Abstract
OBJECTIVE
Venous thrombus resolution sets up an early intense inflammatory reaction, from which vein wall damage results. Tissue response to injury includes matrix metalloproteinase (MMP) activation and extracellular matrix protein turnover. This study sought to determine the effect of exogenous MMP inhibition and its potential attenuation of early vein wall injury.
METHODS
Rats received treatment beginning 24 hours after a stasis venous thrombosis by near occlusive ligation, and until harvest at day 7. Three groups were evaluated: 1). Vehicle saline controls (NaCl); 2). LMWH (Lovenox, 3 mg/Kg per day SQ); 3). Doxycycline (DOXY; 30 mg/Kg per day PO). Thrombus size (mg/mm), levels of TNFα and d-Dimer by colorimetric assay, and ED-1 counts by immunohistochemistry were assessed. Vein wall assessment included stiffness by tensiometry, ILβ protein levels by ELISA, MMP2 and -9 by zymography, and histological analysis of intimal thickness (IT). Comparisons were by t-Test to control. A P < .05 was considered significant.
RESULTS
Thrombi sizes were similar at both days 2 and 7 for all three groups, while thrombus TNFα was increased in 2d LMWH and DOXY treated groups (NaCl = 1.0±.8, LWMH = 9 ±3*, DOXY = 27±5*, pg/mg protein, N = 6 - 8, P < .05); and at 7d in the DOXY group (NaCl = 3.0±2.5, DOXY = 23±4.2*, pg/mg protein, N = 5, P < .05). Vein wall stiffness was less with LMWH treatment at 7d, but not with DOXY, as compared with controls (NaCl = .33±.05, LMWH =.17±.03*, DOXY = .43±.09 N/mm, N = 5-7, P < .05). Vessel-wall IL-1β was reduced only in the DOXY group at 7d (NaCl = 26±3, LMWH = 38±17, DOXY = 6±3* pg/mg protein, N = 4 - 6, P < .05) as was the IT score versus controls (NaCl = 2.2±.6, LMWH =1.7±.3, DOXY = 0.8 ± .20*, IT score, N = 4 -6, P < .05). Zymographic MMP9 activity was significantly reduced at 2 days in the LMWH and DOXY groups (NaCl = 85±24, LMWH = 23±7*, DOXY = 13±5* U/mg protein, N = 6 - 8, P < .05). MMP2 zymographic activity, thrombi monocyte cell counts, and d-Dimer activity were not significantly different across groups.
CONCLUSIONS
Treatment with LMWH or DOXY did not alter size of DVT, mildly altered thrombus composition, and differentially affected vein wall injury, despite similar reductions in early MMP9 activity. Whether exogenous MMP inhibition affects long-term vein wall fibrosis will require further study.
Introduction
A common sequelae of deep vein thrombosis (DVT) is vein wall injury, termed post thrombotic syndrome (PTS), commonly manifesting as swelling, pain, hyperpigmentation, and ulceration. This is an insidious process that may develop over years and is due to vein wall injury and valve destruction.1 The disability from this process is significant and it may affect younger working age patients as compared with atherosclerosis. Early and consistent use of compression stockings can decrease but not eliminate PTS, but may not always be prescribed or appropriately used by the patient.
Adequate anticoagulation is proven to significantly decrease the risk of recurrent DVT and occurrence of pulmonary embolism (PE).2 However, long term anticoagulation, primarily Vitamin K antagonists, have bleeding risks.3 More importantly, these agents may not alter the natural history of the PTS outside of their providing protection from recurrent DVT. PTS is worsened by delayed native thrombolysis,4 as well as prolonged stasis shown experimentally.5 Other factors may increase the risk of developing PTS, including lack of prompt anticoagulation, extensive initial thrombus burden, chronic obstruction in the venous system, obesity, and recurrent thrombosis.6, 7
Matrix metalloproteinase’s (MMP) are major factors in vascular remodeling after injury, particularly MMP2 and 9.8 These proteinases are elastinolytic and collagenolytic, with overlapping but unique substrates.9 In models of abdominal aortic aneurysms and cardiac failure, MMP2 and 9 play critical roles in the pathogenesis, mediating tissue turnover.10, 11 Prior data from our laboratory has also shown correlation between venous thrombosis resolution, vein wall injury, and MMP expression.12-14 The exact role of these MMP’s has not been fully elucidated in the venous system, and whether these proteinases are associated with vein wall damage is not known. However, human studies of varicose veins, though often not associated with a thrombus, suggest a role of MMP’s in its pathogenesis.15
Both direct and pleotropic effects of low molecular weight heparin (LMWH) can modulate vein wall injury.16 The mechanisms of these effects are not clearly delineated, although preservation of the medial smooth muscle cell layer and endothelial cell layer preservation may play a role.17 Prior work in our laboratory has also shown that direct P-selectin inhibition is associated with decreased vein wall injury, manifested by both less vein wall stiffness and less intimal thickening.18
In this study, we sought to determine the role of broad MMP inhibition on several measures of vein wall injury, and compare this with the standard therapy of low molecular weight heparin (LMWH).
