Abstract
In this review article, we provide a detailed and comprehensive discussion of the rationale for using modern IMRT techniques to spare the subgranular zone of the hippocampus during cranial irradiation. We review the literature on neurocognitive effects of cranial irradiation; discuss clinical and preclinical data associating damage to neural progrenitor cells located in subgranular zone of the hippocampus with radiation-induced neurocognitive decline, specifically in terms of short-term memory formation and recall; and present a review of our pilot investigations into the feasibility and risks of sparing the subgranular zone of the hippocampus during whole-brain radiotherapy for brain metastases. We also introduce our phase II cooperative group clinical trial (RTOG 0933) designed to prospectively evaluate the postulated neurocognitive benefit of hippocampal subgranular zone sparing and scheduled to open in 2010.
Neurocognitive Toxicity after Cranial Irradiation
Cranial irradiation is an effective therapeutic modality in multiple different settings of oncologic management: whole-brain radiotherapy (WBRT) for brain metastases, prophylactic cranial irradiation (PCI) for small cell lung cancer (and controversially for non-small-cell lung cancer), and cranial or craniospinal irradiation for pediatric central nervous system malignancies. The benefit of cranial irradiation in these settings largely arises from 1) the inadequate penetration of systemic therapies across the blood-brain barrier and 2) the ability of cranial irradiation to effectively target microscopic and/or gross intracranial disease.
Several prospective randomized trials have shown that withholding WBRT in patients with brain metastases leads to a 70–300% increase in the relative risk of developing brain metastases, compared to delivering up-front WBRT at the time of diagnosis of brain metastases, and this observation was reinforced in the recently completed (but as yet unpublished) EORTC trial (1–4). For example, in the landmark Patchell et al. study (2), the absolute risk of any intracranial failure with and without up-front WBRT was 18% vs 70%, implying an absolute increase of 52% in any brain failure without WBRT over a baseline rate of 18% with WBRT. This translates to a relative increase in risk of just under 300% (52/18 × 100 = 289%). Previous trials have all been relatively small, adequately powered to detect this difference in intracranial failure but not a difference in survival. The recent EORTC trial (4) was much larger and provided data over a longer time frame, inclusive of brain relapse rates out to two years. In the surgical arm of this trial, regional failure in the brain at two years, excluding the increase in local failure at the original tumor site in the brain, was 23% vs. 42% (p < 0.008) with and without WBRT, resulting in an excess regional only failure rate of 19%, which translates into an increased relative risk of 83%. Though compelling, this dramatic reduction in the risk of subsequent development of brain metastases with WBRT comes at the cost of potential neurocognitive toxicity.
Neurocognitive toxicity represents a spectrum of different toxicities, and the time course of these can vary significantly. Most authors have generally focused on severe dementia and described this as a “late toxicity,” occurring several months to years following cranial irradiation. DeAngelis et al. (5) suggested that as many as 11% of long-term brain metastases survivors (>12 months) treated with cranial irradiation develop severe dementia, but only with the use of larger dose-per-fraction schedules. Long-term serious and permanent adverse effects, including cognitive deterioration in other domains and cerebellar dysfunction, have also been described (6). However, recent clinical evidence in the setting of WBRT for brain metastases suggests a component of early neurocognitive decline occurring within the first 1–4 months (3, 7, 8). This early component primarily reflects verbal and short-term memory recall (9–11). However, whether this early decline in memory is associated with long-term and/or permanent decline has not been adequately studied, and some preliminary data suggest a possible late rebound, implying the presence of an early-responding cellular compartment with at least some repair capacity. Furthermore, the analysis of neurocognitive decline in brain metastases can be confounded by several effects, including: 1) patients with brain metastases tend to have reduced neurocognition at the time of presentation, which is frequently not evaluated; 2) disease-progression, both intra- and extra-cranially, will negatively skew population distributions of neurocognitive scores; and 3) the effects of therapeutic interventions such as chemotherapy, anticonvulsants, steroids, opiates, etc., remain inadequately documented.
