Abstract
The effects of two valerian extracts (aqueous and hydroalcoholic) were investigated through [3H]Glutamate ([3H]Glu) and [3H]Fluorowillardine ([3H]FW) receptor binding assays using rat synaptic membranes in presence of different receptor ligands. In addition, the extract stability was monitored spectrophotometrically. Both extracts demonstrated interaction with ionotropic glutamate receptors (iGluRs). However, the extracts displayed considerable differences in receptor selectivity. The hydroalcoholic extract selectively interacted with quisqualic acid (QA), group I metabotropic glutamate receptor (mGluR) ligand, while the aqueous extract did not alter the binding of QA. The stability of the extracts was examined during several weeks. Freshly prepared extract inhibited 38–60% of [3H]FW binding (AMPA). After 10 days, the aqueous extract inhibited 85% of [3H]FW binding while the hydroalcoholic extract markedly potentiated (200%) [3H]FW binding to AMPA receptors. Thus, our results showed that factors such as extraction solvent and extract stability determine the selectivity for glutamate receptor (GluR) interactions.
1. Introduction
Valeriana officinalis, commonly known as Valerian, is one of approximately 250 Valerian species from the Valerianaceae family, and it is used for the preparation of phytomedicines with sedative, anxiolytic, and spasmolytic properties. The therapeutic benefits of Valerian have always been in controversy due to the inconsistent clinical results [1–3]. The discrepancies among clinical trials may be due to methodological limitations and differences in the Valerian preparations (method of extraction and extraction solvent). Many studies use water as the extraction solvent because traditionally natural product preparations (i.e., tea) are prepared in water. Aqueous extracts of Valerian have different spectrum of active constituents than extracts that have been processed with ethanol [4].
The specific mechanism(s) of action responsible for the pharmacological effects of Valerian root extracts have not been fully elucidated although it is thought that Valerian root extracts stimulate the GABA transmission [2, 3, 5–10]. On the other hand, few studies have investigated the effects of Valeriana officinalis in the excitatory glutamate-mediated neurotransmission [11].
Based on these studies, the possible effects of Valerian extracts on glutamate-mediated (excitatory) neurotransmission were assessed using receptor binding assays with rat synaptic membranes in presence of aqueous and hydroalcoholic Valerian extracts and glutamate receptors ligands (MK-801 for NMDA, AMPA or [3H]Fluorowillardiine for AMPA, kainic acid for KA, and quisqualic acid (QA) for Group I metabotropic glutamate receptors (mGluRs)).
2. Materials and Methods
2.1. Chemicals
L-[2,3,4-3H]-Glutamic acid (60 Ci/mmol) and [3H]Fluorowiillardiine (80 Ci/mmol) were obtained from American Radiolabeled Chemicals, Inc (St. Louis, MO). AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid) was obtained from Tocris Cookson, Inc. (Ellisville, MO). Potassium Chloride was purchased from Matheson Coleman & Bell (Norwood, OH). UniverSol ES was obtained from MP Biomedicals (Solon, OH). All other reagents were obtained from Sigma Chemical Company (St. Louis, MO).
2.2. Valerian Extracts
Organically grown and certified Valerian dry powdered roots (Lot. 1111H-OUP), harvested in 2004, were obtained from Pacific Botanicals (LLC Grants Pass, Oregon). Commercial Valerian. Nature's Resources Extract (Lot. LD11282N) and Nature's Resource Herb (Lot. MG11258) were purchased from a local pharmacy. Valerian was extracted in ultra pure water or ethyl alcohol (EtOH) 70% (1 : 10 w/v) at ~23°C, stirred for 1 hour, and filtered through a 12.5 cm Whatman Qualitative no. 1 filter. Aliquots were centrifuged at 6,700 g to remove particulates and stored at 4°C.
