Abstract
Presenilin is the catalytic component of γ-secretase, a complex aspartyl protease and a founding member of intramembrane-cleaving proteases. γ-Secretase is involved in the pathogenesis of Alzheimer’s disease and a top target for therapeutic intervention. However, the protease complex processes a variety of transmembrane substrates, including the Notch receptor, raising concerns about toxicity. Nevertheless, γ-secretase inhibitors and modulators have been identified that allow Notch processing and signalling to continue, and promising compounds are entering clinical trials. Molecular and biochemical studies offer a model for how this protease hydrolyzes transmembrane domains in the confines of the lipid bilayer. Progress has also been made toward structure elucidation of presenilin and the γ-secretase complex by electron microscopy as well as by studying cysteine-mutant presenilins. The signal peptide peptidase (SPP) family of proteases are distantly related to presenilins. However, the SPPs work as single polypeptides without the need for cofactors and otherwise appear to be simple model systems for presenilin in the γ-secretase complex. SPP biology, structure, and inhibition will also be discussed.
Keywords: amyloid, Notch receptor, peptidomimetics, signal peptide peptidase, substrate analogues, substrate recognition
Introduction
Intramembrane-cleaving proteases (I-CLiPs) are multi-pass membrane proteins that process the transmembrane regions of their substrate and have their active sites lying within the confines of the lipid bilayer (Wolfe, 2009). So far, I-CLiPs have been identified that are metalloproteases (the S2P family), serine proteases (the rhomboid family) and aspartyl proteases (the presenilin and SPP families). These proteases are found in all forms of life and play a variety of roles in biology and disease. Presenilin is part of a large protease complex called γ-secretase, involved in Alzheimer’s disease, cell signalling, and membrane protein degradation (Selkoe and Wolfe, 2007). Members of the SPP family require no additional protein factors and are otherwise simpler enzymes compared to the distantly related presenilins (Fluhrer et al., 2009). In this review, we will discuss the role of presenilin and the γ-secretase complex in Alzheimer’s disease and in basic cell biology, how this enzyme hydrolyzes transmembrane substrates, the prospects of developing Alzheimer therapeutics that target γ-secretase, progress toward elucidating the structure of γ-secretase, and how the SPP family may provide insight into presenilin structure and function in the γ-secretase complex.
Presenilin in Alzheimer’s Disease
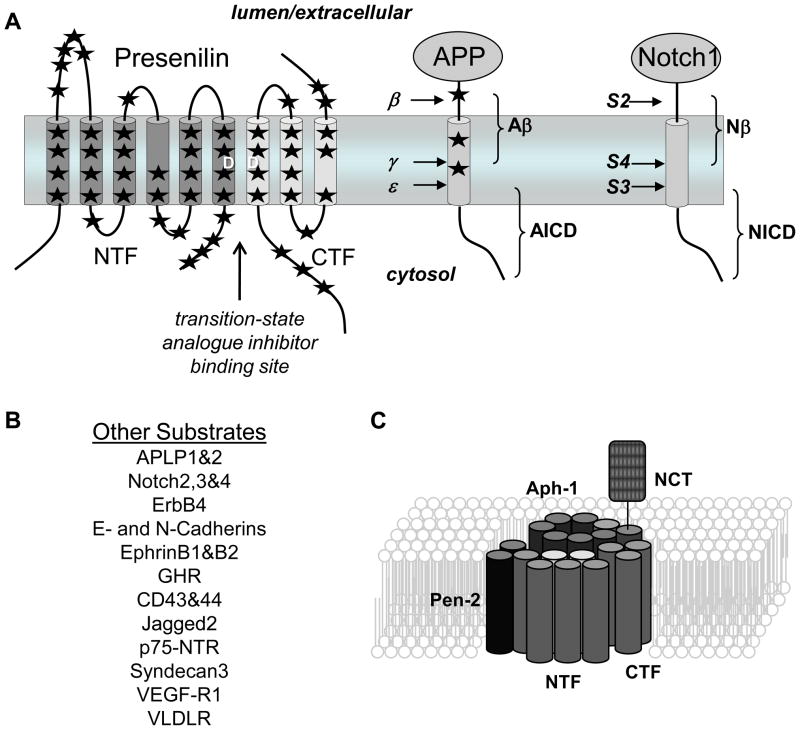
A key step in the pathogenesis of Alzheimer’s disease is proteolysis of the amyloid β-protein precursor (APP) that results in the formation of the amyloid β-protein (Aβ) (Figure 1A), the principle protein component of the characteristic cerebral plaques of the disease (Hardy and Selkoe, 2002; Tanzi and Bertram, 2005). The N-terminus of Aβ is produced from APP first by the action of β-secretase, a membrane-tethered pepsin-like aspartyl protease (Cole and Vassar, 2008). This proteolysis leads to membrane shedding of the large luminal/extracellular APP domain. The 99-residue membrane-bound remnant is then cleaved by γ-secretase in the middle of its transmembrane region at the γ site, releasing Aβ, also near the inner leaflet at the ε site to release the APP intracellular domain (AICD)(Weidemann et al., 2002)(see APP in Figure 1A). Rare mutations in the APP gene, found in and around the small Aβ region, cause familial early-onset Alzheimer’s disease, and these mutations alter the production of Aβ or its aggregation properties, important evidence for the amyloid hypothesis of Alzheimer pathogenesis (Hardy and Selkoe, 2002; Tanzi and Bertram, 2005).
Figure 1.
Presenilin, the γ-secretase complex, and the proteolysis of APP and Notch. A, Presenilin is endoproteolytically cleaved into two polypeptides, an N-terminal fragment (NTF) and a C-terminal fragment (CTF) that remain associated. Each fragment contributes one aspartate to the γ-secretase active site (denoted by D’s). APP is first cleaved in the extracellular domain by β-secretase, and the membrane-bound remnant is cleaved at least twice (at the γ and ε sites) within the membrane by γ-secretase to produce Aβ and the APP intracellular domain (AICD). The Notch extracellular is shed via proteolysis at the S2 site by ADAM17 and then at the S3 and S4 sites by γ-secretase to produce Nβ and the Notch intracellular domain (NICD). Stars indicate general locations of missense mutations in Presenilin and APP that are associated with autosomal dominant, early-onset familial Alzheimer’s disease. B, Other substrates known to be cleaved by the γ-secretase complex. C, Components of the γ-secretase complex. Presenilin assembles with Nicastrin (NCT), Aph1, and Pen-2, whereupon Presenilin is endoproteolytically cleaved to NTF and CTF. Only one of each component is required for γ-secretase activity.
