Abstract
To better define the molecular mechanisms underlying leptin release from adipocytes, we developed a novel protocol that maximizes leptin production from 3T3-L1 adipocytes. The addition of a PPARγ agonist to the Isobutylmethylxanthine/Dexamethasone/Insulin differentiation cocktail increased leptin mRNA levels by 5-fold, maintained insulin sensitivity, and yielded mature phenotype in cultured adipocytes. Under these conditions, acute insulin stimulation for 2 h induced a two-fold increase in leptin secretion, which was independent of new protein synthesis, and was not due to alterations in glucose metabolism. Stimulation with insulin for 15 min induced the same level of leptin release and was blocked by Brefeldin A. Inhibiting PI 3-kinase with wortmannin had no effect on insulin stimulation of leptin secretion. These studies show that insulin can stimulate leptin release via a PI3K independent mechanism and provide a cellular system for studying the effect of insulin and potentially other mediators on leptin secretion.
Keywords: Leptin, Regulated secretion, 3T3-L1 adipocytes, PI3 kinase independent, Adipokines, Insulin
Introduction
Leptin is a 16 kD hormone, secreted by adipocytes, that plays a key role in regulating food intake, body weight, and energy homeostasis. Although the amount of body fat is the most important determinant of circulating leptin levels, the acute production and secretion of leptin is also modulated by numerous extrinsic factors. For example, fasting decreases and feeding and/or insulin increases serum leptin acutely over intervals that have little impact on adipose tissue mass [1–5]. Previous studies have suggested that fluctuations in leptin production are regulated at a transcriptional level, as insulin increased the amount of leptin mRNA in isolated adipocytes from rats as well as in different cultured adipocyte cell lines [6–10]. However, those earlier reports focused on the long-term effects (24–48 h) of insulin treatment on leptin secretion. While other studies, looking at short time periods of insulin action, suggested that leptin production might also be regulated at a post-transcriptional level [11–16], in these studies it has not been clear to what extent these effects are dependent on protein synthesis. Indeed, while some studies reported that cyclohex-amide had no effect on insulin-induced leptin secretion [12,14,15,17] others showed that protein synthesis is required for the induction of leptin release making it unclear whether insulin can stimulate leptin secretion [18,19]. This issue is important not just for leptin release but also because the role of regulated protein secretion in adipocytes is controversial [20,21]. In addition to leptin, adipocytes secrete other circulating peptides (e.g. adiponectin), which are collectively known as adipokines [22]. Thus studies of protein secretion in adipocytes are also likely to be relevant for other adipokines including adiponectin, TNFα, resistin and others.
Leptin is released by adipocytes and is transported through the general secretory pathway. Although leptin has been detected in the endoplasmic reticulum (ER) as well as the Golgi complex and in small vesicles underneath the plasma membrane [13,19,23], studies of leptin secretion have been hampered by a lack of well-characterized leptin antibodies that can be used at an immunohistochemical level. In addition, technical challenges presented by primary adipocytes have made it difficult to define the precise intracellular compartment that contains leptin. Adiponectin has been shown to be partially localized to the ER; the majority of the protein is distributed throughout the cytosol in peripheral storage vesicles [20]. A more complete understanding of the molecular mechanisms that determine the intracellular compartmentalization and trafficking of adipokines may shed light on their different functions and responses among the growing list of factors secreted by adipocytes.
3T3-L1 adipocytes are a well-established cell culture system that can be used to study adipogenesis, fatty acid metabolism, as well as insulin-regulated trafficking. 3T3-L1 fibroblasts differentiated with the standard Isobutylmethylxanthine/Dexamethasone/Insulin (Ibmx/Dex/Ins) protocol differentiate into mature adipocytes but express only small amounts of leptin, a fact that has limited their utility in studying leptin secretion [24]. Thus studies of leptin secretion in 3T3-L1 adipocytes will require modifications to the protocols that have been used previously. While the differentiation cocktail of Indomethacin/Insulin (Indo/Ins) has been shown to markedly enhance leptin production [25], this system has not been modified to render it suitable for studies of leptin secretion or other factors secreted by adipocytes.
In this report we have developed a novel in vitro protocol suitable for studying leptin secretion. 3T3-L1 adipocytes differentiated with Ibmx/Dex/Ins plus a PPARγ agonist displayed not only an increased level of leptin gene expression but also a highly significant 2-fold induction of leptin secretion within 15 min of insulin stimulation. The effect of insulin on leptin exocytosis was blocked by Brefeldin A but not by the PI 3-kinase inhibitor wortmannin or the protein synthesis inhibitor cyclohexamide, suggesting that leptin is targeted to a regulatory secretory compartment in 3T3-L1 adipocytes, where its release is stimulated by insulin via a PI 3-kinase independent mechanism.
Materials and methods
Ligands and chemicals
Antibodies against phospho-Akt (Ser473), and Akt were from Cell Signaling Technology (Beverly, MA). PI 3-kinase inhibitor wortmannin was purchased from Calbiochem (San Diego, CA). All other chemicals were purchased from Sigma.
