Abstract
Inflammatory processes within the cornea are known to be associated with corneal neovascularization (CN). We examined the effects of inflammatory mediators on the expression of angiogenic factors by corneal cells. TNF-α and IL-1 induced VEGF-A secretion by corneal fibroblasts (HCRF) and this was inhibited significantly by IFN-γ. Constitutively secreted VEGF-A by corneal epithelial cells (HCE) was not affected by these cytokines. Moreover, sVEGF-R1(sFlt-1) secretion by HCRF was stimulated significantly by IFN-γ. JAK-STAT pathway inhibitor reversed the effects of IFN-γ on VEGF-A and sFlt-1 secretion by HCRF. RT-PCR analysis showed that IFN-γ influences the expression of VEGF-A and sFlt-1 by affecting their mRNA level. IFN-γ inhibited TGF-β induced VEGF-A secretion but not sVEGF-R1secretion. This is the first report demonstrating the inhibitory and stimulatory effects of IFN-γ on VEGF-A and sFlt-1 secretion, respectively. Our results suggest that IFN-γ acts as an anti-angiogenic cytokine in the human cornea.
Keywords: IFN-γ, VEGF-A, sVEGF-R1, Cornea, Fibroblasts, Corneal neovascularization, Inflammation, IL-1, TNF-α
Corneal transparency is essential for optimal transmission of light signals for clear vision. Corneal neovascularization (CN) due to a variety of pathological insults results in the development of corneal opacity and may lead to loss of visual function [1,2]. Corneal avascularity, regulated by VEGF-A and sVEGF-R1 (sFlt-1), primarily contributes to transparency [1,3]. The critical role of vascular endothelial growth factors (VEGFs) and its receptors in CN has been demonstrated in both human patients and in animal models [3–8]. In most of the cases, inflammation precedes and is closely associated with the development of new blood vessels in CN [4,6]. Furthermore, immunohistochemical analysis of tissue from patients with CN, have shown infiltration of neutrophils, macrophages, and lymphocytes in the inflamed human cornea [4–6]. In addition, IFN-γ, TNF-α, IL-1α, IL-1β, and TGF-β were detected in epithelial and stromal cell layers in neovascularized human cornea [1,2,4–6].
Vascular endothelial growth factors (VEGF) play critical roles in neovascurization under pathological conditions [1,3–6,9]. Since leukocyte trafficking and their inflammatory secretion products almost always precede CN [4–6], we evaluated the role of selected inflammatory cytokines in the regulation of the expression of VEGF and its receptors. In the present study, we show that IFN-γ inhibits VEGF-A and enhances sFlt-1 secretion by human corneal stromal fibroblast cells. This is the first report to demonstrate contrasting effects of IFN-γ on VEGF-A and sFlt-1 expression.
Materials and methods
Materials
Fetal bovine serum, cell culture media, and VEGF-A Elisa kits were obtained from Invitrogen, San Diego, CA. Human recombinant cytokines, growth factors, and soluble VEGF-R1 (sFlt-1) and VEGF-R2 Elisa kits were purchased from R&D Systems, Minneapolis, MN. JAK inhibitor 1, TGF-β R1 kinase inhibitors (ALK-5 inhibitor) were obtained from Calbiochem, San Diego, CA. RNA PCR kits and PCR supplies were obtained from Applied Biosystems, Foster City, CA.
Cell cultures
The human corneal epithelial cell line (HCE) was obtained from RIKEN Cell Bank (Japan). These SV40-Adenovirus transformed cells were well characterized and were shown to express many of the corneal epithelial specific markers and functions [10]. Human corneal fibroblast (HCRF) cells were prepared from corneal buttons or from cornea obtained from donor eyes [11]. For each study, the primary cell lines of HCRF derived from 3 to 5 donor corneas were used at passages 3 to 6. HCE and HCRF cells were grown in minimum essential medium supplemented with 10% fetal bovine serum, non-essential amino acids, and antibiotic–antimycotic mixture. Serum free medium (SFM) is similar to the above medium except that it lacks fetal bovine serum.
