Abstract
The Wistar-Kyoto (WKY) rat strain has been described as an animal model of depressive behavior that consumes significantly greater amounts of alcohol compared to the Wistar (WIS) rat strain. Since the mesolimbic dopamine (DA) type-2 (D2) receptors mediate reward-related behaviors, the present study measured the binding of [125I]-Iodosulpiride to D2 receptors in the brains of WKY versus WIS rats following 24 days of voluntary alcohol or water consumption. Alcohol consuming WKY rats showed a significant increase in D2 receptor binding in several regions of the mesolimbic and nigrostriatal systems. In contrast, alcohol consuming WIS rats showed a reduction in D2 receptor binding in DA cell body areas. The differential regulation of D2 receptors by voluntary alcohol consumption in the two rat strains suggests that D2 receptor mediated neurotransmission may be playing a role in the increased alcohol drinking behavior reported in WKY rats.
Keywords: Dopamine, Dopamine-2 receptor, Wistar-Kyoto rats, Alcohol, Quantitative autoradiography
Introduction
Alcohol use accounts for 4% of global disease burden and is related to approximately 1.8 million deaths each year [1]. In the United States alone, an estimated 7.9% of the population over the age of 12 is in need of treatment for alcoholism [2]. Although physiological systems involved in and affected by alcoholism have been extensively studied, the neurobiological mechanisms that contribute to ethanol intake and abuse are not well understood.
Dopamine (DA) is a neurotransmitter believed to be involved in the reinforcing effects of alcohol [3, 4]. DA pathways mediate numerous physiological and psychological functions including cognition, anxiety, motivation, locomotion and reward [5–7]. Specifically, the mesolimbic DA pathway originating in the ventral tegmental area (VTA) projects to the nucleus accumbens (NAc) and plays a role in reinforcement or motivational behavior [8, 9]. In vivo microdialysis studies have shown increased extracellular DA levels in the NAc and VTA after systemic and local alcohol administration, suggesting these regions may be prime substrates for alcohol reinforcement [10–12]. Furthermore, drugs that block the effects of DA, specifically at DA type-2 (D2) receptor sites, have been shown to reduce alcohol intake in animal as well as human studies [13–15].
The D2 receptors are important in mediating reward related behaviors and are intimately involved in alcohol abuse. Earlier studies have suggested the involvement of the human D2 receptor gene (DRD2) in alcoholism, more specifically the DRD2 TaqI A1 allele [16, 17]. The D2 receptors have two distinct functions based on their localization. In cell body areas such as the VTA and the substantia nigra (SN), these receptors function as autoreceptors [18–20]. They inhibit impulse flow, neurotransmitter synthesis, and release. D2 receptors in the terminal regions such as NAc and caudate putamen (CPu) represent both autoreceptors (on axons) and postsynaptic inhibitory G-protein coupled receptors [18, 20]. Since mice with a genetic depletion targeting D2 receptors demonstrate a reduction in alcohol preference and sensitivity [21], availability of this receptor site may be a critical factor in determining alcohol drinking behavior.
The Wistar-Kyoto (WKY) rat strain has been proposed as an animal model of depressive behavior [22–25]. Several studies have noted that WKY rats differ from other strains in their behavioral, physiological, and neuroendocrine responsiveness to environmental as well as pharmacological challenges [22, 24–28]. For example, compared to outbred control strains, WKY rats exhibit decreased activity in the Open Field Test (OFT), hypolocomotion in the Elevated Plus-Maze (EPM), freezing behavior in the Forced Swim Test (FST), learned helplessness with inescapable shock, and an enhanced adreno-corticotropin releasing hormone response to stressful stimulation [22, 24, 27, 29]. These previous studies indicate that WKY rats are more depressed, anxious and behaviorally inhibited compared to other rat strains. Interestingly, drugs that enhance synaptic levels of DA alleviate these depressive symptoms in WKY rats [25, 30]. Moreover, compared to their outbred counterparts, Wistar (WIS) rats, WKY rats show reduced reward from nicotine as measured by a decrease in conditioned place preference and locomotor response to nicotine administration, indicating a possible DA deficiency in these animals [31]. WKY rats also show reduced levels of sucrose pellet and nicotine self administration [32], further supporting an abnormal reward system in these animals. Consistent with these behavioral studies, we have recently reported strain differences in D1, D2 and DA transporter (DAT) sites in WKY versus WIS rats that were suggestive of an inherent DA deficiency in the mesolimbic pathway [33–35]. This was also supported by findings from De la Graza and Mahoney, which showed reduced DA levels in the pre-frontal cortex and increased DA turnover in the striatum under basal conditions [36].
With evidence suggesting that a reduction in dopaminergic tone, specifically within the mesolimbic DA system, is associated with abnormal alcohol seeking behavior, we recently investigated voluntary alcohol consumption in these two rat strains and reported that WKY rats consume 200% more alcohol than WIS rats under basal conditions [37, 38]. When examining DAT sites following chronic alcohol consumption, DAT binding was increased in a greater number of mesolimbic brain regions, and also in the nigrostriatal regions which plays a prominent role in the expression of motor behavior in WKY compared to WIS rats [39–41]. Since DAT sites play an important role in regulating extracellular DA levels, it is possible that DA neurotransmission may be differentially altered in these two rat strains by alcohol consumption, potentially regulating DA receptor sites in select brain regions in WKY compared to WIS rats. Therefore, the present study was designed to map the density and distribution of D2 receptor sites following voluntary alcohol consumption using the technique of quantitative autoradiography (QAR). We hypothesized that WKY rats would demonstrate differential modulation of D2 receptor sites following voluntary alcohol consumption compared to the WIS rat strain.
