Summary
Accumulating lines of experimental evidence have revealed that the malignant transformation of multipotent tissue-resident adult stem/progenitor cells into cancer stem/progenitor cells endowed with a high self-renewal capacity and aberrant multilineage differentiation potential may be at origin of the most types of human aggressive and recurrent cancers. Based on new cancer stem/progenitor cell concepts of carcinogenesis, it is suggested that a small subpopulation of highly tumorigenic and migrating cancer stem/progenitor cells, also designated as cancer- and metastasis-initiating cells, can provide critical roles for primary tumor growth, metastases at distant tissues and organs, treatment resistance and disease relapse. Particularly, cancer initiation and progression to locally invasive and metastatic stages is often associated with a persistent activation of distinct developmental signaling pathways in these immature cells during epithelial-mesenchymal transition program. The signaling cascades that are often deregulated in cancer stem/progenitor cells include hedgehog, epidermal growth factor receptor (EGFR), Wnt/β-catenin, NOTCH, polycomb gene product BMI-1 and/or stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4). Importantly, the results from recent investigations have also indicated that different cancer subtypes may harbor distinct subsets and/or number of cancer-initiating cells during cancer progression as well as before or after therapy initiation and disease recurrence. Therefore, the identification of the molecular transforming events that frequently occur in cancer- and metastasis-initiating cells versus their differentiated progenies is of immense interest to develop new targeting approach for improving current therapies against aggressive, metastatic, recurrent and lethal cancers.
Keywords: Cancer stem/progenitor cells, Solid tumors, Epithelial-mesenchymal transition, Invasion, Metastases, Treatment resistance, Molecular therapeutic targets, Cancer therapies
Introduction
Major advances in the adult stem/progenitor cell biology have allowed researchers to identify certain specific physiological functions of these immature cells and their early progenies endowed with a self-renewal and multilineage differentiation potential (Kim et al., 2005; Bryder et al., 2006; Mimeault and Batra, 2006; Rizo et al., 2006; Wilson and Trumpp, 2006; Arai and Suda, 2007; Mimeault et al., 2007a; Zhao et al., 2008). The tissue-resident adult stem/progenitor cells with a long longevity generally provide critical roles in the replenishment of cells in homeostatic conditions and after intense injuries along the lifespan of individuals (Fig. 1) (Kim et al., 2005; Bryder et al., 2006; Rizo et al., 2006; Wilson and Trumpp, 2006; Mimeault and Batra, 2006; Arai and Suda, 2007; Mimeault et al., 2007a; Zhao et al., 2008). In counterpart, a growing body of experimental evidence has revealed that an accumulation of genetic abnormalities in tissue-resident adult stem/progenitor cells or their more committed progenies endowed with a self-renewal potential concomitant with the changes in their niches, may result in their malignant transformation into leukemic or tumorigenic cancer stem/progenitor cells (Figs. 1, 2) (Huntly et al., 2004; Bapat et al., 2005; Kim et al., 2005; Rizo et al., 2006; Ginestier et al., 2007; Mimeault et al., 2007b, 2008b; Nicolis, 2007; Nijhof et al., 2007; Vaish, 2007; Aubert and Lansdorp, 2008; Matsui et al., 2008; Mimeault and Batra, 2008a,b; Young et al., 2008; Barker et al., 2009; Zhu et al., 2009). Accordingly with the cancer stem/progenitor cell hypothesis, it is suggested that these immature cancer cells, endowed with a high self-renewal potential and aberrant differentiation ability, and which are also designated as cancer- or tumor-initiating cells, can provide critical roles for primary tumor growth, metastatic spread at distant tissues, resistance to current conventional therapies and disease relapse (Hope et al., 2004; Jamieson et al., 2004; Singh et al., 2004; Bao et al., 2006; Liu et al., 2006a; Ginestier et al., 2007; Hermann et al., 2007; Mimeault et al., 2007a,b; Nicolis, 2007; Eramo et al., 2008; Fillmore and Kuperwasser, 2008; Huang et al., 2008; Matsui et al., 2008; Mimeault and Batra, 2008c; Schatton et al., 2008; Wright et al., 2008; Yang et al., 2008a; Young et al., 2008; Zhang et al., 2008b). In support with these new concepts of carcinogenesis suggesting a major implication of cancer stem/progenitor cells in cancer initiation and progression, the small subpopulations of immature cancer cells with stem cell-like properties, comprising about 0.1–3% of the total cancer cell mass, have been isolated from primary malignant tissues of cancer patients and established cancer cell lines (Al-Hajj and Clarke, 2004; Hope et al., 2004; Jamieson et al., 2004; Matsui et al., 2004; Singh et al., 2004; Fang et al., 2005; Kim et al., 2005; Ponti et al., 2005; Maitland et al., 2006; Salmaggi et al., 2006; Ginestier et al., 2007; Hermann et al., 2007; Mimeault et al., 2007b; Ricci-Vitiani et al., 2007; Prince et al., 2007; Eramo et al., 2008; Schatton et al., 2008; She et al., 2008; Yang et al., 2008a; Zhang et al., 2008a; Huang et al., 2009). The cancer stem/progenitor cells typically expressed several stem cell-like markers including telomerase, aldehyde dehydrogenase (ALDH), CD133, CD44, CXC chemokine receptor 4 (CXCR4), stem cell factor (SCF) receptor KIT, ATP binding-cassette (ABC) multidrug transporters, and/or transcription factors such as OCT-3/4, Nanog and SOX2. The highly leukemic or tumorigenic cancer stem/progenitor cells were able to give rise in vitro and in vivo to the total mass of differentiated cancer cells that recapitulated the complex morphological characteristics and heterogeneous phenotype of original patient’s tumors.