Cell culture
3T3-L1 fibroblast were obtained from the American Type Culture Collection (ATCC, Bethesda, MD) and were cultured in Dulbecco's Eagle's medium (DMEM) with 10% fetal bovine serum (Atlanta Biologicals, Inc.), and 1500 mg/l sodium bicarbonate at 37 °C in 5% CO2/air. For differentiation cells were grown to confluence and carried for 2 to 4 days without fresh medium. At this point, referred to as day 0, 3T3-L1 cells were differentiated by incubation in culture medium with 1 μg/ml insulin, 500 μM methyl-isobutyl-xanthine, 250 nM dexamethasone, with and without 1 μM of a PPARγ agonist, a synthetic thiazolidinedione (Tzd), Troglitazone, a compound. Cells were fed 72 h later (day 3) with culture medium plus 1 μg/ml insulin and then every 48 h with culture medium. For the insulin/indomethacin method confluent and starved 3T3-L1 fibroblasts were differentiated with 5 μg/ml insulin, and 125 μM indomethacin (day 0) and fed 72 h later with culture medium plus 5 μg/ml insulin. 3T3-L1 adipocytes are kept in culture medium plus insulin until day 7 and then fed every 48 h in regular medium without insulin. Experiments were performed on day 14 and 15 of differentiation.
Cell lysates and immunoblots
For immunoblots, cells were washed with 150 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 5 mM KCl, 1 mM MgCl2, pH 7.2 and lysed in Laemmli buffer. Cells were harvested by scraping, and the lysates were sheared through a Q26G5/8 syringe. Proteins were resolved in SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies by using protocols provided by the suppliers.
RT-PCR
Total RNA was isolated from 3T3-L1 adipocytes by TRIZOL reagent (Invitrogen). Quantitative RT-PCR was performed by the Taqman system (Applied Biosystems) according to the manufacturer's instructions. Oligos were designed by the PrimerExpress software. Threshold cycles (Ct) for the internal control (cyclophilin) and target genes were determined from raw fluorescence. The amount of each gene was derived from its Ct and normalized with the amount of cyclophilin. Samples are in duplicate.
Sequences of the Taqman primers and probes are the following:
cyclophilin-F, 5′-TGTGCCAGGGTGGTGACTT; cyclophilin-R, 5′-TCAAATTTCTCTCCGTAGATGGACTT; cyclophilin-probe, 5′-ACACGCCATAATGGCACTGGTGG; leptin-F, 5′-TCCCTGCCTCAGACCAGTG; leptin-R, 5′-TAGAGTGAGGCTTCCAGGACG; leptin-probe, 5′-CATCCAGGCTCTCTGGCTTCTGCAG.
Leptin secretion assay
3T3-L1 adipocytes were cultured in 6-well plates until day 14 of differentiation at 37 °C in 5% CO2/air. Culture medium was changed to minimal amounts (0.5 ml) for 15 h (over night) in order to allow leptin accumulation to occur. Leptin secretion was measured in basal and insulin-stimulated states (170 nM) for various time points. Time points of 0 (over night), 15, and 120 min were chosen to focus on the earlier stages of leptin release. For studies involving the characterization of the transcriptional and secretional regulatory mechanisms mediating leptin synthesis and secretion, cells were additionally pre-incubated for 30 min either with or without 5 μg/ml Brefeldin, 10 μg/ml Cyclohexamide, or 100 nM or 1 μM wortmannin and then incubated for 15 min or 2 h with 170 nM insulin in the presence or absence of the inhibitors. At the end of the incubation period, the medium was collected and frozen at −80 °C for subsequent determination of leptin concentration. Three wells of a 6-well plate were collected per time point or condition to account for inter-well variations. Medium samples were concentrated using a Millipore YM-10 centrifugal filter and assayed for leptin levels using a mouse leptin immunoassay kit (R&D Systems, Minneapolis, MN) according to the manufacture's instructions. Cells were solubilized in PBS, pH 7.2 containing protease inhibitors (protease inhibitor cocktail complete, Roche Diagnostics, Mannheim, Germany) with 1% Triton-X-100 in order to measure total protein content. The amount of leptin released into the medium at each time point was normalized to total protein content using Biorad procedures [26]. Leptin values were expressed as pg/ml/mg protein over stated time periods.
Hexose transport
Hexose transport activity in 3T3-L1 adipocytes was measured by the uptake of [3H]2-deoxy-glucose as described previously [26].
Results
The effect of different differentiation methods on leptin production, secretion, and fat accumulation
In order to study leptin secretion from 3T3-L1 adipocytes, it was necessary to develop culture conditions in which leptin production was increased above the constitutively low levels typically expressed by these cells. We thus evaluated the effect of three differentiation methods on fat accumulation, leptin production, and secretion in 3T3-L1 adipocytes: 1) standard cocktail (Ibmx/Dex/Ins), 2) standard cocktail (Ibmx/Dex/Ins) plus PPARγ agonists (Tzds), and 3) Indomethacin and Insulin (Indo/Ins).
