Abstract
In the central nervous system (CNS) insulin mediates a variety of effects including feeding, metabolism and cognition. The cognitive enhancing effects of insulin are proposed to be mediated through activation of insulin receptors in the hippocampus, an important integration center for learning and memory in the mammalian brain. Since less is known regarding insulin signaling events in the hippocampus, the aim of the current study was to determine whether insulin stimulates similar signaling cascades and GLUT4 translocation in the rat hippocampus as has been described in peripheral tissues. Intracerebroventricular administration of insulin increases hippocampal insulin levels and also stimulates the phosphorylation of Akt in a time-dependent manner. Insulin also stimulates the translocation of GLUT4 to hippocampal plasma membranes in a time course that mirrors the increases in glucose uptake observed during the performance of hippocampal-dependent tasks. Insulin stimulated phosphorylation of Akt and translocation of GLUT4 were blocked by pre-treatment with the PI3-kinase inhibitor LY294002. Confocal immunofluorescence determined that insulin stimulated phosphorylation of Akt was localized to neurons and colocalized with the insulin receptor and GLUT4 in the rat hippocampus, thereby identifying the functional anatomical substrates of insulin signaling in the hippocampus. These results demonstrate that insulin-stimulated translocation of GLUT4 to the plasma membrane in the rat hippocampus occurs via similar mechanisms as described in peripheral tissues and suggests that insulin mediated translocation of GLUT4 may provide a mechanism through which hippocampal neurons rapidly increase glucose utilization during increases in neuronal activity associated with hippocampal-dependent learning.
Keywords: glucose, Akt, cognition, diabetes, confocal microscopy
INTRODUCTION
The insulin receptor (IR) is a heterotetrameric protein consisting of two extracellular α subunits that provide the insulin-binding domain and the transmembrane-spanning β subunits (Jones and Clemmons, 1995). Insulin binding stimulates the tyrosine kinase activity of the β subunit, leading to the activation of intracellular signaling events. In peripheral tissues such as skeletal muscle, cardiac muscle and adipose cells, IR activation stimulates glucose uptake (Saltiel and Pessin, 2002). Cloning and characterization of an insulin-sensitive glucose transporter, GLUT4, helped to elucidate the mechanisms through which IR activation and signaling elicited increases in glucose uptake (Birnbaum, 1989; Charron et al., 1989). In particular, activation of the IR promotes the tyrosine phosphorylation of the IR substrate proteins, leading to the activation of phosphatidylinositol 3-kinase (PI3-K) and the generation of phosphatidylinositol (3,4,5)-triphosphate (PIP3) (Saltiel and Pessin, 2002). PIP3 activates phosphoinositide-dependent protein kinase (PDK), which phosphorylates and activates Akt (also known as protein kinase B). Additional studies determined that insulin stimulated translocation of GLUT4 and increases in glucose uptake are PI-3 kinase-dependent in peripheral tissues (Pessin et al., 1999).
The IR is also expressed in discrete neuronal populations in the CNS, including the hippocampus (Doré et al., 1997; Kar et al., 1993; Marks et al., 1991), an important integration center for learning and memory in the mammalian brain (McEwen and Sapolsky, 1995). Insulin improves cognitive performance and these cognitive enhancing properties of insulin may be mediated through IRs expressed in the hippocampus. The IR and the GLUT4 exhibit overlapping distributions in the rat brain, suggesting that GLUT4 trafficking may be mediated by similar mechanisms in the CNS as has been described previously for peripheral tissues. In support of this hypothesis, we have previously demonstrated that glucose mediated increases in plasma insulin levels stimulates GLUT4 translocation from the cytosol to the plasma membrane in the rat hippocampus (McEwen and Reagan, 2004; Piroli et al., 2007a). However, such analyses cannot differentiate between the potential effects of glucose and/or insulin or whether phosphorylation of Akt is an important intermediate in these trafficking events in the hippocampus, as it is in peripheral tissues. In order to more accurately address these questions, we examined the ability of icv insulin to stimulate GLUT4 trafficking to the plasma membrane and whether these signaling events were PI3-kinase dependent. In addition, we also examined the co-localization of the IR and GLUT4 with phospho-Akt (pAkt) immunoreactivity in the rat hippocampus following intracerebroventricular (icv) administration of insulin.