In an attempt to disentangle these confounding effects, our research group recently published a detailed analysis of the time course of neurocognitive decline in eight prospectively measured domains in 208 brain metastases patients treated with 30 Gy of WBRT and enrolled on a prospective, international multi-institutional randomized trial, with pre-specified and standardized neurocognitive evaluations in association with prospective MR imaging (7). Neurocognitive function (NCF), assessed by tests of memory, executive function, and fine motor coordination, was correlated with metastasis volume regression as measured by magnetic resonance imaging. NCF and survival were compared in 135 patients evaluable at 2 months with tumor shrinkage less than (poor responders) and greater than (good responders) the population median. The mean NCF scores and brain metastasis volume at 4 and 15 months were compared. Good responders experienced significantly improved survival (unidirectional p = 0.03). For all tests, the median time to NCF deterioration was longer in the good than in the poor responders, with statistical significance seen for executive and fine motor functions (Table 1). In long-term survivors, defined as patients surviving more than 15 months, tumor shrinkage was significantly correlated with preservation of executive function and fine motor coordination (r = 0.68–0.88).
Table 1.
Median Time (± 1 SE) to NCF Deterioration in “Good” Versus “Poor” Responders in Days
| Group | Memory | Verbal fluency (COWA) | Pegboard | Executive Function | ||||
|---|---|---|---|---|---|---|---|---|
| Recall | Recognition | Delayed Recall | DH | NDH | Trailmaking A | Trailmaking B | ||
| Good responders | 416 ± 37 | 374 ± 37 | 431 ± 37 | 512 ± 30 | 380 ± 38 | 401 ± 38 | 391 ± 37 | 462 ± 35 |
| Poor responders | 355 ± 41 | 322 ± 38 | 372 ± 39 | 441 ± 39 | 287 ± 37 | 291 ± 38 | 386 ± 36 | 331 ± 38 |
| Net gain (days) | 61 | 52 | 59 | 71 | 93 | 110 | 5 | 131 |
| P | .205 | .478 | .315 | .243 | .049 | .021 | .237 | .017 |
| No. of patients | 131 | 131 | 131 | 131 | 132 | 132 | 132 | 131 |
NOTE: Patients were classified as good versus poor responders based on whether their indicator lesion volume reduction at 2 months was above or below the population median reduction of 45%.
Abbreviations: NCF, neurocognitive function; COWA, controlled oral word association; DH, Dominant Hand; NDH, Nondominant Hand.
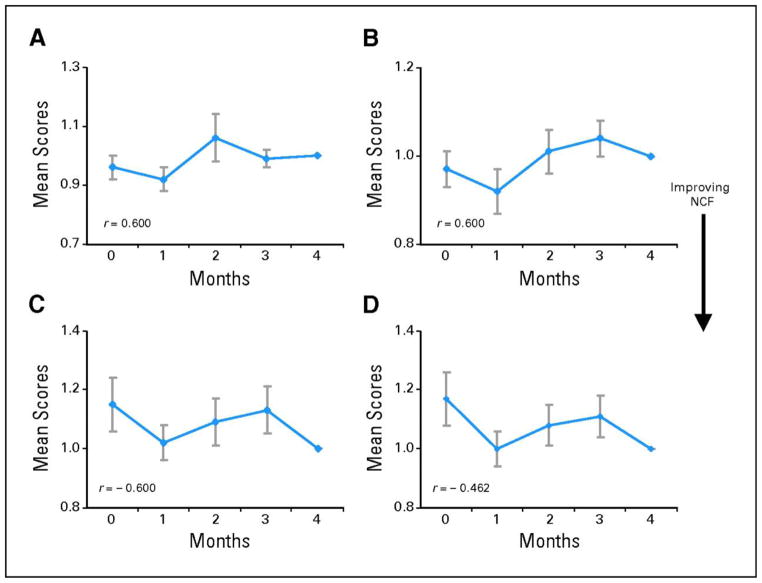
These findings support two important possibilities. First, achieving local control with WBRT was integral to both improving survival and preserving certain neurocognitive domains. Second, an intriguing exception to these findings was memory function, specifically recall and delayed recall as assessed with the Hopkins Verbal Learning Test (HVLT). While executive and fine motor functions demonstrated statistical significance for longer time to NCF deterioration in good compared to poor responders, this statistical difference was not appreciated for the memory functions of recall and delayed recall (Table 1). In addition, amongst patients surviving to four months, these memory domains showed a statistically significant NCF decline at four months compared to baseline (Figure 1). NCF scores for executive and fine motor functions did not show a similar decline (Figure 2). Thus, these data suggest that memory-related neurocognitive domains may have a weaker association with tumor reduction and may be most susceptible to early decline, even in patients with non-progressing brain metastases, implying the selective effect of WBRT in preserving certain domains over others and the differential sensitivity of certain domains to radiation effects.