METHODS
Animal Model
Male Sprague-Dawley rats (350-450 gm) were used for all studies, and all protocols were approved by the University of Michigan Animal Care Protocol. For all surgical procedures, the rats underwent general anesthesia with isoflorane/O2 with full physiological monitoring. Thrombosis was induced by a modified stenosis IVC ligation model (Figure 1).18, 19 Briefly, a midline laparotomy was made, the IVC dissected, posterior lumbar branches ligated, and a 3.0 silk ligature was placed parallel to the IVC. The IVC was then ligated with a 6-0 polypropylene suture to create a near total occlusion. The 3.0 silk suture was then removed once the 6.0 Prolene IVC ligature had been secured. A consistent thrombus was produced by this method.
Figure 1.
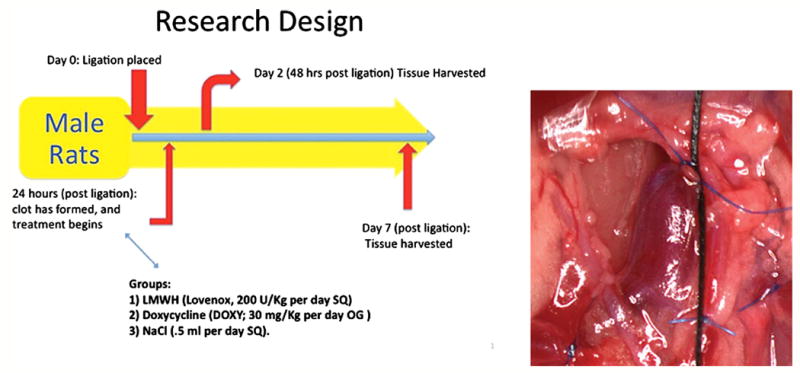
Experimental Protocol. Rats underwent a controlled vena caval stenosis; at 24 hours, either LMWH, doxycycline, or vehicle were administered. Then, at 2d or 7 day post therapy, the rats were sacrificed and tissue analysis completed.
Animals were treated with LMWH (Lovenox, 3 mg/Kg per day SQ, Sanofi-Aventis)18; Doxycycline (DOXY; 30 mg/Kg per day OG, Sigma)20-22 and vehicle control, NaCl (.5 ml per day SQ) at 24 hours after the IVC ligation. For certain assays, rats underwent a midline laparotomy and IVC dissection, but no ligature was placed and served as sham controls, harvested at 7 days. These doses were chosen based on the prior experimental effects as early and midpoint injury responses, based on prior experiments.5 At two and 7 days post ligation, the rats were sacrificed and tissue was obtained for histological analysis, while tissue studies were done after thrombus-vein wall separation.
Tensiometric vein wall analysis
Each harvested IVC was divided longitudinally and placed in iced PBS. Force extension curves were generated for each IVC using an Instron Tensiometer (model 5542, Instron Corporation, Canton, MA), equipped with a 5N static-load cell set at a crosshead speed of 10 mm per minute as described.5, 18, 23 Data analysis was performed using the Merlin materials testing software package (Instron Corporation) to determine the biomechanical property of stiffness—the slope of the linear elastic region of the force-extension curve (Newton (N)/mm), which is the approximate inverse of compliance.
Immunohistochemical Analysis
Immunohistochemical staining was performed on the paraffin embedded tissue sections (10 μm) as described.5, 13, 24 In brief, after blocking nonspecific sites with normal species-specific serum, the sections were incubated with either control serum or IgG (all from Vector Laboratories, Inc. Burlingame CA), or rat primary antibody for thirty minutes as follows with anti ED-1 (1:100; Serotec, Oxford, UK) to denote monocytes. A species-specific DAB peroxidase kit for rabbit, goat or mouse (Vector Laboratories, Inc., Burlingame CA) was used according to the manufacturer’s instructions for the secondary antibody and subsequent steps. The slides were then counterstained with hematoxylin and cover-slipped.
Intima-media Thickness Analysis
The vein wall intima layer extends from the surface endothelium to the internal elastic membrane, present in large veins.25 Paraffin-embedded IVC tissue was sectioned (3 μm) and stained with H&E. The vein wall sections were evaluated for intimal thickness scoring as described, by a blinded pathologist, using light microscopy evaluating five segments around the circumference of the vein with a standardized scoring system.18 A consistent midproximal thrombosed IVC segment was used for all histological analysis.17 Intimal thickness scoring criteria was done as follows: 0: Intima appears as just a potential space with space occupied only by endothelial cells; 1: Very small spaces present between endothelial cells and the internal elastic lamina. Intima still appears generally as thick as the nuclei of normal spindle-shaped endothelial cells; 2: Intima is at least twice as thick as an endothelial nucleus (about same as an RBC diameter) at its widest point in the HPF; 3. Intima is at least 5 times the thickness of an RBC diameter at its widest point in the HPF. Intimal thickness tends to be highly variable and may contain cells other than endothelial cells; 4. Intima is greatly thickened and contains either fibroblasts, white blood cells and/or hemorrhage at its widest point.