Critical clues to the identity of γ-secretase included: (1) Genes encoding the multi-pass membrane proteins presenilin-1 and presenilin-2 are, like APP, associated with familial, early-onset Alzheimer’s disease (Levy-Lahad et al., 1995; Rogaev et al., 1995; Sherrington et al., 1995). The disease-causing missense mutations were found to alter how γ-secretase cuts APP, leading to increased proportions of longer, more aggregation-prone forms of Aβ (Citron et al., 1997; Duff et al., 1996; Lemere et al., 1996; Scheuner et al., 1996). (2) Knockout of presenilin genes eliminates γ-secretase cleavage of APP (De Strooper et al., 1998; Herreman et al., 2000; Zhang et al., 2000). (3) Peptidomimetics that inhibit γ-secretase contain moieties typically found in aspartyl protease inhibitors (Shearman et al., 2000; Wolfe et al., 1999b). These findings led to the identification of two conserved transmembrane aspartates in the multi-pass presenilins that are critical for γ-secretase cleavage of APP (Figure 1A), evidence that presenilins are aspartyl proteases (Steiner et al., 1999; Wolfe et al., 1999c).
Presenilin is endoproteolytically cleaved into two polypeptides, an N-terminal fragment (NTF) and a C-terminal fragment (CTF) (Figure 1A) (Podlisny et al., 1997; Ratovitski et al., 1997), the formation of which is regulated (Thinakaran et al., 1997), metabolically stable (Capell et al., 1998; Podlisny et al., 1997; Ratovitski et al., 1997; Yu et al., 1998; Zhang et al., 1998), and part of a high-molecular weight complex (Capell et al., 1998; Yu et al., 1998), suggesting that the NTF-CTF heterodimer is the biologically active form (Laudon et al., 2004). NTF and CTF each contribute one of the essential and conserved aspartates, and transition-state analogue inhibitors of γ-secretase, compounds designed to interact with the active site of the protease, bind directly to presenilin NTF and CTF (Esler et al., 2000; Li et al., 2000b). While this evidence suggests that presenilin is an aspartyl protease, presenilins do not work alone and are part of a larger multi-protein complex that constitutes γ-secretase (see below).
Notch and Other γ-Secretase Substrates
Presenilins are also required for Notch signaling (Levitan and Greenwald, 1995), a pathway essential for cell differentiation during development and beyond (Selkoe and Kopan, 2003). After Notch is synthesized in the ER, the receptor is cleaved in its extracellular domain during its passage through the secretory pathway, and the two pieces so generated remain associated (Logeat et al., 1998). Upon interaction with a cognate ligand, Notch becomes susceptible to a second extracellular proteolysis, by a membrane-tethered metalloprotease, ADAM17, near the membrane (Brou et al., 2000; Mumm et al., 2000) (Figure 1A). The membrane-associated remnant is then cleaved within its transmembrane domain by a presenilin-dependent γ-secretase-like protease (De Strooper et al., 1999), releasing the Notch intracellular domain (NICD). NICD translocates to the nucleus and activates transcription after associating with the nuclear partner CSL (Schroeter et al., 1998).
Since the discovery that Notch is cleaved by γ-secretase, many other substrates have been identified, including Erb-B4, E- and N-cadherins, CD44, the low density lipoprotein receptor, Nectin-1, and the Notch ligands Delta and Jagged (Beel and Sanders, 2008; De Strooper, 2003) (See Figure 1B). While cellular function can be ascribed in some cases, the ability of γ-secretase to cleave so many different substrates and its apparently poor sequence specificity raise the question of whether a major role of this enzyme is to serve as a general degrading protease for membrane-bound protein remnants (Kopan and Ilagan, 2004).
The γ-Secretase Complex
The highly conserved role of γ-secretase in Notch signalling and its importance in development led to genetic screens in Caenorhabditis elegans that identified three other integral membrane proteins besides presenilin that modify Notch signaling, nicastrin, APH-1, and Pen-2 (Francis et al., 2002; Goutte et al., 2000; Goutte et al., 2002). Nicastrin was independently isolated biochemically as a presenilin-associated protein and found to be essential for γ-secretase processing of both APP and Notch (Yu et al., 2000). All four proteins (presenilin, nicastrin, Aph-1, and Pen-2) associate with one another (Kimberly et al., 2003; Takasugi et al., 2003) and with an immobilized γ-secretase inhibitor (Esler et al., 2002; Kimberly et al., 2003). Moreover, their coexpression increased γ-secretase activity in both Drosophila and mammalian cells (Kimberly et al., 2003; Takasugi et al., 2003) and reconstituted activity in yeast (Edbauer et al., 2003). This quartet of proteins (Figure 1C) is necessary and sufficient for γ-secretase activity, formally demonstrated through purification of the protease complex to virtual homogeneity (Fraering et al., 2004).
The stoichiometry of these four proteins in the γ-secretase complex has been a matter of some controversy, with discrepancies in the reported size of the complex and in the number of presenilin molecules per complex. Sizes of 100–150 KDa to 2 MDa have been reported (Capell et al., 1998; Edbauer et al., 2002; Evin et al., 2005; Kimberly et al., 2003; Li et al., 2000a; Yu et al., 1998), and several studies suggested that two presenilins reside at the catalytic core of the protease complex (Cervantes et al., 2004; Clarke et al., 2006; Schroeter et al., 2003). However, rigorous biochemical and biophysical experiments have shown that isolated, active complexes contain only one of each component (Sato et al., 2007) and, consistent with this stoichiometry, that the size of the purified enzyme is ~230 kDa, as determined by scanning electron microscopy (Osenkowski et al., 2009).