Figure 1. Decline in memory-related neurocognitive domains at 4 months after WBRT.
Change of mean normalized neurocognitive function (NCF) test scores in patients who were surviving at the fourth month. The scores were normalized to individual patient’s own baseline. Figure represents NCF tests where higher scores reflect better function. (A) Recall, (B) delayed recall, (C) recognition, and (D) controlled oral word association (COWA). Each data point represents the mean NCF score ± SE. r represents Spearman’s correction coefficient between mean test scores and time. (*) P value < 0.05 is considered statistically significant.
Figure 2. No change in executive function and fine motor coordination neurocognitive domains at 4 months after WBRT.
Change of mean normalized neurocognitive function (NCF) test scores in patients who were surviving at the fourth month. The scores were normalized to individual patient’s own baseline. Figure represents NCF tests where higher scores reflect better function. (A) Pegboard Dominant Hand, (B) Pegboard Nondominant Hand, (C) Trailmaking A, and (D) Trailmaking B. Each data point represents the mean NCF score ± SE. r represents Spearman’s correction coefficient between mean test scores and time. (*) P value < 0.05 is considered statistically significant.
Further evidence of the early susceptibility of memory function to WBRT was recently demonstrated by Chang and colleagues (3). They reported a single-institution phase III trial of stereotactic radiosurgery (SRS) with or without WBRT in patients with 1–3 brain metastases, with the principal objective of comparing neurocognitive decline between the two arms. Utilizing HVLT as a neurocognitive metric for learning and memory, they defined NCF decline as a >5 point drop 4 months from baseline. Their study was halted early due to an interim observation of a two-fold increase in the mean posterior probability of neurocognitive decline (52%, SRS+WBRT, vs. 24%, SRS alone). Similar findings were reported by Welzel et al., who observed a decline in verbal memory function, as assessed by the Auditory Verbal Learning Test (AVLT) 6–8 weeks after the completion of WBRT for brain metastases (8).
In long-term survivors, a trend towards some rebound in the memory domains has also been observed. RTOG 0214 is a phase III comparison of prophylactic cranial irradiation (PCI) versus observation in patients with locally-advanced non-small-cell lung cancer. In this study, the use of PCI significantly diminished the overt development of brain metastases, without a categorical survival improvement. Despite not reaching target accrual, this trial also demonstrated a significantly greater decline in immediate recall and delayed recall, as assessed by HVLT in the PCI arm at 3, 6 and 12 months follow-up. Interestingly, the largest magnitude of decline in these memory domains occurred 3 months after PCI, with some recovery of recall appreciated over time (12). In our analysis of neurocognition in brain metastases patients, a similar biphasic pattern of memory decline was also observed (7). The summation of these data provides hypothesis-generating observations that these memory domains may be mediated by an early-responding cellular compartment with at least some repair capacity.
Recent work from our group has shown that neurocognition and quality of life are correlated in patients with brain metastases receiving WBRT (13). We found that deterioration in neurocognitive function preceded self-reported quality of life decline by up to 153 days. Hence, there is likely to be a sequential association between neurocognitive decline and deterioration in self-reported quality of life for patients with brain metastasis. The sum of these and our findings suggest that although achievement of intracranial disease control is an important aim of cranial irradiation, strategies meant to preserve memory-related neurocognition warrant further investigation.
Clinical and Preclinical Rationale for Avoiding the Hippocampus
The central role of the hippocampus in supporting memory function was first understood more than fifty years ago, in the case study of H.M., a gentleman who underwent a bilateral medial temporal lobectomy for the relief of medically intractable epilepsy. Immediately following the procedure, H.M. showed a severe anterograde amnesia characterized by impairment in declarative memory (the conscious recollection of facts and events). H.M.’s amnesia, however, did not include the remaining components of his neurocognition, including perception, intelligence, working memory, and motor skill learning, all of which remained largely intact (14, 15). Building on these observations, subsequent neuropsychology studies of median temporal lobe lesions have demonstrated that declarative memory impairment due to hippocampal injury occurs regardless of the sensory modality in which information is presented (16–19) and without affecting immediate memory, perception, and intellectual functions (20–22).
