Abstract
Purpose
The pneumococcal capsule is required for pathogenesis in systemic infections, yet reports show most conjunctivitis outbreaks are caused by nonencapsulated pneumococci, while keratitis infections are caused by encapsulated strains. This study aims to determine the effect of capsule in pneumococcal keratitis and conjunctivitis in rabbit models of infection.
Methods
A capsule-deficient isogenic mutant was created using homologous transformation. Parent and mutant strains were injected within the upper bulbar conjunctiva (conjunctivitis) or into the corneal stroma (keratitis) of New Zealand white rabbits. Clinical examinations were performed 24 and 48 hr post-infection at which time corneas or conjunctivae were removed, homogenized, and plated to determine the recovered bacterial load. Whole eyes were removed for histological examination. The neuraminidase activity was determined following in vitro and in vivo growth.
Results
There were no significant differences in clinical scores between the eyes infected with the parent or mutant for either infection, nor was there a difference in the amount of bacteria recovered from the cornea. In the conjunctivae, however, the mutant strain was cleared by the host faster than the parent strain. Histological examination showed slightly more infiltrating polymorphonuclear leukocytes (PMN) and macrophages in the conjunctivae infected with the parent strain. The neuraminidase activity of both strains was not significantly different when the strains were grown in vitro. However, the neuraminidase activity of the parent was significantly less than that of the mutant at 3 and 12 hr post conjunctival infection.
Conclusions
Although more outbreaks of pneumococcal conjunctivitis are tied to nonencapsulated S. pneumoniae strains, this study showed that an encapsulated strain was capable of establishing conjunctivitis in a rabbit injection model and survive attack by the host immune system longer than its nonencapsulated isogenic mutant. Nonetheless, the nonencapsulated pneumococci had an increased neuraminidase activity level in vivo when compared to the parent strain.
Keywords: Capsule, Conjunctivitis, Keratitis, Neuraminidase, Streptococcus pneumoniae
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is an important worldwide pathogen responsible for causing a variety of systemic diseases including meningitis, pneumonia, and bacteremia.1 This bacterium is also a common cause of ocular infections including keratitis and conjunctivitis.2–6 Keratitis, the inflammation of the cornea, and conjunctivitis, the inflammation of the conjunctiva, are caused by a variety of pathogens including bacteria, viruses, and fungi. Conjunctivitis can also be caused by noninfectious causes, such as allergens and mechanical means.7 Bacterial keratitis is commonly caused by Staphylococcus aureus, Pseudomonas aeruginosa, as well as S. pneumoniae2,3,8,9 and can lead to permanent scarring of the cornea and loss of vision.9–12 S. pneumoniae keratitis often follows surgery or trauma to the eye and is more common in patients with coexisting ocular disease,3,12–14 while S. pneumoniae conjunctivitis is commonly associated with outbreaks involving people in close living quarters, such as college dorms, nursing homes, military bases, and daycare centers.4–6 Symptoms of bacterial conjunctivitis include redness of the eye, purulent discharge lasting all day, eyelid edema, and discomfort or pain.7 Pneumococcus, along with Haemophilus influenzae and S. aureus, is one of the top causes of bacterial conjunctivitis and is often the second most common cause of acute conjunctivitis in children.15–19 In addition to being an inconvenience and source of pain for patients, bacterial conjunctivitis has a negative impact on the economy. Direct and indirect costs for treating patients with bacterial conjunctivitis is an estimated $589 million per year in the United States alone.20
Many virulence factors contribute to the severity of pneumococcal infections including pneumolysin, pneumococcal surface protein A, neuraminidase (Nan), and capsule.1 The polysaccharide capsule is the best characterized of these virulence factors and is the basis of both currently approved pneumococcal vaccines.21 The capsule allows the bacterium to evade the host immune system by inhibiting interaction of complement components with their receptors on phagocytes, thus preventing its killing inside the macrophage.1 The capsule also reduces binding of IgG and C-reactive protein to S. pneumoniae;22 and given that capsule-specific antibody can enhance the transfer of pneumococci from erythrocytes to macrophages via Fc receptors,23 capsule likely functions to inhibit this interaction. With over 90 serotypes, the diversity of capsule serotypes also helps the bacterium escape the host immune system. The role of capsule in systemic pneumococcal disease is well established. Noncapsular derivatives require more than ten million colony-forming units (CFUs) to reach the LD50 in a mouse intraperitoneal infection model compared to 1 CFU required for encapsulated pneumococci.24 Additionally, nonencapsulated strains of S. pneumoniae fail to colonize the nasopharynx in mouse models of carriage while reduction in capsule production to approximately 20% of the parental strain diminishes the ability of the bacteria to cause invasive disease, indicating that capsule is required for colonization and infection.25 The role of capsule in ocular infections, however, is less established.
Most reported cases of pneumococcal keratitis are caused by encapsulated bacteria, though the specific capsular serotype is rarely reported.3,13,14,26 Reed et al. (2005) showed that pneumococcal capsule was not an important virulence factor in the rabbit cornea but was required for virulence in a mouse intraperitoneal infection.27 This study, however, used a non-ocular strain (D39, or Avery’s type 2 strain) and its nonencapsulated counterpart (R6), which had not been constructed as an isogenic mutant by modern molecular means, but rather by passage from D39 via two intermediate “rough” strains, R36 and R36A.
Outbreaks of pneumococcal conjunctivitis are often caused by nonencapsulated, nontypeable S. pneumoniae strains.4–6,28,29 While one study aimed at elucidating the mechanism of adherence of nontypeable S. pneumoniae to human conjunctival epithelial cells has shown that neuraminidases may play a role in bacterial adherence,30 no research has been reported regarding the importance of capsule or neuraminidases in vivo for ocular infections. Pneumococcal neuraminidases have been shown to be important virulence factors in other nonocular disease models, such as the colonization and infection of the upper and lower respiratory tract31 and invasion of the brain endothelium.32 S. pneumoniae encodes up to three neuraminidases, NanA, NanB, and NanC,33 though NanA is the best characterized. NanC, which has low sequence identity to NanA and high identity to NanB, is found in approximately 50% of all pneumococcal strains.34 Both NanA and NanB contain signal peptides for export, however, only NanA has a C-terminal cell surface anchorage domain.35 It is thought that NanA and NanB function as sialidases cleaving the sialic acid from cell surface glycans and mucins, thus promoting pneumococcal colonization.1 This study evaluated the importance of pneumococcal capsule in rabbit models of keratitis and conjunctivitis using an isogenic capsule-deficient mutant while examining changes in neuraminidase activity levels.
METHODS
Bacterial Strains and Growth Conditions
A clinical ocular strain of S. pneumoniae (K1544) was obtained from Regis Kowalski (Charles T. Campbell Laboratory, University of Pittsburgh, Pittsburgh, Pennsylvania, USA) for use in this study. Oligonucleotide primers used in this study are listed in Table 1. K1544, a clinical keratitis strain, was determined to be capsular type 38 by the multiplex polymerase chain reaction (PCR) method of Pai et al. (2006).36 Bacteria were grown on a blood agar base containing 5% sheep erythrocytes. Individual colonies were selected from the plates and grown in Todd Hewitt Broth (BD, Sparks, Maryland, USA) supplemented with 0.5% yeast extract (THY) at 37°C and 5% CO2 overnight. The overnight cultures were then diluted 1:100 in fresh THY and grown to an optical density (A600) corresponding to 108 CFU/mL. Serial dilutions of each inoculum were cultured on 5% blood agar to verify the accuracy of the inoculum CFU.
TABLE 1.