Cytokine/Clotting Components ELISA
After thrombus-vein wall separation, the IVC and clot were separately placed in complete lysis buffer (Complete Protease Inhibitor Cocktail Tabs, Roche, Nutley, NJ), then homogenized and sonicated for 30 seconds each, centrifuged at 0°C at 10,000 × g for 30 min and the supernatant collected. Quantification of peptide mediators was normalized to total protein in the sample. Total protein was quantified by a modified Bradford assay per manufacture’s instructions (Pierce, Inc., Rockford, II) with serial dilutions of bovine serum albumin (BSA, Sigma Chemical, St. Louis, MO) as standards. Tissue homogenate ELISA’s for rat tumor necrosis factor-alpha (TNFα), interleukin-1beta (IL-1β) (both from R and D, Minneapolis, MN), urokinase plasminogen activator (uPA), and plasminogen activator inhibitor (PAI-1) were performed according to manufacturer’s instructions (Innovative Research, Novi, MI). D-dimer was assessed in the thrombus homogenate according to manufacturer’s instructions and standardized to total protein (Diagnostica Stago, Parsippany, NJ).
SDS-PAGE Gelatin Zymography
As described,5, 13, 23 homogenized IVC and clot tissue were subjected to substrate zymography for MMP-2 and -9 using pre-cast 10% SDS-polyacrylamide gels containing 1 mg/ml of gelatin (unless otherwise stated, all zymography supplies were from Novex, San Diego, CA). Densitometry analysis was performed using a FOTO/Analyst CCD CAMERA (Fotodyne, Hartland, WI) and GEL-Pro Analyzer software version 3.1 (Media Cybernetics, Silver Springs, MD). Pro and active MMP-2 and -9 activity optical densities were summed and normalized to mg of IVC and clot total protein.
Statistical Analysis
All data are represented as mean ± SE. Unpaired Student’s T-test and one-way ANOVA were used as appropriate for comparison between the groups at their individual time points and appropriate controls (Sigma Stat, SPSS, Inc. Chicago, IL). A P < .05 was assigned significance
Results
Doxycycline and LWMH Do Not Alter Acute Experimental Thrombus Resolution
Thrombus weights are a simple and reliable measure of thrombus resolution.5, 17, 26 Neither doxycycline nor LMWH affected thrombus resolution in the time frames analyzed. Thrombi sizes were not altered at day 2 (NaCl 6 ± 0.5, LMWH 6.4 ± .05, doxycycline 6.0 ± 0.4 mg/mm, N = 6-8, P > .05), or at day 7 (NaCl 5.9 ± 2.3, LMWH 4.4 ± 1.2, doxycycline 5.4 ± 2.6 mg/mm; P > .05, N=8). This is consistent with prior experimental observations for LMWH with this model.17, 18
The thrombus is composed of leukocytes, fibrin matrix, platelets, and certain cytokines. There were no differences in thrombus fibrinolysis degradation products, measured indirectly by D-Dimer levels at 2 days (NaCL 2.4 ± 1.0, LMWH 2.5 ± 0.8; Doxy 3.3 ± 0.8, ng/mg protein, P >.05, N = 6 - 8), or thrombus monocytes (ED -1 + cells) at 7 days (NaCL 76 ± 12, LMWH 66 ± 6; Doxy 82 ± 11 cells/5 hpf, P > .05, N = 5). Monocytes were not assessed at 2 days, as few are present at this time point.23 TNFα is a cytokine involved in the regulation of immune cells, although it may have an anticoagulant effect.27 Thrombus TNFα levels were elevated 9 to 27 fold in the 2 day LMWH and doxycycline treated groups, respectively, as compared with vehicle controls (P < .05, N = 6)(Figure 2a). At 7 days, the doxycycline group had 8-fold higher TNFα levels as compared with vehicle (P < .05, N = 6) (Figure 2b), while LMWH did not have any significant effect.
Figure 2.
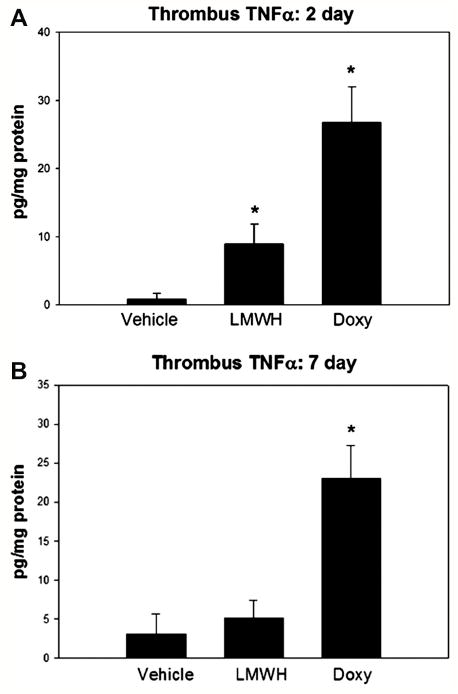
Thrombus cytokine response was altered with both agents. A). At 2d, TNFα was significantly elevated in the LMWH and doxycycline groups. B). At 7 days, only doxycycline was associated with elevated thrombus TNFα.