Recent clinical studies suggest that radiation-induced damage to the hippocampus plays a considerable role in the neurocognitive decline of patients after cranial irradiation. In particular, deficits in learning, memory, and spatial processing observed in patients who have received WBRT are thought to be related to hippocampal injury (23). Moreover, irradiation of the hippocampus has been associated with pronounced cognitive impairment in the learning and memory domain in patients receiving radiation therapy for nasopharyngeal tumors (24, 25), maxillary tumors (26), pituitary tumors (27), and base of skull tumors (28). Preliminary results from a recent MD Anderson study of low-grade or anaplastic brain tumors treated with radiotherapy have observed a dose-response phenomenon, wherein the maximum radiation dose to the left hippocampus was correlated with subsequent decline in learning (p = 0.014) and delayed recall (p = 0.01) (29). Similarly, Jalali and colleagues prospectively evaluated intelligence quotient (IQ) scores in patients with benign and low-grade brain tumors and observed a significant correlation association between IQ decline and dose to the left temporal lobe (30). These clinical observations therefore place the hippocampal anatomy at the center of radiation-induced short-term memory decline, and the traditional belief was that this reflected injury to specific neuronal pathways.
However, recent preclinical work by Michelle Monje and colleagues and others has begun to challenge this “anatomic” explanation, in favor of a “stem-cell compartmental” hypothesis. Memory function has been associated with the pyramidal and granule cells located in the dentate gyrus of the hippocampus (31). In all adult mammals, including humans, new granule cells are generated from mitotically active neural stem cells (NSCs), which are located in the subgranular zone of the dentate gyrus and which migrate into the granular cell layer (32–38). Monje et al. have demonstrated that the pathogenesis of radiation-induced neurocognitive deficit may involve radiation-induced injury to this NSC compartment (39, 40). It has been found that relatively modest doses of radiation cause apoptosis and a sharp and prolonged decline in neurogenesis in the subgranular zone of young rats and mice, and that this compartmental cell loss is associated with extinction of short-term memory, with increasing failure rates on hippocampal-dependent tasks (40–44). On the other hand, little to no apoptosis is observed in other areas of the cerebrum (43), and no loss of function is observed in hippocampal-independent tasks (44). Monje and colleagues went on to show that neurogenesis is inhibited by inflammation in the area surrounding the NSCs (45). This inhibition occurred whether the inflammation was induced by radiation injury or by bacterial lipopolysaccharide. Hence, inflammatory injury to the proliferating subgranular NSC compartment of the hippocampus putatively represents one possible mechanism for the domain-wise differential benefit in neurocognitive function and the temporal sequence of events following cranial irradiation. Of course, none of these observations rule out the contribution of other possible modes of radiation injury as being causative and/or contributory to neurocognitive decline.
To test the hippocampal stem cell hypothesis, we propose to conformally avoid the hippocampus during cranial irradiation to putatively limit the radiation-induced “early” inflammation of the hippocampal region and subsequent alteration of the microenvironment of the NSCs. We hypothesize that avoiding the hippocampus during cranial irradiation may delay or reduce the onset, frequency, and/or severity of neurocognitive decline, without compromising intracranial disease control, and have sought to subject this testable hypothesis to a clinical trial.
Feasibility of Avoiding the Hippocampus
Avoiding the hippocampus during cranial irradiation, while allowing for uniform dose delivery to the remainder of the brain, poses important challenges given the central location and unique anatomic shape of the hippocampus. Recently, we demonstrated the ability of modern intensity-modulated radiotherapy (IMRT) techniques, including helical tomotherapy and LINAC-based IMRT, to allow for the delivery of highly conformal dose distributions (46). For a prescription dose of 30 Gy in 10 fractions to the whole brain, our technique is able to reduce 1) the mean dose per fraction to the hippocampus (normalized to 2-Gy fractions) by 87% to 0.49 Gy2 using helical tomotherapy and by 81% to 0.73 Gy2 using LINAC-based IMRT; and, 2) the maximum dose to the hippocampus to 12.8 Gy using helical tomotherapy and 15.3 Gy using LINAC-based IMRT. Sparing of the hippocampus with these IMRT modalities is accomplished with acceptable target coverage and homogeneity. Using a rat model Michelle Monje and colleagues have observed a radiation dose-dependent effect on neurogenesis, with a single fraction of 10 Gy inducing a 62% reduction in neural stem cell proliferation and a 97% reduction in hippocampal neurogenesis (39, 40). Based on these data, we postulate that sparing the hippocampus could yield clinically significant neurocognitive benefit.