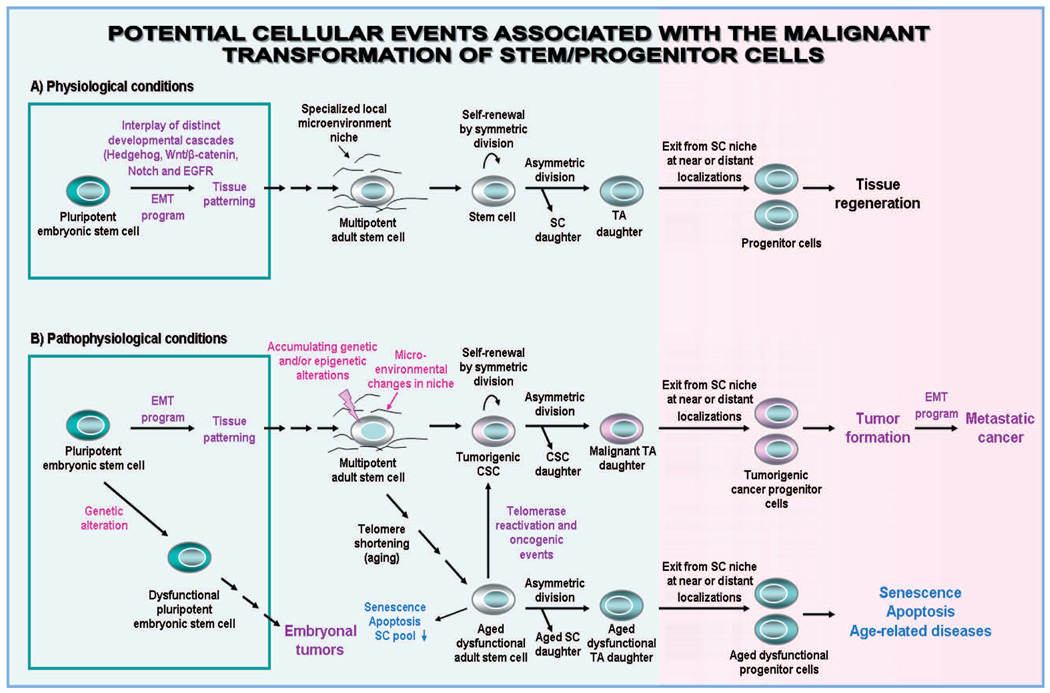
Fig. 1.
Hierarchical model of the clonal expansion and differentiation of adult stem/progenitor cells during epithelial tissue regeneration in physiological conditions and cancer initiation and progression through their malignant transformation. This scheme shows (A) the tissue patterning derived through the differentiation of pluripotent embryonic stem cells during normal embryonic development. The symmetric or asymmetric division of normal tissue-resident adult stem cells (SC) into transit-amplifying (TA)/intermediate cells that in turn can regenerate the bulk mass of poorly, moderately and terminally differentiated cells in the tissue from which they originate in homeostatic conditions or after tissue injury is also illustrated. Moreover, this scheme shows (B) the possibility of embryonal tumor formation derived of the malignant transformation of pluripotent embryonic stem cells. The malignant transformation of adult stem/progenitor cells into cancer stem/progenitor cells (CSCs), which may be induced through genetic and/or epigenetic alterations in these immature cells and changes in their local microenvironments including activated stromal cells. The tumorigenic cancer stem/progenitor cells endowed with an aberrant differentiation potential may give rise to malignant TA cells that in turn can generate the bulk mass of poorly, moderately and/or terminally differentiated cancer cells forming tumor. In addition, the chronological aging of tissue-resident adult stem/progenitor cells concomitant with telomere shortening, which may lead to their dysfunction and loss via senescence and apoptosis and age-related diseases is also indicated. In contrast, the re-activation of telomerase and accumulation of genetic and/or epigenetic alterations in adult stem/progenitor cells with advancing age may result in their malignant transformation and cancer development.