Doxycycline and LMWH are associated with decreased MMP9 activity and alter uPA/PAI-1 balance
To assess doxycycline and LMWH effects on MMP2 and 9, zymography was done on the thrombus and vein wall homogenate. Both doxycycline and LMWH were associated with reduced vein wall total MMP-9 activity at 2 days by 3 - 6 fold (P < .05, N = 6 - 8) (Figure 3). Sham levels were negligible at 0.11 ± .08 AU/mg protein (N = 3). At 7 days, vein wall total MMP9 activity was not significantly different between groups (NaCl 40 ± 11, Doxy 31 ± 24, LMWH 90 ± 47, P > .05, N = 4-5). Note that little active MMP9 was detected, and is essentially all proform.
Figure 3.
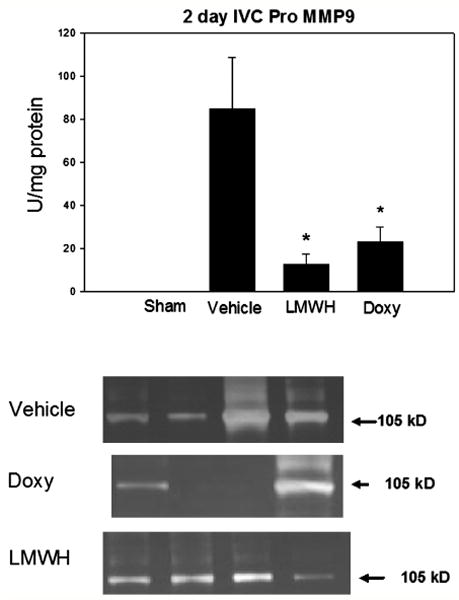
Both LMWH and doxycycline were associated with significantly decreased MMP9 activity as measured by substrate zymography. Pro MMP-9 is at 105 kD. Note * = P < .05.
Total MMP2 activity in the vein wall was not significantly different between groups at 2 days (NaCl 45 ± 14, Doxycycline 23 ± 3, LMWH 49 ± 13, Sham 60 ± 18 AU/mg protein, P > .05, N = 5-8).
Neither thrombus MMP9 nor MMP2 were significantly different between the treatments and control at 7 days (data not shown).
Fibrinolysis is primarily an uPA mediated process in rodents.28 To assess a major proximal activator of MMPs, we assayed uPA and PAI-1 by ELISA. Reflecting the local fibrinolytic balance,12, 29 uPA:PAI-1 ratios were determined by ELISA. Vein wall uPA/PAI-1 Ag ratio was elevated at 7 days in both the LMWH and doxycycline treated animals, increased by 40 – 80%, respectively ( P < .05, N = 6 ) (Figure 4).
Figure 4.
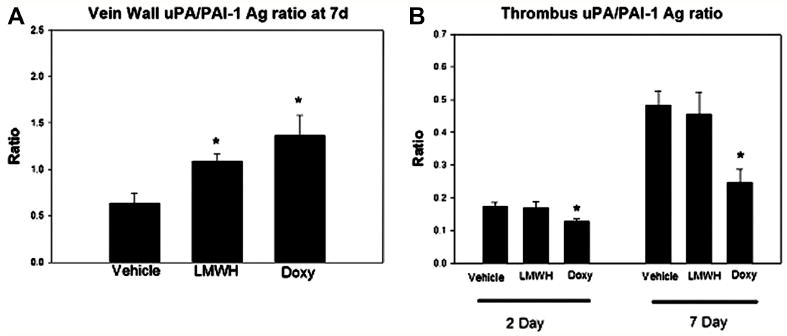
The local vein wall and thrombus fibrinolytic antigen levels were affected by both agents. Vein wall uPA/PAI-1 antigen ratio was significantly increased with both LMWH and doxycycline. Conversely, doxycycline was associated with reduced thrombus uPA/PAI-1 ratio at 2 and 7 days. Note * = P < .05.
Doxycycline was associated with a lower thrombus uPA/PAI-1 antigen (Ag) ratio than the vehicle, reduced by 20% (P < .05, N = 5). No difference was observed with LMWH administration at 2 or 7 days.
Doxycycline and LMWH Differentially Affect Vein Wall Remodeling
Experimental vein wall injury after DVT is characterized by local inflammation with monoctye influx, and elevated proinflammatory factors.5, 23 IL-1β is a pro-inflammatory cytokine which has profibrotic properties.30 Vein wall IL-1β at 7 days was reduced 4 fold in the doxycycline treated animals as compared with vehicle (P < .05, N = 4 - 6) (Figure 5). No difference in IL-1β was observed with LMWH administration as compared with vehicle. Lastly, no difference in vein wall ED-1 positive cells were found comparing all treatment groups (data not shown).
Figure 5.
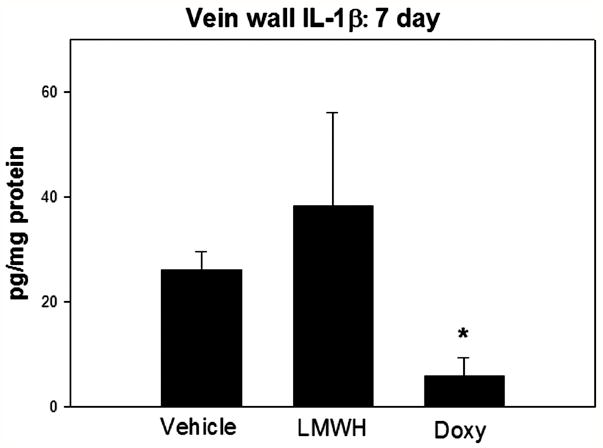
At 7d, IL-1β was significantly decreased with doxycycline administration. Note * = P < .05.