In addition, modern IMRT techniques allow for the simultaneous integrated boost of intracranial metastases, without compromising hippocampal sparing. Recently, our group demonstrated the capability of helical tomotherapy to conformally avoid the hippocampus, and still deliver radiosurgical–quality dose distributions to multiple metastases and a homogeneous dose distribution to the whole brain—all in a single treatment plan (47). A similar study from Hsu and colleagues demonstrated a similar capability using volumetric arc therapy (48). These IMRT techniques thus have the potential to allow for the optimal balance between intracranial disease control, achieved with WBRT and simultaneous boosting of radiographically evident disease, and preservation of neurocognition, theoretically achieved with hippocampal avoidance.
Risk of Perihippocampal Disease Progression
Sparing the hippocampus and perihippocampal region (hippocampus + 5mm margin) of therapeutic doses of radiation poses the theoretical risk of intracranial disease progression in these regions. The degree of this risk is largely dependent on the volume of brain tissue that is spared. The hippocampus consists of two U-shaped interlocking laminae: the cornu ammonus and the dentate gyrus. It is a component of the entire limbic circuit, which includes white matter tracts such as the fimbriae and fornices (the primary efferent system of the hippocampus) and gray matter structures such as the amygdala and parahippocampal gyrus. Preclinical evidence has associated neurogenesis within the dentate gyrus with normal cognitive function (49–51). Cranial irradiation in rat models has been observed to induce apoptosis of these precursor cells and alter their differentiation towards a gliogenic, rather than a neurogenic fate, resulting in a significant reduction in hippocampal neurogenesis (39, 52) and associated cognitive impairment (42).
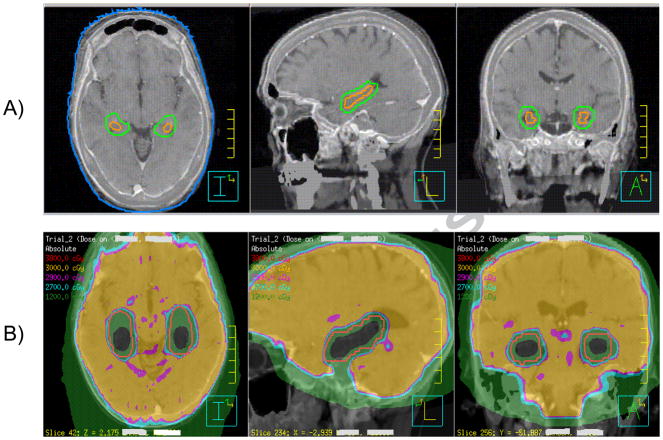
Based on these data, we propose a targeted approach to avoiding the hippocampus, focusing on the dentate gyrus and cornu ammonus, rather than comprehensively avoiding the entire limbic circuit (Figure 1). In other words, we are forgoing the anatomic neuronal circuit damage model for the compartmental NSC model. This approach avoids a clinically unacceptable risk of disease progression. Recently, we detailed our approach to contouring the hippocampus using axial, sagittal, and coronoal sections of a gadolinium-enhanced T1 weighted MRI (Figure 1) (46). Using this approach to targeted hippocampal contouring, we reviewed 371 patients who presented with 1133 metastases (53). In this comprehensive multi-institution analysis, we observed a metastasis within the hippocampal avoidance region (hippocampus plus 5mm margin) in 8.6% of patients, with 11.5% as the upper limit of the 95% confidence interval. No patient presented with a metastasis within the hippocampus proper. These data corroborate the results of our earlier, single-institution study, in which we reviewed 100 patients with brain metastases and observed 8% of patients to have a perihippocampal metastasis at presentation (54). The similar incidence of perihippocampal metastases between the two studies points to the reproducibility of our methodology in reviewing two separate institutional databases. However, enlarging our patient database has allowed us to reduce the standard error and, in so doing, improved the accuracy of our risk estimate.