Oligonucleotide primers used in this study
Creation of Capsule Mutant
A capsule-negative isogenic mutant (K1544ΔCAP) was created using homologous transformation of donor DNA, in which the capsule biosynthesis locus was replaced with the bicistronic Janus cassette containing a gene for resistance to kanamycin (Kan) and a gene conferring sensitivity to streptomycin.37 The strain was transformed as previously described.38 Briefly, K1544 was grown in competence medium (CM) to A600 of 0.6. The culture was diluted 1:50 in fresh CM. After 200 ng of competence-stimulating peptides 1 and 238 were added, the culture was incubated at 37°C and 5% CO2 for 12 min. Following the addition of 1 µg of Janus cassette DNA (kindly provided by Larry S. McDaniel, University of Mississippi Medical Center, Jackson, Mississippi, USA), the mixture was incubated for 4 hr at 37°C and 5% CO2. A double-crossover event occurred in which the capsule locus was replaced with the Kan-resistance cassette of the donor DNA. Transformants were selected on 5% sheep blood agar containing 200 µg/mL kanamycin. The deletion was verified by PCR, as previously described.36,37 Capsule production was confirmed in strain K1544 and absent in the mutant strain K1544ΔCAP using the Pneumoslide kit (BD, Franklin Lanes, New Jersey, USA).
Rabbit Challenge
New Zealand White rabbits (Harlan Rabbitry, Indianapolis, Indiana, USA) were used in this study. They were maintained according to the guidelines of the University of Mississippi Medical Center Institutional Animal Care and Use Committee (IACUC) and the tenets of the Association for Research in Vision and Ophthalmology (ARVO) Resolution on the Use of Animals in Ophthalmic and Vision Research. Rabbits were anesthetized by subcutaneous injection of a mixture of xylazine and ketamine hydrochloride. Proparacaine hydrochloride was topically applied to each eye. S. pneumoniae K1544 or K1544ΔCAP (106 CFU in 20 µL) was injected either within the upper bulbar conjunctivae of naive rabbits for conjunctivitis infections or 105 CFU in 10 µL into the corneal stroma for corneal infections. Two observers, masked to the identity of the rabbit groups, examined the course of the infection at 24 and 48 hr post-infection using four parameters for conjunctivitis: palpebral injection, chemosis, bulbar injection, and eyelid edema, and seven parameters for keratitis: injection, chemosis, iritis, fibrin, hypopyon, corneal edema, and corneal infiltrate.39 All conjunctivitis parameters were assigned grades based on gross examination. The first three keratitis parameters were based on gross examination, and the last four were assigned grades with the aid of a slit lamp biomicroscope (Topcon Corporation, Tokyo, Japan). The sum of all grades resulted in a final score. The scores were averaged for the two observers resulting in an overall score ranging from 0 (normal) to a maximum of 16 or 28 (most severe), for conjunctivitis or keratitis, respectively. At 48 hr post-infection, rabbits were euthanized by an intravenous overdose of pentobarbital sodium. Conjunctivae or corneas were removed, dissected, and homogenized in sterile PBS. Homogenates were serially diluted and plated in triplicate on 5% sheep blood agar. Plates were incubated at 37°C for 24 hr. Colonies were counted and bacterial CFUs were determined and expressed in mean logarithmic units ± SEM. The experiments were performed twice, yielding similar results, and the data from the two experiments were combined.
In a separate experiment, rabbits were infected and conjunctivae removed at 3 and 12 hr post-infection to determine rate of bacterial clearance. Conjunctivae were removed from euthanized rabbits and bacterial CFUs were determined as described above. In another separate experiment, rabbit corneas were infected and the corneas removed 20 hr post-infection to determine whether the bacteria grew above the inoculum in keratitis.
Histopathology
Whole eyes were removed at 24, 48, or 168 hr post-infection. Histologic sectioning and hematoxylin and eosin staining of the rabbit eyes were performed by Excalibur Pathology, Inc. (Moore, Oklahoma, USA). Eyes were fixed in 4% paraformaldehyde and processed to paraffin. Five micron sections were deparaffinized through xylene and rehydrated gradually in water containing decreasing concentrations of alcohol. Nonspecific binding sites on the eye sections were blocked for 40 min in ImmPRESS Blocking Solution (Vector Laboratories, Burlingame, California, USA). Monocloncal antibody (MAC387; Abcam, Cambridge, Massachusetts, USA) specific for the L1 protein found on macrophages and granulocytes, was diluted 1:200 and applied to sections for 2 hr. The sections were then rinsed three times in PBS. ImmPRESS peroxidase reagent anti-mouse Ig (Vector Laboratories) was applied for 40 min and the sections were again washed in PBS. The sections were incubated with AEC chromagen (Vector Laboratories) for 8 min followed by one wash with distilled water. Gill III Hematoxylin (StatLab Medical, McKinney, Texas, USA) was then applied for 1 min and the sections were subjected to bluing reagent. Immunohistomount (Immunobioscience Corp, Mukilteo, Washington, USA) was applied to cover sections and air dried. The sections were then coverslipped with resin.
Neuraminidase Activity Assay
Neuraminidase activity of the pneumococcal strains was detected both in vitro and in vivo using an Amplex red neuraminidase assay kit according to manufacturer’s instructions (Invitrogen, Carlsbad, California, USA). In vitro bacterial neuraminidase activity was determined in strains that were grown in THY to mid-log phase, while in vivo bacterial neuraminidase activity was determined by infecting and removing conjunctivae at 3 and 12 hr post-infection. For in vivo assays, conjunctival homogenates were first centrifuged for 1 min to separate host tissue from bacteria. For both in vitro and in vivo assays, the bacteria were then collected by centrifugation and the supernatant removed. The pellet was subsequently suspended in reaction buffer (supplied by kit), and serially diluted. In triplicate, 50 µL of the sample was mixed with 50 µL of Amplex Red reagent buffer in a 96-well flat bottom plate. The plate was pre-incubated at 37°C for 30 min. Neuraminidase activity was colorimetrically measured at 590 nm. Bacteria were also enumerated by plate counts as described above.
Statistics
Data were analyzed using the Statistical Analysis System (SAS) program for computers (Cary, North Carolina, USA). Clinical scores were analyzed using non-parametric one-way ANOVA and CFU data were analyzed using general linear model of least square means. A student’s t-test was used to analyze the data from the neuraminidase activity assay. A p value < 0.05 was considered significant.
RESULTS
Creation of Capsule Mutant
K1544ΔCAP was created using homologous recombination. Colonies that grew on blood agar containing kanamycin were analyzed using two specific primer sets; one for the universal capsule gene (cpsA) and one for the Janus cassette. PCR analysis showed that the parental strain (K1544) contained cpsA but not the Janus cassette, while analysis of the mutant strain lacked cpsA and contained the Janus cassette.
The presence and absence of capsule in K1544 and K1544ΔCAP, respectively, was then confirmed using latex agglutination of antibodies specific for pneumococcal polysaccharide. Polyvalent pneumococcal polysaccharide served as a positive control for agglutination. K1544 exhibited agglutination when mixed with an S. pneumoniae antibody latex-coated bead suspension while K1544ΔCAP failed to show agglutination. Both strains and positive control were also mixed with a nonspecific antibody latex-coated bead suspension. All three failed to show agglutination with nonspecific control antibody indicating that the agglutination was due to capsular polysaccharide alone (data not shown).