Experimental Procedures
All experimental protocols were reviewed and approved by the Veterans Medical Center, Perry Point, Maryland VAH Institutional Review Committee for the use of animal subjects.
Animals
Since WKY rats tend to weigh less than WIS rats, 4 month old male WKY rats and 3 month old male WIS rats were used in this study (weight range ∼ 290–340 g). Rats within each strain were assigned into a control group which received no alcohol and an alcohol group (n = 8 per group). All groups were equated on the basis of body weight. WIS rats were purchased from Harlan (Indianapolis, IN). WKY rats were raised at the Veterans Medical Center, Perry Point, MD from a breeding stock initially obtained from Charles River Laboratories (Kingston, NY). Animals were individually housed at 22°C and placed on a 12-h light/dark cycle, with lights on between 06:00 and 18:00 h. Animals were allowed to acclimate for 2 weeks prior to the start of the experiment and were handled daily for 1 week prior to the start of the experiment. Standard Laboratory chow and water were kept available during the entire day throughout the experimental period.
Procedures
The alcohol preference method as described by Sandbak and Murison was used with minor modifications [42]. Briefly, a progressive 3–7% (volume/volume) alcohol solution was used to elicit voluntary alcohol consumption. During the first 7 day period (day 1–7) a 3% alcohol solution was presented to the animals followed by a 5% solution during the second 7 day period (day 8–14), and finally a 7% solution throughout the remaining time (day 15–24). Both alcohol and water were freely available throughout the experimental period until rats were sacrificed. The alcohol group was offered two sipper-type drinking tubes with tap water in one and alcohol solution in the other. The position of the tubes was switched daily (left/right) and no additional flavors were added to either bottle in order to eliminate the possibility of location and flavor preference. The control group was offered two sipper tubes containing water. Body weight and alcohol consumption measurements were recorded daily at the same time each day. Alcohol consumption was recorded in terms of grams alcohol per kilogram body weight. At the end of the 24-day experimental period, WKY rats were found to consume an average 2.2 g/kg/day alcohol compared to 0.8 g/kg/day consumed by the control WIS strain. No significant changes in body weights were recorded among WKY and WIS rats following alcohol consumption when compared to control groups (data not shown). On day 25, animals were sacrificed by rapid decapitation between 0800 and 1000, the brains removed and stored at −80°C till use.
Brain Section Preparation
Brains were sectioned (16 μm) at −18°C in a cryostat microtome and mounted on gelatin-coated microscope slides. Sections were sliced from plates 12 and 42 according to the Brain Atlas of Paxinos and Watson [43] and all levels were verified using Nissl staining. Sections were then chosen from plate 12 to represent terminal regions (CPu and NAc) and plate 42 to represent the cell body areas (VTA and SN). All sections, clearly depicting the region of interest, were chosen using the same level for each animal within each strain. For each animal, two adjacent brain sections were used for the binding assay and averaged for statistical analysis.
[125I]-Iodosulpiride Binding Assay
D2 receptors were labeled with [125I]-Iodosulpiride using the method of Stefanski et al. with minor modifications based on our preliminary experiments as described below and in previous publications [35, 44]. To label D2 receptors, adjacent sections were preincubated for 15 min in a buffer solution [50 mM Tris–HCL, 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2] at a PH of 7.4. Following preincubation, sections were incubated in the previously described buffer solution containing 0.1 nM [125I]-Iodosulpiride (2000 Ci/mmol, Amersham Biosciences) and 5 nM PD 28,907 (to block D3 receptors) at room temperature for 30 min. To determine non-specific binding, adjacent sections were incubated in 0.1 nM [125I]-Iodosulpiride, 5 nM PD 28,907, and 1 μM domperidone for 30 min. Following incubation, the sections were rinsed twice in cold buffer for 5 min each, followed by a dip in cold deionized water. The slides were then dried at room temperature, transferred into cassettes, and exposed to Kodak BioMax MS film along with [3H] standards. The exposure time for plate 12 was 4 days and for plate 42 it was 7 days. Films were developed using Kodak GBX developer.
Quantification
The images were quantified using the Brain 3.0 computerized brain image analysis program for Macintosh. Non-specific binding was subtracted from the total binding to provide the specific binding in the regions of interest. Data were expressed as mean ± SEM specific binding (fmol/mg brain protein).
Statistics
Statistical analysis was performed with SigmaStat program for Windows. Differences between means in individual regions were analyzed using two-way analysis of variance (ANOVA), with strain (two levels) and treatment (two levels) as independent variables. Where significant main effects of strain and treatment as well as the strain × treatment interactions were reported (P < 0.05), a post hoc Tukey's test, with HSD P < 0.05, was conducted to locate significant differences between groups. All values of binding density were expressed as mean ± SEM and graphed as percent change from water-drinking control animals.