Inhibitors and Modulators
Designed inhibitors have proven to be useful tools in understanding the mechanism of γ-secretase and substrate recognition. As pointed out above, transition-state analogue inhibitors (e.g., compound 1, Figure 2) provided the first clear clue that the enzyme is an aspartyl protease, leading to the identification of two conserved and essential aspartates in presenilin. Moreover, affinity labelling with transition-state analogue inhibitors showed binding at the interface between the presenilin NTF and CTF subunits, consistent with the active site residing at this interface, with each presenilin subunit contributing one of the essential aspartates.
Figure 2.
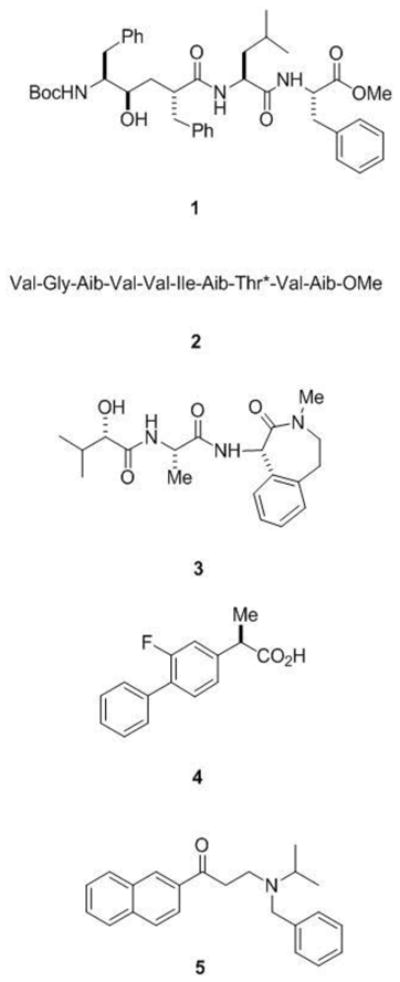
Inhibitors and modulators of γ-secretase. Transition-state analogue inhibitors such as the peptidomimetic 1 include hydroxyl-containing moieties that interact with the catalytic aspartates of aspartyl proteases. Helical peptide inhibitors include α-aminoisobutyric acid (Aib)-containing substrate mimics such as 2 (*denotes that the threonine residue contains an O-benzyl group). These helical peptides mimic the APP transmembrane domain and interact with the substrate docking site on the protease. The potent benzodiazepine inhibitor 3 (LY-450,139) is in late-stage clinical trials for the treatment of Alzheimer’s disease. NSAID-like modulator 4 (R-Flurbiprofen or tarenflurbil), which recently failed in late-stage clinical trials for Alzheimer’s disease, shifts where γ-secretase cuts APP, reducing the aggregation-prone Aβ42 and elevating more soluble Aβ38. Naphthyl ketone 5 inhibits total Aβ production without interfering with the ability of γ-secretase to cleave Notch receptor substrates.
Among the more intriguing questions about the entire emerging family of I-CLiPs is how they handle substrates and cleave within their TMDs, and again, small-molecule inhibitors have helped answer this question. Integral membrane substrates should require docking on the outer surface of the protease, with lateral gating to bring the substrate into the internal active site (Wolfe et al., 1999a). Initial evidence for such a mechanism came from isolation of the γ-secretase complex with an immobilized transition-state analogue inhibitor: γ-secretase complex members copurified with an endogenous APP substrate of the enzyme (Esler et al., 2002). Because the protease active site was blocked by the immobilized transition-state analogue inhibitor, this result suggested the existence of a separate substrate binding site.
Designed peptides based on the transmembrane domain of APP and constrained in a helical conformation (e.g., compound 2, Figure 2) can potently inhibit γ-secretase, apparently by interacting with this docking site (Das et al., 2003). Conversion of these helical peptide inhibitors into affinity labeling reagents led to the localization of the substrate docking site to the presenilin NTF/CTF interface (Kornilova et al., 2005). Transition-state analogue inhibitors also bind directly to the NTF/CTF interface but at a site distinct from that of helical peptide inhibitors. These findings suggest a pathway for γ-secretase substrate from docking site to active site: upon binding to the outer surface of presenilin at the NTF/CTF interface, the substrate can pass between these two presenilin subunits to access the internal active site.
γ-Secretase has in many ways been an attractive target for Alzheimer therapeutics (Wolfe, 2008), with one inhibitor (compound 3, Figure 2) now in advanced clinical trials (Siemers et al., 2007). However, interference with Notch processing and signalling may lead to toxicities that preclude clinical use of such inhibitors. Long-term treatment with γ-secretase inhibitors causes severe gastrointestinal toxicity and interferes with the maturation of B- and T-lympocytes in mice, effects that are indeed due to inhibition of Notch processing and signaling (Searfoss et al., 2003; Wong et al., 2004). However, compounds that can modulate the enzyme to alter or block Aβ little or no effect on Notch would bypass this potential roadblock to therapeutics. Such compounds have indeed been identified that can alter substrate selectivity and the sites of substrate proteolysis, both in cells and in purified biochemical systems.
Certain non-steroidal anti-inflammatory drugs (NSAIDs) can reduce the production of the highly aggregation-prone Aβ42 peptide and increase a 38-residue form of Aβ (Weggen et al., 2001). The alteration of the proteolytic cleavage site is observed with isolated or purified γ-secretase (Fraering et al., 2004; Takahashi et al., 2003; Weggen et al., 2003), indicating that the compounds can interact directly with the protease complex to exert these effects. The site of cleavage within the Notch transmembrane domain may be similarly affected, but this subtle change does not inhibit the release of the intracellular domain and thus does not affect Notch signaling (Okochi et al., 2006). One compound, (R)-flurbiprofen (tarenflurbil; Flurizan; compound 4 in Figure 2) entered late-stage clinical trials but failed due to lack of efficacy (Green et al., 2009). The exact target and mechanism of action of these NSAIDs in altering γ-secretase cleavage of APP remains unsettled (Beher et al., 2004; Sato et al., 2006b; Takahashi et al., 2003). However, a recent study suggests that the substrate itself is the target, specifically in the region of APP substrate at the extracellular/membrane junction (Kukar et al., 2008).