2.3. Cerebral Cortex Synaptic Membranes
Analytical Biological Services, Inc. (Wilmington, DE) prepared the synaptic membranes as follows: female rats of approximately two months of age were decapitated and the brain promptly removed. The cortex was dissected and homogenized (1 : 10 w/v) in ice-cold 10 mM Tris-HCl buffer pH 7.4. The homogenate was centrifuged twice at 2,500 g for 10 min. The resulting supernatant was centrifuged at 12,500 g for 20 min. The pellet was washed twice with ice-cold 10mM Tris-HCl buffer pH 7.4 (1 : 10 w/v) and centrifuged at 12,500 g for 20 min. The pellet (synaptical membrane, P2) was resuspended in 10 mM Tris-HCl buffer pH 7.4 and freeze thawed at least three times before being stored at −80°C until used. Protein concentration was determined using the Bradford assay [12] using bovine serum albumin (BSA) as reference standard.
2.4. Receptor Binding Assays
2.4.1. [3H]Glutamate Binding
Receptor binding competition assays were done using synaptic membranes of cerebral cortex from Analytical Biological Services, Inc. (Wilmington, DE). The reaction was initiated by the addition of tissue (100 μg protein) to tubes containing 1 mM of (MK-801 for NMDA, AMPA, kainic acid for KA, and quisqualic acid (QA) for Group I metabotropic glutamate receptors (mGluR) and 20 nM [3H]Glutamic Acid ([3H]Glu) in a final volume of 500 μL of 50 mM Tris HCl/100 mM KCl buffer, pH 7.4). The nonspecific binding was determined in the presence of 1 mM nonradioactive glutamate. Total binding was determined in the presence of Valerian extracts in different solvents (H2O and EtOH) in order to do competition studies. All samples were incubated on ice (0–4°C) for 40 minutes. The assay was stopped by centrifugation for 30 min at 6,764 g; the supernatant was extracted and the pellet washed two times with 1 mL of ice-cold buffer. The pellet was resuspended in 500 μL of buffer. Radioactivity of the samples was quantified in a Beckman LS 6500 Multipurpose Scintillation Counter with 1 mL of UniverSol ES scintillation cocktail. Results are shown as percentage of total binding (±SEM).
2.4.2. [3H]Fluorowillardine Binding
Assays were done using synaptic membranes of cerebral cortex from Analytical Biological Services, Inc. (Wilmington, DE). The reaction was initiated by the addition of tissue (100 μg protein) to tubes containing 15 nM [3H] Fluorowillardine ([3H]FW) in a final volume of 500 μL of 50 mM Tris HCl/100 mM KCl buffer, pH 7.4. Nonspecific binding was determined in the presence of 10 mM glutamate. Total binding was determined in the presence of Valerian extracts in different solvents (H2O and EtOH) in order to do competition studies. All samples were incubated on ice (0–4°C) for 40 minutes. The assay was stopped by centrifugation for 30 min at 6,764 g. Then, the supernatant was removed and the pellet washed two times with 1 mL of ice-cold buffer. The pellet was resuspended in 500 μL of buffer. Radioactivity of the samples was quantified in a Beckman LS 1800 counter with 1 mL of UniverSol scintillation cocktail. Results are shown as percentage of total binding (±SEM).
2.5. Wavelength Scans
Spectrophotometric analyses were done with a Beckman DU Series 500 Spectrophotometer (Fullerton, CA). A volume of 1 mL of 10 mg/mL Valerian extract was placed in Elkay Ultra-Vu Disposable Cuvettes from Elkay Products, Inc. (Shrewsbery, MA). The scans were done from 190 nm to 320 nm in steps of 1.0 nm.
2.6. Statistical Analysis
Data are expressed as mean values ± the standard error of the mean (SEM) of at least three experiments. The differences between the experimental groups were tested for significance using one way analysis of variance followed by Tukey-Kramer Multiple Comparisons Test, with P < .05. Statistics for the experimental group versus total binding are not shown for clarity.