Rabbit Challenge
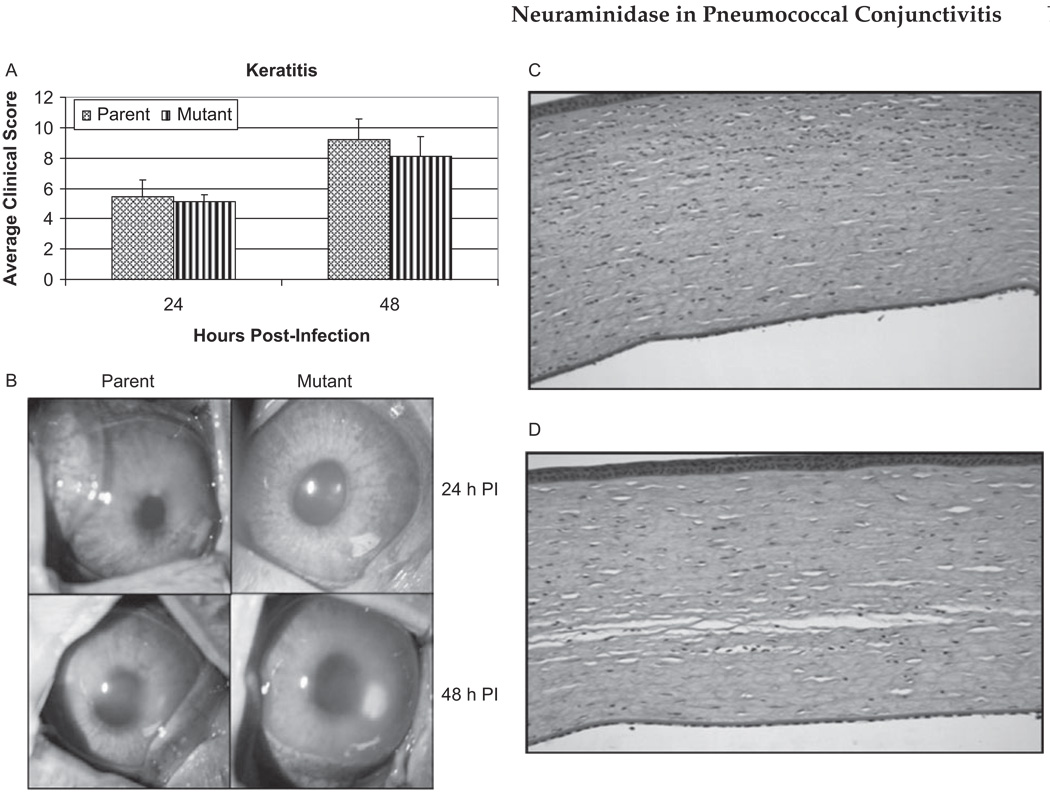
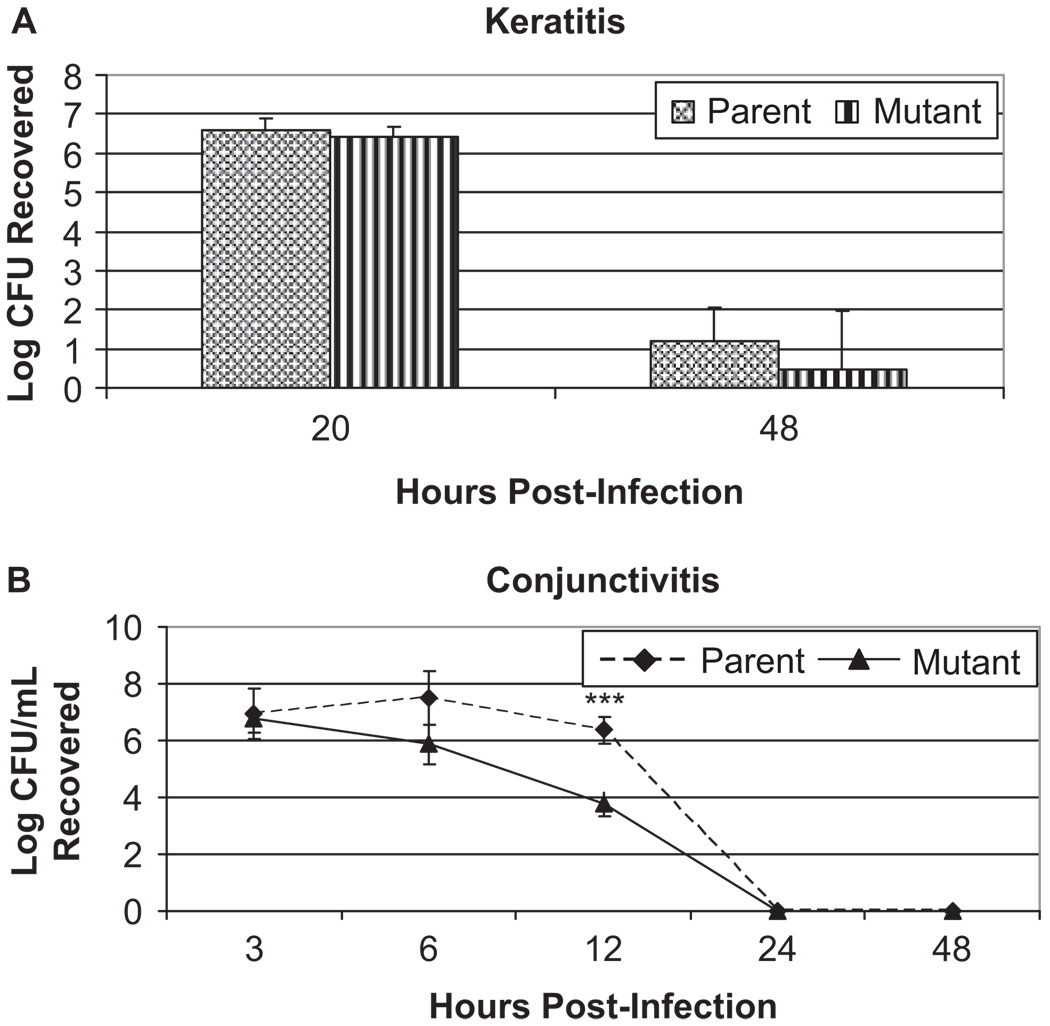
Clinical scores were used to quantify the differences in symptoms between the parent and isogenic capsule mutant strains of S. pneumoniae. Corneas infected with parent strain K1544 (n = 11) had mean clinical scores of 5.48 ± 1.09 and 9.18 ± 1.39 at 24 and 48 hr post-infection, respectively, while corneas infected with mutant strain K1544ΔCAP (n = 10) had scores of 5.15 ± 0.43 and 8.09 ± 1.30 (Figure 1A and 1B). At neither time point were there any statistical differences in the scores between corneas infected with the parent and mutant strains (p > 0.05). Additionally, there were no differences between strains in CFU recovered from the cornea 48 hr post-infection (1.22 ± 0.82 and 0.48 ± 1.51, p > 0.05, Figure 2A). To confirm that the bacterial strains grew similarly in this model of infection, corneas were removed at 20 hr post-infection and bacterial load was determined. At 20 hr post-infection, there was no difference in recovered log10CFU between the strains (p > 0.05). Additionally, both strains grew above the initial inoculum of 105 CFU reaching 6.59 ± 0.30 and 6.40 ± 0.27 log10CFU for the parent and mutant at 20 hr post-infection, respectively (Figure 2A).
FIGURE 1.
(A) Average clinical scores of keratitis infection at 24 and 48 hr post-infection caused by S. pneumoniae parent strain K1544 and mutant strain K1544ΔCAP. p > 0.05 at both time points. SLE scores are reported as the mean scores ± standard errors of the means. (B) Pictures show representative eyes with keratitis caused by S. pneumoniae parent strain K1544 and its isogenic mutant strain K1544ΔCAP. Eyes were scored and photographed 24 and 48 hr post-infection. Representative histology pictures of eyes infected intrastromally with the (C) parent strain K1544 or (D) mutant strain K1544ΔCAP at 48 hr post-infection.