Other allosteric modulators resemble kinase inhibitors and interact with a nucleotide binding site on the γ-secretase complex. The discovery that ATP can increase Aβ production in membrane preparations prompted the testing of a variety of compounds that interact with ATP binding sites on other proteins (Netzer et al., 2003). In this focused screen, the Abl kinase inhibitor Gleevec emerged as a selective inhibitor of Aβ production in cells without affecting the proteolysis of Notch. In light of these findings, ATP and other nucleotides were tested for effects on purified γ-secretase preparations and found to selectively increase the proteolytic processing of a purified recombinant APP-based substrate without affecting the proteolysis of a Notch counterpart (Fraering et al., 2005). Furthermore, certain compounds known to interact with ATP binding sites (e.g., compound 5 in Figure 2) were found to selectively inhibit APP processing vis-à-vis Notch in purified protease preparations. These and other results suggest that the γ-secretase complex contains a nucleotide binding site and that this site allows allosteric regulation of γ-secretase processing of APP with respect to Notch. Whether this regulation is physiologically important is unclear, but the pharmacological relevance is profound and may lead to new therapeutic candidates for Alzheimer’s disease.
Towards the Structure of γ-Secretase
The purification of the γ-secretase complex (Fraering et al., 2004) allowed the first glimpse into its structure. Electron microscopy (EM) with negative heavy-atom staining and single-particle analysis reveals that the complex has a globular structure that at low resolution (10–15 ) appears rather amorphous (Lazarov et al., 2006). Nevertheless, two important features were gleaned. The first is a rather large low-density interior cavity of ~ 20 diameter that is presumably where the active site resides. The second is the presence of two small openings that may be the site of entry for water.
Recently, an improved structure of γ-secretase was determined by cryo-EM at 12 Å resolution (Osenkowski et al., 2009) (Figure 3). Cryo-EM reveals a protein structure itself, rather than a surface of the protein structure coated by a heavy metal stain. The new cryo-EM structure reveals three smaller low-density interior regions, regions that do not coalesce to form a single chamber as observed in the negative stain structure (Lazarov et al., 2006). The cryo-EM structure has better definition than the previous negative-stained structure in that the ~4 nm thick transmembrane region and four extracellular density domains are resolved (Osenkowski et al., 2009). The structure reveals an apparent groove that may be the initial substrate docking site.
Figure 3.
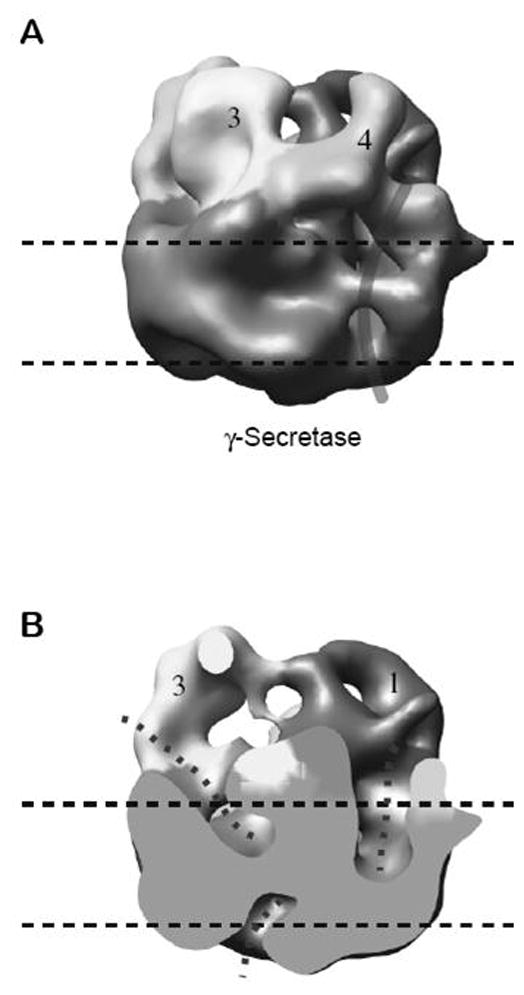
A 12 Å resolution structure of γ-secretase by cryo-electron microscopy. A, Surface-rendered side view of the γ-secretase structure. The thick curved line indicates an apparent membrane surface groove that is hypothesized to represent the approximate position at which a transmembrane substrate might bind. Horizontal dashed lines represent the boundaries of the cell membrane. B, Cut-open view of the structure shown in A. The dotted lines indicate the potential water-accessible spaces.
Other structural features have been revealed by cysteine mutagenesis with cross-linking of chemical probes (Sato et al., 2006a; Tolia et al., 2006). A cysteine-less version of presenilin retained the ability to assemble with other complex members, undergo endoproteolysis to NTF and CTF, and process APP, and this allowed incorporation of single cysteine resides at various sites near the key aspartates. Disulfide formation with thiol-containing reagents then provided information about the relative accessibility of these sites from the aqueous milieu, allowing the construction of a model in which water can funnel down to where the aspartates reside. Furthermore, simultaneous mutation of the two conserved transmembrane aspartates to cysteine and apparent intramolecular crosslinking provided the first evidence that these two aspartates are indeed in close proximity (Tolia et al., 2006). which is required for them to coordinate and serve catalytic function. Using this same approach (cysteine mutagenesis and crosslinking), two more recent studies suggest that TMD 9 serves as a gatekeeper for lateral entry of the substrate TMD (Sato et al., 2008; Tolia et al., 2008). Still another cysteine-crosslinking study suggested that TMD1 is in direct contact with TMD8 (Kornilova et al., 2006). More detailed information will likely require a crystal structure of presenilin or a presenilin homolog.