Results
Effects of Alcohol on D2 Receptor Sites
The binding of [125I]-Iodosulpiride was measured in several regions of the mesolimbic and nigrostriatal pathways in WKY and WIS rats as depicted in Fig. 1. As previously described [35], [125I]-Iodosulpiride binding to D2 receptor sites was observed in similar terminal and cell body regions of WKY and WIS rats. Within the striatum, D2 receptor binding was strongest in the CPu (140–260 ng/mg brain protein) followed by the NAc (70–120 ng/mg brain protein). Within the cell body areas, the binding of [125I]-Iodosulpiride to D2 receptors was strongest in the VTA (90–160 ng/mg brain protein), with moderate levels of receptor binding observed in the SN (75–120 ng/mg brain protein).
Fig. 1.
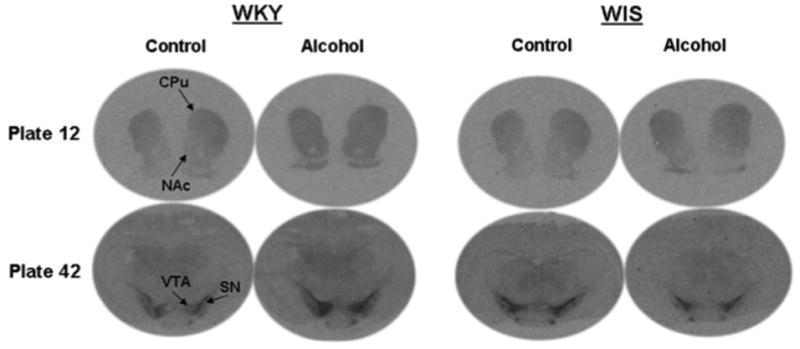
Autoradiographs representing increased D2 receptor binding in terminal and cell body regions of Wistar-Kyoto (WKY) rats and cell body regions of Wistar (WIS) rats. CPu caudate putamen, NAc nucleus accumbens, VTA ventral tegmental area, SN substantia nigra
Terminal Regions
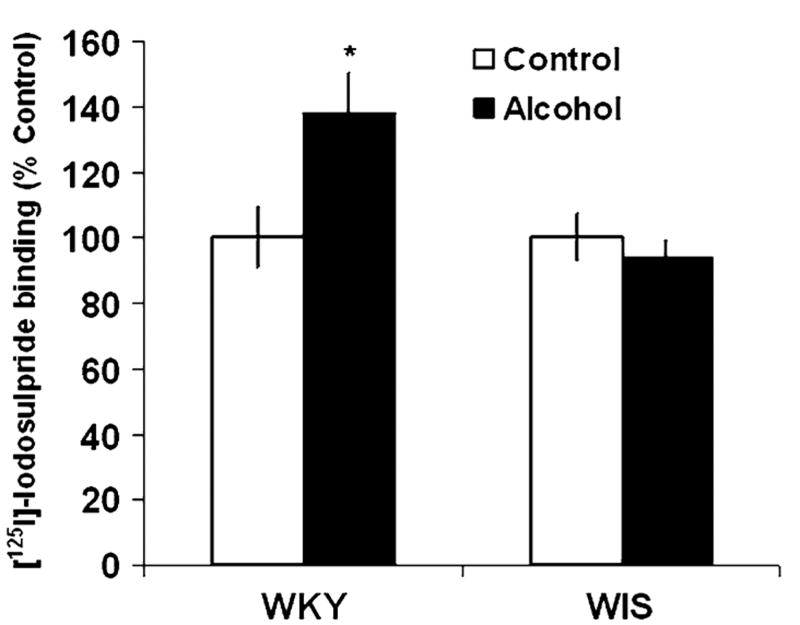
In WKY rats, the specific binding of [125I]-Iodosulpiride to D2 sites was increased by 37% in the CPu following chronic alcohol consumption, F(1, 28) = 4.856, P < 0.05, Tukey HSD test, P < 0.05, while WIS rats showed a slight, non-significant reduction (−6%) in binding values with alcohol exposure (Fig. 2). In the NAc, alcohol consumption significantly increased D2 receptor binding by 55%, F(1, 26) = 5.25, P < 0.05, Tukey HSD test, P < 0.05 in WKY rats (Fig. 3). However, similar to the effects observed in the CPu, WIS rats only demonstrated a nonsignificant downward trend (−3%) in receptor binding following chronic alcohol consumption.
Fig. 2.
Effects of alcohol consumption on the binding of [125I]-Iodosulpiride to D2 receptor sites in the caudate putamen (CPu) of WKY and WIS rats. Data are expressed as the mean ± SEM of measurements from eight rats from each strain, with determinations made in duplicate sections from each brain. Binding data are graphed as percent change from the water control group of each respective rat strain. Asterisk represents significant differences between treatment groups within strain, P < 0.05. WKY Wistar-Kyoto, WIS Wistar
Fig. 3.