Analysis of VEGF-A and sVEGF-R1(sFlt-1) secretion by HCE and HCRF
HCE and HCRF cells were grown to confluence in 24 well plates in medium containing 10% FBS. Then cultures were washed with SFM and incubated in SFM overnight. Cultures were incubated in SFM containing various cytokines, as indicated in the figure legends, for 24 h. Culture supernatants were then collected and the levels of VEGF-A and sVEGF-R1 were determined by ELISA. The following cytokines, IFN-γ (100 U/ml), TNF-α (10 ng/ml), IL-1α (10 ng/ml), and IL-1β (10 ng/ml) were used. The mean detectable levels of VEGF-A and sVEGF-R1 are 5 and 3.5 pg/ml, respectively. With sVEGF-R1 assay, no significant cross-reactivity was reported in the presence of VEGF-A121aa, VEGF-A 165aa, VEGF-C, VEGF-D, VEGF-R2, Flt-3, and Flt-4.
Effect of JACK-STAT pathway inhibitor on VEGF-A and sVEGF-R1 secretion by HCRF
Confluent cultures of HCRF were grown in 24 well plates as described above and appropriate wells were pretreated for 30 min with JAK inhibitor 1 dissolved in DMSO. Then cultures were treated with various cytokine preparations in the absence or presence of JAK inhibitor. After 24 h incubation, supernatants were collected and used for the determination of the levels of secreted VEGF-A and sVEGF-R1 by ELISA.
Effect of TGF-β on VEGF-A and sVEGF-R1 secretion by HCE and HCRF
Confluent cultures grown in 24 well plates were washed and incubated overnight in SFM. SFM was replaced by fresh SFM containing TGF-β1 or TGF-β2 (10 ng/ml). After 24 h incubation, culture supernatants were collected and the levels of VEGF-A and sVEGF-R1 were analyzed by ELISA. For TGF-β inhibition studies, cultures were pretreated for 30 min with TGF-β R1 kinase inhibitor I followed by the addition of TGF-β and the inhibitor.
RT-PCR analysis of VEGF-A and sVEGF-R1 mRNA expression
The following primers were used for PCR [12,13]. The numbers in the parenthesis indicate the size of PCR products.
36B4-F: 5′-TGG GCT CCA AGC AGA TGC-3′
36B4-R: 5′-GGC TTC GCT GGC TCC CAC-3′ (413 bp)
VEGF-A-F: 5′-CCA TGA ACT TTC TGC TGT CTT-3′
VEGF-A-R: 5′-TCG ATC GTT CTG TAT CAG TCT-3′ (516, 648, 720, 771 bp)
mbFlt-1-F: 5′-GCA CCT TGG TTG TGG CTG AC-3′
mbFlt-1-R: 5′-TGG AAT TCG TGC TGC TGC TTC CTG GTC C-3′ (587 bp)
sFlt-1-F: 5′-CCA GGA ATG TAT ACA CAG G-3′
sFlt-1-R: 5′-CAA CAA ACA CAG AGA AGG-3′(393 bp)
VEGF-R1-F: 5′-GTC ACA GAA GAG GAT GAA GGT GTC TA-3′
VEGF-R1-R: 5′-CAC AGT CCG GCA CGT AGG TGA TT-3′ (414 bp).
HCRF cultures were grown to confluence in 60 mm dishes and treated with various cytokines and/or inhibitors in SFM for 8 h. Total cellular RNA was prepared from the cells by using commercially available kits (RNAeasy mini kit, Quiagen Sciences, MD or RNA STAT-60, Tel-Test, Friendswood, TX). RNA PCR kit (Applied Biosystems, Foster City, CA) was used for reverse transcription and polymerase chain reaction according to the protocols provided by the manufacturer and procedure described by us earlier [11,13]. We used 36B4 gene as a control gene, the expression of which was unchanged in all our control and treatment samples. The density of sample bands were normalized to corresponding 36B4 values and expressed as fold changes. Densitometric analyses of RT-PCR bands were performed by using the National Institutes of Health imageJ Program (http://rsb.info.nih.gov/ij/).