RESULTS
Time course of insulin mediated GLUT4 signaling events in the hippocampus
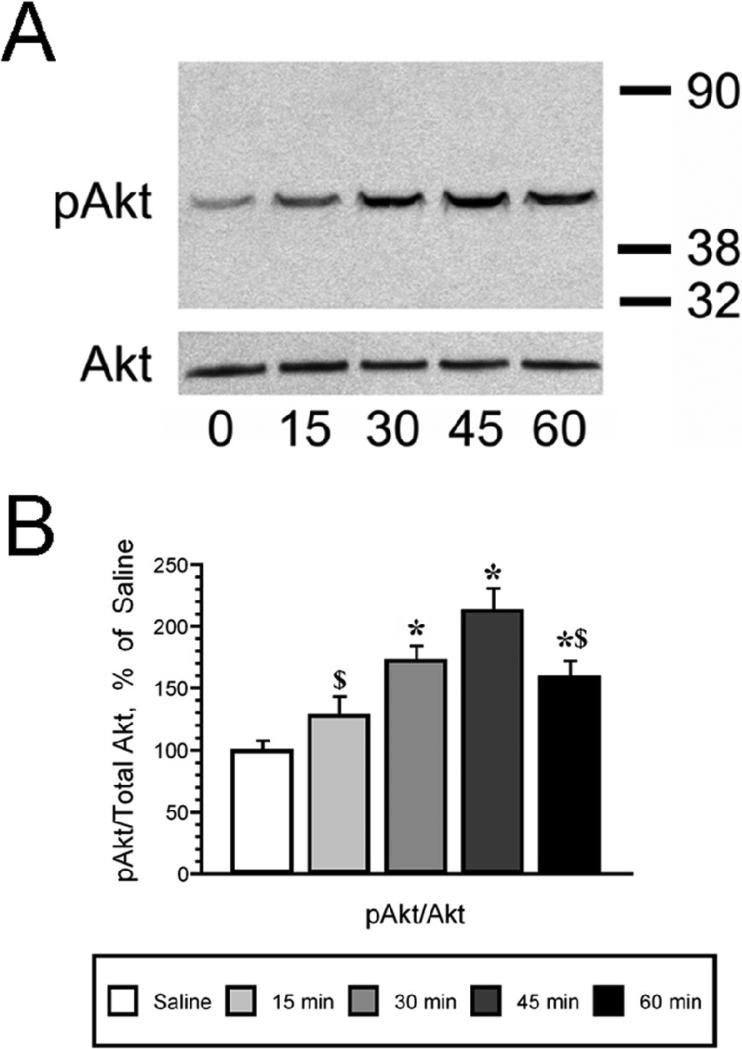
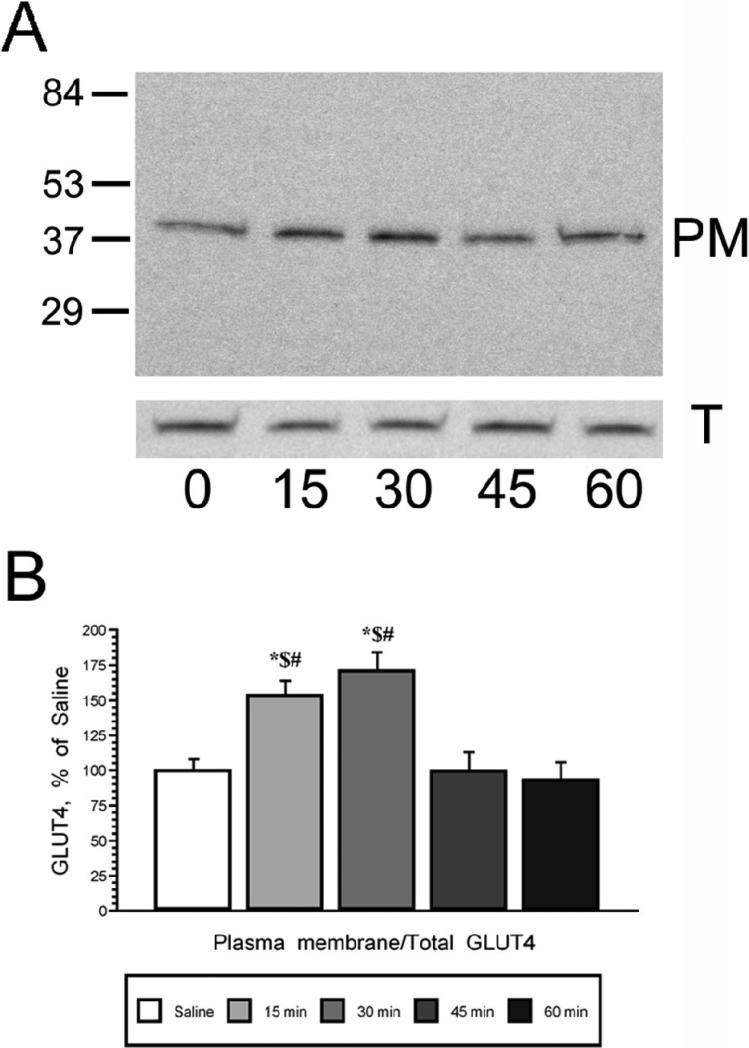
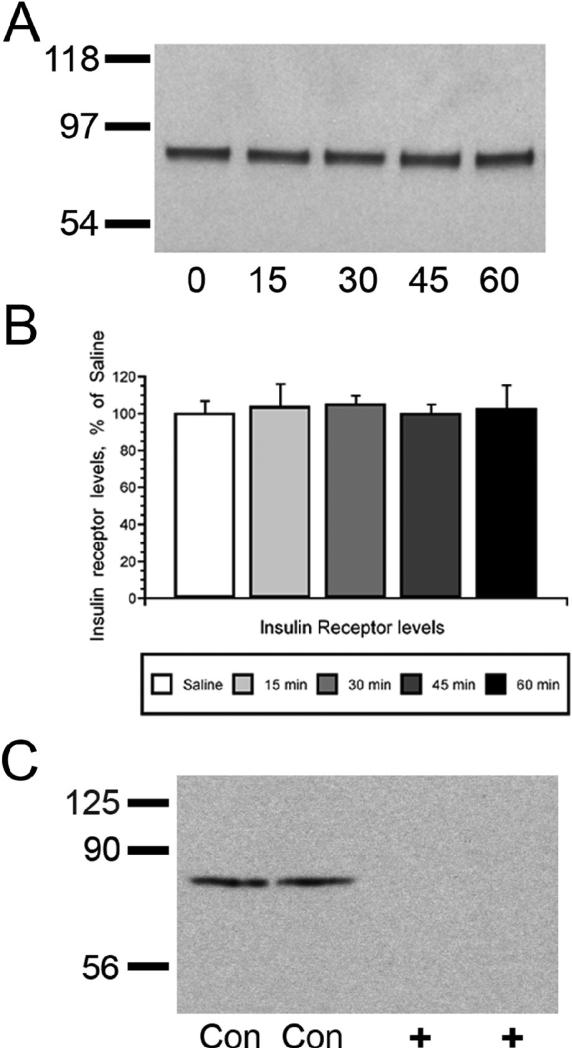
The time course of insulin mediated phosphorylation of Akt in the hippocampus was examined after icv insulin injection using Western blotting techniques. As shown in Figure 1, insulin exerted a long lasting elevation in pAkt levels, which started with a non-significant increase observed 15 minutes following icv insulin administration, followed by significant increases between 30 and 60 minutes post-icv insulin. Maximal increases in pAkt levels were observed 45 minutes following insulin administration (Figure 1, Panel A). No changes in total Akt levels were observed during the same period of time. Autoradiographic analysis revealed that the pAkt/total Akt ratio was significantly increased between 30 and 60 minutes after icv insulin injection, with peak increases in the phosphorylation of Akt observed 45 minutes following insulin administration (Figure 1, Panel B). Using plasma membrane preparations from the same animals, the time course of GLUT4 translocation to the plasma membrane was determined (Figure 2, Panel A). Insulin rapidly increases GLUT4 plasma membrane association in the rat hippocampus, with maximal increases observed at 15 and 30 minutes following insulin treatment; GLUT4 levels in the total membrane fraction did not change (Figure 2, Panel B). Autoradiographic analysis revealed that plasma membrane association of GLUT4 returns to basal levels at the 45 and 60 minute time points. These insulin-mediated changes in Akt phosphorylation and translocation of GLUT4 occurred in the absence of changes in the expression of the insulin receptor (Figure 3, Panels A and B). Specificity of the insulin receptor antisera was demonstrated by pre-absorbing the primary antisera with the peptide immunogen (Figure 3, panel C).
Figure 1.
Intracerebroventricular insulin administration increases phosphorylation of Akt (pAkt) in the rat hippocampus in a time-dependent manner. Panel A. Representative immunoblot sof time-dependent increases in pAkt levels in total membrane extracts of rat hippocampus (top panel); icv insulin did not modulate total Akt levels in rat hippocampus (bottom panel). The zero time point rats were injected with saline. Panel B. Autoradiographic image analysis revealed that insulin significantly increased pAkt levels 30 minutes following icv injection, increases that were also observed at the 45 and 60 minute time points. Molecular weight standards are shown on the right. Data in Panel B are expressed as the pAkt/total Akt ratio and are based upon at least 6 rats/group. * = p ≤ 0.005 compared to saline control rats (zero time point); $ = p ≤ 0.02 compared to 45 minute time point.
Figure 2.
Intracerebroventricular insulin administration increases plasma membrane association of GLUT4 in the rat hippocampus. Panel A. Representative immunoblots of GLUT4 immunoreactive bands in hippocampal plasma membrane fractions (PM; top panel) and total membrane fractions (T; bottom panel) 15, 30, 45 and 60 minutes following icv insulin treatment; zero time point rats were injected with saline. Panel B. Autoradiographic image analysis revealed that insulin increased plasma membrane association of GLUT4 in the rat hippocampus at the 15 and 30 minute post-insulin time points, increases that returned to baseline at the 45 minute time point. Molecular weight standards are shown on the left. Data in Panel B are expressed as the plasma membrane GLUT4/total membrane GLUT4 ratio and are based upon at least 6 rats/group. * = p ≤ 0.001 compared to saline control rats; $ = p ≤ 0.001 compared to 45 minute insulin group; # = p ≤ 0.001 compared to 60 minute insulin group.
Figure 3.
Insulin receptor expression is unaffected by intracerebroventricular insulin administration. Panel A. Representative immunoblot of insulin receptor expression in hippocampal plasma membrane fractions isolated from icv-treated rats. Panel B. Autoradiographic analysis of hippocampal plasma membrane fractions of icv-treated rats revealed that insulin receptor expression is not modulated by insulin administration. Panel B. Pre-absorption of primary antisera with the peptide immunogen eliminated the 90 kDa band detected in immunoblot analysis. Con lanes indicate control conditions (i.e. primary antisera in the absence of blocking peptide); + indicates primary antisera pre-absorbed with blocking peptide. Data are expressed as percentage of saline-treated rats. Molecular weight standards are shown on the left.