FIGURE 2.
Log CFU recovered from (A) corneal homogenates and (B) conjunctival homogenates throughout the course of infection with S. pneumoniae parent strain K1544 and S. pneumoniae mutant strain K1544ΔCAP. (***) indicates significant difference in recovered bacterial load between the two strains, p < 0.05.
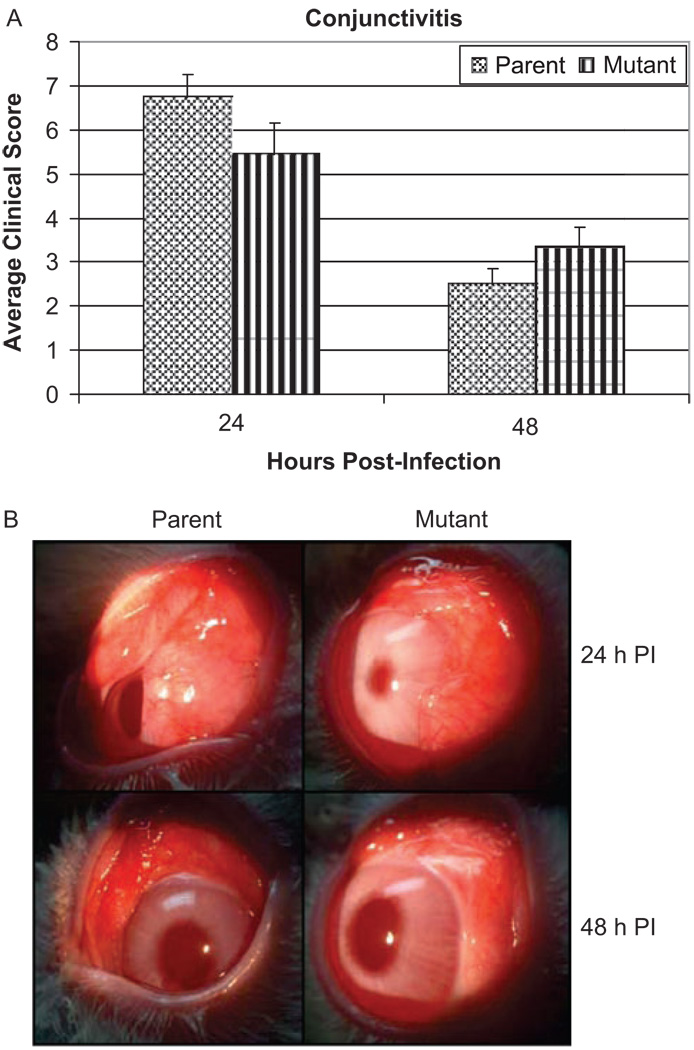
Upper bulbar conjunctivae of rabbits were also infected with S. pneumoniae strain K1544 and K1544ΔCAP. At 24 hr post-infection (n = 16), clinical scores were 6.74 ± 0.51 and 5.44 ± 0.37 for rabbits infected with strains K1544 and K1544ΔCAP, respectively, while at 48 hr post-infection (n = 10) clinical scores were 2.50 ± 0.73 and 3.33 ± 0.46 (Figure 3A). Differences in clinical scores were not significant at either 24 hr post-infection (p = 0.05) or at 48 hr post-infection (p = 0.35). Figure 3B shows representative eyes infected with each strain. Conjunctivae infected with either parent strain K1544 or mutant strain K1544ΔCAP had more swelling and conjunctival injection at 24 hr post-infection than at 48 hr post-infection.
FIGURE 3.
(A) Average clinical scores of conjunctival infection at 24 and 48 hr post-infection caused by S. pneumoniae parent strain K1544 and mutant strain K1544ΔCAP. There were no significant differences in scores between the groups, p ≥ 0.05. Scores are reported as the mean scores ± standard errors of the means. (B) Representative eyes with conjunctivitis caused by S. pneumoniae parent strain K1544 and its isogenic mutant K1544ΔCAP. Eyes were scored and photographed 24 and 48 hr post-infection.
Log10CFU recovered from the conjunctivae differed depending on strain. At 3 hr post-infection, both the parent and the mutant strains grew above the initial inoculum (6.93 ± 0.88 and 6.76 ± 0.49 log10CFU, respectively; n = 6). By 6 hr post-infection, however, the parent strain continued to grow above the inoculum (7.42 ± 0.93; n = 6) while the capsule-negative mutant began to be cleared by the host (5.88 ± 0.73; n = 6). At 12 hr post-infection, the difference between recovered bacteria was statistically significant with the mutant strain (3.77 ± 0.41; n = 6) being cleared better than the parent strain (6.37 ± 0.48; p = 0.002; n = 6). By 24 hr post-infection, rabbits were able to clear both bacterial strains and all conjunctivae were sterile (n = 6; Figure 2B). At 168 hr post-infection, the conjunctivae showed no clinical signs of infection (data not shown).
Histopathology
Eyes infected intrastromally (keratitis) with parent strain K1544 or mutant strain K1544ΔCAP showed similar severity of PMN infiltrate and stromal edema at 48 hr post-infection (Figure 1C and 1D).
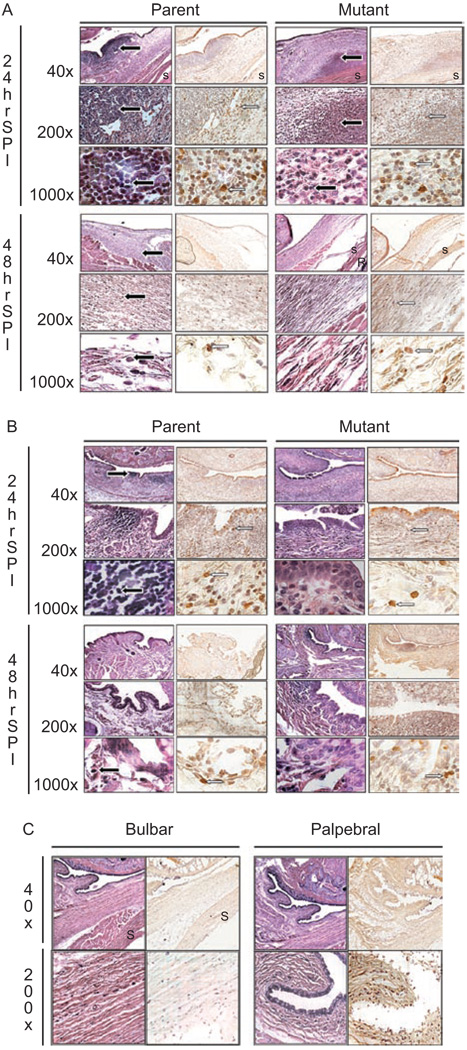
Conjunctivae infected with parent strain K1544 appeared to have more infiltrating PMNs and macrophages present in the bulbar and palpebral conjunctivae than those infected with mutant strain K1544ΔCAP (Figure 4). Additionally, the amount of macrophages and granulocytes present was highest at 24 hr post-infection in the palpebral conjunctivae (Figure 4B) and 48 hr post-infection in bulbar conjunctivae (Figure 4A) regardless of which strain was used for the infection and despite the bacteria being injected into the bulbar conjunctivae. This is likely due to the influx of PMNs from circulation passing into the palpebral conjunctivae first before spreading to the bulbar conjunctivae. Germinal follicles could be seen in the palpebral conjunctivae infected with either strain (Figure 4B). By 168 hr post-infection, the apparent amount of macrophages was reduced to levels lower than 24 hr post-infection (data not shown).