Results
VEGF-A and sVEGF-R1 secretion by human corneal epithelial cells (HCE)
HCE cells constitutively secreted 220 pg/ml of VEGF-A. Treatment of the cells with IFN-γ, TNF-α, IL-1α or IL-1β did not alter VEGF-A secretion significantly (data not shown). Secretion of sVEGF-R1 (sFlt-1) was not observed in control and cytokine treated HCE. TGF-β did not have any effect on VEGF-A and sVEGF-R1secretion.
VEGF-A and sVEGF-R1 secretion by human corneal fibroblasts (HCRF)
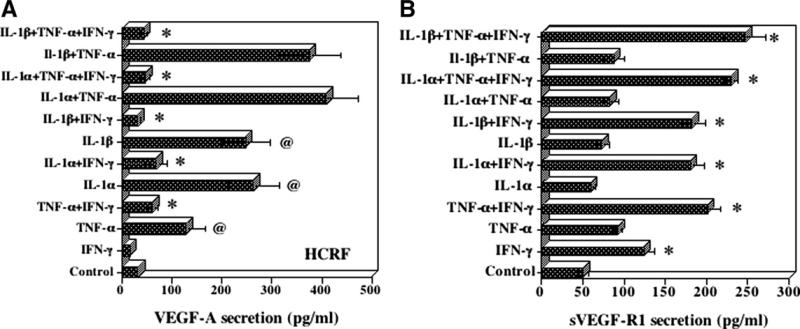
HCRF cells constitutively secreted 31 pg/ml of VEGF-A. Incubation of cells with TNF-α, IL-1α or IL-1β, and combinations of these cytokines significantly elevated VEGF-A secretion (Fig. 1A). In contrast, the addition of IFN-γ resulted in a significant decrease (p < 0.01–0.001) in VEGF-A secretion. HCRF cells constitutively secreted 50 pg/ml of sVEGF-R1 that increased to 124 pg/ml in the presence of IFN-γ (Fig. 1B). TNF-α, IL-1α, and IL-1β did not significantly increase sVEGF-R1 but addition of IFN-γ to these cytokines significantly enhanced (p < 0.01) the secretion of sVEGF-R1. Thus IFN-γ is the primary stimulant for sVEGF-R1 secretion. VEGF-R2 was not secreted by HCRF stimulated by these cytokines.
Fig. 1.
Effect inflammatory cytokines on (A) VEGF-A and (B) sVEGF-R1(sFlt-1) secretion by human corneal fibroblast cells (HCRF). HCRF cultures were incubated in the presence of various cytokines (IFN-γ, 100 U/ml; TNF-α,10 ng/ml; IL-1α,10 ng/ml; IL-1β, 10 ng/ml) in serum free medium for 24 h. Levels of VEGF-A and sVEGF-R1 secreted into the culture supernatants were determined by ELISA. The results are means ± SE for six experiments each with duplicate samples. (A) *decrease p < 0.01, @increase p < 0.01; (B) *increase p < 0.01.
JAK-1 inhibitor blocks IFN-γ effects on VEGF-A and sVEGF-R1 secretion by HCRF
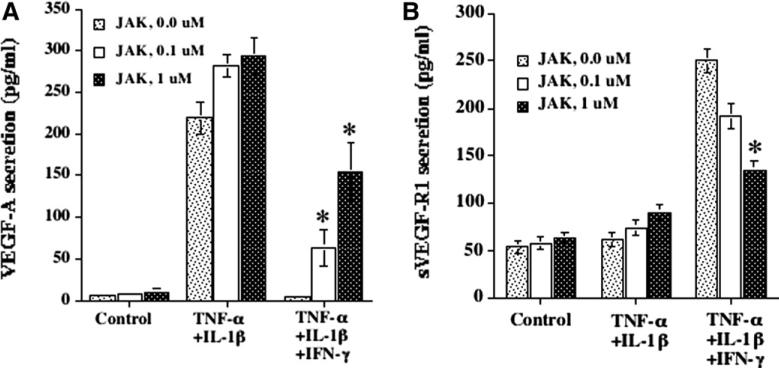
The above experiments demonstrated that IFN-γ inhibited TNF-α + IL-1β induced secretion of VEGF-A by more than 80%. This IFN-γ induced inhibition was reversed significantly (p < 0.01) in the presence of JAK-1 inhibitor [14,15] at 0.1 and 1 μM concentrations (Fig. 2A). At these concentrations, JAK-1 inhibitor had no effect on TNF-α + IL-1β induced VEGF-A secretion (Fig. 2A). We have also observed that IFN-γ enhanced sVEGF-R1 secretion by more than 4-fold when compared to HCRF cultures treated with media and TNF-α + IL-1β. In the presence of JAK-1 inhibitor, sVEGF-R1 secretion was reversed significantly (Fig. 2B). The results from these experiments provide a strong support that IFN-γ acts through JAK-1 signal transduction pathway to inhibit VEGF-A and enhance sVEGF-R1secretion by HCRF.