Assuming that the risk of developing subsequent brain metastasis within the hippocampal avoidance region scales in the same proportion as that at presentation, we estimate that a patient treated with hippocampal sparing during whole-brain radiotherapy (WBRT) will derive 91.4% of the relative benefit of WBRT in terms of preventing the emergence of radiographically visible intracranial lesions, with a lower 95% confidence limit of 88.5% (53). This modest increase in risk of intra-cranial progression with hippocampal avoidance may be partially compensated by the possibility of salvage with radiosurgery, which remains to be validated. Should salvage radiosurgery be used for a perihippocampal recurrence, we expect that given the very steep radiation dose falloff with stereotactic radiosurgery, only some but not all of the potential neurocognitive benefit of hippocampal avoidance will be lost. Based on these data, we conclude that hippocampal sparing during WBRT should be tested clinically.
RTOG 0933: A Phase II Trial of Hippocampal Sparing
To prospectively evaluate the neurocognitive benefit of hippocampal sparing, the RTOG has developed a phase II clinical trial (RTOG 0933) to test hippocampal sparing during WBRT in patients with brain metastases (Table 2). The primary endpoint will be delayed recall assessed using HVLT at 4 months after treatment, and a planned statistical comparison will be made to an historical control of patients who received WBRT without hippocampal avoidance on a recent phase III trial (PCI-P120-9801). (55, 56).
Table 2. Schema for RTOG 0933, a phase II trial of hippocampal avoidance during WBRT (HA-WBRT) for patients with brain metastases.
The primary endpoint will be delayed recall, assessed using the Hopkins Verbal Learning Test at 4 months after HA-WBRT. A planned statistical comparison will be made to an historical control of patients who received WBRT without hippocampal avoidance.
|
For Patients with MRI Evidence of Brain Metastasis Within 1 Month of WBRT | ||
|---|---|---|
| REGISTER1 |
Within 2 Weeks Prior to Treatment |
Radiation Therapy |
|
WBRT with Hippocampal Avoidance using IMRT (30 Gy in 10 Fractions)4 | |
Abbreviations: WBRT, whole-brain radiotherapy; 3D SPGR MRI, three-dimensional spoiled-gradient magnetic resonance imaging; IMRT, intensity-modulated radiotherapy.
Summary and Future Directions
In summary, preclinical and clinical evidence suggests that radiation dose received by the neural stem cells of the subgranular zone in the hippocampus may play a role in radiation-induced neurocognitive decline, specifically memory recall. Although neurocognitive assessment in patients receiving WBRT can be confounded by intracranial metastatic disease, analyses from our group and others suggest a differential sensitivity to WBRT of various memory-related neurocognitive domains, such as delayed recall. This provides the rationale to explore the clinical feasibility of hippocampal sparing during WBRT. We and others have demonstrated the dosimetric capabilities of intensity-modulated radiotherapy to conformally avoid the hippocampus without detriment to the radiation dose received by the remainder of the brain. Through retrospective analyses, we have also estimated the theoretical risk of perihippocampal disease progression with hippocampal avoidance. Given the overall aim of preventing neurocognitive decline, and the possibility of salvaging hippocampal and perihippocampal recurrences with radiosurgery, we hypothesize that hippocampal sparing during WBRT may provide a net gain in this endpoint. To prospectively evaluate this hypothesis, we have developed a phase II clinical trial through RTOG (RTOG 0933). Given the experimental nature of this hypothesis, at this point, hippocampal sparing should not be used outside of clinical trials, such as RTOG 0933 and others as they evolve. Efforts are ongoing at our institution to develop similar clinical trial concepts for the use of hippocampal sparing in prophylactic cranial irradiation in small-cell lung cancer and cranial and craniospinal irradiation in pediatric CNS tumors.
Figure 3. Targeted approach to avoiding the hippocampus.