FIGURE 4.
Representative histology pictures of (A) bulbar conjunctivae or (B) palpebral conjunctivae that were infected with parent strain K1544 and mutant strain K1544ΔCAP at 24 and 48 hr post-infection. A non-infected negative control (C) is also shown. The first column at each time point is stained with hematoxylin and eosin while the second column is stained with a mouse anti-rabbit monoclonal antibody specific for macrophages and granulocytes. Black arrow indicates PMN. White arrow indicates macrophage. S: sclera; R: retina.
Neuraminidase Activity Assay
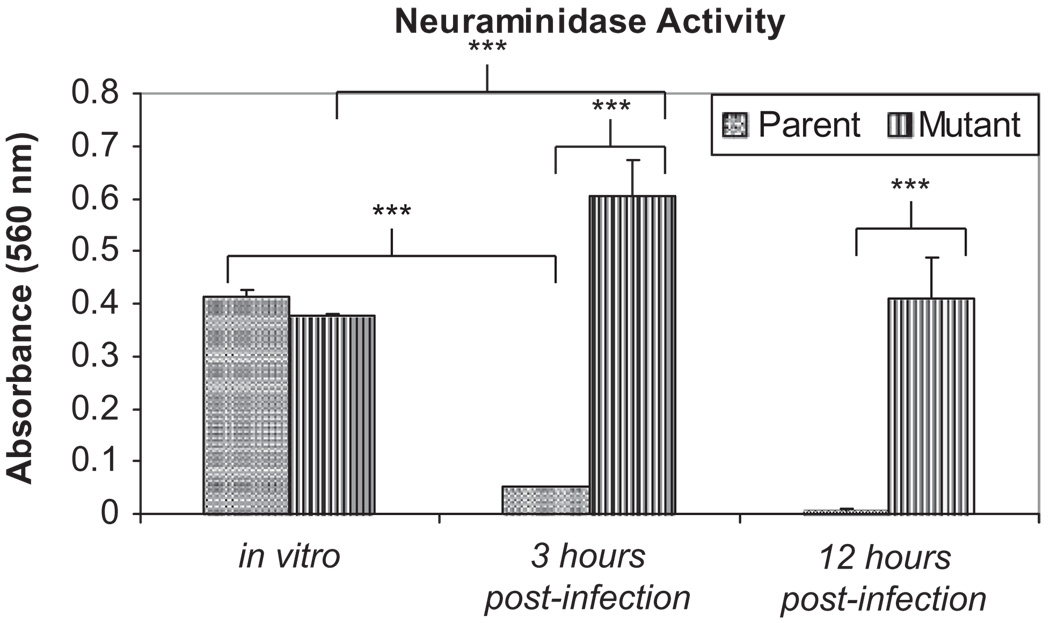
A neuraminidase activity assay was performed to assess differences in the neuraminidase activity of the encapsulated and nonencapsulated isogenic mutant form of the bacteria both in vitro and in vivo. Both the parent K1544 strain and the capsule-deficient mutant strain, K1544ΔCAP, had similar levels of neuraminidase activity when grown to mid-log phase in broth culture (A560 = 0.41 ± 0.013 and 0.38 ± 0.0034, respectively, p = 0.065; Figure 5). However, when conjunctivae infected with each strain were removed 3 hr post-infection, the neuraminidase activity levels differed significantly with the capsule-deficient mutant strain having much higher levels of neuraminidase activity than the parent strain (A560 = 0.61 ± 0.069 and 0.051 ± 0.001, respectively, p = 0.0073; Figure 5). At 12 hr post-infection, the neuraminidase activity levels were also higher in the conjunctiva infected with the mutant strain (A560 = 0.41 ± 0.076) than the conjunctiva infected with the parent strain (A560 = 0.0050 ± 0.0033, p = 0.018; Figure 5). Additionally, the neuraminidase activity of the parent strain was significantly lower in vivo at 3 hr post-infection than after growth in vitro (p = 0.00054) while the mutant strain was significantly higher when compared to growth in vitro (0.043). The neuraminidase activity of naive conjunctivae removed at 3 and 12 hr post-infection were subtracted from the scores of infected conjunctivae to control for host neuraminidases.
FIGURE 5.
Neuraminidase activity of parent strain K1544 and mutant strain K1544ΔCAP following growth in THY or recovered from infected conjunctivae 3 and 12 hr post-infection. After growth in THY and at 3 hr post-infection, there were 1 × 107 CFU/mL per strain. At 12 hr post-infection, there were 1 × 104 CFU/mL per strain. A student t-test was used to analyze the data. (***) indicates significant differences in the absorbance between groups, p < 0.05. Results are reported as the mean absorbances ± standard errors of the means.
DISCUSSION
The study presented herein showed that pneumococcal capsule did not appear to play a role in the clinical severity of conjunctivitis in the rabbit subconjunctival injection model. Absence of capsule did, however, allow the host to clear the bacteria more quickly. Additionally, the progression of a keratitis infection was unaffected by the presence or absence of capsule.
This study was the first to investigate the role of capsule in the course of keratitis or conjunctivitis infection using an isogenic capsule-deficient mutant along with its parent strain. We chose a strain isolated from a human keratitis infection for use in this study based on two reasons: the parental strain was a clinical isolate and it produced capsule. S. pneumoniae is an opportunistic pathogen able to colonize or invade numerous sites within a host by regulating expression of capsule, toxins, or adhesins produced in response to host-specific signals.40–42 A keratitis strain, specifically, was chosen rather than a conjunctivitis strain because the majority of S. pneumoniae strains isolated from conjunctivitis outbreaks are already nonencapsulated,29 while those isolated from keratitis infections produce capsule.
Our model of pneumococcal conjunctivitis is, to the best of our knowledge, the first in vivo model of its type. Although this model allows for studying host-pathogen interactions beyond the current confines of in vitro studies, it is limited in that it requires direct injection of bacteria into the ocular tissue rather than application of the bacteria topically as would more likely occur in nature. A topical infection was attempted to circumvent this problem but could not be established. Rabbit topical models of ocular infections using gram positive bacteria are often difficult to establish, and thus, studies using Staphylococcus and Streptococcus species typically rely on injection models.43–45
Differences in the glycan structures of ocular surface mucins may contribute to the difficulty of establishing a topical infection in rabbits that mimics a natural human infection. Royle et al. (2008) showed that human ocular mucins were predominantly negatively charged and terminated with sialic acid, whereas ocular mucins in rabbits were mostly neutral terminating in either α 1–2 fucose and/or 1–3 N-acetylgalactosamine.46 Unlike gram positive bacteria, P. aeruginosa is capable of establishing a keratitis infection after scratching and topically applying the bacteria to the cornea.47 While P. aeruginosa pili have been shown to bind α 2–6 sialic acid linkages in corneal and tear glycoproteins,48 P. aeruginosa also has a fucose receptor49 and it has been suggested that the bacteria may bind other motifs in the tear fluid.50 The ability of P. aeruginosa to bind sialic acid and fucose may allow the bacterium to establish topical infections in both humans and rabbits while S. pneumoniae cannot.