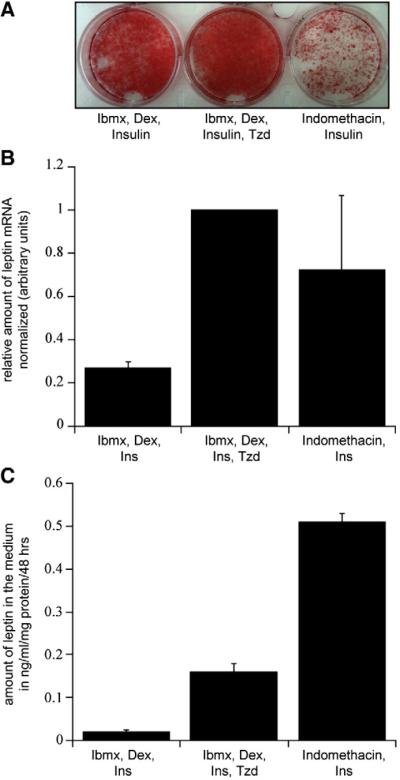
Production of lipid droplets is generally used as an indication of adipocyte differentiation. Therefore we compared the amount of lipid accumulation in 3T3-L1 adipocytes using the same three sets of conditions. The Ibmx/Dex/Ins plus Tzd method produced a near 100% efficiency of lipid droplet production in day 14 adipocytes (Fig. 1A, middle panel). When the PPARγ agonist was omitted, a less impressive lipid droplet production was evident (left panel). In contrast, differentiation with the Indo/Ins cocktail yielded a less profound amount of adipocyte differentiation with only partial formation of lipid droplets (Fig. 1A, right panel).
Fig. 1.
Effects of different differentiation methods on fat accumulation, leptin expression, and secretion in day 14 3T3-L1 adipocytes. (A) Oil red O staining in fully differentiated adipocytes. (B) Leptin mRNA levels, relative to cyclophilin A mRNA levels were determined using quantitative real-time PCR. Each bar is the mean±SE of 5 independent experiments normalized to the differentiation with Ibmx, Dex, Ins, and Tzd. (C) Representative experiment of leptin accumulation into the medium over 48 h normalized to total protein. Data of three wells of a 6-well culture dish were combined for each condition. The experiment was repeated over 8 times all with equivalent data.
Adipocytes differentiated with the standard cocktail produced low amounts of leptin mRNA and failed to show detectable secretion of leptin, consistent with previous reports (Figs. 1B and C) [24]. Interestingly, adipocytes differentiated with Ibmx/Dex/Ins plus Tzd or Indo/Ins exhibited a 4 to 5 fold increase in leptin mRNA levels (Fig. 1B). While both methods yielded similar amounts of leptin transcript, the Indo/Ins method also increased basal leptin release in the medium over a 48-hour period in adipocytes 14 days post-differentiation (Fig. 1C). Although differentiation with Ibmx/Dex/Ins plus Tzd increased leptin mRNA expression to the same level as the Indo/Ins method, it only induced one-third of the increase in basal leptin release in the medium over the same 48-hour time window.
Thus while the Indo/Ins cocktail could induce an increase in leptin production and its constitutive basal release into the medium, it only led to an incomplete differentiation of 3T3-L1 adipocytes. Based on this we decided to use the protocol with the standard Ibmx/Dex/Ins plus Tzd method to investigate leptin secretion from adipocytes.
Insulin-stimulated leptin secretion in cells differentiated by Ibmx/Dex/Ins plus Tzd method is independent of protein synthesis and involves a secretion based mechanism
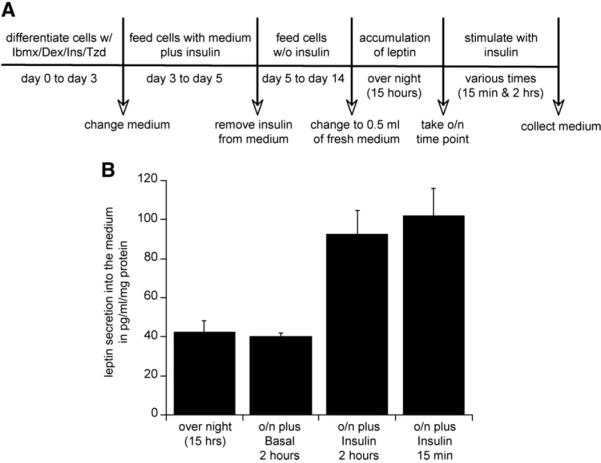
We employed a short-term secretion assay to study the effects of acute insulin stimulation on leptin secretion in cells differentiated with Ibmx/Dex/Ins and Tzd. To ensure that the leptin measurements were within the detection limits of the ELISA assay, an experimental design was used as described in Fig. 2A. Fully differentiated 3T3-L1 adipocytes (day14) were incubated in 0.5 ml medium over night in order to allow leptin to accumulate. After a 15-hour incubation leptin concentration in the medium was measured to establish a baseline for leptin release. The application of insulin (170 nM) to the medium induced a 2-fold increase in leptin secretion over a 2-hour period relative to the accumulation for the preceding 15 h (Fig. 2B). There was no statistically significant change of leptin release over these 2 h in the absence of insulin confirming the previous observation that basal secretion of leptin under these conditions is low. Therefore, the observed 2-fold induction of leptin secretion by insulin, is in reality much larger but impossible to measure directly. In these cells, treatment with insulin for only 15 min induced the release of the same pool of intracellular leptin as within 2 h, indicating a rapid mechanism of secretion (Fig. 2B).
Fig. 2.