Effects of alcohol consumption on the binding of [125I]-Iodosulpiride to D2 receptor sites in the nucleus accumbens (NAc) of WKY and WIS rats. Data are expressed as the mean ± SEM of measurements from eight rats from each strain, with determinations made in duplicate sections from each brain. Binding data are graphed as percent change from the water control group of each respective rat strain. Asterisk represents significant differences between treatment groups within strain, P < 0.05. WKY Wistar-Kyoto, WIS Wistar
Cell Body Regions
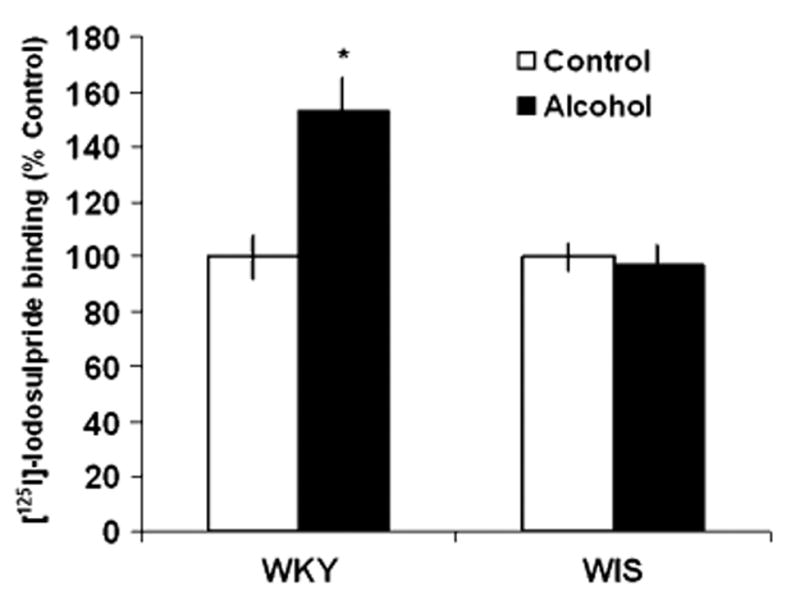
In the SN, alcohol consumption significantly increased the binding of [125I]-Iodosulpiride to D2 receptor sites by 36% in WKY rats and decreased binding by 22% in WIS rats, F(1, 26) = 11.58, P < 0.05, Tukey HSD test, P < 0.05 (Fig. 4) when compared to their respective controls. Furthermore, a significant increase (+57%) in the specific binding of [125I]-Iodosulpiride to D2 sites was observed in the VTA of WKY rats following alcohol consumption, F(1, 25) = 7.26, P <0.05, Tukey HSD test, P <0.05. In contrast, chronic alcohol intake led to reduced (−20%) levels of D2 receptor binding in WIS rats when compared to control rats (P < 0.05) (Fig. 5).
Fig. 4.
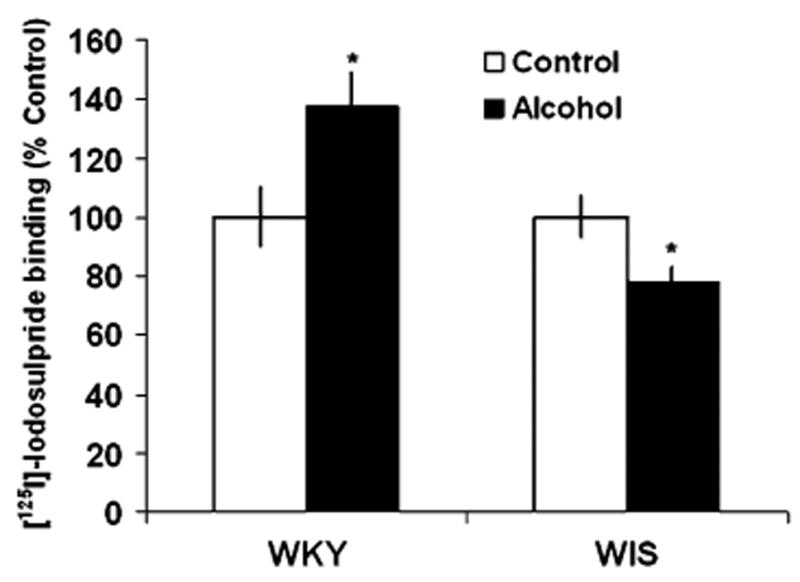
Effects of alcohol consumption on the binding of [125I]-Iodosulpiride to D2 receptor sites in the substantia nigra (SN) of WKY and WIS rats. Data are expressed as the mean ± SEM of measurements from eight rats from each strain, with determinations made in duplicate sections from each brain. Binding data are graphed as percent change from the water control group of each respective rat strain. Asterisk represents significant differences between treatment groups within strain, P < 0.05. WKY Wistar-Kyoto, WIS Wistar
Fig. 5.