Insulin mediated translocation of GLUT4 is PI3-kinase dependent
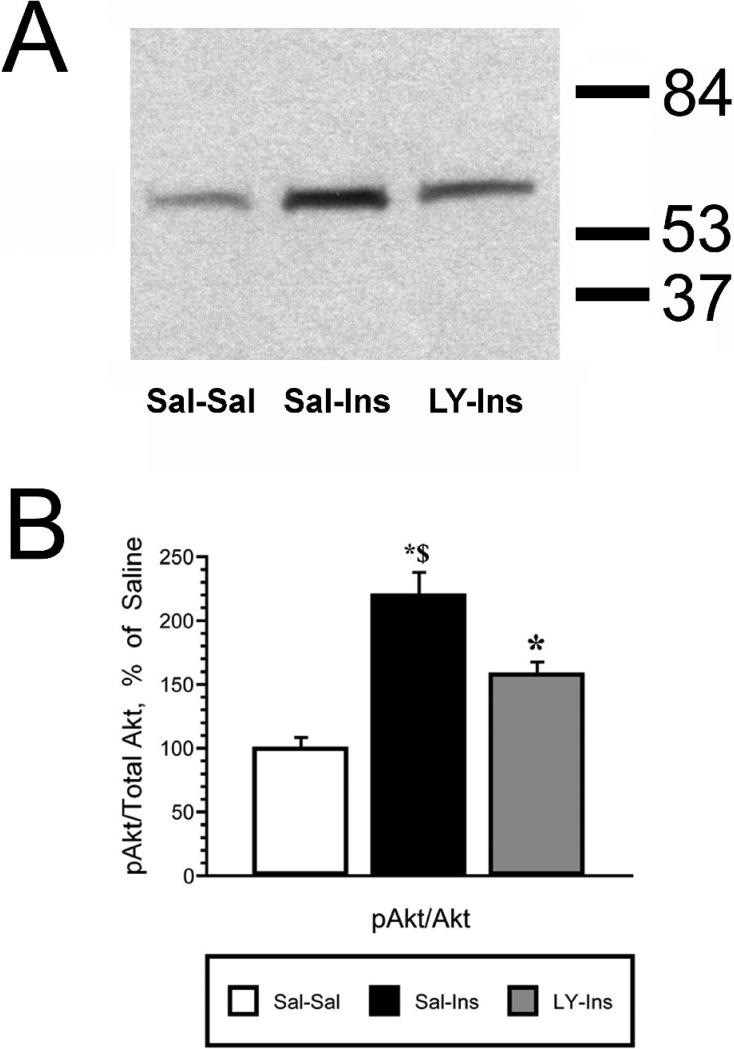
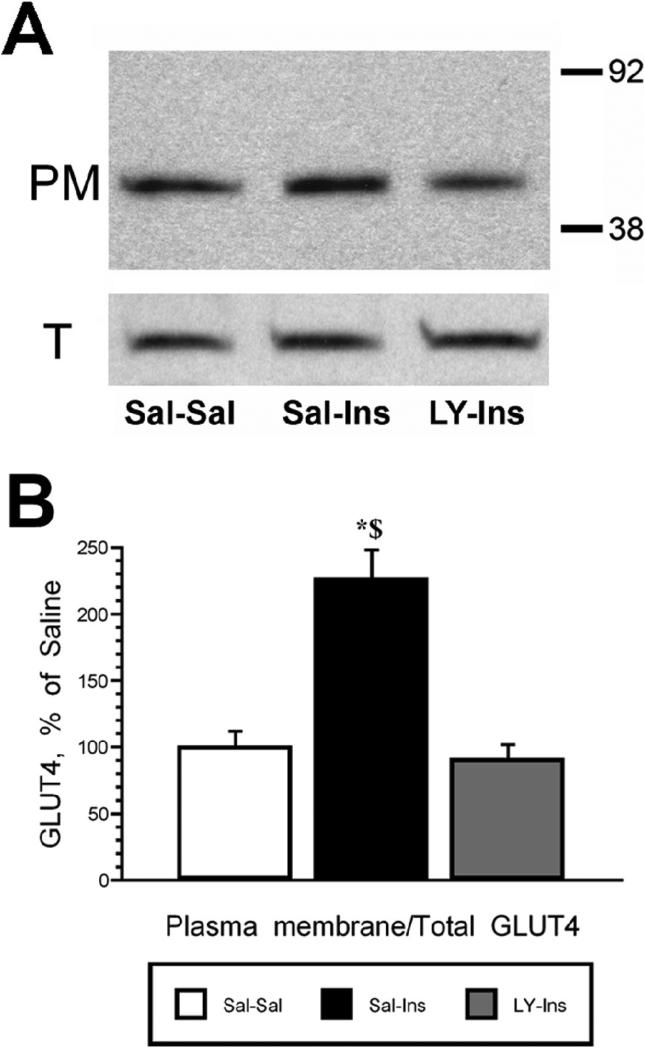
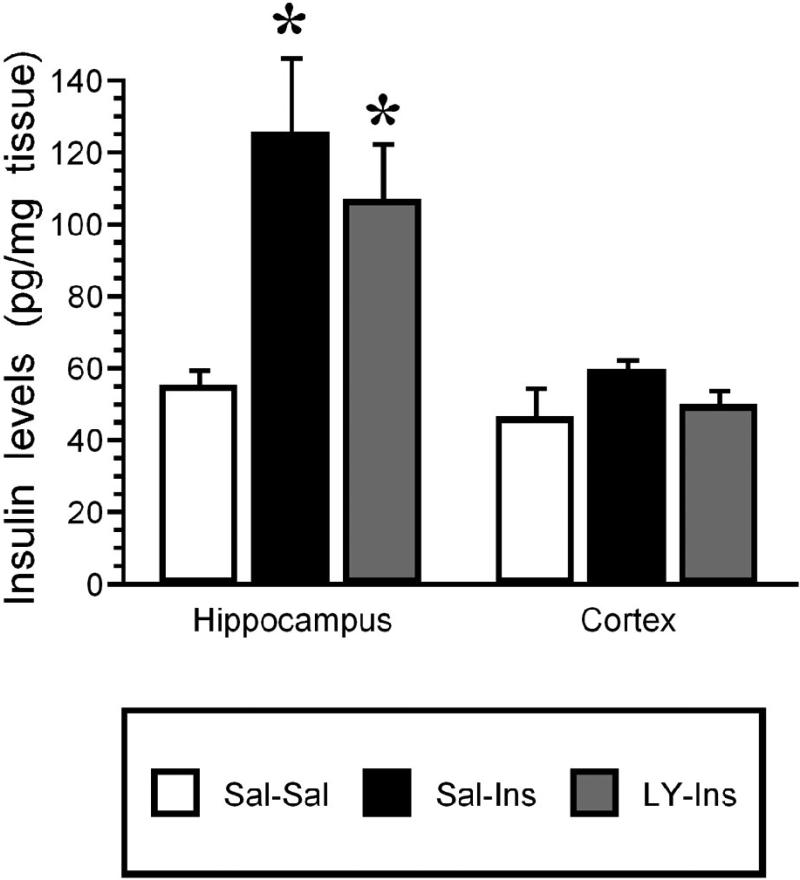
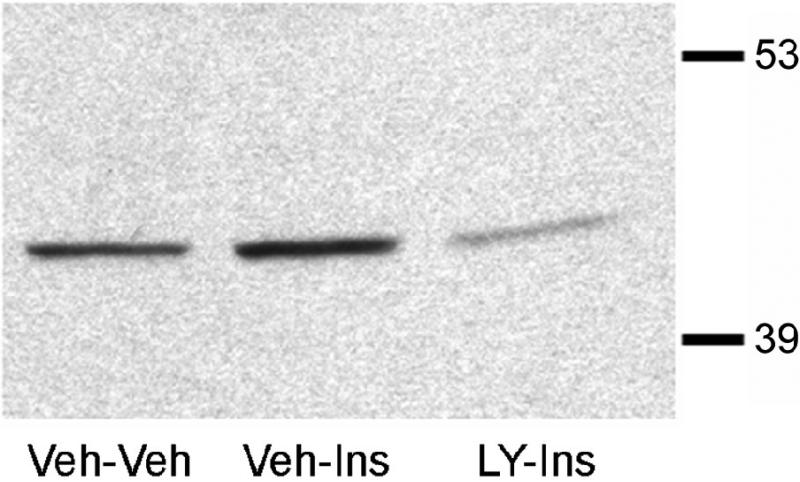
To determine the role of PI3-kinse in insulin stimulated phosphorylation of Akt and GLUT4 translocation, the PI3-kinase inhibitor LY294002 was administered 15 minutes prior to the insulin treatment. Following this pretreatment, insulin was administered and the hippocampus isolated 30 minutes later. The 30 minute post-insulin time point was selected based upon the time course experiment described above (See Figures 1 and 2). While total Akt levels were unchanged, insulin stimulated increases in pAkt levels were significantly reduced by LY294002 pretreatment (LY-Ins group) when compared to saline-insulin-treated rats (Sal-Ins group; Figure 4). Plasma membrane association of GLUT4 was also evaluated in this paradigm. While total GLUT4 levels did not change, insulin treatment elicited a significant increase in GLUT4 levels in the plasma membrane fraction (Sal-Ins), increases that were completely inhibited by LY294002 pretreatment (LY-Ins; Figure 5). To verify that hippocampal insulin levels were increased following icv administration, radioimmunoassay analysis was performed for insulin in hippocampal extracts from the Sal-Sal, Sal-Ins and LY-Ins treated rats. As shown in Figure 6, insulin-treated rats exhibited significant increases in hippocampal insulin levels following icv administration when compared to saline-treated controls, increases not observed in the cortex. Importantly, increases in hippocampal insulin levels were similar in rats pre-treated with saline (Sal-Ins) and pre-treated with LY294002 (LY-Ins). These results suggest that decreases in insulin mediated phosphorylation of Akt and translocation of GLUT4 resulted from inhibition of PI3-kinase and were not due to failure to increase hippocampal insulin levels. In order to further verify these actions of insulin upon GLUT4 translocation, the effects of insulin treatment was examined in hippocampal slice preparations. Hippocampal slices isolated from control rats were incubated with either vehicle (Veh-Veh), with 1 uM insulin in the absence of LY294002 (Veh-Ins) or 1 uM insulin in the presence of LY294002 (LY-Ins). Immunoblot blot analysis revealed that 15 minute stimulation with insulin increased GLUT4 association to the plasma membrane in hippocampal slice preparations, an effect that was blocked by LY294002 (Figure 6).