Acute insulin-induced leptin secretion from adipocytes differentiated with Ibmx/Dex/Ins plus PPARγ agonist. (A) Scheme of a designed secretion assay with short-term insulin stimulation (170 nM). 3T3-L1 fibroblasts were differentiated with Ibmx/Dex/Ins plus Tzd cocktail and cultured until fully differentiated (day 14). On the night before the experiment medium was changed to minimal amounts (0.5 ml) in order to allow leptin to accumulate for 15 h overnight. In the morning, an overnight point was taken as a starting concentration for leptin secretion and cells were stimulated with 170 nM insulin for 2 h. Medium was collected from 3 wells of a 6-well plate, concentrated using Millipore filter units and leptin content was assayed using a Quantikine Elisa kit. Accumulated leptin was normalized to total amount of protein from each well. (B) Representative experiment of insulin-induced leptin secretion over 15 min and 2 h from adipocytes. The experiment was repeated over 10 times all with equivalent data.
To determine whether the insulin-stimulated leptin secretion in 3T3-L1 adipocytes is independent of de novo protein synthesis, as would be expected if there was regulated secretion of leptin, the protein synthesis inhibitor cyclohexamide was applied. Cyclohexamide (CHX) (10 μg/ml) had no effect on basal or insulin-induced secretion of leptin over 2 h, suggesting that insulin can recruit a pool of intracellular leptin independent of de novo protein synthesis (Fig. 3A).
Fig. 3.
Leptin secretion from 3T3-L1 adipocytes is independent of protein synthesis and requires trafficking through the Golgi apparatus. (A) Effect of cyclohexamide (CHX) on insulin-induced leptin secretion over 2 h. Cells were pretreated with 10 μg/ml cyclohexamide for 30 min before stimulated with 170 nM insulin for 2 h. Each bar is a mean± SE of 3 independent experiments normalized to the insulin values. (B) Effect of Brefeldin A (BFA) on short-term insulin-regulated leptin secretion. Cells were treated with 5 μg/ml Brefeldin A for 30 min before 15-min insulin stimulation. Each bar is a mean ± SE of 6 independent experiments normalized to the insulin-stimulated state. *p = 0.001.
We next investigated whether trafficking through the Golgi complex was necessary for insulin-induced leptin secretion. Brefeldin A blocks transport through the Golgi by inhibiting the nucleotide exchange factor for the small GTPase ADP-ribosylation factor 1 (ARF1), which acts to recruit coat proteins (COPI) involved in intra-Golgi transport as well as trafficking between Golgi and the endoplasmic reticulum (ER) [27–30]. By blocking the function of ARF1, all vesiculation at the Golgi stops, leading to a collapse of the Golgi into the ER. The insulin-stimulated secretion of leptin that is evident after 15 min of insulin treatment in 3T3-L1 adipocytes was completely blocked by Brefeldin A (5 μg/ml) (Fig. 3B). These data show that intracellular trafficking through the Golgi is necessary for acute insulin-stimulated regulation of leptin secretion and thereby suggest that leptin is not recruited from a post-Golgi storage compartment.
Insulin stimulation of leptin secretion is independent of glucose metabolism and mediated by a PI 3-kinase independent mechanism
The data above do not address whether the insulin-induced secretion of leptin is a direct effect of insulin signal transduction on leptin vesicle trafficking or if the stimulation is a result of secondary changes in glucose metabolism. To address this question, we cultured 3T3-L1 adipocytes in glucose-free medium for 2 h and measured insulin-stimulated leptin secretion. As shown in Fig. 4A, insulin induced a two-fold induction in leptin secretion in glucose-free medium, similar to the effect of insulin on leptin secretion in medium with 25 mM glucose. These data indicate that the stimulation of insulin-regulated leptin secretion is not mediated through changes in glucose metabolism but is rather dependent on insulin per se.
Fig. 4.
Insulin-induced leptin secretion from 3T3-L1 adipocytes is independent of the PI 3-kinase signaling pathway and of glucose metabolism. (A) 3T3-L1 cells were cultured and differentiated with Ibmx/Dex/Ins plus Tzd as described in Fig. 2 until day 15, at which stage the medium was changed to either standard DMEM containing 4.5 g/l glucose or glucose-free DMEM both supplemented with 10% FBS. After 2 h with or without 170 nM insulin the medium was collected, concentrated and assayed for secreted leptin. Data of three wells of a 6-well culture dish were combined for each condition. The experiment was repeated 4 times all with equivalent data. (B) Wortmannin (WT) does not block insulin-induced secretion of leptin over 2 h. Cells were treated with 100 nM or 1 μM wortmannin for 30 min prior to 2-hour insulin stimulation. Each bar is a mean±SE of 5 independent experiments normalized to the insulin-stimulated state values. (C) 3T3-L1 adipocytes were treated with or without 100 nM or 1 μM wortmannin for 30 min before insulin stimulation for 2 h. Cell lysates were analyzed in immunoblots by using noted antibodies.
Glucose uptake into adipocytes is mediated by the PI 3-kinase signaling pathway. We next examined the effect of a PI 3-kinase inhibitor wortmannin on insulin-stimulated secretion of leptin. Cells were pretreated with wortmannin (100 nM) for 30 min before 2-hour insulin stimulation. Wortmannin did not block insulin-stimulated leptin secretion (Fig. 4B), even though it potently inhibited insulin-induced activation of AKT as shown by its inhibition of AKT phosphorylation (Fig. 4C). At even higher concentration, wortmannin (1 μM) had no effect on insulin-stimulated leptin secretion further supporting a PI 3-kinase independent mechanism.