Fig. 2.
Reversal of IFN-γ effect on (A) VEGF-A and (B) sVEGF-R1 secretion by JAK-1 inhibitor. HCRF cultures were pretreated with JAK-1 inhibitor (inhibitor of JAK-STAT pathway of IFN-γ) for 30 min. Then cultures were incubated in the presence of cytokines without or with JAK-1 inhibtor for 24 h in serum free medium. VEGF-A and sVEGF-R1 levels in the culture supernatants were determined by ELISA. Results are means ± SD from one representative experiment, representing three other experiments, with triplicate samples. *p < 0.01.
TGF-β stimulates VEGF-A and sVEGF- R1 secretion by HCRF cells
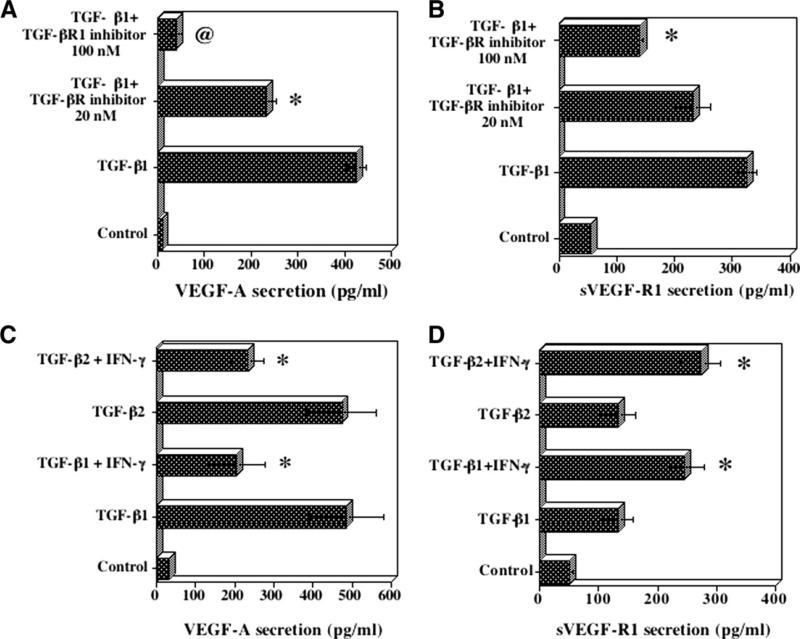
The incubation of HCRF cells with TGF-β1 resulted in a significant stimulation of secretion of VEGF-A and sVEGF-R1(Fig. 3A and B). TGF-β Receptor 1 kinase inhibitor [16] at 100 nM abolished the effects of TGF-β1(Fig. 3A). sVEGF-R1 secretion induced by TGF-β1 was partially but significantly (p < 0.01) inhibited by TGF-β Receptor 1 kinase inhibitor (Fig. 3B). IFN-γ inhibited TGF-β1 and TGF-β2 induced secretion of VEGF-A by 50% (Fig. 3C). In contrast, the stimulatory effects of IFN-γ and TGF-β are additive in the secretion of sVEGF-R1(Fig. 3D). Thus IFN-γ reduces VEGF-A levels while elevating sVEGF-R1 levels in HCRF in the presence of TGF-β. These effects of IFN-γ favor an anti-angiogenic environment within the cornea.