Figure 4.
Insulin mediated increases in pAkt in the rat hippocampus are inhibited by the PI3-kinase inhibitor LY294002. Panel A. Representative immunoblot of pAkt levels in total hippocampal membrane extracts isolated from saline-saline rats (Sal-Sal, open bars), saline-insulin rats (Sal-Ins, black bars) and LY294002-insulin rats (LY-Ins, grey bars). Panel B. Autoradiographic image analysis revealed that icv insulin significantly increased pAkt levels in rat hippocampus, increases that were attenuated by pre-treatment with the PI3-kinase inhibitor. Molecular weight standards are shown on the right. Data in Panel B are expressed as the pAkt/total Akt ratio and are based upon at least 6 rats/group. * = p ≤ 0.01 compared to Sal-Sal control rats; $ = p ≤ 0.01 compared to LY-Ins group.
Figure 5.
LY294002 inhibits insulin stimulated GLUT4 translocation in the rat hippocampus. Panel A. Representative immunoblots of hippocampal GLUT4 in plasma membrane fractions (PM; top panel) and total membrane fractions (T; bottom panel) in saline-saline rats (Sal-Sal, open bars), saline-insulin rats (Sal-Ins, black bars) and LY294002-insulin rats (LY-Ins, grey bars). Panel B. Autoradiographic analysis revealed that insulin-stimulated translocation of GLUT4 to the plasma membrane was inhibited by the PI3-kinase inhibitor LY294002. Molecular weight standards are shown on the right. Data in Panel B are expressed as the plasma membrane GLUT4/total membrane GLUT4 ratio and are based upon at least 6 rats/group. * = p ≤ 0.05 compared to Sal-Sal control rats; $ = p ≤ 0.05 compared to LY-Ins group.
Figure 6.
Radioimmunoassay (RIA) of insulin content in hippocampus and cortex following icv saline or insulin treatment. Tissue extracts were prepared for insulin RIA from saline-saline rats (Sal-Sal, open bars), saline-insulin rats (Sal-Ins, black bars) and LY294002-insulin rats (LY-Ins, grey bars). Hippocampal insulin levels were significantly increased in Sal-Ins rats and LY-Ins rats compared to control (Sal-Sal) rats. Conversely, insulin levels in rat cortex were unaffected by icv administration. Data based upon at least 6 rats per group. * = p ≤ 0.01 compared to saline-saline controls.