Discussion
Here, we present the development of an in vitro protocol and validated it for the study of insulin-regulated leptin secretion in 3T3-L1 adipocytes. Differentiating cells with Ibmx/Dex/Ins plus Tzd increases leptin gene expression and leads to the formation of an intracellular storage pool of leptin, which can be released upon acute insulin stimulation. These results clearly indicate that leptin can be released from adipocytes via a regulated pathway for protein secretion and also provide a potential means for studying secretion of other adipokines. Using this method we also showed that the effect of insulin on stimulating leptin secretion is independent of insulin-induced changes in glucose metabolism and on insulin-mediated PI 3-kinase activation.
The differentiation of 3T3-L1 adipocytes with the standard Ibmx/Dex/Ins cocktail with or without Tzds is a well-established in vitro cellular system for studying adipogenesis, fatty acid metabolism, and insulin-regulated trafficking [31–35]. In this report, we further show that this system can be adapted to study insulin-induced leptin secretion. Most of the previous studies have focused on transcriptional and translational regulation of leptin production, but did not evaluate if leptin can be acutely regulated, e.g. if there is a storage pool that can be released upon stimulation [6,7,10,36,37]. Such analyses would be important as it has become evident that circulating leptin levels change acutely in response to starvation and re-feeding in rats and humans [38–40]. These latter results indicate that the molecular mechanisms that regulate leptin release within minutes are likely to be of physiological significance.
The use of PPARγ agonist with the standard Ibmx/Dex/Ins method leads to an increase in leptin gene expression while forming an intracellular pool of leptin that can be released upon insulin stimulation (Fig. 1). Other reports observed a negative effect of PPARγ agonists on leptin production in 3T3-L1 adipocytes only when adding the Tzds for 24 h to already fully differentiated day 16 adipocytes [41]. Here we include the Tzd in the differentiation cocktail; a method, which further enhances adipogenesis and insulin sensitivity as shown in studies of adipocyte differentiation and insulin-regulated GLUT4 trafficking (unpublished data) [42,43].
In contrast, 3T3-L1 adipocytes differentiated by the Indo/Ins method seem to fail to form this intracellular pool of leptin, but rather secrete leptin continuously. We currently do not know the reason for this difference; if this is because those cells do not have the retention pathway or because they do not make enough leptin. However, our study focuses on understanding acute leptin secretion rather than different differentiation procedures, therefore future studies will be required to characterize the leptin-containing compartment in 3T3-L1 adipocytes on a molecular level.
We further show that the intracellular pool of leptin in 3T3-L1 adipocytes released by insulin is independent of protein synthesis, confirming that this is a post-transcriptional mechanism (Fig. 3A). These data are consistent with findings from some [13–15,17,44], but not other studies of primary cultured adipocytes from rats and humans [18,19]. Although our data have been obtained using inhibitors rather than by measuring leptin biosynthesis directly, the results clearly demonstrate that the intracellular stores of leptin can be recruited upon insulin stimulation independently of protein synthesis. This is further supported by the observation that insulin stimulates leptin release within 15 min, making the involvement of protein synthesis in regulated leptin secretion unlikely.
As most of insulin's effects are mediated through a PI 3-kinase signaling pathway, we investigated the effect of a PI 3-kinase inhibitor wortmannin on leptin exocytosis (Fig. 4B). Surprisingly wortmannin did not block basal or insulin-regulated leptin release, although it successfully inhibited the phosphorylation of AKT, a downstream substrate of PI 3-kinase. In contrast, Bradley and Cheatham observed a reduction of insulin-induced leptin secretion by inhibitors of the PI 3-kinase pathway (LY294002), the MEK pathway (PD98059) as well as with the immunosuppressant rapamycin, suggesting a complex system that mediates insulin-regulated leptin secretion [12]. The basis for this discrepancy is unclear, because using higher amounts of wortmannin (1 μM), an amount which has been shown to completely block PI 3-kinase signaling, had no effect on insulin-induced leptin secretion in our system, further supporting a PI 3-kinase independent mechanism at least in 3T3-L1 adipocytes.
PI 3-kinase independent signaling by insulin has been previously shown in other settings including the insulin-stimulated recruitment of the glucose transporter GLUT4, to the plasma membrane of adipocytes. In some cases, this PI 3-kinase independent signaling pathway involves recruitment of a CAP/Cbl complex to the insulin receptor and subsequently activation of a small Rho GTPase called TC10 [45,46]. TC10 has been shown to be involved in insulin-induced actin remodeling near the plasma membrane and to interact with one of the components of the exocyst complex, Exo70, in a GTP-dependent fashion [47]. Further studies will be required to determine whether this pathway provides a link between insulin signaling and the induction of leptin secretion.