Fig. 3.
Effect of TGF-β1 on (A) VEGF-A and (B) sVEGF-R1 secretion by HCRF cells and its inhibition by TGF-β receptor 1 kinase inhibitor. HCRF cells were incubated with TGF-β1(10 ng/ml) for 24 h in serum free medium. For inhibitor studies, cultures were pretreated with the inhibitor for 30 min. Culture supernatants were used for analysis of VEGF-A and sVEGF-R1 by ELISA. Results are means ± SD from one representative experiment with triplicate samples. Effect of IFN-γ on TGF-β1 and TGF-β2 induced VEGF-A (C) and sVEGF-R1 (D) secretion by HCRF cells. Cultures were incubated with IFN-γ (100 U/ml) and TGF-β (10 ng/ml) in serum free medium for 24 h. Levels of VEGF-A and sVEGF-R1 in culture supernatants were determined by ELISA. Results are means ± SE for five experiments each with duplicate samples. *p < 0.01.
Analysis of the expression of VEGF-A and VEGF-R1 mRNA in HCRF cells
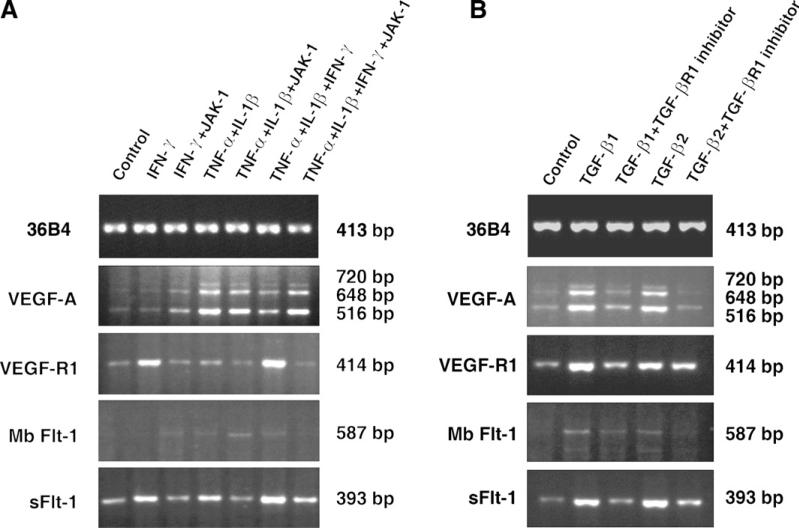
To determine if IFN-γ is affecting the expression of VEGF-A and sVEGF-R1 at transcription level, we examined the steady state mRNA levels of VEGF-A and sVEGF-R1 by RT-PCR. The primers used for VEGF-A amplify four isoforms of its mRNA [13]. HCRF cells constitutively expressed very low levels of three PCR products of 510, 648, and 720 bp size that corresponds to VEGF-A121, 165 and 189 amino acid forms (Fig. 4 A, panel 2). Highly soluble form of VEGF-A 121 aa is the predominant form expressed followed by 165aa and 189aa in all the treatment groups. TNF-α and IL-1 enhanced the density of the PCR product by 21-fold when compared to control and addition of IFN-γ to TNF-α and IL-1 reduced the band density to 11-fold suggesting inhibitory effects of IFN-γ. Moreover, when the cells were treated with JAK-1 inhibitor, selective inhibitor of IFN-γ JAK-STAT signal transduction pathway [14,15], the inhibition by IFN-γ was completely reversed (22-fold compared to control). These RT-PCR results corroborate with the VEGF-A secretion studies (Fig. 1A) thus indicating IFN-γ, TNF-α, and IL-1 affects VEGF-A secretion by influencing at the transcription level. RT-PCR analysis of VEGF-R1 (common for both mbFlt-1 and sFlt-1), mbFlt-1 and sFlt-1 are shown in Fig. 4A (panel 3, 4, and 5). Both VEGF-R1 and sFlt-1 PCR products band densities are significantly higher (data not shown) in IFN-γ, and TNF-α, IL-1 and IFN-γ treatment groups. The stimulatory effects of IFN-γ on sFlt-1 and VEGF-R1 are confirmed by the results from JAK-1 treated samples that showed 3- to 5-fold decrease in band densities. The expression of mbFlt-1(membrane bound form of VEGF-R1) appears to be very weak in comparison to sFlt-1. RT-PCR results of sFlt-1(Fig. 4) clearly support that sVEGF-R1 secretion (Fig. 1B) enhanced by IFN-γ is due to increased mRNA levels.