It has been previously shown that the presence of capsule is not required in a rabbit model S. pneumoniae keratitis.27 However, prior to the study reported herein, no one had reported using a parent bacterial strain and its isogenic capsule-deficient mutant to qualify the differences in an infection. Using this isogenic pair, we were able to conclude that the absence of capsule did not affect the severity of a keratitis infection in the rabbit intrastromal model. Additionally, the absence of capsule did not affect the rate at which the bacteria were cleared from the cornea, contrasting with what was found previously when significantly fewer CFUs were recovered from corneas infected with noncapsular strain R6 when compared to capsular strain D3927. Complement-mediated clearance has been shown to vary depending on the pneumococcal serotype.51 It is possible that the differences in bacterial clearance from the cornea observed in our study and the previous study are due to an inherent difference in the ability of complement to bind the two serotypes. It is interesting to note, however, that while a capsule-deficient mutant was able to establish keratitis in the study, there are no published reports of human cases of pneumococcal keratitis caused by nonencapsulated strains.
The results for keratitis were in contrast with what was observed for conjunctivitis. Although the presence of pneumococcal capsule did not affect the severity of conjunctivitis, pneumococci lacking capsules were cleared from the conjunctivae more quickly that those expressing capsules. Even though our data suggested nonencapsulated pneumococci were more quickly cleared from the conjunctiva, most reported outbreaks of pneumococcal conjunctivitis were caused by nonencapsulated strains.4–6,28,29,52 It is possible that differences in the initial binding steps between encapsulated and nonencapsulated strains play an important role in understanding these contrasting results.
It is well established that pneumococcal capsule has the ability to mask pneumococcal adhesins and that nonencapsulated pneumococci may have an advantage in colonization by allowing the exposure of important surface proteins.42,53,54 Pneumococcal mutants that produce only a portion of capsule that their parent strains produce have been shown to be as effective at colonization in the nasopharynx of a mouse with little effect on their virulence in an intraperitoneal infection model. However, these same bacteria had significant reduction in their virulence in an intravenous infection model.25 It is possible that in the conjunctiva the benefit of increased exposure of adhesins on a nonencapsulated pneumococcus outweighs the benefit of capsule in the reduction in killing due to opsonophagocytosis.
Our data underscore that the eye is a unique site of infection for pneumococci. Results observed for keratitis and conjunctivitis are in stark contrast to all other pneumococcal infections in which capsule is necessary to cause pathology. In the cornea and conjunctiva, the main virulence factors are non-capsular components. This important difference in the basic mechanism of pneumococcal infection should be considered in analyses of pneumococcal virulence such that generalizations are not made for all host infection sites.
Previous work by Williamson et al. (2007) indicates a role for pneumococcal neuraminidases in the initial binding of nonencapsulated pneumococci to immortalized human conjunctival epithelial cells. Using microarray analysis, they showed that nonencapsulated conjunctivitis isolates upregulated the expression of neuraminidases in vitro. Furthermore, these isolates had reduced binding to human conjunctival epithelial cells that expressed high levels of mucin compared to conjunctival epithelial cells that expressed low levels of mucin. Moreover, this reduced binding was enhanced with the addition of exogenous neuraminidases. The authors proposed that mucin may act as a barrier to pneumococcal binding at the ocular surface but that the upregulation of neuraminidases in conjunctivitis isolates may contribute to the adherence of the bacterium to the conjunctival surface by degrading host mucin in vivo.30
This previous study, however, only examined the in vitro effect of neuraminidases and mucin on the binding and invasion abilities of nonencapsulated conjunctivitis isolates. While the presence of neuraminidases may affect binding and invasion in vitro, the importance of neuraminidases in vivo often depends on the host tissue and location of the infection. Likewise, host immune responses cannot fully be examined in cultured conjunctival cells. The effect of NanA on virulence has been studied in a variety of model systems with mixed results. No differences in pathology were found when BALB/c mice were challenged intraperitoneally,55 however, when outbred MF1 mice were challenged intravenously or intranasally, NanA mutants were unable to persist and caused ongoing infection.31 Additionally, the in vitro results of conjunctival epithelial cell infection by various nonencapsulated conjunctivitis strains and encapsulated laboratory strains did not control for differences in the genetic background with the use of an isogenic pair.30
Our study used an isogenic capsule mutant and parent and an in vivo approach to better elucidate the role of pneumococcal capsule and neuraminidase production in the conjunctiva. The nonencapsulated strain had similar neuraminidase activity compared to the parent strain when grown in vitro; however, had significantly greater activity than the parent following conjunctival infection in the rabbit. Interestingly, the neuraminidase activity of the parent strain after infection in vivo was significantly less than the activity after growth in vitro, whereas the neuraminidase activity of the mutant strain after infection was significantly more than after growth in vitro (Figure 5). Because only the bacterial pellet was assayed for neuraminidase activity, we believe that the increased activity seen in vivo can be attributed solely to NanA, as NanA is the only pneumococcal neuraminidase which contains the C-terminal cell surface anchorage domain and, therefore, is the only neuraminidase found in the bacterial pellet.35
Most research regarding pneumococcal NanA expression is performed on encapsulated pneumococci, probably because in most infections types, capsule is required for pathology. In the conjunctivae, the nonencapsulated strain was cleared more rapidly although it possessed significantly higher NanA activity than the encapsulated strain. It is possible that the model system used (injected bacteria versus topically applied bacteria) fails to fully account for the role of NanA, which we hypothesize to be important in the initial binding of the bacteria. It is possible that in the conjunctivae, where capsule is unimportant to disease severity, a nonencapsulated strain may have some kind of selective advantage that allows it to increase NanA production, thus allowing the bacteria to utilize ocular mucins as a carbon source. This would permit the pneumococci to reach higher levels of growth at the conjunctival surface and possibly allow for more opportunities to invade the conjunctival epithelium and, thus, establish an infection. Another possible explanation for the increase in neuraminidase activity observed in the nonencapsulated strain may be that the lack of capsule exposes a receptor that when stimulated by some host factor causes the bacterium to increase its neuraminidase production. Our preliminary in vivo data combined with the in vitro data from Williamson et al. (2008)30 seem to indicate a role for NanA in the initial steps of pneumococcal conjunctivitis. Additional studies in vivo are underway to better elucidate this role.
Additional bacterial receptors may play a significant role in the initial binding of nonencapsulated pneumococci to conjunctival epithelial cells. Pneumococcal adherence to epithelial cells, including conjunctival epithelial cells, was shown to be reduced in bacteria not expressing a recently described receptor, plasminogen- and fibronectin-binding protein B (PfbB). Bacterial adherence to epithelial cells was reduced in both encapsulated and nonencapsulated strains that lacked PfbB.56 The roles of pneumococcal adhesins need to be further explored in order to fully understand the initial steps of conjunctivitis. Our data underscores the idea that isolation of noncapsular strains in conjunctivitis could be due to an initial binding event since our model cannot yet accommodate a topical infection.
An alternative theory explaining the prevalence of noncapsular strains as the etiological agent of pneumococcal conjunctivitis could be that rather than having differences at the initial binding step, encapsulated strains are establishing the infection and then “losing” their capsules in vivo, such that predominantly nonencapsulated variants are recovered from an infected eye. This loss of capsule could hypothetically be due to an unknown down-regulation of capsule biosynthesis locus genes in response to an environmental signal. This hypothesis was tested using the Pneumoslide kit in which the presence of the bacterial capsule was confirmed prior to infection and then assessed again on bacteria recovered from the conjunctivae at 3 and 12 hr post-infection. Our results showed that capsule production was unaffected in vivo and bacteria recovered from conjunctivae infected with an encapsulated strain continued to produce capsule. This indicates that the predominance of nonencapsulated pneumococci found in conjunctivitis may be due to an initial binding step. However, this capsule assay simply assessed presence or absence and failed to take into account the amount of capsule produced. Moreover, the use of specific pathogen-free animals might have affected the outcome as interactions between pneumococci and normal ocular flora that may contribute to variations in capsule production were missing.