Signal Peptide Peptidase (SPP) and SPP-like Proteases
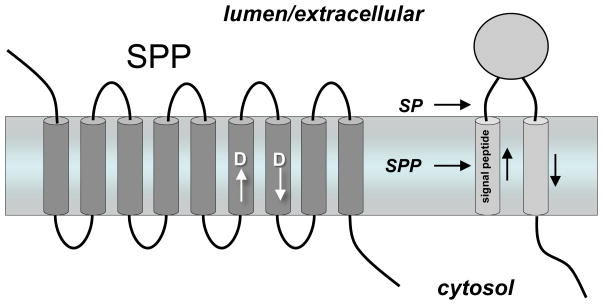
The concept of presenilin as the catalytic component for γ-secretase was considerably strengthened when signal peptide peptidase (SPP) was found to be a similar intramembrane aspartyl protease (Figure 4). SPP clears remnant signal peptides from the membrane after their production by signal peptidase. However, this process apparently also plays a role in immune surveillance, in which signal peptides from the major histocompatibility complex (MHC) type I are cleaved by SPP. The resultant peptide products are then presented on the cell surface as an indication to natural killer cells whether MHC synthesis is proceeding normally (Lemberg et al., 2001). In addition, SPP is exploited by the hepatitis C virus for the maturation of its core protein, suggesting that this protease may be a suitable target for antiviral therapy (McLauchlan et al., 2002).
Figure 4.
The presenilin homolog signal peptide peptidase (SPP). Signal peptides are removed from membrane proteins by signal peptidase (SP), and the remnant peptides are released from the membrane by SPP-mediated intramembrane proteolysis. SPP, like presenilin, contains two aspartates essential for protease activity, but the conserved aspartate-containing motifs are in the opposite orientation compared with their presenilin counterparts. Consistent with the flipped orientation of SPP vis-à-vis presenilin, the substrates of these two proteases also run in the opposite direction. Unlike presenilin, SPP apparently does not require other protein cofactors or cleavage into two subunits for proteolytic activity.
SPP was identified by affinity labeling with a peptidomimetic inhibitor, and the protein sequence displayed similarities with presenilin (Weihofen et al., 2002). SPP contains two conserved aspartates, each predicted to lie in the middle of a transmembrane domain, and the aspartate-containing sequences resemble those found in presenilins. The predicted topology of SPP also resembles that of presenilins, placing the key aspartates in the same relative position to each other in the membrane. However, the orientation of the aspartate-containing transmembrane domains of SPP is apparently opposite that of presenilins, in correlation with the orientation of SPP substrates, which is opposite that of γ-secretase substrates. Interestingly, prior to the identification of SPP, a computational search for presenilin-like proteins netted an entire family of so-called presenilin homologs (PSHs) (Ponting et al., 2002); however, it is not yet clear if all of these proteins have catalytic activity. Two homologs, SPP-like proteases, SPPL2a and SPPL2b, apparently cleave tumor necrosis factor α (Fluhrer et al., 2006) and the dementia-associated Bri2 protein (Martin et al., 2008), although the biological roles of these proteolytic events are unknown. SPP has also been identified in malarial parasites and may represent a worthwhile therapeutic target (Nyborg et al., 2006).
SPP appears to be less complicated than γ-secretase. Expression of human SPP in yeast reconstituted the protease activity, suggesting that the protein has activity on its own and does not require other mammalian protein cofactors (Weihofen et al., 2002) This has been confirmed by the expression of various SPP orthologs in E. coli and purification of active enzyme to homogeneity (Narayanan et al., 2007). Moreover, unlike presenilins, SPP is not processed into two pieces. Thus, SPP may be a more tractable enzyme for understanding intramembrane aspartyl proteases and may shed light on γ-secretase structure and function. Indeed, the catalytic sites of the two proteases appear remarkably similar: their activities are inhibited by some of the same active site-directed peptidomimetics (Kornilova et al., 2003; Weihofen et al., 2003) and helical peptides (Sato et al., 2006b), and activity can be modulated by the same NSAIDs that affect γ-secretase (Sato et al., 2006b). The ability to express SPP as a single protein in bacteria and purify it in active form suggests that this presenilin-like protease may be amenable to crystallization and high-resolution structure determination, as has been accomplished for the serine protease rhomboid (Ben-Shem et al., 2007; Lemieux et al., 2007; Wang and Ha, 2007; Wang et al., 2006; Wu et al., 2006) and the metalloprotease S2P (Feng et al., 2007).
Conclusions
Aspartyl I-CLiPs are found in all forms of life and play essential roles in biology and disease. How these enzymes carry out hydrolysis in the membrane is a fascinating question that is not entirely resolved, but evidence suggests an initial substrate docking site and a lateral gate into a pore where water and the active site aspartates reside. Designed inhibitors have been critical in elucidating these mechanisms, but inhibitors targeting γ-secretase for the treatment of Alzheimer’s disease must avoid interfering with Notch signaling. Such compounds have been identified and represent a promising class of drug candidates. Aspartyl I-CLiPs have so far eluded atomic-resolution structure determination, but EM and mutagenesis studies have offered information about the general shape and contours of the γ-secretase complex. SPP may represent a convenient model system that will more easily allow crystallization and detailed structure elucidation. In any event, the biochemistry and biology of the SPP family are worthy of study in their own right, and these presenilin-like proteases may prove to be worthwhile therapeutic targets as well.
Acknowledgments
This work was supported by grants from the National Institutes of Health to MSW (AG17574, NS41355, GM79555) and Dennis J. Selkoe (AG15379).