Fig. 4.
RT-PCR analysis of VEGF-A, VEGF-R1 (Flt-1), MbFlt-1, and sFlt-1 mRNA in HCRF cells. (A) Effects of IFN-γ (100 U/ml), TNF-α (10 ng/ml), IL-1β (10 ng/ml), and JAK-1 inhibitor (1 μM). HCRF cultures were incubated in serum free medium for 8 h in the presence of various combinations as indicated in the figure. Total RNA prepared was used for RT-PCR as described in the Methods section. (B) Effect of TGF-β1(10 ng/ml), TGF-β2 (10 ng/ml) and TGF-βR1 inhibitor (100 nM). HCRF cultures were incubated in serum free medium for 8 h in the presence of various combinations as indicated in the figure and RT-PCR performed as described above. PCR reactions in (A) and (B) were performed for 25 (36B4) or 30 (all others) cycles. Each panel is amplified to different levels to visualize the bands; therefore, comparisons may not be made between the panels.
TGF-β enhances VEGF-A and VEGF-R1 mRNA expression in HCRF cells
TGF-β1 and β2 enhanced the density of PCR products of 516, 648, and 720 bp corresponding to of VEGF-A isoforms 121aa, 165aa, and 189aa (Fig. 4B). Soluble form of VEGF-A 121aa is the predominant form followed by 165aa and 189aa as revealed by the densitometric analysis (data not shown). The specificity of TGF-β effect on VEGF-A is confirmed by decrease in band densities by 2- to 4-fold in the presence of TGF-β receptor 1 inhibitor [16] that blocks TGF-β signal transduction pathway. VEGF-R1 (amplifying both mbFlt-1 and sFlt-1) and sFlt-1 mRNA levels were enhanced by TGF-β1 and TGF-β2 as seen by the density of the bands that increased by 3- to 5-fold. In the presence of TGF-β receptor1 inhibitor, TGF-β1 induced VEGF-R1 and sFlt-1 bands were decreased by 1.6- and 3.4-folds, respectively. In contrast, faint bands were seen for mbFlt-1 (Fig. 4B, panel4) indicating predominant expression of sFlt-1 mRNA. Gene expression studies presented above support the concept that VEGF-A and sFlt-1 secretion enhanced by TGF-β (Fig. 3A and B) is the result of elevated mRNA levels.
Discussion
We used corneal epithelial (HCE) and stromal fibroblast cells (HCRF) to delineate the role of inflammatory mediators in corneal neovascularization (CN). IFN-γ down regulated constitutively produced as well as TNF-α, IL-1, and TGF-β enhanced VEGF-A secretion and mRNA levels. On other hand, sVEGF-R1(sFlt-1) secretion and mRNA levels were upregulated by IFN-γ constitutively and in the presence of TNF-α, IL-1, and TGF-β. These contrasting effects of IFN-γ on VEGF-A and sVEGF-R1 expression have not been reported earlier in corneal and/or in any other cell types. Thus these studies indicate that IFN-γ acts as a double-edged sword controlling angiogenesis by inhibiting angiogenic agent VEGF-A expression, and simultaneously enhancing sFlt-1expression that acts as anti-angiogenic agent by sequestering VEGF-A.