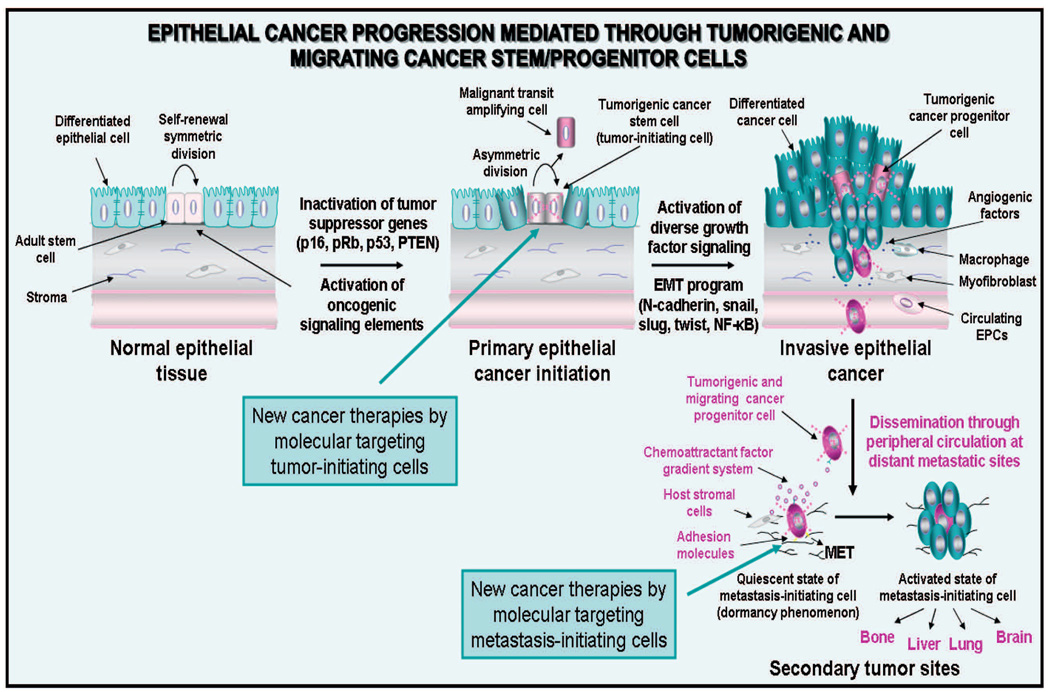
Fig. 2.
Model of epithelial cancer initiation and progression mediated through tumorigenic and migrating cancer stem/progenitor cells. The scheme shows the cancer initiation through the accumulation of genetic abnormalities in tissue-resident adult stem cells. The asymmetric division of cancer stem cells localized in the basal compartment into transit-amplifying (TA) cancer progenitor cells can generate the bulk mass of differentiated cancer cells constituting the solid tumor. Furthermore, the transformation of tumorigenic stem/progenitor cells into migrating cancer stem/progenitor cells, which may be induced by the sustained activation of distinct growth factor signaling pathways during the epithelial-mesenchymal transition (EMT) program, is also shown. The possible invasion of certain tumorigenic and migrating cancer stem/progenitor cells in the activated stroma which may lead to their dissemination through the peripheral circulation at distant sites along chemoattractant ligand gradient systems such as SDF-1/CXCR4, and adhesion to ECM components is also illustrated. Moreover, the possible loss of the migratory phenotype of cancer stem/progenitor cells via the occurrence of mesenchymal-epithelial transition (MET) at secondary tumor sites is indicated. The dormancy phenomenon of metastasis-initiating cells and their possible re-activation associated with the formation of secondary tumor formation under specific microenvironmental conditions at distant sites is also indicated. The new cancer therapies by molecular targeting of tumor- and metastasis-initiating cells to counteract cancer progression and metastases at distant sites are also indicated.