References
- Beel AJ, Sanders CR. Substrate specificity of gamma-secretase and other intramembrane proteases. Cell Mol Life Sci. 2008;65:1311–1334. doi: 10.1007/s00018-008-7462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Clarke EE, Wrigley JD, Martin AC, Nadin A, Churcher I, Shearman MS. Selected non-steroidal anti-inflammatory drugs and their derivatives target gamma-secretase at a novel site. Evidence for an allosteric mechanism. J Biol Chem. 2004;279:43419–43426. doi: 10.1074/jbc.M404937200. [DOI] [PubMed] [Google Scholar]
- Ben-Shem A, Fass D, Bibi E. Structural basis for intramembrane proteolysis by rhomboid serine proteases. Proc Natl Acad Sci U S A. 2007;104:462–466. doi: 10.1073/pnas.0609773104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israël A. A Novel Proteolytic Cleavage Involved in Notch Signaling: The Role of the Disintegrin-Metalloprotease TACE. Molecular Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- Capell A, Grunberg J, Pesold B, Diehlmann A, Citron M, Nixon R, Beyreuther K, Selkoe DJ, Haass C. The proteolytic fragments of the Alzheimer's disease-associated presenilin-1 form heterodimers and occur as a 100–150-kDa molecular mass complex. J Biol Chem. 1998;273:3205–3211. doi: 10.1074/jbc.273.6.3205. [DOI] [PubMed] [Google Scholar]
- Cervantes S, Saura CA, Pomares E, Gonzalez-Duarte R, Marfany G. Functional implications of the presenilin dimerization. Reconstitution of gamma -secretase activity by assembly of a catalytic site at the dimer interface of two catalytically inactive presenilins. J Biol Chem. 2004;279:36519–36529. doi: 10.1074/jbc.M404832200. [DOI] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, et al. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Clarke EE, Churcher I, Ellis S, Wrigley JD, Lewis HD, Harrison T, Shearman MS, Beher D. Intra- or intercomplex binding to the gamma-secretase enzyme. A model to differentiate inhibitor classes. J Biol Chem. 2006;281:31279–31289. doi: 10.1074/jbc.M605051200. [DOI] [PubMed] [Google Scholar]
- Cole SL, Vassar R. The role of APP processing by BACE1, the beta-secretase, in Alzheimer's disease pathophysiology. J Biol Chem. 2008;283:29621–29625. doi: 10.1074/jbc.R800015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das C, Berezovska O, Diehl TS, Genet C, Buldyrev I, Tsai JY, Hyman BT, Wolfe MS. Designed helical peptides inhibit an intramembrane protease. J Am Chem Soc. 2003;125:11794–11795. doi: 10.1021/ja037131v. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Haass C, Steiner H. Presenilin and nicastrin regulate each other and determine amyloid beta-peptide production via complex formation. Proc Natl Acad Sci U S A. 2002;99:8666–8671. doi: 10.1073/pnas.132277899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai JY, Rahmati T, Xia W, Selkoe DJ, Wolfe MS. Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nature Cell Biology. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Ye W, Diehl TS, Selkoe DJ, Wolfe MS. Activity-dependent isolation of the presenilin/γ-secretase complex reveals nicastrin and a γ substrate. Proc Natl Acad Sci USA. 2002;99:2720–2725. doi: 10.1073/pnas.052436599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evin G, Canterford LD, Hoke DE, Sharples RA, Culvenor JG, Masters CL. Transition-state analogue gamma-secretase inhibitors stabilize a 900 kDa presenilin/nicastrin complex. Biochemistry. 2005;44:4332–4341. doi: 10.1021/bi0481702. [DOI] [PubMed] [Google Scholar]
- Feng L, Yan H, Wu Z, Yan N, Wang Z, Jeffrey PD, Shi Y. Structure of a site-2 protease family intramembrane metalloprotease. Science. 2007;318:1608–1612. doi: 10.1126/science.1150755. [DOI] [PubMed] [Google Scholar]
- Fluhrer R, Grammer G, Israel L, Condron MM, Haffner C, Friedmann E, Bohland C, Imhof A, Martoglio B, Teplow DB, et al. A gamma-secretase-like intramembrane cleavage of TNFalpha by the GxGD aspartyl protease SPPL2b. Nat Cell Biol. 2006;8:894–896. doi: 10.1038/ncb1450. [DOI] [PubMed] [Google Scholar]
- Fluhrer R, Steiner H, Haass C. Intramembrane proteolysis by signal peptide peptidases: a comparative discussion of GXGD-type aspartyl proteases. J Biol Chem. 2009;284:13975–13979. doi: 10.1074/jbc.R800040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraering PC, Ye W, Lavoie MJ, Ostaszewski BL, Selkoe DJ, Wolfe MS. gamma -Secretase substrate selectivity can be modulated directly via interaction with a nucleotide binding site. J Biol Chem. 2005;280:41987–41996. doi: 10.1074/jbc.M501368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraering PC, Ye W, Strub JM, Dolios G, LaVoie MJ, Ostaszewski BL, Van Dorsselaer A, Wang R, Selkoe DJ, Wolfe MS. Purification and Characterization of the Human gamma-Secretase Complex. Biochemistry. 2004;43:9774–9789. doi: 10.1021/bi0494976. [DOI] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, et al. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Goutte C, Hepler W, Mickey KM, Priess JR. aph-2 encodes a novel extracellular protein required for GLP-1-mediated signaling. Development. 2000;127:2481–2492. doi: 10.1242/dev.127.11.2481. [DOI] [PubMed] [Google Scholar]
- Goutte C, Tsunozaki M, Hale VA, Priess JR. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A. 2002;99:775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. Jama. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proc Natl Acad Sci U S A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- Kornilova AY, Bihel F, Das C, Wolfe MS. The initial substrate-binding site of gamma-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A. 2005;102:3230–3235. doi: 10.1073/pnas.0407640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilova AY, Das C, Wolfe MS. Differential effects of inhibitors on the gamma-secretase complex. Mechanistic implications. J Biol Chem. 2003;278:16470–16473. doi: 10.1074/jbc.C300019200. [DOI] [PubMed] [Google Scholar]