3. Results
3.1. Extraction Solvents Change Ligand Binding to Glutamate Receptors
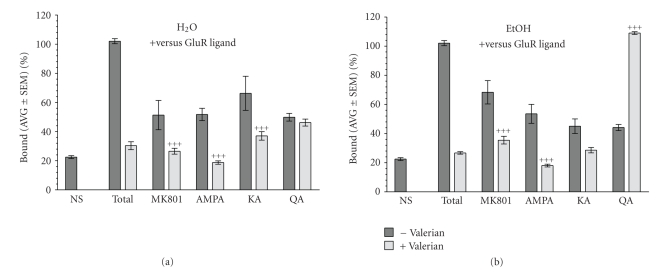
Figure 1 shows the effects of different Valerian extracts (1 mg/mL) on [3H]Glutamate binding to rat synaptic membranes in the presence of 1 mM GluR ligands (NMDA, AMPA, KA, or QA). The aqueous extract (a) decreases the effects of the NMDA (MK-801), AMPA, and kainate (KA), but it did not alter the effects of quisqualic acid (QA), a Group I metabotropic glutamate receptor (mGluR) ligand. The hydroalcoholic extract (b) has similar effects as the aqueous extracts on NMDA, AMPA, and KA treated membranes. However, the hydroalcoholic extract potentiates the effects of QA, suggesting a strong effect on group I mGluR.
Figure 1.
Effects of different fresh Valerian extracts (1 mg/mL) on [3H]Glutamate binding to rat synaptic membranes in the presence of 1 mM glutamate receptor (GluR) ligands. (a) The aqueous extract decreases the effects of the NMDA (MK-801), AMPA, and Kainate (KA), but it did not alter the effects of quisqualic acid (QA), a group I metabotropic glutamate receptor (mGluR) ligand. (b) The hydroalcoholic extract also decreases the effects of NMDA, AMPA, and KA. In contrast, Valerian reverses the inhibitory effects of QA. + agonist versus agonist + Valerian, P < .05; ++ P < .01; +++ P < .001.
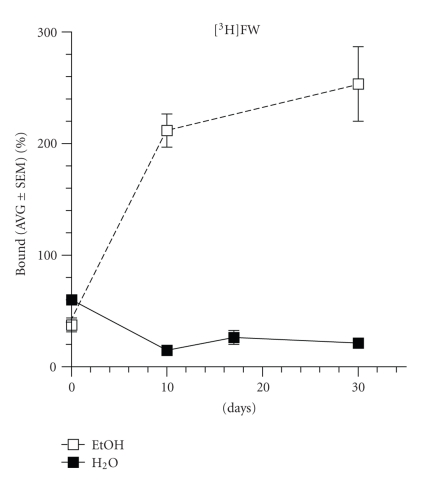
Similarly, the effects of aqueous and hydroalcoholic extracts on AMPA were evaluated by binding assays using [3H]FW. Initially, the aqueous and hydroalcoholic extracts (Figure 2) have 60% and 38% inhibitory effects on [3H]FW binding, respectively. However, with time (4 weeks), the aqueous extract inhibits [3H]FW to 85% while the hydroalcoholic extract markedly increases [3H]FW binding (200%) to AMPA receptors.
Figure 2.
Changes in 3[H]Fluorowillardine binding with time. Initially, a 60% of inhibition is observed with the aqueous extracts while the hydroalcoholic extract inhibits only a 38%. However, with time, the inhibitory effects of the aqueous extract increase to 85% whereas the hydroalcoholic extract markedly potentiates (200%) 3[H]Fluorowillardine ([3H]FW) binding to AMPA receptors.
3.2. Extract Stability Alters Ligand Binding to Glutamate Receptor
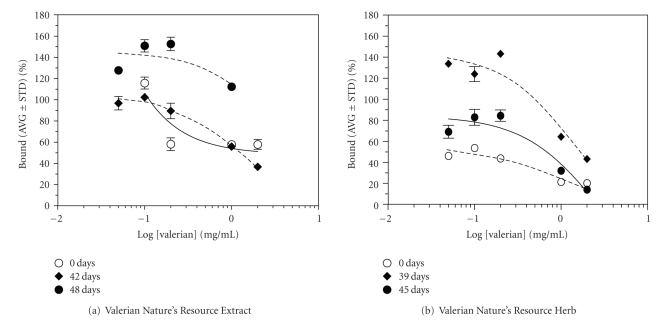
The effect of two Valerian commercial products (prepared as aqueous extracts) was evaluated on AMPA binding ([3H]FW). Figure 3 shows the effect of time in [3H]FW binding. (a) At time zero, Valerian Nature's Resource Extract and Nature's Resource Herb inhibit [3H]Fluorowillardine binding. (b) After a period of time (42 days (Extract) or 39 days (Herb)), the extracts loose the inhibitory effects. Further in time (48 days (Extract) or 45 days (Herb)), they potentiate [3H]Fluorowillardine binding.