CN is caused by a number of pathological insults such as viral, bacterial, and protozoan infections as well as due to trauma, contact lens wear, alkali burns and graft versus host diseases [1,2,17,18]. Cornea consists of 5–6 layers of epithelial cells, followed by stroma that contributes to about 90% of the corneal tissue. Under normal conditions, corneal tissue is devoid of blood vessels, which end in limbal layers that surround corneal tissue [1,2,18]. During CN, new angiogenic vessels sprout from limbal layers in to the cornea leading to loss of corneal transparency and corneal blindness [1–4,6].
Immunohistochemical studies with human corneal buttons obtained from a number of patients with CN revealed the presence of macrophages, T lymphocytes and neutrophils predominantly in the stroma [4–6]. These infiltrating inflammatory cells are known to produce TNF-α, IL-1, IFN-γ, TGF-β, GM-CSF, and others that initiate further cascade of reactions for CN [1,2,5,6]. The demonstration of elevated levels of VEGF in the corneal extracts and the presence of TGF-β and inflammatory mediators strongly support their role in CN [1,2,5,6]. The critical roles of IL-1, TNF-α, and TGF-β in CN in animal models have also been demonstrated [17,19–21]. In animal models of CN, treatment with anti-VEGF antibodies or with VEGF trap decoy, soluble VEGF-R1 (sFlt-1) prevented CN [7,8]. Soluble VEGF-R1 (sFlt-1), that lacks membrane anchor, is an alternatively spliced variant form of membrane anchored VEGF-R1 (mbFlt-1) [12,22]. sFlt-1can sequester unbound VEGF-A isoforms present in the circulation and in extracellular environment, thereby preventing binding of VEGF-A to its cognate membrane receptors [9,12,22]. Thus balance between VEGF-A and sFlt-1 is critical factor in the regulation of angiogenesis in CN.
The patterns of trafficking of infiltrating cells, T lymphocytes and macrophages in human CN with different etiologies have not been clearly delineated [1,2,4,6]. IFN-γ is primarily produced by T lymphocyts and NK cells, while TNF-α, IL-1, and TGF-β are produced by macrophages and corneal resident HCE and HCRF cells during CN and herpetic stromal keratitis [5,6,17,23]. The complex interplay of migrating cells, their secretory products and their influence on resident corneal cells should be taken together to understand CN. Since it is not feasible to analyze these events in detail in vivo, we used in vitro approach to get some insights in to possible pathways and mechanisms of CN.
Although IFN-γ has been shown to inhibit angiogenesis in a mouse model of bFGF induced corneal neovascularization, the cellular and molecular basis of the mechanisms are not analyzed [24]. TGF-β may act as pro-angiogenic or anti-angiogenic agent depending on the pathological conditions of the tissue involved [19,20]. In HCRF, TGF-β enhances expression of both VEGF-A and sVEGF-R1 that acts as pro and anti-angiogenic molecules respectively. Here, we show that IFN-γ exhibits inhibitory and stimulatory effects on VEGF-A and sVEGF-R1 expression, respectively in corneal stromal fibroblasts, that make up bulk of the cornea. Furthermore, IFN-γ down regulates TGF-β induced VEGF-A while enhancing sVEGF-R1 secretion by HCRF. Thus IFN-γ acts as an anti-angiogenic cytokine by inhibiting the expression of angiogenic agent VEGF-A, and at the same time enhancing the expression of sVEGF-R1 that acts as anti-angiogenic molecule by traping soluble VEGF-A.
Acknowledgments
This research was supported (in part) by the Intramural Research Program of the National Eye Institute, National Institutes of Health.