Vein wall stiffness is the approximate inverse of vein wall compliance and is a biomechanical measure of vein wall injury following DVT.5, 18, 23 At 7 days, tensiometry measurements showed the LWMH group to have 2-fold lower vein wall stiffness than vehicle control (P < .05, N = 5 - 7). No significant difference in stiffness parameters was found with doxycycline administration as compared with vehicle (Figure 6).
Figure 6.
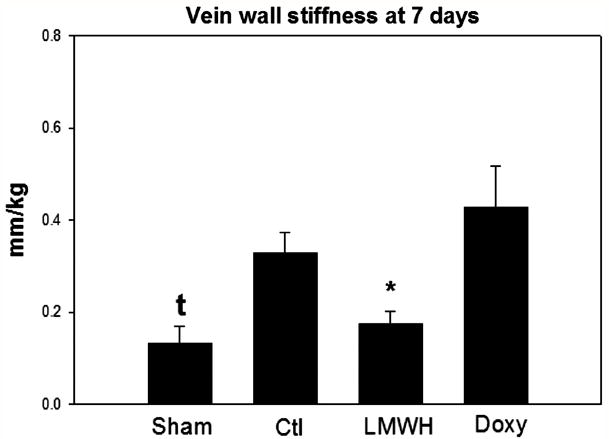
Vein wall stiffness was measured by microtensiometry at 7 days. Only LMWH was associated with significantly reduced stiffness. Note * = P < .05.
Intimal thickness reflects response to vessel injury, and is best characterized in arterial models of injury31 but also the venous system.18, 23 As measured by IT score, doxycycline was associated with 3 fold less intimal thickening as compared with vehicle control (P < .05, N = 4 - 6) (Figure 7). No significant difference was found with LMWH as compared with vehicle control.
Figure 7.
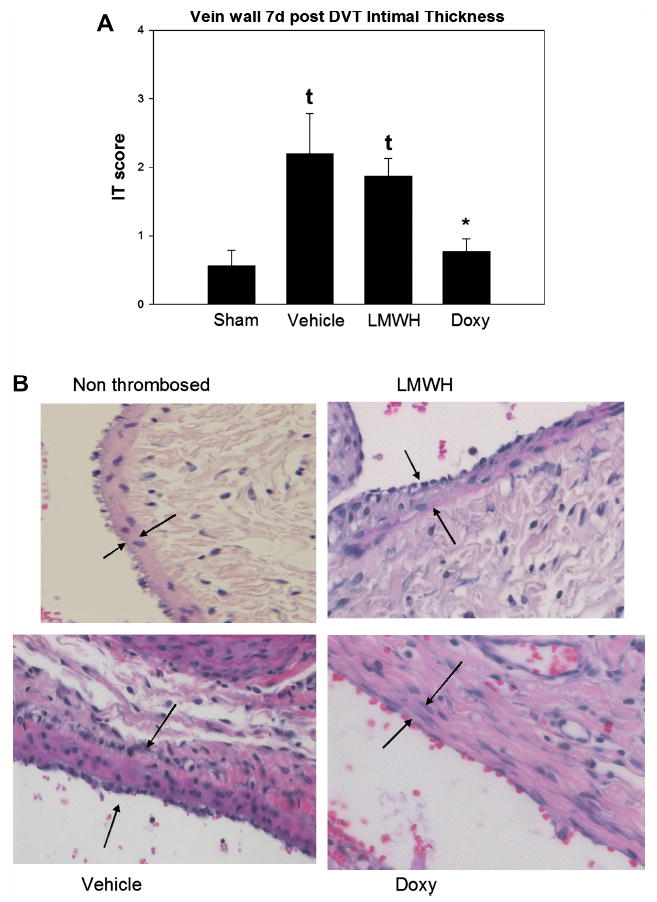
A). Vein wall intimal thickness was significantly reduced in the doxycycline group as compared with the vehicle control. No difference was observed with the LMWH. B). Photomicrographs of the vehicle, LMWH and doxycycline are shown, at 200X. Arrows denote intimal layer. Note * = P < .05 compared with vehicle. τ = P < .05 compared with sham non thrombosed control.
Discussion
Thrombus resolution occurs as a result of interaction between the vein wall and thrombus, often leaving the vein wall scarred and permanently damaged.4 Matrix metalloproteinase’s have been shown to be associated with vessel wall damage after thrombosis,12 and causal in other models of injury.8, 22 This study examined the effect of exogenous MMP inhibition and its potential attenuation of early vein wall injury. Three main conclusions can be drawn from the study; namely, post venous thrombosis treatment with LMWH and doxycycline: 1. inhibited MMP9 but not MMP2 activity in the vein wall in the early period after thrombosis; 2. did not directly affect thrombus resolution; and 3. differentially affected vein wall injury.