Confocal immunofluorescence for IR, GLUT4 and pAkt
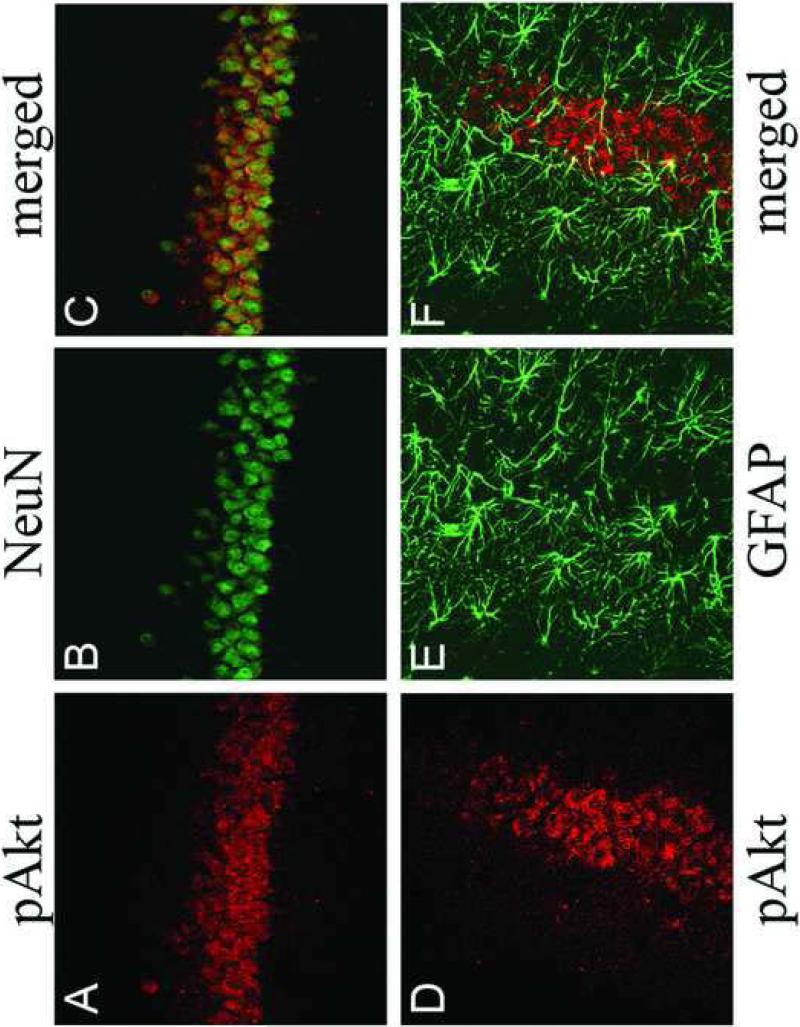
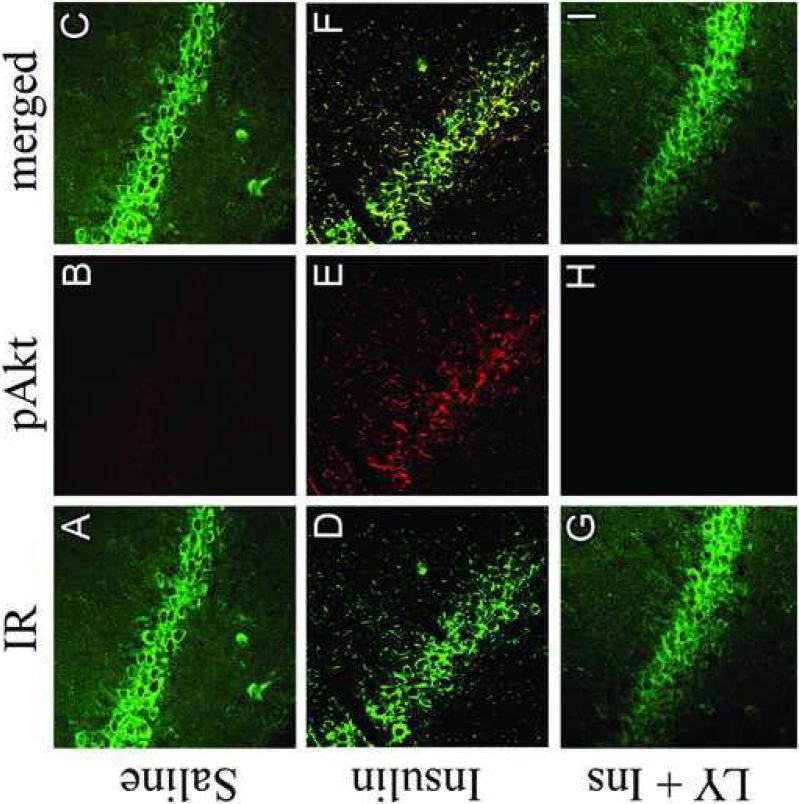
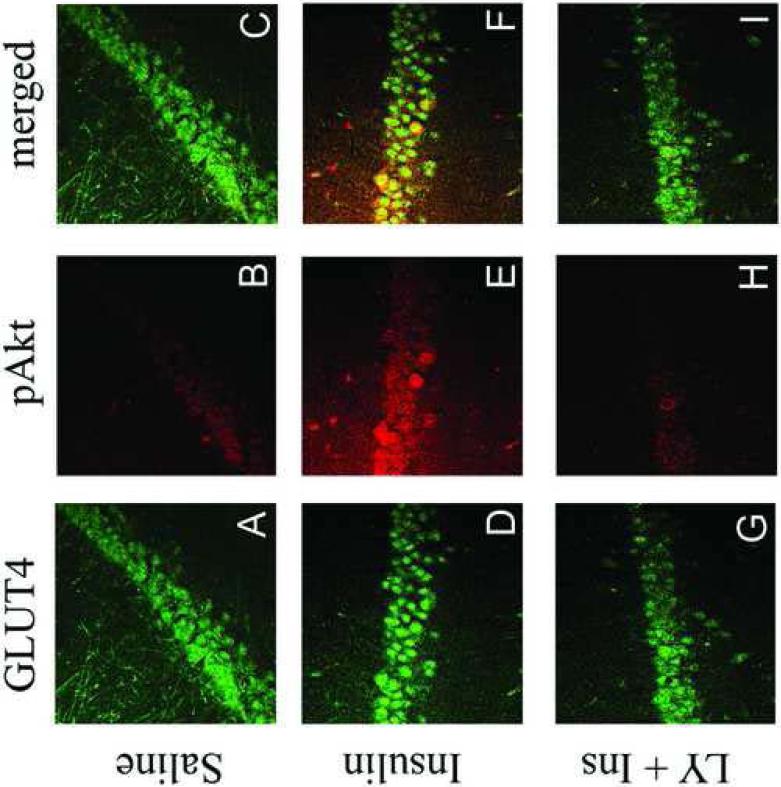
In order to characterize the phenotype of pAkt-positive cells following insulin administration, confocal immunofluorescence was performed in the rat hippocampus following intracerebroventricular administration of insulin. As shown in Figure 8, icv insulin administration stimulated phosphorylation of Akt in the rat hippocampus (red fluorescence, Panels A and D), immunofluorescence that was co-localized with the neuronal marker NeuN (green fluorescence, Panel C), but not glial fibrillary acidic protein (GFAP; green fluorescence, Panel F). These results demonstrate that insulin stimulated phosphorylation of Akt is limited to neurons and not glial cells in the rat hippocampus. The co-localization of IR with insulin-stimulated phosphorylation of Akt in the hippocampus was examined by confocal immunofluorescence. As shown in Figure 9, the IR exhibited the expected distribution in principal and non-principal cell bodies in the CA3 region of the rat hippocampus (green fluorescence, Panels A, D and G). Immunofluorescence for pAkt was very low in rats given icv saline (red fluorescence, Panel B); conversely, pAkt immunoreactivity was detected in the CA3 region 30 minutes following icv administration of insulin (Panel E). Insulin-stimulated phosphorylation of Akt was blocked by pre-treatment with LY-294002 (Panel H). The merged images demonstrate that insulin-stimulated pAkt exhibits nearly complete overlap with the IR in the rat hippocampus (Panel F), co-localization that is not observed in saline-treated rats (Panel C) or rats pre-treated with LY 294002 (Panel I).
Figure 8.
Confocal immunofluorescence for pAkt, NeuN and GFAP in the hippocampus of insulin-treated rats demonstrates that insulin stimulated phosphorylation of Akt is localized to neurons in the rat hippocampus. Panels A and D illustrate insulin stimulated pAkt (red fluorescence) in the CA3 region of the rat hippocampus. Panel B illustrates immunofluorescence for the neuron-specific marker NeuN (green fluorescence). Panel E depicts GFAP immunofluorescence in the CA3 region of the rat hippocampus (green fluorescence). Merged images demonstrates that pAkt is colocalized with NeuN (Panel C), while GFAP immunofluorescence exhibits little colocalization with pAkt (Panel F).
Figure 9.
LY294002 inhibits insulin stimulated colocalization of pAkt with the insulin receptor in the rat hippocampus. The insulin receptor (IR; green immunofluorescence) exhibits the expected immunofluorescence profile in the CA3 region of the rat hippocampus in saline-treated rats (Panel A), insulin-treated rats (Panel D) and rats pre-treated with the PI3-kinase inhibitor LY294002 prior to insulin treatment (Panel G). Insulin treatment increases pAkt immunofluorescence in the CA3 region of the hippocampus (Panel E; red fluorescence), increases not observed in rats pre-treated with LY294002 (Panel H). The phosphorylated form of Akt was not detected in saline-treated rats (Panel B). The merged images illustrate that pAkt exhibits colocalization with the IR in the hippocampus of insulin-treated rats (Panel F).