References
- 1.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans. Am. Ophthalmol. Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang SX, Ma JX. Ocular neovascularization: implication of endogenous angiogenic inhibitors and potential therapy. Prog. Retin. Eye Res. 2007;26:1–37. doi: 10.1016/j.preteyeres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Ambati BK, Patterson E, Jani P, Jenkins C, Higgins E, Singh N, Suthar T, Vira N, Smith K, Caldwell R. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br. J. Ophthalmol. 2007;91:505–508. doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cursiefen C, Kuchle M, Naumann GO. Angiogenesis in corneal diseases: histopathologic evaluation of 254 human corneal buttons with neovascularization. Cornea. 1998;17:611–613. doi: 10.1097/00003226-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Cursiefen C, Rummelt C, Kuchle M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1 in human corneas with neovascularization. Cornea. 2000;19:526–533. doi: 10.1097/00003226-200007000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest. Ophthalmol. Vis. Sci. 2000;41:2514–2522. [PubMed] [Google Scholar]
- 7.Lai CM, Brankov M, Zaknich T, Lai YK, Shen WY, Constable IJ, Kovesdi I, Rakoczy PE. Inhibition of angiogenesis by adenovirus-mediated sFlt-1 expression in a rat model of corneal neovascularization. Hum. Gene Ther. 2001;12:1299–1310. doi: 10.1089/104303401750270959. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, Wu J, Li H, Wang Z, Chen X, Tian Y, Yi M, Ji X, Ma J, Huang Q. Inhibition of corneal neovascularization by recombinant adenovirus-mediated sFlk-1 expression. Biochem. Biophys. Res. Commun. 2007;361:946–952. doi: 10.1016/j.bbrc.2007.07.114. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 10.Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest. Ophthalmol. Vis. Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 11.Djalilian AR, Nagineni CN, Mahesh SP, Smith JA, Nussenblatt RB, Hooks JJ. Inhibition of inflammatory cytokine production in human corneal cells by dexamethasone, but not cyclosporin. Cornea. 2006;25:709–714. doi: 10.1097/01.ico.0000208815.02120.90. [DOI] [PubMed] [Google Scholar]
- 12.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagineni CN, Samuel W, Nagineni S, Pardhasaradhi K, Wiggert B, Detrick B, Hooks JJ. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: involvement of mitogen-activated protein kinases. J. Cell. Physiol. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR, Cameron PM, Meinke PT, Liverton N, Weng Y, DeMartino JA. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg. Med. Chem. Lett. 2002;12:1219–1223. doi: 10.1016/s0960-894x(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 15.Nagineni CN, Cherukuri KS, Kutty V, Detrick B, Hooks JJ. Interferon-gamma differentially regulates TGF-beta1 and TGF-beta2 expression in human retinal pigment epithelial cells through JAK-STAT pathway. J. Cell. Physiol. 2007;210:192–200. doi: 10.1002/jcp.20839. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer JS, Anderson BD, Beight DW, Campbell RM, Jones ML, Herron DK, Lampe JW, McCowan JR, McMillen WT, Mort N, Parsons S, Smith EC, Vieth M, Weir LC, Yan L, Zhang F, Yingling JM. Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J. Med. Chem. 2003;46:3953–3956. doi: 10.1021/jm0205705. [DOI] [PubMed] [Google Scholar]
- 17.Biswas PS, Rouse BT. Early events in HSV keratitis—setting the stage for a blinding disease. Microbes Infect. 2005;7:799–810. doi: 10.1016/j.micinf.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv. Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 19.Friling R, Yassur Y, Levy R, Kost J, Schwartz B, Mikhailowsky R, Lamprecht SA. A role of transforming growth factor-beta 1 in the control of corneal neovascularization. In Vivo. 1996;10:59–64. [PubMed] [Google Scholar]
- 20.Sakamoto T, Ueno H, Sonoda K, Hisatomi T, Shimizu K, Ohashi H, Inomata H. Blockade of TGF-beta by in vivo gene transfer of a soluble TGF-beta type II receptor in the muscle inhibits corneal opacification, edema and angiogenesis. Gene Ther. 2000;7:1915–1924. doi: 10.1038/sj.gt.3301320. [DOI] [PubMed] [Google Scholar]
- 21.Nakao S, Hata Y, Miura M, Noda K, Kimura YN, Kawahara S, Kita T, Hisatomi T, Nakazawa T, Jin Y, Dana MR, Kuwano M, Ono M, Ishibashi T, Hafezi- Moghadam A. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am. J. Pathol. 2007;171:1058–1065. doi: 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis. 2006;9:225–230. doi: 10.1007/s10456-006-9055-8. (discussion 231) [DOI] [PubMed] [Google Scholar]
- 23.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J. Leukoc. Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 24.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J. Natl. Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]