Adipocytes secrete other adipokines in addition to leptin such as adiponectin [20,48–50]. Since leptin and adiponectin exhibit different roles in metabolism, and their expression is inversely correlated, it will be important to establish whether adiponectin is also secreted by a regulated pathway. If so, it would suggest that distinct trafficking pathways for protein secretion function in adipocytes and could provide different ways of regulation for the release of these adipokines. In the studies performed thus far, adiponectin appears to be constitutively secreted in the basal state from 3T3-L1 adipocytes with only a small fraction being stimulated by insulin [20]. In this case, in contrast to leptin, the effect of insulin was mediated by a PI 3-kinase dependent mechanism, where wortmannin and LY294002 blocked insulin-stimulated adiponectin secretion from 3T3-L1 adipocytes [20]. This observation adds further evidence in support of the possibility that there are different cellular mechanisms of vesicle trafficking in regulating leptin and adiponectin secretion.
Although leptin has been mainly localized to the ER by immunofluorescent studies [13], it is also distributed through the Golgi and in small vesicles near the plasma membrane in white rat adipocytes [19,23,51]. The acute effect of insulin within 15 min on leptin exocytosis is completely blocked by Brefeldin A (Fig. 3B), indicating that at least trafficking through the Trans Golgi Network (TGN)/Golgi is required for full response. This is in agreement with an earlier study where insulin-induced leptin release was also inhibited by disruption of the Golgi complex in isolated rat adipocytes [52]. The TGN has been shown to secrete and sort proteins for different destinations within the cell and to participate in trafficking loops for certain secretory proteins [53–56]. It is yet unclear whether the insulin-regulated rapidly releasable pool of intracellular leptin is recruited directly from the TGN or if more medial-Golgi compartments participate in this response. Some cellular fractionation studies revealed an increase of leptin with insulin stimulation in the high-density membrane fractions [52], however contamination of isolated fractions by other membranes greatly reduces the specificity of this assay.
Taken together our data show for the first time that 3T3-L1 adipocytes possess an insulin-responsive secretory pathway that regulates leptin release and from where leptin is rapidly recruited for secretion. The induction of leptin secretion is mediated by insulin itself, as no involvement of glucose metabolism has been observed. This in vitro system recapitulates findings from isolated primary cells from rats and humans and presents a useful system for studying adipocyte secretion in molecular detail. Future studies using this cell culture system described here will provide new insights into understanding the connection between insulin signal transduction and leptin secretion, as shown by the independence of insulin-stimulated leptin exocytosis on the PI 3-kinase signaling pathway.
Acknowledgments
We thank members of the Friedman and McGraw laboratories for helpful discussions and suggestions, and Sam Cushman for useful comments on the manuscript. We thank Susan Korres for help with preparing the manuscript. This work was supported by National Institutes of Health Grant DK52852 (T.E.M.) and DK041096 (J.M.F.).
REFERENCES
- [1].Bado A. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- [2].Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, Myint M, Caro JF. Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes. 1996;45(11):1511–1515. doi: 10.2337/diab.45.11.1511. [DOI] [PubMed] [Google Scholar]
- [3].Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J. Clin. Endocrinol. Metabol. 1996;81(9):3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- [4].Attoub S, Levasseur S, Buyse M, Goiot H, Laigneau J, Moizo L, Hervatin F, Le Marchand-Brustel Y, Lewin J, Bado A. Physiological role of cholecystokinin B/gastrin receptor in leptin secretion. Endocrinology. 1999;140:4406–4410. doi: 10.1210/endo.140.10.7079. [DOI] [PubMed] [Google Scholar]
- [5].Saad M, Khan A, Sharma A, Michael R, Riad-Gabriel M, Boyadjian R, Jinagouda S, Steil G, Kamdar V. Physiological insulinemia acutely modulates plasma leptin. Diabetes. 1998;47:544–549. doi: 10.2337/diabetes.47.4.544. [DOI] [PubMed] [Google Scholar]
- [6].Leroy P, Dessolin S, Villageois P, Moon BC, Friedman JM, Ailhaud G, Dani C. Expression of ob gene in adipose cells. J. Biol. Chem. 1996;27(5):2365–2368. doi: 10.1074/jbc.271.5.2365. [DOI] [PubMed] [Google Scholar]
- [7].MacDougald OA, Hwang CS, Fan H, Lane MD. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. 1995;92(20):9034–9037. doi: 10.1073/pnas.92.20.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moreno-Aliaga M, Stanhope K, Havel P. Transcriptional regulation of the leptin promoter by insulin-stimulated glucose metabolism in 3t3-l1 adipocytes. Biochem. Biophys. Res. Commun. 2001;283:544–548. doi: 10.1006/bbrc.2001.4822. [DOI] [PubMed] [Google Scholar]