The co-localization of GLUT4 with insulin-stimulated pAkt was also examined by confocal immunofluorescence. In agreement with our previous studies (McEwen and Reagan, 2004), GLUT4 is localized to cell bodies of principal and non-principal cells in the CA3 region of the rat hippocampus (green fluorescence; Figure 10, Panels A, D and G). Levels of pAkt were low in saline-treated rats (red fluorescence; Panel B). Insulin administration increased pAkt levels in the CA3 region (Panel E), increases that were inhibited by pre-treatment with LY294002 (Panel H). The merged images illustrate that insulin-stimulated increases in the phosphorylated form of Akt are co-localized with GLUT4 (Panel F), co-localization not observed in saline-treated rats (Panel C) or rats pretreated with LY294002 (Panel I).
Figure 10.
Insulin stimulated phosphorylation of Akt is colocalized with GLUT4 in the rat hippocampus. GLUT4 (green immunofluorescence) exhibits the expected immunofluorescence distribution in the CA3 region of the rat hippocampus in saline-treated rats (Panel A), insulin-treated rats (Panel D) and rats pre-treated with the PI3-kinase inhibitor LY294002 prior to insulin treatment (Panel G). Insulin treatment increases pAkt immunofluorescence in the CA3 region of the hippocampus (Panel E; red fluorescence), increases not observed in rats pre-treated with LY294002 (Panel H). The phosphorylated form of Akt is not detected in saline-treated rats (Panel B). The merged images illustrate that pAkt exhibits colocalization with GLUT4 in the hippocampus of insulin-treated rats (Panel F).
DISCUSSION
The results of the current study demonstrate that intracerebroventricular administration of insulin stimulates the translocation of the insulin sensitive glucose transporter GLUT4 to the plasma membrane in the rat hippocampus in a time and PI3-kinase-dependent manner. Additionally, unlike the sustained increases in the pAkt levels, insulin-stimulated translocation of GLUT4 to the plasma membrane is transient and returns to baseline levels approximately 45 minutes after icv insulin administration. Lastly, fluorescence immunohistochemistry revealed that insulin-stimulated pAkt is co-localized with the insulin receptor and GLUT4, demonstrating the functional anatomical substrates of insulin signaling in the rat hippocampus. These results support the hypothesis that insulin mediates both transient as well as long-term effects in the hippocampus ranging from enhancing metabolic activities and plasticity of hippocampal neurons to activating changes in gene expression. Additionally, these results suggest that impairments in these functional activities of insulin in the hippocampus may contribute to the neurological complications of diabetes/obesity phenotypes in the central nervous system, including cognitive deficits and increased risk of co-morbid neurological disorders such as depression and Alzheimer's disease (Reagan, 2007).
Previous studies have provided evidence to suggest that insulin regulates plasma membrane levels of GLUT4 in the CNS via similar mechanisms as have been described in peripheral tissues. For example, peripheral glucose administration, which increases plasma insulin levels, rapidly stimulates the translocation of GLUT4 translocation to the plasma membrane in the rat hippocampus (McEwen and Reagan, 2004; Piroli et al., 2007a; Piroli et al., 2007b). Conversely, plasma membrane association of GLUT4 is modulated in the cerebellum, cortex (Vannucci et al., 1998) and hippocampus (Reagan, 2002; Winocur et al., 2005) in rodent models of type 1 and type 2 diabetes. However, these observations cannot exclusively point towards a role for insulin in GLUT4 trafficking events due to the variety of pathophysiological alterations associated with diabetes phenotypes (Gispen and Biessels, 2000; McCall, 1992). Indeed, while these studies provide provocative data to support the hypothesis that insulin stimulates GLUT4 trafficking in the CNS, it is important to note that diabetes phenotypes and glucose administration are associated with changes in plasma glucose and plasma insulin levels. The results of the current study provide more convincing evidence that insulin is the stimulus responsible for the translocation of GLUT4 to the plasma membrane in the rat hippocampus using similar mechanisms as have been described in muscle and adipose tissue.
In peripheral tissues, activation of the IR promotes insulin stimulated translocation of GLUT4 and increases in glucose uptake in muscle cells and adipose cells that are PI-3 kinase-dependent (Pessin et al., 1999). There is significant debate whether insulin mediates similar activities in the CNS in general and the hippocampus in particular. For example, previous studies suggest that the majority of glucose utilization in the hippocampus is mediated through GLUT1 expressed in microvessels and glial cells and GLUT3 expressed in neurons (Duelli et al., 2001). However, while these GLUTs are responsible for the majority of glucose uptake, they cannot account for all the glucose metabolism in the hippocampus. Moreover, GLUT3 is localized to neuronal processes and not neuronal cell bodies (McCall et al., 1994), even though glucose uptake in hippocampal principle cell layers is greater compared to the neuropil. Therefore, additional transport systems localized to neuronal cell bodies may contribute to neuronal glucose uptake and utilization. The insulin-sensitive GLUTs, namely GLUT4 and GLUT8 (McEwen and Reagan, 2004), may serve this critical function in the adult hippocampus.
Another important consideration is that these autoradiographic studies do not provide the temporal or spatial resolution that is required to identify rapid and transient changes in glucose metabolism that may occur during periods of increased neuronal activity. More simply, autoradiographic studies using radiolabeled glucose provide a snapshot rather than a movie of ongoing hippocampal glucose utilization. It is likely that glucose uptake and utilization is a dynamic process that fluctuates during periods of increased metabolic demand. In support of this hypothesis, studies by McNay and co-workers demonstrated that extracellular levels of glucose decrease in the hippocampus during the performance of hippocampal-dependent tasks, decreases not observed in the striatum (McNay et al., 2000). These rapid and transient changes in extracellular glucose levels are presumably mediated by increases in neuronal activity during task learning and suggest that neurons possess the capacity to rapidly modulate their metabolic activity. These changes in glucose uptake occur in a time frame that precludes the participation of transcriptional/translational events and suggests that a readily mobolizable pool of glucose transporters respond to increases in neuronal activity to enhance glucose metabolism. The results of the current study support the hypothesis that insulin mediated translocation of GLUT4 is responsible for, or contributes to, increase glucose utilization of the hippocampus during learning paradigms. Indeed, the time-dependent, insulin-stimulated increases in plasma membrane association of GLUT4 temporally mirror the changes in glucose flux observed during performance of hippocampal dependent tasks (McNay and Gold, 2002).
More recent studies by Fernando et al provide additional support for a role of GLUT4 in hippocampal glucose uptake and utilization (Fernando et al., 2008). This study demonstrated that insulin-regulated aminopeptidase (IRAP) is co-localized in GLUT4-containing vesicles in the hippocampus and that glucose uptake is reduced in hippocampal slices prepared from IRAP-knockout mice. It is important to note this same study revealed that while insulin increased glucose uptake in hippocampal slice preparations, these increases did not achieve statistical significance. However, hippocampal slices were incubated with insulin for shorter time periods than described in the current study, suggesting that longer incubation periods may have identified insulin mediated increases in glucose uptake. Studies performed in human neuroblastoma cells indicate that insulin-stimulated GLUT4 translocation enhances glucose uptake in a PI3-kinase-dependent manner (Benomar et al., 2006). More recent studies revealed that insulin stimulates GLUT4 translocation in a PI3-kinase dependent manner in primary cultures of cerebellar neurons (Bakirtzi et al., 2009). While there are limitations associated with in vitro studies, these results support the hypothesis that insulin stimulated translocation of GLUT4 contributes to hippocampal glucose utilization.