Figure 3.
Effect of time in 3[H]Fluorowillardine binding from two Valerian commercial products. (a) At time zero, aqueous extracts of Nature's Resource Extract and Nature's Resource Herb inhibit [3[H]]Fluorowillardine binding. (b) After a period of time (42 days (Extract) or 39 days (Herb)), the extracts loose the inhibitory effects. Further in time (48 days (Extract) or 45 days (Herb)), they potentiate [3[H]]Fluorowillardine binding.
3.3. Spectrophotometric Analysis of Aqueous and Hydroalcoholic Extracts
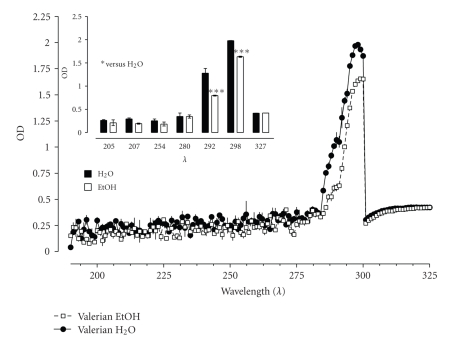
The spectra of different Valerian extracts (10 mg/mL) prepared in H2O and EtOH were evaluated spectrophotometrically as shown in Figure 4. The insert in Figure 4 shows a list of wavelength and only two, 292 and 298 nm, demonstrate significant differences.
Figure 4.
Spectra and absorbance from aqueous and hydroalcoholic Valerian extracts (10 mg/mL). Significant differences at 292 and 298 nm were observed. H2O versus EtOH, P < .05; ++ P < .01; +++ P < .001.
4. Discussion
Valerian is a well-recognized plant used in the folkloric medicine for its sedative and anxiolytic properties [2]. Numerous studies evaluate the use of Valerian root extracts to induce and improve sleep quality [13–15] while others evaluate Valerian anxiolytic effects [16–18]. In humans, Valerian extracts reduced latency to fall asleep as effectively as small doses of benzodiazepines [19]. Although Valerian's anxiolytic properties are well documented in mice and rats [20], there is no scientific evidence that could support the anxiolytic properties of the plant in humans, due to intrinsic methodological problems such as variation in Valerian doses and preparations, number of patients, and length of treatment [1].
It is not fully understood which constituents of Valeriana officinalis are responsible for the pharmacological actions, but many manufacturers standardize Valerian preparations according to the valerenic acid content as recommended by the German Commission E. Studies suggested that valerenic acid is the principal active component responsible for Valerian's anxiolytic properties [21, 22]. However, other Valerian constituents such as borneol, lignans, and flavonoids (hesperidin, linarin, and 6-methylapigenin) have also been found to have anxiolytic and sedative activity [23–27]. Moreover, other Valerian species with low valerenic acid concentration (e.g., Valeriana edulis and Valeriana sitchensis) have similar pharmacological profiles [19, 28–30]. Thus, there is no evidence showing that valerenic acid is exclusively responsible for the sedative and anxiolytic effects attributed to Valeriana officinalis.