The presence of capsule has little effect on the severity of pneumococcal conjunctivitis; however, not much is known about additional pneumococcal virulence factors that may be at play. Pneumolysin, a cholesterol dependent cytotoxin, is a known virulence factor associated with systemic as well as ocular infections. Johnson et al. (1990) showed that rabbits infected intrastromally with pneumococci lacking pneumolysin had significantly reduced clinical pathology than those infected with a parent strain.39 Pneumolysin, which possesses a complement activation domain and a pore-forming cytolytic domain, causes severe inflammation in the cornea.39,57 The complement activation domain contributes to ocular inflammation via the recruitment of polymorphonuclear leukocytes (PMNs) to the tissues.58–61 Histological sections of infected corneas and conjunctivae show PMN infiltration in both infections caused by the encapsulated parent and the nonencapsulated mutant strains (Figures 1 and 4). This influx of PMNs may be, at least in part, due to pneumolysin though it could be also be due to other factors or a combination of other factors and pneumolysin. Further study needs to ascertain the importance of pneumolysin to this infection and possibly address the effectiveness of therapies aimed at the toxin.
In conclusion, the present study demonstrates the varied role of capsule in ocular infections. While its presence does not affect the pathology or amount of bacteria recovered after a keratitis infection, the absence of capsule causes a reduction in the number of bacteria recovered from an infected conjunctiva. Additionally, the nonencapsulated pneumococci had increased neuraminidase activity levels in vivo when compared to the parent strain. As more knowledge is acquired regarding the unique nature of pneumococcal conjunctivitis and the predominance of nonencapsulated strains as the etiologic agents of this disease, pneumococcal and host-site-specific therapies can be developed to reduce cost and recovery time.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Larry McDaniel for providing the Janus cassette and Ms. Sherrina Dixon for serotyping the original bacterial strain. We would also like to thank Mr. Regis Kowalski for providing the original bacterial strain and Dr. Ed Swiatlo for critical review of the manuscript. This study was supported in part by Public Health Services Grant RO1 EY016195 (M.E.M.), National Eye Institute, National Institutes of Health, and by the University of Mississippi Medical Center.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev. 2001;65:187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhave P, Chamie G. Streptococcus pneumoniae keratitis. J Hosp Med. 2008;3:353. doi: 10.1002/jhm.306. [DOI] [PubMed] [Google Scholar]
- 3.Bharathi MJ, Ramakrishnan R, Vasu S, et al. Epidemiology of bacterial keratitis in a referral centre in South India. Indian J Med Microbiol. 2003;21:239–245. [PubMed] [Google Scholar]
- 4.Martin M, Turco JH, Zegans ME, et al. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniae. N Engl J Med. 2003;348:1112–1121. doi: 10.1056/NEJMoa022521. [DOI] [PubMed] [Google Scholar]
- 5.Crum NF, Barrozo CP, Chapman FA, et al. An outbreak of conjunctivitis due to a novel unencapsulated Streptococcus pneumoniae among military trainees. Clin Infect Dis. 2004;39:1148–1154. doi: 10.1086/424522. [DOI] [PubMed] [Google Scholar]
- 6.Buck JM, Lexau C, Shapiro M, et al. A community outbreak of conjunctivitis caused by nontypeable Streptococcus pneumoniae in Minnesota. Pediatr Infect Dis J. 2006;25:906–911. doi: 10.1097/01.inf.0000238143.96607.ec. [DOI] [PubMed] [Google Scholar]
- 7.Tarabishy AB, Jeng BH. Bacterial conjunctivitis: A review for internists. Cleve Clin J Med. 2008;75:507–512. doi: 10.3949/ccjm.75.7.507. [DOI] [PubMed] [Google Scholar]
- 8.Dada T, Sharma N, Dada VK, et al. Pneumococcal keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:460–461. doi: 10.1016/s0886-3350(99)00349-1. [DOI] [PubMed] [Google Scholar]
- 9.Jhanji V, Moorthy S, Vajpayee RB. Microbial keratitis in patients with down syndrome: A retrospective study. Cornea. 2009;28:163–165. doi: 10.1097/ICO.0b013e3181861d3a. [DOI] [PubMed] [Google Scholar]
- 10.Holden BA, Sweeney DF, Sankaridurg PR, et al. Microbial keratitis and vision loss with contact lenses. Eye Contact Lens. 2003;29:S131–S134. doi: 10.1097/00140068-200301001-00035. [DOI] [PubMed] [Google Scholar]
- 11.Saeed A, D’Arcy F, Stack J, et al. Risk factors, microbiological findings, and clinical outcomes in cases of microbial keratitis admitted to a tertiary referral center in Ireland. Cornea. 2009;28:285–292. doi: 10.1097/ICO.0b013e3181877a52. [DOI] [PubMed] [Google Scholar]
- 12.Wagoner MD, Al-Ghamdi AH, Al-Rajhi AA. Bacterial keratitis after primary pediatric penetrating keratoplasty. Am J Ophthalmol. 2007;143:1045–1047. doi: 10.1016/j.ajo.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Norina TJ, Raihan S, Bakiah S, et al. Microbial keratitis: Aetiological diagnosis and clinical features in patients admitted to Hospital Universiti Sains Malaysia. Singapore Med J. 2008;49:67–71. [PubMed] [Google Scholar]
- 14.Bharathi MJ, Ramakrishnan R, Meenakshi R, et al. Microbial keratitis in South India: Influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61–69. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 15.Buznach N, Dagan R, Greenberg D. Clinical and bacterial characteristics of acute bacterial conjunctivitis in children in the antibiotic resistance era. Pediatr Infect Dis J. 2005;24:823–828. doi: 10.1097/01.inf.0000178066.24569.98. [DOI] [PubMed] [Google Scholar]
- 16.Di BS, Higa M, Janer M, et al. [Neonatal conjunctivitis in a hospital at Gran Buenos Aires. Last 5 years up-date] Rev Argent Microbiol. 2005;37:139–141. [PubMed] [Google Scholar]
- 17.Patel PB, Diaz MC, Bennett JE, et al. Clinical features of bacterial conjunctivitis in children. Acad Emerg Med. 2007;14:1–5. doi: 10.1197/j.aem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Epling J. Bacterial conjunctivitis. Clin Evid (Online) 2007 [PubMed] [Google Scholar]
- 19.Bodor FF. Conjunctivitis-otitis syndrome. Pediatrics. 1982;69:695–698. [PubMed] [Google Scholar]
- 20.Smith AF, Waycaster C. Estimate of the direct and indirect annual cost of bacterial conjunctivitis in the United States. BMC Ophthalmol. 2009;9:13. doi: 10.1186/1471-2415-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49:1–35. [PubMed] [Google Scholar]
- 22.Hyams C, Camberlein E, Cohen JM, et al. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Szalai AJ, Hollingshead SK, et al. Antibody to the type 3 capsule facilitates immune adherence of pneumococci to erythrocytes and augments their transfer to macrophages. Infect Immun. 2009;77:464–471. doi: 10.1128/IAI.00892-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson DA, Musher DM. Interruption of capsule production in Streptococcus pneumonia serotype 3 by insertion of transposon Tn916. Infect Immun. 1990;58:3135–3138. doi: 10.1128/iai.58.9.3135-3138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magee AD, Yother J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun. 2001;69:3755–3761. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed JM, O’Callaghan RJ, Girgis DO, et al. Ocular virulence of capsule-deficient Streptococcus pneumoniae in a rabbit keratitis model. Invest Ophthalmol Vis Sci. 2005;46:604–608. doi: 10.1167/iovs.04-0889. [DOI] [PubMed] [Google Scholar]