- Kornilova AY, Kim J, Laudon H, Wolfe MS. Deducing the transmembrane domain organization of presenilin-1 in gamma-secretase by cysteine disulfide cross-linking. Biochemistry. 2006;45:7598–7604. doi: 10.1021/bi060107k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukar TL, Ladd TB, Bann MA, Fraering PC, Narlawar R, Maharvi GM, Healy B, Chapman R, Welzel AT, Price RW, et al. Substrate-targeting gamma-secretase modulators. Nature. 2008;453:925–929. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudon H, Mathews PM, Karlstrom H, Bergman A, Farmery MR, Nixon RA, Winblad B, Gandy SE, Lendahl U, Lundkvist J, et al. Co-expressed presenilin 1 NTF and CTF form functional gamma-secretase complexes in cells devoid of full-length protein. J Neurochem. 2004;89:44–53. doi: 10.1046/j.1471-4159.2003.02298.x. [DOI] [PubMed] [Google Scholar]
- Lazarov VK, Fraering PC, Ye W, Wolfe MS, Selkoe DJ, Li H. Electron microscopic structure of purified, active gamma-secretase reveals an aqueous intramembrane chamber and two pores. Proc Natl Acad Sci U S A. 2006;103:6889–6894. doi: 10.1073/pnas.0602321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg MK, Bland FA, Weihofen A, Braud VM, Martoglio B. Intramembrane proteolysis of signal peptides: an essential step in the generation of HLA-E epitopes. J Immunol. 2001;167:6441–6446. doi: 10.4049/jimmunol.167.11.6441. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Lopera F, Kosik KS, Lendon CL, Ossa J, Saido TC, Yamaguchi H, Ruiz A, Martinez A, Madrigal L, et al. The E280A presenilin 1 Alzheimer mutation produces increased A beta 42 deposition and severe cerebellar pathology. Nat Med. 1996;2:1146–1150. doi: 10.1038/nm1096-1146. [DOI] [PubMed] [Google Scholar]
- Lemieux MJ, Fischer SJ, Cherney MM, Bateman KS, James MN. The crystal structure of the rhomboid peptidase from Haemophilus influenzae provides insight into intramembrane proteolysis. Proc Natl Acad Sci U S A. 2007;104:750–754. doi: 10.1073/pnas.0609981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D, Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995;377:351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Li YM, Lai MT, Xu M, Huang Q, DiMuzio-Mower J, Sardana MK, Shi XP, Yin KC, Shafer JA, Gardell SJ. Presenilin 1 is linked with gamma -secretase activity in the detergent solubilized state. Proc Natl Acad Sci U S A. 2000a;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000b;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Fluhrer R, Reiss K, Kremmer E, Saftig P, Haass C. Regulated intramembrane proteolysis of Bri2 (Itm2b) by ADAM10 and SPPL2a/SPPL2b. J Biol Chem. 2008;283:1644–1652. doi: 10.1074/jbc.M706661200. [DOI] [PubMed] [Google Scholar]
- McLauchlan J, Lemberg MK, Hope G, Martoglio B. Intramembrane proteolysis promotes trafficking of hepatitis C virus core protein to lipid droplets. Embo J. 2002;21:3980–3988. doi: 10.1093/emboj/cdf414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A Ligand-Induced Extracellular Cleavage Regulates Gamma-Secretase-like Proteolytic Activation of Notch1. Molecular Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Narayanan S, Sato T, Wolfe MS. A C-terminal region of signal peptide peptidase defines a functional domain for intramembrane aspartic protease catalysis. J Biol Chem. 2007;282:20172–20179. doi: 10.1074/jbc.M701536200. [DOI] [PubMed] [Google Scholar]
- Netzer WJ, Dou F, Cai D, Veach D, Jean S, Li Y, Bornmann WG, Clarkson B, Xu H, Greengard P. Gleevec inhibits beta-amyloid production but not Notch cleavage. Proc Natl Acad Sci U S A. 2003;100:12444–12449. doi: 10.1073/pnas.1534745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyborg AC, Ladd TB, Jansen K, Kukar T, Golde TE. Intramembrane proteolytic cleavage by human signal peptide peptidase like 3 and malaria signal peptide peptidase. Faseb J. 2006;20:1671–1679. doi: 10.1096/fj.06-5762com. [DOI] [PubMed] [Google Scholar]
- Okochi M, Fukumori A, Jiang J, Itoh N, Kimura R, Steiner H, Haass C, Tagami S, Takeda M. Secretion of the Notch-1 Abeta-like peptide during Notch signaling. J Biol Chem. 2006;281:7890–7898. doi: 10.1074/jbc.M513250200. [DOI] [PubMed] [Google Scholar]
- Osenkowski P, Li H, Ye W, Li D, Aeschbach L, Fraering PC, Wolfe MS, Selkoe DJ, Li H. Cryoelectron microscopy structure of purified gamma-secretase at 12 A resolution. J Mol Biol. 2009;385:642–652. doi: 10.1016/j.jmb.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny MB, Citron M, Amarante P, Sherrington R, Xia W, Zhang J, Diehl T, Levesque G, Fraser P, Haass C, et al. Presenilin proteins undergo heterogeneous endoproteolysis between Thr291 and Ala299 and occur as stable N- and C-terminal fragments in normal and Alzheimer brain tissue. Neurobiol Dis. 1997;3:325–337. doi: 10.1006/nbdi.1997.0129. [DOI] [PubMed] [Google Scholar]
- Ponting CP, Hutton M, Nyborg A, Baker M, Jansen K, Golde TE. Identification of a novel family of presenilin homologues. Hum Mol Genet. 2002;11:1037–1044. doi: 10.1093/hmg/11.9.1037. [DOI] [PubMed] [Google Scholar]
- Ratovitski T, Slunt HH, Thinakaran G, Price DL, Sisodia SS, Borchelt DR. Endoproteolytic processing and stabilization of wild-type and mutant presenilin. J Biol Chem. 1997;272:24536–24541. doi: 10.1074/jbc.272.39.24536. [DOI] [PubMed] [Google Scholar]
- Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, Chi H, Lin C, Holman K, Tsuda T, et al. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Sato C, Morohashi Y, Tomita T, Iwatsubo T. Structure of the catalytic pore of gamma-secretase probed by the accessibility of substituted cysteines. J Neurosci. 2006a;26:12081–12088. doi: 10.1523/JNEUROSCI.3614-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Takagi S, Tomita T, Iwatsubo T. The C-terminal PAL motif and transmembrane domain 9 of presenilin 1 are involved in the formation of the catalytic pore of the gamma-secretase. J Neurosci. 2008;28:6264–6271. doi: 10.1523/JNEUROSCI.1163-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Diehl TS, Narayanan S, Funamoto S, Ihara Y, De Strooper B, Steiner H, Haass C, Wolfe MS. Active gamma-secretase complexes contain only one of each component. J Biol Chem. 2007;282:33985–33993. doi: 10.1074/jbc.M705248200. [DOI] [PubMed] [Google Scholar]