Circosta and colleagues [31] conducted the biological and chemical characterization of two Valerian extracts (aqueous and hydroalcoholic) and found remarkable differences in the content of valepotriates. Similar results were found by Occhiuto et al. in 2008 [32]. Both studies, from the same group of researchers, revealed that the phytochemical analysis of the hydroalcoholic extract and the aqueous extract was “similar” in the valerenic acid composition (aqueous extracts being half of the hydroalcoholic extract content), but the concentration of the valepotriates was much lower in the aqueous extract than in the hydroalcoholic extract. In the biological assays, Circosta and colleagues [31] observed a lower percent of animals with arrhythmias when the hydroalcoholic and not the aqueous extracts were given at a dose of 50 mg/kg (80% versus 60%, resp.). Similarly, Occhiuto et al. [32] reported that the concentration causing 50% inhibition of the amplitude of contractions (uterorelaxant effect) was 29.5 ± 3.40 and 68.7 ± 5.20 μg/mL for the hydroalcoholic and aqueous extracts, respectively. Therefore, the biological analysis by these studies demonstrated that the Valerian extracts possess coronary dilatory, hypotensive, and bronchodilatory properties as well as relaxing effects on human myometrial muscle at different concentrations; the hydroalcoholic extracts being always more potent. These differences in potency of the hydroalcoholic and aqueous extracts may be related to differences in the extraction solvent and procedure, raw material storage conditions, growing techniques, harvesting conditions, and age of the plant, among other factors.
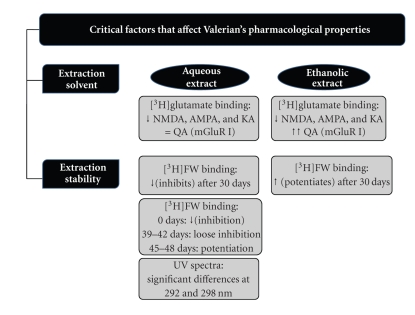
Previously, modest effects of commercial aqueous Valerian extracts on NMDA receptor [3H]MK-801 binding have been reported [33]. Similarly, Malva and colleagues [11] reported AMPA-mediated neuroprotective effects of Valerian. Our experiments show that aqueous and hydroalcoholic extracts from Valeriana officinalis have different biological activity as demonstrated by the GluR binding assays. Aqueous and hydroalcoholic Valerian extracts interact with NMDA, AMPA, and KA receptors. Moreover, Valerian extracts effects on different receptors change with time as seen with AMPA binding assays. On the other hand, the aqueous extracts did not have effect in QA while a strong interaction with group I mGluR is observed with the hydroalcoholic extracts (see Figure 5). Previous results from our lab (not shown) had demonstrated that when valerenic acid was used, as a pure compound, in the same type of experiments, a similar response (potentiation) was obtained. These results suggested that the hydroalcoholic Valerian extract reverses the inhibitory effects of QA probably due to a higher content of valerenic acid. Further studies should be done to verify the valerenic acid content in the different extracts.
Figure 5.
Summary of the aqueous and ethanolic Valerian extract effects in receptor binding assays and spectrophotometric absorbance.
Previous studies by different researchers suggest that mGluR may have an important role in anxiety [34–36]. In particular, there is evidence involving group II mGluR (mGlu2/3) and group I mGluR (mGlu5) receptor agonists as potential anxiolytic agents [37, 38]. This mGluR role could suggest a possible mechanism by which Valerian extracts produce their effects. A more detailed study of these Valerian interactions with mGluR is being examined by our lab.
Navarrete et al. (2006) [29] used HPLC and detection at different wavelengths to identify Valerian components (i.e., chlorogenic acid, lignans, flavonoids, valerenic acids, and valepotriates) in different species. Similarly, we screened for possible spectrographic differences between the aqueous and hydroalcoholic extracts. A qualitative analysis of the absorbance spectra (at different wavelengths) of the extracts revealed significant differences at 292 and 298 nm. Further studies should be done in order to determine the relation between spectra changes and binding selectivity.
A survey made by ConsumerLab.com reported that 4 of 17 Valerian products had no detectable Valerian compounds, four had half of the expected levels, two had lead contamination, and one had cadmium contamination (http://www.consumerlab.com) [39]. This particular situation occurred with commercial natural products manufactured by various processes using different solvents. In addition, the low stability of the compounds, mainly valepotriates, could result in the absence of these compounds in the different pharmaceutical dosage forms since they may decompose during the manufacturing process or upon storage [40].