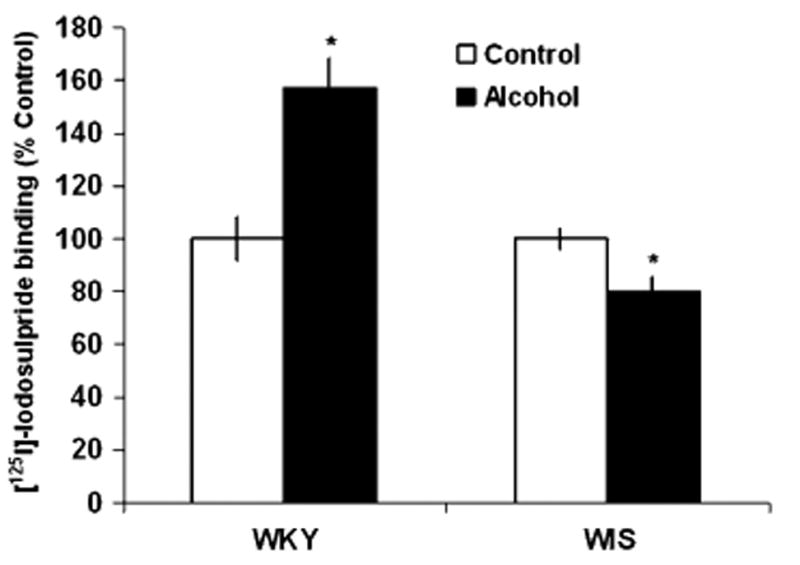
Effects of alcohol consumption on the binding of [125I]-Iodosulpiride to D2 receptor sites in the ventral tegmental area (VTA) of WKY and WIS rats. Data are expressed as the mean ± SEM of measurements from eight rats from each strain, with determinations made in duplicate sections from each brain. Binding data are graphed as percent change from the water control group of each respective rat strain. Asterisk represents significant differences between treatment groups within strain, P < 0.05. WKY Wistar-Kyoto, WIS Wistar
Discussion
Much of the current literature supports the idea that DA alterations may underlie increased alcohol drinking behavior. Previous reports have suggested that the reinforcing effects of alcohol are in part due to the stimulation of the mesolimbic DA pathway, and that a disruption in the normal functioning of this pathway may lead to a predisposition towards alcohol abuse [7, 45, 46]. Abnormal dopaminergic neurotransmission is thought to be one of the underlying mechanisms of increased alcohol consumption. Studies involving selectively bred alcohol preferring rats have reported lower baseline levels of DA in the NAc of these animals compared to their non-preferring counterpart [47] and Di Chiara and Imperato have suggested that since alcohol preferring rats have low basal DA tone, they consume more alcohol in order to re-establish or increase DA levels in the mesolimbic (reward) pathway [10]. When examining the neurochemical profile of WKY rats, De La Garza and Mahoney reported a reduction in DA levels in the pre-frontal cortex and an increase in DA turnover in the striatum and NAc of WKY compared to WIS animals under basal conditions [36]. These data may suggest reduced DA levels in the terminal field regions due to more active turnover processes in WKY rats. More recently we have noted a decrease in DA turnover in the VTA of WKY rats [48], suggesting less DA activity in this cell body region.
In support of the current literature surrounding DA deficiency as a precedent to increased alcohol consumption, we have also observed that WKY rats consumed 200% more alcohol than WIS rats when given a free choice of alcohol or water [38]. Thus, the aim of this report was to examine the effects of chronic alcohol consumption on D2 receptors in WKY compared to WIS rats using QAR.
In the current study, chronic alcohol consumption increased the binding of [125I]-Iodosulpiride to D2 receptor sites in the CPu, NAc, SN and VTA of WKY rats, but not WIS rats. Since, D2 autoreceptors inhibit impulse flow, neurotransmitter synthesis and release, the observed increase in D2 receptor density in the cell body areas (SN and VTA) following alcohol consumption may represent inhibition of extracellular DA levels [18, 19]. In the terminal regions (CPu and NAc), the noted D2 receptor up-regulation may be a consequence of lower DA levels in areas projecting from the SN and VTA. A previous study from our laboratory also reported increased DAT sites in the CPu and NAc regions of WKY rats following chronic alcohol consumption [37]. Since DAT sites are responsible for the clearance of synaptic DA, one explanation may be that increased DAT sites would lower levels of DA in the synaptic cleft following chronic alcohol consumption, thus contributing to the observed up-regulation of D2 receptor sites in the CPu and NAc. In agreement with our findings, Sari and colleagues reported that 14 weeks of continuous alcohol exposure increased D2 receptor density in the NAc region of inbred alcohol-preferring rats [49]. Similarly, Kim et al. reported an increase in D2 receptor mRNA in the NAc and CPu following prolonged alcohol consumption [50]. Previous studies of receptor binding have attributed an up-regulation of D2 receptor sites to a reduction in dopaminergic activity in several brain regions [51–53]. Therefore, an increase in D2 receptor sites in all brain regions measured of WKY rats may represent reduced DA levels as a result of chronic alcohol consumption, thus leading to increased consummatory behavior to re-establish baseline DA levels. Our results suggest that the differential regulation of D2 receptor binding in these two rat strains may be related to the differences in their daily alcohol intake values. Thus, the reduction in D2 receptor binding in cell body areas (VTA and SN) of WIS rats may indicate reduced DA auto-inhibition therefore leading to increased DA levels in the mesolimbic and nigrostriatal pathways of WIS animals.
While the increased alcohol drinking behavior in WKY rats may seem to contradict previously reported reductions in sucrose and nicotine self administration in WKY rats [32], we believe that this is not the case. One proposed explanation is that the previously reported learning and cognitive impairments and an inability to learn discrimination in an operant setting [54] in these animals may limit their ability to learn and perform in a self administration paradigm. Another possibility is that alcohol may be more reinforcing when compared with nicotine, as suggested by previous findings with conditioned place preference [55–58] and therefore WKY rats may consume more alcohol solution for its greater rewarding effects. However, this possibility does not fit with the sucrose findings as this substance has been shown to have high reinforcing value in a similar paradigm [59].
The greater density of D2 receptor sites in WKY rats following alcohol consumption may facilitate the previously reported increase in drinking behavior in this rat strain [37]. A functional role of D2 receptor sites in alcohol consumption is supported by findings that administration of a D2 like antagonist directly into the VTA or NAc reduces alcohol responding in rats [60, 61], and D2 knockout mice consume less alcohol than WT mice [21]. Recent evidence suggests that D2 receptor antagonists decrease alcohol consumption in clinical as well as in preclinical studies [13–15]. Clinical reports suggest the use of olanzapine (D2 antagonist) for substance dependence disorder [62], while in alcohol preferring rats, repeated olanzapine treatment significantly reduced alcohol consumption [15]. These studies suggest that targeting D2 receptors may provide a novel therapeutic approach to treating alcohol abuse and lend further support to the idea that D2 receptor mediated neurotransmission may be important in the increased alcohol drinking behavior observed in WKY rats.