One question that remains to be determined is the functional impact of activation of insulin stimulated cascades in the hippocampus. A number of clinical and pre-clinical studies support the hypothesis that increases in brain insulin levels enhances cognitive performance. A comprehensive review of this literature is beyond the scope of this discussion (See (Reagan, 2007)), but a few of the previous studies should be highlighted in relation to the current findings. For example, acute hyperinsulinemic/euglycemic clamp conditions increased memory recall in normal healthy volunteers (Kern et al., 2001), which supports the hypothesis that increases in brain insulin levels facilitate cognition. Craft and co-workers previously illustrated that acute hyperinsulinemic conditions also increases memory performance in Alzheimer's disease patients (Craft et al., 1999). Acute activation of insulin signaling in the CNS may include translocation of GLUT4 to the plasma membrane and increased glucose uptake and availability, which could facilitate neuronal function during increased metabolic demand. Chronic intranasal insulin administration also improves cognitive performance in healthy individuals (Benedict et al., 2004), as well as memory recall and functional status in AD patients (Reger et al., 2008). The results from these studies suggest that chronic insulin administration strengthens hippocampal insulin signaling and thereby enhances cognitive performance, a hypothesis supported by animal studies (Zhao et al., 1999; Zhao et al., 2004). The important message from these studies is that insulin may facilitate cognition via rapid mechanisms that may include translocation of GLUT4 to the plasma membrane, as well as via mechanisms that initiate long lasting changes in neuronal structure and function.
In summary, the results of the current study demonstrate that insulin stimulated translocation of GLUT4 to the plasma membrane in the rat hippocampus is time- and PI3-kinase dependent. These results suggest that the mechanisms through which insulin improves cognitive performance may include GLUT4 mediated increases in hippocampal glucose utilization. Improvement in the acute and chronic activation of insulin signaling cascades that stimulates GLUT4 translocation may represent novel therapeutic approaches for the treatment of cognitive impairments associated with a variety of neurological disorders, including Alzheimer's disease and diabetes.
EXPERIMENTAL PROCEDURES
Animal Protocols
Adult male Sprague Dawley rats (CD strain, Charles River) weighing 200-250 g were housed in accordance with all guidelines and regulations of The University of South Carolina Animal Care and Use Committee. Animals were maintained in a temperature-controlled room, with a light/dark cycle of 12/12 hours (lights on at 0700 hours). Rats were handled daily for 5 days and then underwent stereotaxic surgery. After an overnight fast, rats were anesthetized, placed in the stereotaxic apparatus, and insulin (human recombinant, Sigma), LY294002 (Cell Signaling) or saline were infused into the third ventricle using the following coordinates: AP: −4.3 mm; L: 0.0 mm; DV: −4.2 mm. Drugs were infused with a final volume of 6 μl at a flow rate of 1 μl/minute with a 10 μl Hamilton syringe driven by a motorized stereotaxic injector (Stoelting 53310); the needle was left in place for at least additional 15 minutes. To determine the time course of insulin effects on Akt phosphorylation and GLUT4 trafficking to the plasma membrane, rats received saline or 6 mU of insulin and were sacrificed 15, 30, 45 or 60 minutes later. This does of insulin was selected based upon previous studies that examined the cognitive enhancing activities of insulin (Park et al., 2000). Tissue was then processed for immunoblot analysis as described below. To determine the role of PI3-kinase on insulin effects, rats received either: 1) 15 nmol LY294002 followed 15 minutes later with 6 mU of insulin (LY-Ins group); or 2) saline followed 15 minutes later with 6 mU of insulin (Sal-Ins group). Control rats received saline solution at both time points (Sal-Sal group). Rats were sacrificed 30 minutes after the last infusion and hippocampal extracts were prepared for immunohistochemistry or immunoblot analysis as described below.
Immunohistochemical approaches
Rats were perfused through the ascending aorta sequentially with: 1) 100 ml of saline solution containing 0.1% of heparin and 0.5% NaNO2; 2) 50 ml of 3.75% acrolein/2% paraformaldehyde in 0.1 M PB, pH 7.4; and 3) 200 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Rat brain sections were cut on a freezing microtome at a thickness of 40 μm, washed with phosphate-buffered saline (PBS) and then incubated with primary antisera for the insulin receptor (1:500 dilution; Santa Cruz Biotechnology), pAkt (1:500 dilution, Cell Signaling Technology), GLUT4 (1:1,000; (Piroli et al., 2007a), NeuN (1:1,000, Chemicon), or glial fibrillary acidic protein (GFAP, 1:500, Cell Signaling). Sections were washed and incubated with anti-rabbit IgG or anti-mouse IgG secondary antisera coupled to Alexa 488 (green, 1:200) or Alexa 546 (red, 1:200) (Molecular Probes, Inc., Eugene, OR). Tissue sections were mounted on gelatin-coated slides and coverslipped with AquaMount (Lerner Laboratories). Sections were examined on a Zeiss LSM510 confocal laser scanning microscope.
Membrane preparation
Isolation of membrane-containing fractions was performed as described previously (Piroli et al., 2002; Reagan et al., 2000). Briefly, rats were decapitated and hippocampi were isolated, frozen on dry ice and stored at −70°C until use. One hippocampal hemisphere from each individual rat was homogenized in ice-cold homogenization buffer and centrifuged for 10 minutes at 500g at 4°C. The total membrane fraction (supernatant) was saved; a portion of this fraction was centrifuged at 31,000g for 30 minutes at 4°C. The resulting pellet, which contained the plasma membrane fraction, was resuspended in PBS. Protein concentrations of the total membrane fraction and the plasma membrane fraction were determined by the method of Bradford (Bradford, 1976) using bovine serum albumin (BSA) as a standard.
Immunoblot analysis
Immunoblot analysis was performed as described in our previous studies (Reagan et al., 1993; Reagan et al., 2000; Reagan et al., 2001). Briefly, proteins were separated by SDS/PAGE (10%), transferred to nitrocellulose (NC) membranes and blocked in tris-buffered saline (TBS) plus 10% nonfat dry milk (NFDM) for 60 minutes. NC membranes were incubated with primary antisera to GLUT4 (1:1,000) or pAkT (1:1,000) prepared in TBS/5% NFDM overnight at 4°C with gentle shaking. NC membranes were then washed with TBS plus 0.05% Tween 20 (TBST) and incubated with peroxidase-labeled species-specific secondary antibodies (1:5,000) at room temperature for 60 minutes. NC membranes were then washed with TBST and developed using enhanced chemiluminescence reagents (ECL, Amersham) as described by the manufacturer. For the insulin receptor pre-absorption studies, primary antisera were incubated for several hours at room temperature in the absence (Control lanes) or presence (+ lanes) of the peptide immunogen for the insulin receptor (1 ug/ul; Santa Cruz Biotechnology). Normalization for protein loading was performed using a mouse monoclonal primary antibody selective for actin (Sigma Chemical Company).
Determination of insulin content in the hippocampus and cortex
One hippocampal or cortical hemisphere from each individual rat was homogenized in 1 ml acid mixture (ethanol: water: 12N sulfuric acid at a ratio of 800-179-21) and kept overnight at 4°C. The samples were then centrifuged at 16,000g for 10 minutes at 4°C, the supernatants were transferred to fresh tubes and were evaporated in a SpeedVac centrifuge. Pellets were resuspended in 250 μl PBS, then centrifuged at 16,000g for 10 min 4°C, and the resulting supernatants were used for the determination of insulin by radioimmunoassay at a 1:10 dilution, according to the manufacturer's instructions (Millipore, Billerica, MA).