(A) The hippocampus (orange) was contoured by focusing on the dentate gyrus and cornus ammonus, rather than the entire limbic circuit, using T1-weighted magnetic resonance imaging (MRI) sagittal and coronal sequences. The hippocampal avoidance region (green) was generated by expanding the hippocampal contour by 5 mm volumetrically to account for setup error. (B) Spatial isodose distribution for 1 sample patient at the level of the hippocampi for hippocampal-avoidance during whole-brain radiotherapy (prescription of 30 Gy in 10 fractions) using helical tomotherapy. Orange contour represents the hippocampal avoidance region. Green isodose represents 12 Gy; light blue, 27 Gy; pink, 29 Gy; yellow, 30 Gy; red, 38 Gy, in 10 fractions.
Acknowledgments
None.
Footnotes
Conflicts of Interest Statement: Wolfgang Tome serves as a consultant to and receives research funding from Philips Radiation Oncology Systems. Minesh Mehta serves or has served as a consultant for Adnexus, Bayer, Genentech, Merck, Tomotherapy, and YM BioSciences; has stock ownership in Pharmacyclics and Tomotherapy; and, serves on the Board of Directors of Pharmacyclics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 3.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 4.Mueller RP, Soffietti R, Abacioglu MU, et al. J Clin Oncol. Vol. 27. Chicago, IL: 2009. Adjuvant whole-brain radiotherapy versus observation after radiosurgery for surgical resection of 1-3 cerebral metastases: Results of the EORTC 22952–26001 study. (suppl; abstr 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 6.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Bentzen SM, Renschler M, et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 8.Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. 2008;72:1311–1318. doi: 10.1016/j.ijrobp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 10.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 11.Regine WF, Schmitt FA, Scott CB, et al. Feasibility of neurocognitive outcome evaluations in patients with brain metastases in a multi-institutional cooperative group setting: results of Radiation Therapy Oncology Group trial BR-0018. Int J Radiat Oncol Biol Phys. 2004;58:1346–1352. doi: 10.1016/j.ijrobp.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Movsas B, Kae K, Meyers C, et al. Phase III study of prophylactic cranial irradiation vs. observation in patients with stage III non-small-cell lung cancer: Neurocognitive and quality of life analysis of RTOG 0214. American Society of Radiation Oncology Annual Meeting; Chicago, IL. 2009. p. S1. [Google Scholar]
- 13.Li J, Bentzen SM, Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 14.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corkin S. What’s new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- 16.Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 17.Levy DA, Manns JR, Hopkins RO, et al. Impaired visual and odor recognition memory span in patients with hippocampal lesions. Learn Mem. 2003;10:531–536. doi: 10.1101/lm.66703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray EA, Mishkin M. Severe tactual as well as visual memory deficits follow combined removal of the amygdala and hippocampus in monkeys. J Neurosci. 1984;4:2565–2580. doi: 10.1523/JNEUROSCI.04-10-02565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Squire LR, Schmolck H, Stark SM. Impaired auditory recognition memory in amnesic patients with medial temporal lobe lesions. Learn Mem. 2001;8:252–256. doi: 10.1101/lm.42001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drachman DA, Arbit J. Memory and the hippocampal complex. II. Is memory a multiple process? Arch Neurol. 1966;15:52–61. doi: 10.1001/archneur.1966.00470130056005. [DOI] [PubMed] [Google Scholar]
- 21.Clark RE, West AN, Zola SM, et al. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus. 2001;11:176–186. doi: 10.1002/hipo.1035. [DOI] [PubMed] [Google Scholar]
- 22.Schmolck H, Stefanacci L, Squire LR. Detection and explanation of sentence ambiguity are unaffected by hippocampal lesions but are impaired by larger temporal lobe lesions. Hippocampus. 2000;10:759–770. doi: 10.1002/1098-1063(2000)10:6<759::AID-HIPO1013>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 24.Lee PW, Hung BK, Woo EK, et al. Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry. 1989;52:488–492. doi: 10.1136/jnnp.52.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung SF, Kreel L, Tsao SY. Asymptomatic temporal lobe injury after radiotherapy for nasopharyngeal carcinoma: incidence and determinants. Br J Radiol. 1992;65:710–714. doi: 10.1259/0007-1285-65-776-710. [DOI] [PubMed] [Google Scholar]