Recently, we reported that stressed WKY rats consumed more alcohol than non-stressed animals [38]. Since WKY rats are characterized as hyper-responsive to stress, alcohol consumption may have a stress reducing effect in that alcohol reduces the emotional reactivity of WKY rats. This observation is strengthened because access to alcohol reduced the response latency in the OFT and increased time spent in the open arm in the EPM for WKY rats [63]. While stressed WKY rats exhibited a similar pattern of D2 receptor alterations as seen in the present study [38], it was interesting to observe that alcohol consuming stressed WKY rats showed a reversal in the stress-induced increases in D2 receptor binding [38]. It is possible that prolonged stress exposure could dampen DA signaling in several mesolimbic and mesocortical regions [6, 64], and the addition of alcohol to this regime could function to elevate daily levels of DA and block the stress-induced alterations in DA receptors [10, 12]. These observations would be in agreement with previous reports where alcohol administration was found to block stress induced increases in plasma epinephrine [65], corticosterone [66] as well as decrease the stress induced increases in DA in several brain regions [67] and norepinephrine [68].
Thus, based on our previous and present work, the WKY rat strain provides a suitable rodent model in which to investigate DA mediated mechanisms involved in alcohol consuming behaviors. Not only does the WKY rat strain exhibit depressive-like behavior [27, 29], it also voluntarily consumes 200% more alcohol than WIS rats, suggesting a resemblance to the clinical “self medication” phenotype [37, 38]. Previous reports of increased DAT sites [37] coupled with the present report of increased D2 receptor sites in similar brain regions, provides support for the involvement of DA pathways in the increased alcohol drinking behavior in this rat strain. Since pharmacological agents target D2 receptor sites in the treatment of alcohol abuse in preclinical and clinical settings, future studies will investigate the role of these pharmacological agents in regulating alcohol consumption in this rat strain.
Acknowledgments
This study was supported by USPHS Grant AA 015921 to S.T-B, and research funds from the Office of Research and Development, Medical Research Service, Department of Veteran Affairs (W. P.). The authors wish to thank Drs. William P. Paré and Xilu Jiao for their help with this study.
References
- 1.WHO. Management of substance abuse: Alcohol. 2009 [cited 2009 June 1]; available from: http://www.who.int/substance_abuse/facts/alcohol/en/index.html.
- 2.SAMHSA. Results from the 2002 National Survey on Drug Use and Health: National Findings 2003 [Google Scholar]
- 3.Di Chiara G, Acquas E, Carboni E. Drug motivation and abuse: a neurobiological perspective. Ann N Y Acad Sci. 1992;654:207–219. doi: 10.1111/j.1749-6632.1992.tb25969.x. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 5.Gambarana C, Masi F, Leggio B, et al. Acquisition of a palatable-food-sustained appetitive behavior in satiated rats is dependent on the dopaminergic response to this food in limbic areas. Neuroscience. 2003;121:179–187. doi: 10.1016/s0306-4522(03)00383-x. [DOI] [PubMed] [Google Scholar]
- 6.Gambarana C, Masi F, Tagliamonte A, et al. A chronic stress that impairs reactivity in rats also decreases dopaminergic transmission in the nucleus accumbens: a microdialysis study. J Neurochem. 1999;72:2039–2046. doi: 10.1046/j.1471-4159.1999.0722039.x. [DOI] [PubMed] [Google Scholar]
- 7.Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 8.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF. Hedonic valence, dopamine and motivation. Mol Psychiatry. 1996;1:186–189. [PubMed] [Google Scholar]
- 10.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan QS, Zheng SZ, Feng MJ, et al. Involvement of 5-HT1B receptors within the ventral tegmental area in ethanol-induced increases in mesolimbic dopaminergic transmission. Brain Res. 2005;1060:126–137. doi: 10.1016/j.brainres.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto K, McBride WJ, Lumeng L, et al. Ethanol enhances the release of dopamine and serotonin in the nucleus accumbens of HAD and LAD lines of rats. Alcohol Clin Exp Res. 1992;16:781–785. doi: 10.1111/j.1530-0277.1992.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 13.Hutchison KE, Ray L, Sandman E, et al. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006;31:1310–1317. doi: 10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- 14.Hutchison KE, Swift R, Rohsenow DJ, et al. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology (Berl) 2001;155:27–34. doi: 10.1007/s002130000629. [DOI] [PubMed] [Google Scholar]
- 15.Ingman K, Korpi ER. Alcohol drinking of alcohol-preferring AA rats is differentially affected by clozapine and olanzapine. Eur J Pharmacol. 2006;534:133–140. doi: 10.1016/j.ejphar.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Blum K, Noble EP, Sheridan PJ, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263:2055–2060. [PubMed] [Google Scholar]