Products containing Valerian have managed to keep their place in the natural products market, in spite of the lack of science based evidence for its efficacy and the strong competition with synthetic drugs such as benzodiazepines. Inconsistent and variable pharmacological effects of plant-derived products are one of the challenges of the natural products research. Consequently, a drug derived from natural products could be considered as a rational drug only if it is standardized and proven to meet the standards of quality (prove identity, purity, etc.). Biological screening of plant extracts instead of analysis of individual compound may be a useful tool in clarifying Valerian pharmacological effects because of possible synergism as a mechanism of action(s) of various active compounds. The present study demonstrates that Valerian extracts interact with the GluR, and factors such as the extraction solvent and stability of the extracts are critical to determine changes in selectivity for GluR interaction.
Acknowledgments
This work was supported in part by the Research Centers for Minority Institution (RCMI/NIH Grant no. G12 RR03051), Institutional Minority Biomedical Research Support (MBRS-RISE) Program at the University of Puerto Rico, Medical Sciences Campus (Grant no. 2 R25 GM061838-05), and Puerto Rico Louis Stokes Alliance for Minority Participation (PR-LSAMP) Program at the University of Puerto Rico (Grant no. HRD-0601843).
Abbreviations
- AMPA:
Alpha-amino-3-hydroxy-5-methylisox-azole-4-propionic acid
- BSA:
Bovine serum albumin
- EtOH:
Ethyl alcohol
- [3H]FW:
[3H]Fluorowillardine
- [3H]Glu:
[3H]Glutamate
- GluR:
Glutamate receptors
- Group I mGluR:
Metabotropic glutamate receptor (1/5)
- iGluR:
Ionotropic glutamte receptor
- KA:
Kainic acid
- mGluR:
Metabotropic glutamate receptors
- NMDA:
N-methyl-D-aspartic acid
- QA:
Quisqualic acid, (2S)-2-amino-3-(3,5-dioxo-1,2,4-oxadiazolidin-2-yl) propanoic acid).
References
- 1.Miyasaka LS, Atallah AN, Soares BGO. Valerian for anxiety disorders. Cochrane Database of Systematic Reviews. 2006;(4) doi: 10.1002/14651858.CD004515.pub2. Article ID CD004515. [DOI] [PubMed] [Google Scholar]
- 2.Houghton PJ. The scientific basis for the reputed activity of valerian. Journal of Pharmacy and Pharmacology. 1999;51(5):505–512. doi: 10.1211/0022357991772772. [DOI] [PubMed] [Google Scholar]
- 3.Diaper A, Hindmarch I. A double-blind, placebo-controlled investigation of the effects of two doses of a valerian preparation on the sleep, cognitive and psychomotor function of sleep-disturbed older adults. Phytotherapy Research. 2004;18(10):831–836. doi: 10.1002/ptr.1574. [DOI] [PubMed] [Google Scholar]
- 4.Schulz V, Hänsel R, Blumenthal M, Tyler VE. Rational Phytotherapy: A Reference Guide for Physicians and Pharmacists. 5th edition. Berlin, Germany: Springer; 2004. [Google Scholar]
- 5.Santos MS, Ferreira F, Cunha AP, Carvalho AP, Macedo T. An aqueous extract of valerian influences the transport of GABA in synaptosomes. Planta Medica. 1994;60(3):278–279. doi: 10.1055/s-2006-959476. [DOI] [PubMed] [Google Scholar]
- 6.Santos MS, Ferreira F, Cunha AP, Carvalho AP, Ribeiro CF, Macedo T. Synaptosomal GABA release as influenced by valerian root extract—involvement of the GABA carrier. Archives Internationales de Pharmacodynamie et de Therapie. 1994;327(2):220–231. [PubMed] [Google Scholar]
- 7.Cavadas C, Araujo I, Cotrim MD, et al. In vitro study on the interaction of Valeriana officinalis L. extracts and their amino acids on GABA(A) receptor in rat brain. Arzneimittel-Forschung/Drug Research. 1995;45(7):753–755. [PubMed] [Google Scholar]