Doxycycline treated rats demonstrated reduced vein wall MMP9 levels, consistent with its known mechanism of action.20, 21 Despite repeated dosing, this effect was only seen at 2 days and not the later time point of 7 days. This may be due to other stimulatory mechanisms that override the doxycycline effect, such as the thrombus juxtaposition and plasmin release, a known activator of MMP9.32 Interestingly, LMWH was also associated with a similar pattern of MMP9 inhibition. Heparin can inhibit MMP activity in vitro33 and this may represent a direct effect. Alternatively, this may be due to decreased preformed MMP9 release from degranulating PMNs,34 or other cellular anti-inflammatory effects.35 We recognize the limitations of zymography for assessing in vivo MMP activity, as well as partial as compared with complete MMP activity abrogation. Preliminary experiments with genetically deleted MMP9 and MMP2 mice suggest divergent effects on vein wall remodeling, with MMP2 deletion being associated with less later vein wall fibrosis whereas MMP9 has less striking effects (unpublished data).
Doxycycline did not inhibit thrombus resolution, at least through 7 days, and suggests that MMP9 does not play a role in DVT resolution. Although we have found inhibition of PMN influx into the early thrombus was associated with larger thrombi and correlated with reduced MMP9 activity,23 this is likely due to diminished plasminogen activation. Consistent with this observation is that exogenous plasmin inhibition was associated with larger thrombi and significantly elevated MMP9 activity,14 again suggesting that MMP9 is not crucial for thrombus resolution. Lastly, we have found that genetically deleted MMP9 mice show no difference in thrombus resolution (DVT size) over time (unpublished data).
Despite its effective clinical use as an anticoagulant, LMWH has been shown to have little effect on early thrombus resolution in rodent models.17, 18, 36 This may be an artifact of our model, although in our model flow occurs through the stenosed segment, in contrast to the total stasis model.18 In fact, LMWH treatment is associated with smaller DVT, but only manifests at later time points (e.g. 14 day).17 Although we found that the uPA to PAI-1 balance was affected by both treatments, being decreased in the thrombus with doxycycline and elevated in the vein wall with both LMWH and doxycycline, this also did not appear to affect the overall thrombus size. It may be the direction of these changes cancelled each other out, that plasmin was activated by alternative mechanisms, or the physiological consequences of this difference was not significant.
Only LMWH was associated with decreased vein wall stiffness, and suggests this biomechanical measure of injury may be unrelated to MMP9 activity. Prior evaluation of tropoelastin antigen levels were not directly correlated with stiffness,5 suggesting simple elastinolysis may not be the primary mechanism that this biomechanical measure captures. This was suggested in prior work with exogenous plasmin inhibition, whereby the stiffness was reduced despite significantly upregulated MMP9 activity.14 We speculate, given prior experimental data, that LMWH may work via anti-P-selectin activity as we have shown previously.18 Both LMWH and P-selectin inhibition were associated with significantly reduced vein wall stiffness after experimental DVT, consistent with the current report.
Given that LMWH had a similar decrease in MMP9 as doxycycline, but no decrease in intimal thickness, this argues against intimal reaction as dependent on MMP9. However, doxycycline has broad MMP inhibition activity, and the reduced MMP9 may be a surrogate marker for other MMP activity against vein wall matrix. Inhibition of MMP activity may allow accelerated healing of the intima after mechanical stretch from the thrombus. Although pre treatment with LMWH may protect the venous endothelium, this effect is diminished with delayed administration.17 Impaired endothelialization is associated with intimal hyperplasia in arterial models.31 A thicker intima likely reflects a particular process of sub endothelial response to injury that may be driven by IL-1β, which was not decreased by LMWH as compared with the vehicle control group. Interestingly, MMP2 was not significantly altered with either LMWH or doxycycline, and is consistent with experimental administration of doxycycline as similar dosages to ours in models of aortic aneurismal disease.21 This may be due to the persistent stimulatory nature of the DVT on the vein wall, as well as presence of thrombin and plasmin.8 Smooth muscle cells produce this proteinase, while MMP9 is mainly delivered by the influxing leukocytes. Similarly, MMP2 activity was present in non-thrombosed sham operated rat’s IVC and suggests a homeostatic role. This has been suggested in other vascular beds as well.8
Doxycycline did not affect the vein wall stiffness, but was associated with decreased vein wall intimal thickness, correlating with reduced vein-wall, IL-1β. Prior work has also shown that modulation of the vein wall fibrotic response is possible,36 although MMP inhibition was not tested nor was IL-1β measured. As extrapolated from arterial vascular injury, IL-1β may play a profibrotic role in neointimal hyperplasia as well as being decreased by doxycycline administration.37, 38 Whether a preserved intimal thickness is less thrombogenic is not clear, and cannot be answered by the current model.
Both LMWH and doxycycline treatment were associated with elevated early thrombus TNFα level, while persistence of elevation was only observed with doxcycline. The role of TNFα in thrombogenesis suggests mixed effects.27 It is likely given similar sizes of DVT with the injury in this model, it reflects alteration in cellular processes that probably don’t alter thrombus dissolution directly.
Important clinical applications are possible from the further study of MMP inhibition and vein wall remodeling following DVT as its protective effect may be different than LMWH. Doxycycline is overall well tolerated and is being evaluated in patients with AAA disease (G Upchurch, personal communication 2009). Further work will be needed to clarify the mechanism of action in this model, whether combination of LMWH and doxycycline is additive, and later vein wall analysis to determine whether preservation of the intima is significant from a thrombogenic standpoint.