- 17.Comings DE, Comings BG, Muhleman D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266:1793–1800. [PubMed] [Google Scholar]
- 18.Garris PA, Budygin EA, Phillips PE, et al. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 19.Kita JM, Parker LE, Phillips PE, et al. Paradoxical modulation of short-term facilitation of dopamine release by dopamine autoreceptors. J Neurochem. 2007;102:1115–1124. doi: 10.1111/j.1471-4159.2007.04621.x. [DOI] [PubMed] [Google Scholar]
- 20.Missale C, Nash SR, Robinson SW, et al. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 21.Phillips TJ, Brown KJ, Burkhart-Kasch S, et al. Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 23.Pare WP. Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol Behav. 1989;46:993–998. doi: 10.1016/0031-9384(89)90203-5. [DOI] [PubMed] [Google Scholar]
- 24.Redei E, Pare WP, Aird F, et al. Strain differences in hypothalamic-pituitary-adrenal activity and stress ulcer. Am J Physiol. 1994;266:R353–R360. doi: 10.1152/ajpregu.1994.266.2.R353. [DOI] [PubMed] [Google Scholar]
- 25.Tejani-Butt S, Kluczynski J, Pare WP. Strain-dependent modification of behavior following antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:7–14. doi: 10.1016/s0278-5846(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 26.Athey GR, Iams SG. Cold-restraint induced gastric lesions in normotensive and spontaneously hypertensive rats. Life Sci. 1981;28:889–894. doi: 10.1016/0024-3205(81)90050-3. [DOI] [PubMed] [Google Scholar]
- 27.Pare WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav. 1992;51:1051–1056. doi: 10.1016/0031-9384(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 28.Tejani-Butt SM, Pare WP, Yang J. Effect of repeated novel stressors on depressive behavior and brain norepinephrine receptor system in Sprague–Dawley and Wistar Kyoto (WKY) rats. Brain Res. 1994;649:27–35. doi: 10.1016/0006-8993(94)91045-6. [DOI] [PubMed] [Google Scholar]
- 29.Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 30.Jiao X, Pare WP, Tejani-Butt SM. Antidepressant drug induced alterations in binding to central dopamine transporter sites in the Wistar Kyoto rat strain. Progr Neuropsychopharmacol Biol Psychiatry. 2006;30:30–41. doi: 10.1016/j.pnpbp.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Rauhut AS, Zentner IJ, Mardekian SK, et al. Wistar Kyoto and Wistar rats differ in the affective and locomotor effects of nicotine. Physiol Behav. 2008;93:177–188. doi: 10.1016/j.physbeh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 32.De la Garza R., II Wistar Kyoto rats exhibit reduced sucrose pellet reinforcement behavior and intravenous nicotine self-administration. Pharmacol Biochem Behav. 2005;82:330–337. doi: 10.1016/j.pbb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Jiao X, Pare WP, Tejani-Butt S. Strain differences in the distribution of dopamine transporter sites in rat brain. Progr Neuropsychopharmacol Biol Psychiatry. 2003;27:913–919. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 34.Novick A, Yaroslavsky I, Tejani-Butt S. Strain differences in the expression of dopamine D1 receptors in Wistar-Kyoto (WKY) and Wistar rats. Life Sci. 2008;83:74–78. doi: 10.1016/j.lfs.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaroslavsky I, Colletti M, Jiao X, et al. Strain differences in the distribution of dopamine (DA-2 and DA-3) receptor sites in rat brain. Life Sci. 2006;79:772–776. doi: 10.1016/j.lfs.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 36.De La Garza R, II, Mahoney JJ., III A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: implications for animal models of anxiety and depression. Brain Res. 2004;1021:209–218. doi: 10.1016/j.brainres.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 37.Jiao X, Pare WP, Tejani-Butt SM. Alcohol consumption alters dopamine transporter sites in Wistar-Kyoto rat brain. Brain Res. 2006:1073–1074. 175–182. doi: 10.1016/j.brainres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Yaroslavsky I, Tejani-Butt SM. Voluntary alcohol consumption alters stress-induced changes in dopamine-2 receptor binding in Wistar-Kyoto rat brain. Pharmacol Biochem Behav. 2010;94:471–476. doi: 10.1016/j.pbb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 40.Carli M, Evenden JL, Robbins TW. Depletion of unilateral striatal dopamine impairs initiation of contralateral actions and not sensory attention. Nature. 1985;313:679–682. doi: 10.1038/313679a0. [DOI] [PubMed] [Google Scholar]
- 41.Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- 42.Sandbak T, Murison R. Voluntary alcohol consumption in rats: relationships to defensive burying and stress gastric erosions. Physiol Behav. 1996;59:983–989. doi: 10.1016/0031-9384(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sydney: 1986. [DOI] [PubMed] [Google Scholar]
- 44.Stefanski R, Lee SH, Yasar S, et al. Lack of persistent changes in the dopaminergic system of rats withdrawn from methamphetamine self-administration. Eur J Pharmacol. 2002;439:59–68. doi: 10.1016/s0014-2999(02)01301-8. [DOI] [PubMed] [Google Scholar]