In vitro stimulation assays
Control rats were decapitated, the hippocampi were isolated and sliced into 500 μm sections using a tissue slicer. All incubations were performed at room temperature. Slices were distributed into the inserts of a 6-well tissue culture plate containing artificial cerebrospinal fluid (aCSF: 125 mM NaCl, 2.7 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 10 mM glucose, 0.5 mM CaCl2, 7 mM MgSO4, 20 μM D-APV, pH 7.4 maintained with continuous bubbling of 95% O2, 5% CO2). After a preincubation period of 1 hour, tissue was separated into 3 experimental groups: 1) Ly-Ins group, which was incubated with LY294002 (final concentration 50 μM) for 15 minutes and then with insulin (final concentration 1 μM) for an additional 15 minutes; 2) Veh-Ins group, which was incubated with aCSF for 15 minutes and then with insulin (final concentration 1 μM) for an additional 15 minutes; and 3) Veh-Veh group, which was incubated with aCSF for 30 minutes. Isolation of membrane-containing fractions from these incubations was performed as described above.
Autoradiographic film analysis and statistical analysis
Computer-assisted microdensitometry of autoradiographic images was performed on MCID image analysis system (Imaging Research, Inc., St. Catherines, Canada). For quantitative purposes, microscale 14C standards (Amersham) were exposed on Kodak X-OMAT film and digitized. Grey level/optical density calibrations were performed using a calibrated film strip ladder (Imaging Research, Inc., St. Catherines, Canada). Optical density was plotted as a function of microscale calibration values. Statistical analysis for all studies was performed with a one-way ANOVA, followed by a Student-Newman-Keuls post-hoc test, with P<0.05 as the cutoff value for statistical significance.
Figure 7.
In vitro insulin stimulation of hippocampal slices increases plasma membrane association of GLUT4. Hippocampal slices were prepared and treated with artificial cerebrospinal fluid (Veh-Veh), artificial cerebrospinal fluid plus insulin (Veh-Ins) or artificial cerebrospinal fluid plus LY294002 prior to insulin treatment (LY-Ins). Slices were then used to isolate plasma membrane fractions. Insulin treatment increased hippocampal plasma membrane association of GLUT4, an effect that was blocked by the PI3-kinase inhibitor LY294002.
ACKNOWLEDGEMENTS
Supported by Juvenile Diabetes Research Foundation grant number 2-03-675 (LPR) and NIH grant number NS047728 (LPR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bakirtzi K, Belfort G, Lopez-Coviella I, Kuruppu D, Cao L, Abel ED, Brownell AL, Kandror KV. Cerebellar neurons possess a vesicular compartment structurally and functionally similar to Glut4-storage vesicles from peripheral insulin-sensitive tissues. J. Neurosci. 2009;29:5193–5201. doi: 10.1523/JNEUROSCI.0858-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Benomar Y, Naour N, Aubourg A, Bailleux V, Gertler A, Djiane J, Guerre-Millo M, Taouis M. Insulin and leptin induce Glut4 plasma membrane translocation and glucose uptake in a human neuronal cell line by a phosphatidylinositol 3-kinase- dependent mechanism. Endocrinology. 2006;147:2550–2556. doi: 10.1210/en.2005-1464. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Bradford MA. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Charron MJ, Brosius FC, III, Alper SL, Lodish HF. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc. Natl. Acad. Sci. U. S. A. 1989;86:2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer Disease with insulin and somatostatin, but not glucose. Arch. Gen. Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- Doré S, Kar S, Rowe W, Quirion R. Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]Insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience. 1997;80:1033–1040. doi: 10.1016/s0306-4522(97)00154-1. [DOI] [PubMed] [Google Scholar]
- Duelli R, Maurer MH, Staudt R, Sokoloff L, Kuschinsky W. Correlation between local glucose transporter densities and local 3-O-methylglucose transport in rat brain. Neurosci. Lett. 2001;310:101–104. doi: 10.1016/s0304-3940(01)02060-2. [DOI] [PubMed] [Google Scholar]
- Fernando RN, Albiston AL, Chai SY. The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus--potential role in modulation of glucose uptake in neurones? Eur. J. Neurosci. 2008;28:588–598. doi: 10.1111/j.1460-9568.2008.06347.x. [DOI] [PubMed] [Google Scholar]
- Gispen WH, Biessels G-J. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kar S, Chabot J-G, Quirion R. Quantitative autoradiographic localization of [125I]Insulin-like growth factor I, [125I]Insulin-like growth factor II and [125I]Insulin binding sites in developing and adult rat brain. J. Comp. Neurol. 1993;333:375–397. doi: 10.1002/cne.903330306. [DOI] [PubMed] [Google Scholar]
- Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74:270–280. doi: 10.1159/000054694. [DOI] [PubMed] [Google Scholar]
- Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology. 1991;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- McCall AL. The impact of diabetes on the CNS. Diabetes. 1992;41:557–570. doi: 10.2337/diab.41.5.557. [DOI] [PubMed] [Google Scholar]
- McCall AL, Van Bueren AM, Moholt-Siebert M, Cherry NJ, Woodward WR. Immunohistochemical localization of the neuron-specific glucose transporter (GLUT3) to neuropil in adult rat brain. Brain Res. 1994;659:292–297. doi: 10.1016/0006-8993(94)90896-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur. J. Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr. Opin. Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc. Natl. Acad. Sci. USA. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav. Cogn Neurosci. Rev. 2002;1:264–280. doi: 10.1177/1534582302238337. [DOI] [PubMed] [Google Scholar]
- Park CR, Seely RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol. Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. J. Biol. Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Hoskin EK, Znamensky V, Katz EB, Milner TA, McEwen BS, Charron MJ, Reagan LP. Peripheral glucose administration stimulates the translocation of GLUT8 glucose transporter to the endoplasmic reticulum in the rat hippocampus. J. Comp. Neurol. 2002;452:103–114. doi: 10.1002/cne.10368. [DOI] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Reznikov LR, Adams S, McEwen BS, Charron MJ, Reagan LP. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology. 2007a;85:71–80. doi: 10.1159/000101694. [DOI] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Reznikov LR, Reagan LP. Expression and Functional Activities of Glucose Transporters in the Central Nervous System. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. Springer; New York: 2007b. pp. 387–404. [Google Scholar]
- Reagan LP. Glucose, stress and hippocampal neuronal vulnerability. Int. Rev. Neurobiol. 2002;51:289–324. doi: 10.1016/s0074-7742(02)51009-6. [DOI] [PubMed] [Google Scholar]
- Reagan LP. Insulin signaling effects on memory and mood. Curr. Opin. Pharmacol. 2007;7:633–637. doi: 10.1016/j.coph.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Gorovits N, Hoskin EK, Alves SE, Katz EB, Grillo CA, Piroli GG, McEwen BS, Charron MJ. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proc. Natl. Acad. Sci. USA. 2001;98:2820–2825. doi: 10.1073/pnas.051629798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, Magariños AM, Yee DK, Szweda LI, Van Bueren A, McCall AL, McEwen BS. Oxidative stress and HNE conjugation of GLUT3 are increased in the hippocampus of diabetic rats subjected to stress. Brain Res. 2000;862:292–300. doi: 10.1016/s0006-8993(00)02212-5. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Theveniau M, Yang X, Siemens IR, Yee DK, Reisine T, Fluharty SJ. Development of polyclonal antibodies against Angiotensin Type 2 (AT2) receptors. Proc. Natl. Acad. Sci. USA. 1993;90:7956–7960. doi: 10.1073/pnas.90.17.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. GLUT4 glucose transporter expression in rodent brain: effects of diabetes. Brain Res. 1998;797:1–11. doi: 10.1016/s0006-8993(98)00103-6. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE, Piroli GG, Grillo CA, Reznikov LR, Reagan LP, McEwen BS. Memory Impairment in Obese Zucker Rats: An Investigation of Cognitive Function in an Animal Model of Insulin Resistance and Obesity. Behav. Neurosci. 2005;119:1389–1395. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. J. Biol. Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]