Acknowledgments
We appreciate the administrative assistance of Pam Moss, and the technical assistance of Robert Sigler, DVM, PhD.
Supported by NIH HL083918, Esperion Family Foundation Student award, and a Lifeline Student Research Fellowship.
Footnotes
Presented at the 21st Annual meeting of the American Venous Forum, Phoenix, AZ, February 12, 2009
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Geerts W. Antithrombotic and Thrombolytic Therapy. Chest. (8) 2008;133:381s–451s. ACCP Guidelines. [Google Scholar]
- 3.Hirsh J. Oral anticoagulant drugs. N Engl J Med. 1991;324(26):1865–1875. doi: 10.1056/NEJM199106273242606. [DOI] [PubMed] [Google Scholar]
- 4.Meissner MH, Caps MT, Zierler BK, Polissar N, Bergelin RO, Manzo RA, Strandness DE., Jr Determinants of chronic venous disease after acute deep venous thrombosis. J Vasc Surg. 1998;28(5):826–833. doi: 10.1016/s0741-5214(98)70057-6. [DOI] [PubMed] [Google Scholar]
- 5.Henke PK, Varma MR, Moaveni DK, Dewyer NA, Moore AJ, Lynch EM, Longo C, Deatrick CB, Kunkel SL, Upchurch GR, Jr, Wakefield TW. Fibrotic injury after experimental deep vein thrombosis is determined by the mechanism of thrombogenesis. Thromb Haemost. 2007;98(5):1045–1055. [PubMed] [Google Scholar]
- 6.Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Lamping DL, Johri M, Ginsberg JS. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698–707. doi: 10.7326/0003-4819-149-10-200811180-00004. [DOI] [PubMed] [Google Scholar]
- 7.Henke PK, Ferguson E, Varma M, Deatrick KB, Wakefield GT, Woodrum DT. Proximate versus nonproximate risk factor associated primary deep venous thrombosis: clinical spectrum and outcomes. J Vasc Surg. 2007;45(5):998–1003. doi: 10.1016/j.jvs.2007.01.042. discussion 1003-1004; quiz 1005-1007. [DOI] [PubMed] [Google Scholar]
- 8.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–262. [PubMed] [Google Scholar]
- 9.Galt SW, Lindemann S, Medd D, Allen LL, Kraiss LW, Harris ES, Prescott SM, McIntyre TM, Weyrich AS, Zimmerman GA. Differential regulation of matrix metalloproteinase-9 by monocytes adherent to collagen and platelets. Circ Res. 2001;89(6):509–516. doi: 10.1161/hh1801.096339. [DOI] [PubMed] [Google Scholar]
- 10.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000 July;106(1):55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curci JA, Thompson RW. Adaptive cellular immunity in aortic aneurysms: cause, consequence, or context? J Clin Invest. 2004;114(2):168–171. doi: 10.1172/JCI22309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deatrick KB, Eliason JL, Lynch EM, Moore AJ, Dewyer NA, Varma MR, Pearce CG, Upchurch GR, Wakefield TW, Henke PK. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg. 2005;42(1):140–148. doi: 10.1016/j.jvs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Henke PK, Pearce CG, Moaveni DM, Moore AJ, Lynch EM, Longo C, Varma M, Dewyer NA, Deatrick KB, Upchurch GR, Jr, Wakefield TW, Hogaboam C, Kunkel SL. Targeted Deletion of CCR2 Impairs Deep Vein Thombosis Resolution in a Mouse Model. J Immunol. 2006;177(5):3388–3397. doi: 10.4049/jimmunol.177.5.3388. [DOI] [PubMed] [Google Scholar]
- 14.Dewyer NA, Sood V, Lynch EM, Luke CE, Upchurch GR, Jr, Wakefield TW, Kunkel S, Henke PK. Plasmin inhibition increases mmp-9 activity and decreases vein wall stiffness during venous thrombosis resolution. J Surg Res. 2007;142(2):357–363. doi: 10.1016/j.jss.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodside KJ, Hu M, Burke A, Murakami M, Pounds LL, Killewich LA, Daller JA, Hunter GC. Morphologic characteristics of varicose veins: possible role of metalloproteinases. J Vasc Surg. 2003;38(1):162–169. doi: 10.1016/s0741-5214(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Downing LJ, Strieter RM, Kadell AM, Wilke CA, Greenfield LJ, Wakefield TW. Low-dose low-molecular-weight heparin is anti-inflammatory during venous thrombosis. J Vasc Surg. 1998;28:848–854. doi: 10.1016/s0741-5214(98)70060-6. [DOI] [PubMed] [Google Scholar]
- 17.Moaveni DK, Lynch EM, Luke C, Sood V, Upchurch GR, Wakefield TW, Henke PK. Vein wall re-endothelialization after deep vein thrombosis is improved with low-molecular-weight heparin. J Vasc Surg. 2008;47(3):616–624. doi: 10.1016/j.jvs.2007.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers DD, Jr, Henke PK, Bedard PW, Wrobleski SK, Kaila N, Shaw G, Meier TR, Hawley AE, Schaub RG, Wakefield TW. Treatment with an oral small molecule inhibitor of P selectin (PSI-697) decreases vein wall injury in a rat stenosis model of venous thrombosis. J Vasc Surg. 2006;44(3):625–632. doi: 10.1016/j.jvs.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Gossage JA, Humphries J, Modarai B, Burnand KG, Smith A. Adenoviral urokinase-type plasminogen activator (uPA) gene transfer enhances venous thrombus resolution. J Vasc Surg. 2006;44(5):1085–1090. doi: 10.1016/j.jvs.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Petrinec D, Liao S, Holmes DR, Reilly JM, Parks WC, Thompson RW. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J Vasc Surg. 1996;23(2):336–346. doi: 10.1016/s0741-5214(96)70279-3. [DOI] [PubMed] [Google Scholar]