- 45.Fibiger HC. Neurobiology of depression: focus on dopamine. Adv Biochem Psychopharmacol. 1995;49:1–17. [PubMed] [Google Scholar]
- 46.Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy JM, McBride WJ, Lumeng L, et al. Contents of monoamines in forebrain regions of alcohol-preferring (P) and—nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 1987;26:389–392. doi: 10.1016/0091-3057(87)90134-1. [DOI] [PubMed] [Google Scholar]
- 48.Scholl JL, Renner KJ, Forster GL, et al. Society for Neuroscience. Atlanta: 2007. A neurochemical analysis of central biogenic amine levels in the Wistar-Kyoto rat: a proposed animal model of depressive behavior. [Google Scholar]
- 49.Sari Y, Bell RL, Zhou FC. Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-preferring rats. Alcohol Clin Exp Res. 2006;30:46–56. doi: 10.1111/j.1530-0277.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MO, Lee YK, Choi WS, et al. Prolonged ethanol intake increases D2 dopamine receptor expression in the rat brain. Mol Cells. 1997;7:682–687. [PubMed] [Google Scholar]
- 51.May PC, Osterburg HH, Mandel RJ, et al. Alteration of calmodulin distribution does not accompany dopaminergic supersensitization of the mouse striatum. J Neurosci Res. 1987;17:247–250. doi: 10.1002/jnr.490170307. [DOI] [PubMed] [Google Scholar]
- 52.Severson JA, Robinson HE, Simpson GM. Neuroleptic-induced striatal dopamine receptor supersensitivity in mice: relationship to dose and drug. Psychopharmacology (Berl) 1984;84:115–119. doi: 10.1007/BF00432038. [DOI] [PubMed] [Google Scholar]
- 53.Eisenberg J, Brecher-Fride E, Weizman R, et al. Dopamine receptors in a rat model of minimal brain dysfunction. Neuropsychobiology. 1982;8:151–155. doi: 10.1159/000117891. [DOI] [PubMed] [Google Scholar]
- 54.Pare WP. Learning behavior, escape behavior, and depression in an ulcer susceptible rat strain. Integr Physiol Behav Sci. 1992;27:130–141. doi: 10.1007/BF02698502. [DOI] [PubMed] [Google Scholar]
- 55.Biala G, Langwinski R. Rewarding properties of some drugs studied by place preference conditioning. Pol J Pharmacol. 1996;48:425–430. [PubMed] [Google Scholar]
- 56.Nocjar C, Middaugh LD, Tavernetti M. Ethanol consumption and place-preference conditioning in the alcohol-preferring C57BL/6 mouse: relationship with motor activity patterns. Alcohol Clin Exp Res. 1999;23:683–692. [PubMed] [Google Scholar]
- 57.Parker LA. Place conditioning in a three- or four-choice apparatus: role of stimulus novelty in drug-induced place conditioning. Behav Neurosci. 1992;106:294–306. doi: 10.1037//0735-7044.106.2.294. [DOI] [PubMed] [Google Scholar]
- 58.Shoaib M, Stolerman IP, Kumar RC. Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology (Berl) 1994;113:445–452. doi: 10.1007/BF02245221. [DOI] [PubMed] [Google Scholar]
- 59.Agmo A, Galvan A, Talamantes B. Reward and reinforcement produced by drinking sucrose: two processes that may depend on different neurotransmitters. Pharmacol Biochem Behav. 1995;52:403–414. doi: 10.1016/0091-3057(95)00128-j. [DOI] [PubMed] [Google Scholar]
- 60.Czachowski CL, Santini LA, Legg BH, et al. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28:39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- 61.Eiler WJ, II, June HL. Blockade of GABA(A) receptors within the extended amygdala attenuates D(2) regulation of alcohol-motivated behaviors in the ventral tegmental area of alcohol-preferring (P) rats. Neuropharmacology. 2007;52:1570–1579. doi: 10.1016/j.neuropharm.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sattar SP, Grant K, Bhatia S, et al. Potential use of olanzapine in treatment of substance dependence disorders. J Clin Psychopharmacol. 2003;23:413–415. doi: 10.1097/01.jcp.0000085417.08426.54. [DOI] [PubMed] [Google Scholar]
- 63.Pare AM, Pare WP, Kluczynski J. Negative affect and voluntary alcohol consumption in Wistar-Kyoto (WKY) and Sprague–Dawley rats. Physiol Behav. 1999;67:219–225. doi: 10.1016/s0031-9384(99)00054-2. [DOI] [PubMed] [Google Scholar]
- 64.Cabib S, Puglisi-Allegra S. Different effects of repeated stressful experiences on mesocortical and mesolimbic dopamine metabolism. Neuroscience. 1996;73:375–380. doi: 10.1016/0306-4522(96)00750-6. [DOI] [PubMed] [Google Scholar]
- 65.Vogele C, Steptoe A. Physiological and subjective stress responses in surgical patients. J Psychosom Res. 1986;30:205–215. doi: 10.1016/0022-3999(86)90051-6. [DOI] [PubMed] [Google Scholar]
- 66.Rivier C, Vale W. Interaction between ethanol and stress on ACTH and beta-endorphin secretion. Alcohol Clin Exp Res. 1988;12:206–210. doi: 10.1111/j.1530-0277.1988.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 67.Fadda F, Mosca E, Niffoi T, et al. Ethanol prevents stress-induced increase in cortical DOPAC: reversal by RO 15-4513. Physiol Behav. 1987;40:383–385. doi: 10.1016/0031-9384(87)90065-5. [DOI] [PubMed] [Google Scholar]
- 68.Shirao I, Tsuda A, Ida Y, et al. Effect of acute ethanol administration on noradrenaline metabolism in brain regions of stressed and nonstressed rats. Pharmacol Biochem Behav. 1988;30:769–773. doi: 10.1016/0091-3057(88)90097-4. [DOI] [PubMed] [Google Scholar]