- 28.Shayegani M, Parsons LM, Gibbons WE, Jr, et al. Characterization of nontypeable Streptococcus pneumoniae-like organisms isolated from outbreaks of conjunctivitis. J Clin Microbiol. 1982;16:8–14. doi: 10.1128/jcm.16.1.8-14.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho MG, Steigerwalt AG, Thompson T, et al. Confirmation of nontypeable Streptococcus pneumoniae-like organisms isolated from outbreaks of epidemic conjunctivitis as Streptococcus pneumoniae. J Clin Microbiol. 2003;41:4415–4417. doi: 10.1128/JCM.41.9.4415-4417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson YM, Gowrisankar R, Longo DL, et al. Adherence of nontypeable Streptococcus pneumoniae to human conjunctival epithelial cells. Microb Pathog. 2008;44:175–185. doi: 10.1016/j.micpath.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Manco S, Hernon F, Yesilkaya H, et al. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect Immun. 2006;74:4014–4020. doi: 10.1128/IAI.01237-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchiyama S, Carlin AF, Khosravi A, et al. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J Exp Med. 2009;206:1845–1852. doi: 10.1084/jem.20090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gut H, King SJ, Walsh MA. Structural and functional studies of Streptococcus pneumoniae neuraminidase B: An intramolecular trans-sialidase. FEBS Lett. 2008;582:3348–3352. doi: 10.1016/j.febslet.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Pettigrew MM, Fennie KP, York MP, et al. Variation in the presence of neuraminidase genes among Streptococcus pneumoniae isolates with identical sequence types. Infect Immun. 2006;74:3360–3365. doi: 10.1128/IAI.01442-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lock RA, Paton JC, Hansman D. Comparative efficacy of pneumococcal neuraminidase and pneumolysin as immunogens protective against Streptococcus pneumoniae. Microb Pathog. 1988;5:461–467. doi: 10.1016/0882-4010(88)90007-1. [DOI] [PubMed] [Google Scholar]
- 36.Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol. 2006;44:124–131. doi: 10.1128/JCM.44.1.124-131.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung CK, Li H, Claverys JP, et al. An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol. 2001;67:5190–5196. doi: 10.1128/AEM.67.11.5190-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pozzi G, Masala L, Lannelli F, et al. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: Two allelic variants of the peptide pheromone. J Bacteriol. 1996;178:6087–6090. doi: 10.1128/jb.178.20.6087-6090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson MK, Hobden JA, Hagenah M, et al. The role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr Eye Res. 1990;9:1107–1114. doi: 10.3109/02713689008997584. [DOI] [PubMed] [Google Scholar]
- 40.Kloosterman TG, van der Kooi-Pol MM, Bijlsma JJ, et al. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+-resistance gene czcD in Streptococcus pneumoniae. Mol Microbiol. 2007;65:1049–1063. doi: 10.1111/j.1365-2958.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- 41.Gupta R, Shah P, Swiatlo E. Differential gene expression in Streptococcus pneumoniae in response to various iron sources. Microb Pathog. 2009;47:101–109. doi: 10.1016/j.micpath.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Hammerschmidt S, Wolff S, Hocke A, et al. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun. 2005;73:4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green SN, Sanders M, Moore QC, III, et al. Protection from Streptococcus pneumoniae keratitis by passive immunization with pneumolysin antiserum. Invest Ophthalmol Vis Sci. 2008;49:290–294. doi: 10.1167/iovs.07-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dajcs JJ, Thibodeaux BA, Girgis DO, et al. Corneal virulence of Staphylococcus aureus in an experimental model of keratitis. DNA Cell Biol. 2002;21:375–382. doi: 10.1089/10445490260099656. [DOI] [PubMed] [Google Scholar]
- 45.Guzek JP, Cline DJ, Row PK, et al. Rabbit Streptococcus pneumoniae keratitis model and topical therapy. Invest Ophthalmol Vis Sci. 1998;39:2012–2017. [PubMed] [Google Scholar]
- 46.Royle L, Matthews E, Corfield A, et al. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25:763–773. doi: 10.1007/s10719-008-9136-6. [DOI] [PubMed] [Google Scholar]
- 47.Hyndiuk RA. Experimental Pseudomonas keratitis. Trans Am Ophthalmol Soc. 1981;79:541–624. [PMC free article] [PubMed] [Google Scholar]
- 48.Aristoteli LP, Willcox MD. The adhesion of Pseudomonas aeruginosa to high molecular weight human tear film species corresponds to glycoproteins reactive with Sambucus nigra lectin. Exp Eye Res. 2006;83:1146–1153. doi: 10.1016/j.exer.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Rhim AD, Stoykova L, Glick MC, et al. Terminal glycosylation in cystic fibrosis (CF): A review emphasizing the airway epithelial cell. Glycoconj J. 2001;18:649–659. doi: 10.1023/a:1020815205022. [DOI] [PubMed] [Google Scholar]
- 50.McNamara NA, Andika R, Kwong M, et al. Interaction of Pseudomonas aeruginosa with human tear fluid components. Curr Eye Res. 2005;30:517–525. doi: 10.1080/02713680590969456. [DOI] [PubMed] [Google Scholar]
- 51.Hyams C, Yuste J, Bax K, et al. Streptococcus pneumoniae resistance to complement-mediated immunity is dependent on the capsular serotype. Infect Immun. 2010;78:716–725. doi: 10.1128/IAI.01056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Medeiros MI, Neme SN, da SP, et al. Streptococcus pneumoniae and Haemophilus influenzae as etiological agents of conjunctivitis outbreaks in the region of Ribeirao Preto, SP, Brazil. Rev Inst Med Trop Sao Paulo. 1998;40:7–9. doi: 10.1590/s0036-46651998000100002. [DOI] [PubMed] [Google Scholar]
- 53.Hammerschmidt S. Adherence molecules of pathogenic pneumococci. Curr Opin Microbiol. 2006;9:12–20. doi: 10.1016/j.mib.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Bootsma HJ, Egmont-Petersen M, Hermans PW. Analysis of the in vitro transcriptional response of human pharyngeal epithelial cells to adherent Streptococcus pneumoniae: Evidence for a distinct response to encapsulated strains. Infect Immun. 2007;75:5489–5499. doi: 10.1128/IAI.01823-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berry AM, Paton JC. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun. 2000;68:133–140. doi: 10.1128/iai.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Papasergi S, Garibaldi M, Tuscano G, et al. Plasminogen- and fibronectin-binding protein B is involved in the adherence of Streptococcus pneumoniae to human epithelial cells. J Biol Chem. 2010;285:7517–7524. doi: 10.1074/jbc.M109.062075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson MK, Hobden JA, O’Callaghan RJ, et al. Confirmation of the role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr Eye Res. 1992;11:1221–1225. doi: 10.3109/02713689208999547. [DOI] [PubMed] [Google Scholar]
- 58.Jounblat R, Kadioglu A, Mitchell TJ, et al. Pneumococcal behavior and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin. Infect Immun. 2003;71:1813–1819. doi: 10.1128/IAI.71.4.1813-1819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paton JC, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984;43:1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubins JB, Charboneau D, Fasching C, et al. Distinct roles for pneumolysin’s cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am J Respir Crit Care Med. 1996;153:1339–1346. doi: 10.1164/ajrccm.153.4.8616564. [DOI] [PubMed] [Google Scholar]
- 61.Rubins JB, Janoff EN. Pneumolysin: A multifunctional pneumococcal virulence factor. J Lab Clin Med. 1998;131:21–27. doi: 10.1016/s0022-2143(98)90073-7. [DOI] [PubMed] [Google Scholar]