- 21.Sho E, Chu J, Sho M, Fernandes B, Judd D, Ganesan P, Kimura H, Dalman RL. Continuous periaortic infusion improves doxycycline efficacy in experimental aortic aneurysms. J Vasc Surg. 2004;39(6):1312–1321. doi: 10.1016/j.jvs.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105(11):1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henke PK, Varma MR, Deatrick KB, Drewyer NA, Lynch EM, Moore AJ, Dubay DA, Sukheepod P, Pearce CG, Upchurch GR, Jr, Kunkel SL, Franz MG, Wakefield TW. Neutrophils modulate post-thrombotic vein wall remodeling but not thrombus neovascularization. Thromb Haemost. 2006;95(2):272–281. doi: 10.1160/TH05-02-0099. [DOI] [PubMed] [Google Scholar]
- 24.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL, Upchurch GR, Jr, Wakefield TW. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24(6):1130–1137. doi: 10.1161/01.ATV.0000129537.72553.73. [DOI] [PubMed] [Google Scholar]
- 25.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 26.Wakefield TW, Strieter RM, Wilke CA, Kadell AM, Wrobleski SK, Burdick MD, Schmidt R, Kunkel SL, Greenfield LJ. Venous thrombosis-associated inflammation and attenuation with neutralizing antibodies to cytokines and adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15(2):258–268. doi: 10.1161/01.atv.15.2.258. [DOI] [PubMed] [Google Scholar]
- 27.Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;25(7):1321–1324. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- 28.Singh I, Burnand KG, Collins M, Luttun A, Collen D, Boelhouwer B, Smith A. Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: rescue by normal bone marrow-derived cells. Circulation. 2003;107(6):869–875. doi: 10.1161/01.cir.0000050149.22928.39. [DOI] [PubMed] [Google Scholar]
- 29.Bouzeghrane F, Zhang X, Gevry G, Raymond J. Deep vein thrombosis resolution is impaired in diet-induced type 2 diabetic mice. J Vasc Surg. 2008;48(6):1575–1584. doi: 10.1016/j.jvs.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 30.Hogaboam CM, Steinhauser ML, Chensue SW, Kunkel SL. Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney Int. 1998;54(6):2152–2159. doi: 10.1046/j.1523-1755.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- 31.Gennaro G, Menard C, Michaud SE, Rivard A. Age-dependent impairment of reendothelialization after arterial injury: role of vascular endothelial growth factor. Circulation. 2003;107(2):230–233. doi: 10.1161/01.cir.0000050652.47145.4c. [DOI] [PubMed] [Google Scholar]
- 32.Lijnen HR, Van Hoef B, Lupu F, Moons L, Carmeliet P, Collen D. Function of the plasminogen/plasmin and matrix metalloproteinase systems after vascular injury in mice with targeted inactivation of fibrinolytic system genes. Arterioscler Thromb Vasc Biol. 1998;18(7):1035–1045. doi: 10.1161/01.atv.18.7.1035. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki M, Kashima M, Ito T, Watanabe A, Sano M, Kagaya M, Shioya T, Miura M. Effect of heparin and related glycosaminoglycan on PDGF-induced lung fibroblast proliferation, chemotactic response and matrix metalloproteinases activity. Mediators Inflamm. 2000;9(2):85–91. doi: 10.1080/096293500411541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Brown JR, Varki A, Esko JD. Heparin’s anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J Clin Invest. 2002;110(1):127–136. doi: 10.1172/JCI14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thanaporn P, Myers DD, Wrobleski SK, Hawley AE, Farris DM, Wakefield TW, Henke PK. P-selectin inhibition decreases post-thrombotic vein wall fibrosis in a rat model. Surgery. 2003;134(2):365–371. doi: 10.1067/msy.2003.249. [DOI] [PubMed] [Google Scholar]
- 37.Aird WC. Mechanisms of endothelial cell heterogeneity in health and disease. Circ Res. 2006;98(2):159–162. doi: 10.1161/01.RES.0000204553.32549.a7. [DOI] [PubMed] [Google Scholar]
- 38.Solomon A, Rosenblatt M, Li DQ, Liu Z, Monroy D, Ji Z, Lokeshwar BL, Pflugfelder SC. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Invest Ophthalmol Vis Sci. 2000;41(9):2544–2557. [PubMed] [Google Scholar]
- 39.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28(3):387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]


