub 2006 Feb 8. [DOI] [PubMed] [Google Scholar]
- 16.Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65:745–7. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- 17.Rosas HD, Salat DH, Lee SY, et al. Cerebral cortex and the clinical expression of Huntington's disease: complexity and heterogeneity. Brain. 2008;131:1057–68. doi: 10.1093/brain/awn025. Epub 2008 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strand AD, Baquet ZC, Aragaki AK, et al. Expression profiling of Huntington's disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci. 2007;27:11758–68. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–70. doi: 10.1093/cercor/bhm087. Epub 2007 Jun 14. [DOI] [PubMed] [Google Scholar]
- 21.Unified Huntington's Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 1996;11:136–42. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 22.Shoulson I, Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29:1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin Genet. 2004;65:267–77. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Schippling S, Schneider SA, Bathia KP, et al. Abnormal motor cortex excitability in preclinical and very early Huntington's disease. Biol Psychiatry. doi: 10.1016/j.biopsych.2008.12.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrer I, Blanco R. N-myc and c-myc expression in Alzheimer disease, Huntington disease and Parkinson disease. Brain Res Mol Brain Res. 2000;77:270–6. doi: 10.1016/s0169-328x(00)00062-0. [DOI] [PubMed] [Google Scholar]
- 28.Zuccato C, Ciammola A, Rigamonti D, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293:493–8. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 29.Zuccato C, Liber D, Ramos C, et al. Progressive loss of BDNF in a mouse model of Huntington's disease and rescue by BDNF delivery. Pharmacol Res. 2005;52:133–9. doi: 10.1016/j.phrs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Gines S, Bosch M, Marco S, et al. Reduced expression of the TrkB receptor in Huntington's disease mouse models and in human brain. Eur J Neurosci. 2006;23:649–58. doi: 10.1111/j.1460-9568.2006.04590.x. [DOI] [PubMed] [Google Scholar]
- 31.Gines S, Seong IS, Fossale E, et al. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington's disease knock-in mice. Hum Mol Genet. 2003;12:497–508. doi: 10.1093/hmg/ddg046. [DOI] [PubMed] [Google Scholar]
- 32.Spires TL, Grote HE, Varshney NK, et al. Environmental enrichment rescues protein deficits in a mouse model of Huntington's disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–6. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altar CA, Cai N, Bliven T, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–60. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 34.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–25. doi: 10.1113/jphysiol.2008.159905. Epub 2008 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch G, Kramar EA, Rex CS, et al. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington's disease. J Neurosci. 2007;27:4424–34. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]