- [9].Zhang Y, Proenca P, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- [10].Lee M, Yang R, Gong D, Fried S. Feeding and insulin increase leptin translation. Importance of the leptin mRNA untranslated regions. J. Biol. Chem. 2007;282:72–80. doi: 10.1074/jbc.M609518200. [DOI] [PubMed] [Google Scholar]
- [11].Russell C, Petersen R, Rao S, Ricci M, Prasad A, Zhang Y, Brolin R, Fried S. Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am. J. Physiol. 1998;275:E507–E515. doi: 10.1152/ajpendo.1998.275.3.E507. [DOI] [PubMed] [Google Scholar]
- [12].Bradley RL, Cheatham B. Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes. 1999;48:272–278. doi: 10.2337/diabetes.48.2.272. [DOI] [PubMed] [Google Scholar]
- [13].Barr VA, Malide D, Zarnowski MJ, Taylor SI, Cushman SW. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138(10):4463–4472. doi: 10.1210/endo.138.10.5451. [DOI] [PubMed] [Google Scholar]
- [14].Roh C, Han J, Tzatsos A, Kandror K. Nutrient-sensing mTOR-mediated pathway regulates leptin production in isolated rat adipocytes. Am. J. Physiol. Endocrinol. Metab. 2003;284:E322–E330. doi: 10.1152/ajpendo.00230.2002. [DOI] [PubMed] [Google Scholar]
- [15].Lee M, Fried S. Multilevel regulation of leptin storage, turnover, and secretion by feeding and insulin in rat adipose tissue. J. Lipid. Res. 2006;47:1984–1993. doi: 10.1194/jlr.M600065-JLR200. [DOI] [PubMed] [Google Scholar]
- [16].Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137(9):4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- [17].Lee M, Wang Y, Ricci M, Sullivan S, Russell C, Fried S. Acute and chronic regulation of leptin synthesis, storage, and secretion by insulin and dexamethasone in human adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2007;292:E858–E864. doi: 10.1152/ajpendo.00439.2006. [DOI] [PubMed] [Google Scholar]
- [18].Levy JR, Stevens W. The effects of insulin, glucose, and pyruvate on the kinetics of leptin secretion. Endocrinology. 2001;142:3558–3562. doi: 10.1210/endo.142.8.8313. [DOI] [PubMed] [Google Scholar]
- [19].Cammisotto P, Bukowiecki L, Deshaies Y, Bendayan M. Leptin biosynthetic pathway in white adipocytes. Biochem. Cell. Biol. 2006;84:207–214. doi: 10.1139/o06-032. [DOI] [PubMed] [Google Scholar]
- [20].Bogan J, Lodish H. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J. Cell Biol. 1999;146:609–620. doi: 10.1083/jcb.146.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhong Q, Lin C, Clarke K, Kemppainen R, Schwartz D, Judd R. Endothelin-1 inhibits resistin secretion in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2002;296:383–387. doi: 10.1016/s0006-291x(02)00882-3. [DOI] [PubMed] [Google Scholar]
- [22].Guerre-Millo M. Adipose tissue hormones. J. Endocrinol. Investig. 2002;25:855–861. doi: 10.1007/BF03344048. [DOI] [PubMed] [Google Scholar]
- [23].Bornstein S, Abu-Asab M, Glasow A, Path G, Hauner H, Tsokos M, Chrousos G, Scherbaum W. Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture. Diabetes. 2000;49:532–538. doi: 10.2337/diabetes.49.4.532. [DOI] [PubMed] [Google Scholar]
- [24].Norman D, Isidori A, Frajese V, Caprio M, Chew S, Grossman A, Clark A, Michael Besser G, Fabbri A. ACTH and alpha-MSH inhibit leptin expression and secretion in 3T3-L1 adipocytes: model for a central-peripheral melanocortin-leptin pathway. Mol. Cell. Endocrinol. 2003;200:99–109. doi: 10.1016/s0303-7207(02)00410-0. [DOI] [PubMed] [Google Scholar]
- [25].Slieker LJ, Sloop KW, Surface PL. Differentiation method-dependent expression of leptin in adipocyte cell lines. Biochem. Biophys. Res. Commun. 1998;251:225–229. doi: 10.1006/bbrc.1998.9433. [DOI] [PubMed] [Google Scholar]
- [26].Gibbs E, Lienhard G, Gould G. Insulin-induced translocation of glucose transporters to the plasma membrane precedes full stimulation of hexose transport. Biochemistry. 1988;27:6681–6685. doi: 10.1021/bi00418a006. [DOI] [PubMed] [Google Scholar]
- [27].Ward T, Polishchuk R, Caplan S, Hirschberg K, Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 2001;155:557–570. doi: 10.1083/jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pepperkok R, Whitney J, Gomez M, Kreis T. COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo. J. Cell Sci. 2000;113:135–144. doi: 10.1242/jcs.113.1.135. [DOI] [PubMed] [Google Scholar]
- [29].Ooi C, Dell'Angelica E, Bonifacino J. ADP-ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J. Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Styers M, Kowalczyk A, Faundez V. Architecture of the vimentin cytoskeleton is modified by perturbation of the GTPase ARF1. J. Cell Sci. 2006;119:3643–3654. doi: 10.1242/jcs.03147. [DOI] [PubMed] [Google Scholar]
- [31].Cheung K, Tzameli I, Pissios P, Rovira I, Gavrilova O, Ohtsubo T, Chen Z, Finkel T, Flier J, Friedman J. Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metab. 2007;5:115–128. doi: 10.1016/j.cmet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- [32].Tzameli I, Fang H, Ollero M, Shi H, Hamm J, Kievit P, Hollenberg AN, Flier J. Regulated production of a peroxisome proliferator-activated receptor-gamma ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J. Biol. Chem. 2004;279:36093–36102. doi: 10.1074/jbc.M405346200. [DOI] [PubMed] [Google Scholar]