- 8.Ortiz JG, Nieves-Natal J, Chavez P. Effects of Valeriana Officinalis extracts on 3[H]flunitrazepam binding, synaptosomal 3[H]GABA uptake, and hippocampal 3[H]GABA release. Neurochemical Research. 1999;24(11):1373–1378. doi: 10.1023/a:1022576405534. [DOI] [PubMed] [Google Scholar]
- 9.Yuan C-S, Mehendale S, Xiao Y, Aung HH, Xie J-T, Ang-Lee MK. The gamma-aminobutyric acidergic effects of valerian and Valerenic acid on rat brainstem neuronal activity. Anesthesia and Analgesia. 2004;98(2):353–358. doi: 10.1213/01.ANE.0000096189.70405.A5. [DOI] [PubMed] [Google Scholar]
- 10.Neuhaus W, Trauner G, Gruber D, et al. Transport of a CABAA receptor modulator and its derivatives from Valeriana officinalis L s. I. across an in vitro cell culture model of the blood-brain barrier. Planta Medica. 2008;74(11):1338–1344. doi: 10.1055/s-2008-1081343. [DOI] [PubMed] [Google Scholar]
- 11.Malva JO, Santos S, Macedo T. Neuroprotective properties of Valeriana officinalis extracts. Neurotoxicity Research. 2004;6(2):131–140. doi: 10.1007/BF03033215. [DOI] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Bent S, Padula A, Moore D, Patterson M, Mehling W. Valerian for sleep: a systemic review and meta-analysis. American Journal of Medicine. 2006;119(12):1005–1012. doi: 10.1016/j.amjmed.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leathwood PD, Chauffard F, Heck E, Munoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacology Biochemistry and Behavior. 1982;17(1):65–71. doi: 10.1016/0091-3057(82)90264-7. [DOI] [PubMed] [Google Scholar]
- 15.Stevinson C, Ernst E. Valerian for insomnia: a systematic review of randomized clinical trials. Sleep Medicine. 2000;1(2):91–99. doi: 10.1016/s1389-9457(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 16.Andreatini R, Sartori VA, Seabra MLV, Leite JR. Effect of valepotriates (valerian extract) in generalized anxiety disorder: a randomized placebo-controlled pilot study. Phytotherapy Research. 2002;16(7):650–654. doi: 10.1002/ptr.1027. [DOI] [PubMed] [Google Scholar]
- 17.Leuschner J, Müller J, Rudmann M. Characterisation of the central nervous depressant activity of a commercially available valerian root extract. Arzneimittel-Forschung/Drug Research. 1993;43(6):638–641. [PubMed] [Google Scholar]
- 18.Lindahl O, Lindwall L. Double blind study of a Valerian preparation. Pharmacology Biochemistry and Behavior. 1989;32(4):1065–1066. doi: 10.1016/0091-3057(89)90082-8. [DOI] [PubMed] [Google Scholar]
- 19.Leathwood PD, Chauffard F. Aqueous extract of valerian reduces latency to fall asleep in man. Planta Medica. 1985;(2):144–148. doi: 10.1055/s-2007-969430. [DOI] [PubMed] [Google Scholar]
- 20.Hattesohl M, Feistel B, Sievers H, Lehnfeld R, Hegger M, Winterhoff H. Extracts of Valeriana officinalis L. s.l. show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine. 2008;15(1-2):2–15. doi: 10.1016/j.phymed.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Benke D, Barberis A, Kopp S, et al. GABAA receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology. 2009;56(1):174–181. doi: 10.1016/j.neuropharm.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Hendriks H, Bos R, Woerdenbag HJ, Koster AS. Central nervous depressant activity of valerenic acid in the mouse. Planta Medica. 1985;1:28–31. [PubMed] [Google Scholar]
- 23.Wasowski C, Marder M, Viola H, Medina JH, Paladini AC. Isolation and identification of 6-methylapigenin, a competitive ligand for the brain GABAA receptors, from Valeriana wallichii. Planta Medica. 2002;68(10):934–936. doi: 10.1055/s-2002-34936. [DOI] [PubMed] [Google Scholar]