- 26.Sakata K, Aoki Y, Karasawa K, et al. Analysis of the results of combined therapy for maxillary carcinoma. Cancer. 1993;71:2715–2722. doi: 10.1002/1097-0142(19930501)71:9<2715::aid-cncr2820710905>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Grattan-Smith PJ, Morris JG, Shores EA, et al. Neuropsychological abnormalities in patients with pituitary tumours. Acta Neurol Scand. 1992;86:626–631. doi: 10.1111/j.1600-0404.1992.tb05500.x. [DOI] [PubMed] [Google Scholar]
- 28.Meyers CA, Geara F, Wong PF, et al. Neurocognitive effects of therapeutic irradiation for base of skull tumors. Int J Radiat Oncol Biol Phys. 2000;46:51–55. doi: 10.1016/s0360-3016(99)00376-4. [DOI] [PubMed] [Google Scholar]
- 29.Mahajan A, Dong L, Prabhu S, et al. Application of deformable image registration to hippocampal doses and neurocognitive outomces. Vol. 9. Society of Neuro-Oncology; Dallas: 2007. p. 538. [Google Scholar]
- 30.Jalali R, Mallick I, Dutta D, et al. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int J Radiat Oncol Biol Phys. 77:974–979. doi: 10.1016/j.ijrobp.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Collier TJ, Quirk GJ, Routtenberg A. Separable roles of hippocampal granule cells in forgetting and pyramidal cells in remembering spatial information. Brain Res. 1987;409:316–328. doi: 10.1016/0006-8993(87)90717-7. [DOI] [PubMed] [Google Scholar]
- 32.Bayer SA. Changes in the total number of dentate granule cells in juvenile and adult rats: a correlated volumetric and 3H-thymidine autoradiographic study. Exp Brain Res. 1982;46:315–323. doi: 10.1007/BF00238626. [DOI] [PubMed] [Google Scholar]
- 33.Cameron HA, Woolley CS, McEwen BS, et al. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 34.Gould E, McEwen BS, Tanapat P, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 37.Gould E, Beylin A, Tanapat P, et al. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 40.Mizumatsu S, Monje ML, Morhardt DR, et al. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 41.Ferrer I, Serrano T, Alcantara S, et al. X-ray-induced cell death in the developing hippocampal complex involves neurons and requires protein synthesis. J Neuropathol Exp Neurol. 1993;52:370–378. doi: 10.1097/00005072-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 43.Nagai R, Tsunoda S, Hori Y, et al. Selective vulnerability to radiation in the hippocampal dentate granule cells. Surg Neurol. 2000;53:503–506. doi: 10.1016/s0090-3019(00)00214-7. discussion 506–507. [DOI] [PubMed] [Google Scholar]
- 44.Madsen TM, Kristjansen PE, Bolwig TG, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 45.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 46.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: A “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. International Journal of Radiation Oncology, Biology and Physics. 2010 doi: 10.1016/j.ijrobp.2010.01.039. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutierrez AN, Westerly DC, Tome WA, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int J Radiat Oncol Biol Phys. 2007;69:589–597. doi: 10.1016/j.ijrobp.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu F, Carolan H, Nichol A, et al. Whole Brain Radiotherapy with Hippocampal Avoidance and Simultaneous Integrated Boost for 1–3 Brain Metastases: A Feasibility Study Using Volumetric Modulated Arc Therapy. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 49.Shors TJ, Miesegaes G, Beylin A, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 50.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 51.Lemaire V, Koehl M, Le Moal M, et al. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tada E, Parent JM, Lowenstein DH, et al. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 53.Gondi V, Tome WA, Marsh J, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol. 95:327–331. doi: 10.1016/j.radonc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghia A, Tome WA, Thomas S, et al. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. 2007;68:971–977. doi: 10.1016/j.ijrobp.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 56.Mehta MP, Shapiro WR, Glantz MJ, et al. Lead-in phase to randomized trial of motexafin gadolinium and whole-brain radiation for patients with brain metastases: centralized assessment of magnetic resonance imaging, neurocognitive, and neurologic end points. J Clin Oncol. 2002;20:3445–3453. doi: 10.1200/JCO.2002.07.500. [DOI] [PubMed] [Google Scholar]