- [33].Sun X, Zemel M. Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells. Lipids. 2007;42:297–305. doi: 10.1007/s11745-007-3029-5. [DOI] [PubMed] [Google Scholar]
- [34].Sano H, Equez L, Teruel M, Fukuda M, Chuang T, Chavez J, Lienhard G, McGraw T. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [35].Bose A, Guilherme A, Huang S, Hubbard A, Lane C, Soriano N, Czech M. The v-SNARE Vti1a regulates insulin-stimulated glucose transport and Acrp30 secretion in 3T3-L1 adipocytes. J. Biol. Chem. 2005;280:36946–36951. doi: 10.1074/jbc.M508317200. [DOI] [PubMed] [Google Scholar]
- [36].Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, Rascher W, Teller W, Tornqvist H, Hauner H. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45(10):1435–1438. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- [37].Rentsch J, Chiesi M. Regulation of ob gene mRNA levels in cultured adipocytes. FEBS Lett. 1996;379(1):55–59. doi: 10.1016/0014-5793(95)01485-3. [DOI] [PubMed] [Google Scholar]
- [38].Licinio J, Mantzoros C, Negrao AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat. Med. 1997;3(5):575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- [39].Walker C, Bryson J, Bell-Anderson K, Hancock D, Denyer G, Caterson I. Insulin determines leptin responses during a glucose challenge in fed and fasted rats. Int. J. Obes. (Lond) 2005;29:398–405. doi: 10.1038/sj.ijo.0802884. [DOI] [PubMed] [Google Scholar]
- [40].Yildiz B, Suchard M, Wong M, McCann S, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc. Natl. Acad. Sci. 2004;101:10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kallen C, Lazar M. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. 1996;93:5793–5796. doi: 10.1073/pnas.93.12.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, Vaysse P, Durkin MM, Laz TM, Linemeyer DL, Schaffhauser AO, Whitebread S, Hofbauer KG, Taber RI, Branchek TA, Weinshank RL. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- [43].Zeigerer A, Lampson M, Karylowski O, Sabatini D, Adesnik M, Ren M, McGraw T. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell. 2002;13:2421–2435. doi: 10.1091/mbc.E02-02-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fried SH, Bernard R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- [45].Baumann C, Ribon V, Kanzaki M, Thurmond D, Mora S, Shigematsu S, Bickel P, Pessin J, Saltiel A. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- [46].Chiang S, Baumann C, Kanzaki M, Thurmond D, Watson R, Neudauer C, Macara I, Pessin J, Saltiel A. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature. 2001;410:944–948. doi: 10.1038/35073608. [DOI] [PubMed] [Google Scholar]
- [47].Inoue M, Chang L, Hwang J, Chiang S, Saltiel A. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- [48].Pereira R, Draznin B. Inhibition of the phosphatidylinositol 3'-kinase signaling pathway leads to decreased insulin-stimulated adiponectin secretion from3T3-L1 adipocytes. Metabolism. 2005;54:1636–1643. doi: 10.1016/j.metabol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- [49].Clarke M, Ewart M, Santy L, Prekeris R, Gould G. ACRP30 is secreted from 3T3-L1 adipocytes via a Rab11-dependent pathway. Biochem. Biophys. Res. Commun. 2006;342:1361–1367. doi: 10.1016/j.bbrc.2006.02.102. [DOI] [PubMed] [Google Scholar]
- [50].Xie L, O'Reilly C, Chapes S, Mora S. Adiponectin and leptin are secreted through distinct trafficking pathways in adipocytes. Biochm. Biophys. Acta. 2008;1782:99–108. doi: 10.1016/j.bbadis.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Roh C, Roduit R, Thorens B, Fried S, Kandror K. Lipoprotein lipase and leptin are accumulated in different secretory compartments in rat adipocytes. J. Biol. Chem. 2001;276:35990–35994. doi: 10.1074/jbc.M102791200. [DOI] [PubMed] [Google Scholar]
- [52].Bradley R, Cleveland K, Cheatham B. The adipocyte as a secretory organ: mechanisms of vesicle transport and secretory pathways. Recent Prog. Horm. Res. 2001;56:329–358. doi: 10.1210/rp.56.1.329. [DOI] [PubMed] [Google Scholar]
- [53].Mardones G, Burgos P, Brooks D, Parkinson-Lawrence E, Mattera R, Bonifacino J. The TGN accessory protein p56 promotes long-range movement of GG A/Clathrin-containing transport carriers and lysosomal enzymes sorting. Mol. Biol. Cell. 2007;18(9):3486–3501. doi: 10.1091/mbc.E07-02-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tortorella L, Schapiro F, Maxfield F. Role of an acidic cluster/dileucine motif in cation-independent mannose 6-phosphate receptor traffic. Traffic. 2007;8:402–413. doi: 10.1111/j.1600-0854.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- [55].Scott G, Fei H, Thomas L, Medigeshi G, Thomas G. A PACS-1, GGA3 and CK2 complex regulates CI-MPR trafficking. EMBO J. 2006;25:4423–4435. doi: 10.1038/sj.emboj.7601336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hua W, Sheff D, Toomre D, Mellman I. Vectorial insertion of apical and basolateral membrane proteins in polarized epithelial cells revealed by quantitative 3D live cell imaging. J. Cell Biol. 2006;27:1035–1044. doi: 10.1083/jcb.200512012. [DOI] [PMC free article] [PubMed] [Google Scholar]