- 24.Schumacher B, Scholle S, Hölzl J, Khudeir N, Hess S, Müller CE. Lignans isolated from Valerian: identification and characterization of a new olivil derivative with partial agonistic activity at A1 adenosine receptors. Journal of Natural Products. 2002;65(10):1479–1485. doi: 10.1021/np010464q. [DOI] [PubMed] [Google Scholar]
- 25.Marder M, Viola H, Wasowski C, Fernández S, Medina JH, Paladini AC. 6-Methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS. Pharmacology Biochemistry and Behavior. 2003;75(3):537–545. doi: 10.1016/s0091-3057(03)00121-7. [DOI] [PubMed] [Google Scholar]
- 26.Fernández S, Wasowski C, Paladini AC, Marder M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacology Biochemistry and Behavior. 2004;77(2):399–404. doi: 10.1016/j.pbb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Granger RE, Campbell EL, Johnston GAR. (+)- and (−)-borneol: efficacious positive modulators of GABA action at human recombinant α 1 β 2γ2LGABAA receptors. Biochemical Pharmacology. 2005;69(7):1101–1111. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Gao XQ, Björk L. Valerenic acid derivatives and valepotriates among individuals, varieties and species of Valeriana. Fitoterapia. 2000;71(1):19–24. doi: 10.1016/s0367-326x(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 29.Navarrete A, Avula B, Choi Y-W, Khan IA. Chemical fingerprinting of valeriana species: simultaneous determination of valerenic acids, flavonoids, and phenylpropanoids using liquid chromatography with ultraviolet detection. Journal of AOAC International. 2006;89(1):8–15. [PubMed] [Google Scholar]
- 30.Oliva I, González-Trujano ME, Arrieta J, Enciso-Rodríguez R, Navarrete A. Neuropharmacological profile of hydroalcohol extract of Valeriana edulis ssp. procera roots in mice. Phytotherapy Research. 2004;18(4):290–296. doi: 10.1002/ptr.1389. [DOI] [PubMed] [Google Scholar]
- 31.Circosta C, De Pasquale R, Samperi S, Pino A, Occhiuto F. Biological and analytical characterization of two extracts from Valeriana officinalis. Journal of Ethnopharmacology. 2007;112(2):361–367. doi: 10.1016/j.jep.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Occhiuto F, Pino A, Palumbo DR, et al. Relaxing effects of Valeriana officinalis extracts on isolated human non-pregnant uterine muscle. Journal of Pharmacy and Pharmacology. 2009;61(2):251–256. doi: 10.1211/jpp/61.02.0016. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz JG, Rassi N, Maldonado PM, González-Cabrera S, Ramos I. Commercial Valerian interactions with [3H]flunitrazepam and [3H]MK-801 binding to rat synaptic membranes. Phytotherapy Research. 2006;20(9):794–798. doi: 10.1002/ptr.1960. [DOI] [PubMed] [Google Scholar]
- 34.Chojnacka-Wójcik E, Klodzinska A, Pilc A. Glutamate receptor ligands as anxiolytics. Current Opinion in Investigational Drugs. 2001;2(8):1112–1119. [PubMed] [Google Scholar]
- 35.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacology and Therapeutics. 2007;115(1):116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Swanson CJ, Bures M, Johnson MP, Linden A-M, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nature Reviews Drug Discovery. 2005;4(2):131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 37.Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6(3):189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- 38.Spooren WPJM, Vassout A, Neijt HC, et al. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. Journal of Pharmacology and Experimental Therapeutics. 2000;295(3):1267–1275. [PubMed] [Google Scholar]
- 39.Valerian: product review. http://www.consumerlab.com.
- 40.Bos R, Woerdenbag HJ, Hendriks H, et al. Analytical aspects of phytotherapeutic valerian preparations. Phytochemical Analysis. 1996;7(3):143–151. [Google Scholar]