- Sato T, Nyborg AC, Iwata N, Diehl TS, Saido TC, Golde TE, Wolfe MS. Signal peptide peptidase: biochemical properties and modulation by nonsteroidal antiinflammatory drugs. Biochemistry. 2006b;45:8649–8656. doi: 10.1021/bi060597g. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Ilagan MX, Brunkan AL, Hecimovic S, Li YM, Xu M, Lewis HD, Saxena MT, De Strooper B, Coonrod A, et al. A presenilin dimer at the core of the gamma-secretase enzyme: insights from parallel analysis of Notch 1 and APP proteolysis. Proc Natl Acad Sci U S A. 2003;100:13075–13080. doi: 10.1073/pnas.1735338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Searfoss GH, Jordan WH, Calligaro DO, Galbreath EJ, Schirtzinger LM, Berridge BR, Gao H, Higgins MA, May PC, Ryan TP. Adipsin: a biomarker of gastrointestinal toxicity mediated by a functional gamma secretase inhibitor. J Biol Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131:215–221. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Shearman MS, Beher D, Clarke EE, Lewis HD, Harrison T, Hunt P, Nadin A, Smith AL, Stevenson G, Castro JL. L-685,458, an Aspartyl Protease Transition State Mimic, Is a Potent Inhibitor of Amyloid beta-Protein Precursor gamma-Secretase Activity. Biochemistry. 2000;39:8698–8704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Siemers ER, Dean RA, Friedrich S, Ferguson-Sells L, Gonzales C, Farlow MR, May PC. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-beta after inhibition of gamma-secretase. Clin Neuropharmacol. 2007;30:317–325. doi: 10.1097/WNF.0b013e31805b7660. [DOI] [PubMed] [Google Scholar]
- Steiner H, Duff K, Capell A, Romig H, Grim MG, Lincoln S, Hardy J, Yu X, Picciano M, Fechteler K, et al. A loss of function mutation of presenilin-2 interferes with amyloid beta-peptide production and notch signaling. J Biol Chem. 1999;274:28669–28673. doi: 10.1074/jbc.274.40.28669. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Hayashi I, Tominari Y, Rikimaru K, Morohashi Y, Kan T, Natsugari H, Fukuyama T, Tomita T, Iwatsubo T. Sulindac sulfide is a noncompetitive gamma-secretase inhibitor that preferentially reduces Abeta 42 generation. J Biol Chem. 2003;278:18664–18670. doi: 10.1074/jbc.M301619200. [DOI] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Harris CL, Ratovitski T, Davenport F, Slunt HH, Price DL, Borchelt DR, Sisodia SS. Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J Biol Chem. 1997;272:28415–28422. doi: 10.1074/jbc.272.45.28415. [DOI] [PubMed] [Google Scholar]
- Tolia A, Chavez-Gutierrez L, De Strooper B. Contribution of presenilin transmembrane domains 6 and 7 to a water-containing cavity in the gamma -secretase complex. J Biol Chem. 2006;281:27633–27642. doi: 10.1074/jbc.M604997200. [DOI] [PubMed] [Google Scholar]
- Tolia A, Horre K, De Strooper B. Transmembrane domain 9 of presenilin determines the dynamic conformation of the catalytic site of gamma -secretase. J Biol Chem. 2008;15:15. doi: 10.1074/jbc.M802461200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ha Y. Open-cap conformation of intramembrane protease GlpG. Proc Natl Acad Sci U S A. 2007;104:2098–2102. doi: 10.1073/pnas.0611080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Ha Y. Crystal structure of a rhomboid family intramembrane protease. Nature. 2006;444:179–180. doi: 10.1038/nature05255. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, Golde TE, Koo EH. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity. J Biol Chem. 2003;278:31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Eggert S, Reinhard FB, Vogel M, Paliga K, Baier G, Masters CL, Beyreuther K, Evin G. A Novel var epsilon-Cleavage within the Transmembrane Domain of the Alzheimer Amyloid Precursor Protein Demonstrates Homology with Notch Processing. Biochemistry. 2002;41:2825–2835. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science. 2002;296:2215–2218. doi: 10.1126/science.1070925. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Lemberg MK, Friedmann E, Rueeger H, Schmitz A, Paganetti P, Rovelli G, Martoglio B. Targeting Presenilin-type Aspartic Protease Signal Peptide Peptidase with gamma -Secretase Inhibitors. J Biol Chem. 2003;278:16528–16533. doi: 10.1074/jbc.M301372200. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Gamma-secretase: structure, function, and modulation for Alzheimer's disease. Curr Top Med Chem. 2008;8:2–8. doi: 10.2174/156802608783334024. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Intramembrane-cleaving proteases. J Biol Chem. 2009;3:3. doi: 10.1074/jbc.R800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS, De Los Angeles J, Miller DD, Xia W, Selkoe DJ. Are presenilins intramembrane-cleaving proteases? Implications for the molecular mechanism of Alzheimer's disease. Biochemistry. 1999a;38:11223–11230. doi: 10.1021/bi991080q. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Moore CL, Leatherwood DD, Ostaszewski B, Donkor IO, Selkoe DJ. Peptidomimetic probes and molecular modeling suggest Alzheimer's γ-secretases are intramembrane-cleaving aspartyl proteases. Biochemistry. 1999b;38:4720–4727. doi: 10.1021/bi982562p. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999c;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee HJ, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Yan N, Feng L, Oberstein A, Yan H, Baker RP, Gu L, Jeffrey PD, Urban S, Shi Y. Structural analysis of a rhomboid family intramembrane protease reveals a gating mechanism for substrate entry. Nat Struct Mol Biol. 2006;13:1084–1091. doi: 10.1038/nsmb1179. [DOI] [PubMed] [Google Scholar]
- Yu G, Chen F, Levesque G, Nishimura M, Zhang DM, Levesque L, Rogaeva E, Xu D, Liang Y, Duthie M, et al. The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains beta-catenin. J Biol Chem. 1998;273:16470–16475. doi: 10.1074/jbc.273.26.16470. [DOI] [PubMed] [Google Scholar]
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kang DE, Xia W, Okochi M, Mori H, Selkoe DJ, Koo EH. Subcellular distribution and turnover of presenilins in transfected cells. J Biol Chem. 1998;273:12436–12442. doi: 10.1074/jbc.273.20.12436. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Nadeau P, Song W, Donoviel D, Yuan M, Bernstein A, Yankner BA. Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]