In addition, it has also been observed that the cancer progression to locally advanced and metastatic disease stages is usually associated with the inactivation of diverse tumor suppressor gene products and activation of a complex network of oncogenic signaling pathways in cancer cells including cancer-initiating cells concomitant with the changes in their microenvironment (Bapat et al., 2005; Brabletz et al., 2005a; Bao et al., 2006; Haraguchi et al., 2006; Li and Neaves, 2006; Liu et al., 2006a,b; Tso et al., 2006; Gray-Schopfer et al., 2007; Hermann et al., 2007; Katoh, 2007; Nicolis, 2007; Rich, 2007; Sato et al., 2007; Sengupta et al., 2007; Chiba et al., 2008; Ma et al., 2008; Yang et al., 2008a; Mimeault and Batra, 2009). The stimulation of distinct tumorigenic cascades initiated, in an autocrine or a paracrine manner, through diverse hormones, growth factors and cytokines may contribute to the sustained growth, survival, migration, invasion and treatment resistance of cancer stem/progenitor cells and their differentiated progenies. Particularly, the acquisition of a migratory phenotype by tumorigenic cancer stem/progenitor cells and their progenies during the epithelial-mesenchymal transition (EMT) process concomitant with the changes in the activated stroma may lead to their invasion from primary neoplasms, dissemination through the peripheral circulation and formation of aggressive and metastatic cancers at distant sites (Figs. 1, 2) (Bapat et al., 2005; Brabletz et al., 2005a; Zhou and Hung, 2005; Haraguchi et al., 2006; Thiery and Sleeman, 2006; Tso et al., 2006; Hermann et al., 2007; Mimeault and Batra, 2007b; Moustakas and Heldin, 2007; Shah et al., 2007; Shipitsin et al., 2007; Spaderna et al., 2007; Wang et al., 2007b; Das et al., 2008; Sarkar et al., 2008; Storci et al., 2008; Turley et al., 2008). Consistent with this hypothesis, the tumorigenic and migrating cancer stem/progenitor cells, also designated as metastasis-initiating cells, have been detected at invasion sites in primary tumors as well as isolated from peripheral blood and secondary tumor samples of cancer patients and metastatic cancer cell lines (Galli et al., 2004; Brabletz et al., 2005a; Fang et al., 2005; Ponti et al., 2005; Balic et al., 2006; Patrawala et al., 2006, 2007; Hermann et al., 2007; Wei et al., 2007; Das et al., 2008; Fillmore and Kuperwasser, 2008; Moustakas and Heldin, 2008; Quintana et al., 2008; Schatton et al., 2008; She al., 2008; Shmelkov et al., 2008; Yang et al., 2008a,b; Aktas et al., 2009). Importantly, the results from numerous recent studies have also indicated that the resistance of leukemic or tumorigenic and migrating cancer stem/progenitor cells to current clinical therapies could result in their persistence at primary and/or secondary neoplasms after treatment initiation (Bao et al., 2006; Liu et al., 2006a; Phillips et al., 2006; Hermann et al., 2007; Liu et al., 2007; Mimeault et al., 2007b, 2008a; Shah et al., 2007; Todaro et al., 2007; Wang et al., 2007a; Chen et al., 2008; Chiba et al., 2008; Fillmore and Kuperwasser, 2008; Friel et al., 2008; Johannessen et al., 2008; Levina et al., 2008; Loebinger et al., 2008; Ma et al., 2008; Matsui et al., 2008; Schatton et al., 2008; Sung et al., 2008; Zhang et al., 2008b). Thereby, cancer stem/progenitor cells can be responsible for the leukemia recurrence or tumor re-growth and disease relapse. In regard with this, we described here the recent investigations undertaken to establish the specific deregulated gene products induced in cancer stem/progenitor cells versus their differentiated progenies during cancer etiology and progression to locally invasive and metastatic disease stages. The provided information should help to design new targeting strategies for eradicating the total cancer cell mass including tumor- and metastasis-initiating cells and improving the current cancer treatments against aggressive, metastatic, recurrent and lethal cancers.
Critical functions of cancer stem/progenitor cells in cancer etiopathogenesis and progression to invasive and metastatic disease stages
Numerous factors may influence the risk of developing a cancer including inherited or somatic DNA mutations, intense oxidative stress, tobacco smoking, environmental carcinogens, radiation exposure, chronic inflammatory and fibrotic atrophies and age of individuals (Kim et al., 2005; Mimeault et al., 2007b; Nijhof et al., 2007; Sato et al., 2007; Vaish, 2007; Aubert and Lansdorp, 2008; Catassi et al., 2008; Friedman, 2008; Gumucio et al., 2008; Mimeault and Batra, 2008b; Mimeault et al., 2008b; Widera et al., 2008). Although the precise etiological causes responsible of cancer initiation remain not precisely established, the cancer development is usually associated with a cumulative genotoxic stress in cells that may cause chromosomal instability leading to the changes in the expression levels and/or activity of many deregulated gene products (Mimeault et al., 2007b; Shiras et al., 2007; Tchernev and Orfanos, 2007; Vaish, 2007; Zhou et al., 2007; Gumucio et al., 2008; Marusyk and DeGregori, 2008; Widera et al., 2008). In this matter, it is worth mentioning that certain inherited genetic aberrations may notably result in developmental defects and a predisposition to develop certain cancer types in post-natal life (Fig. 1) (MacDonald et al., 2003; Tiffin et al., 2003; Nakagawara and Ohira, 2004; Walton et al., 2004; Tostar et al., 2006; Mannelli et al., 2007; Mimeault et al., 2007b; Park et al., 2007; Ross and Spengler, 2007). For instance, the germinal mutations in PTCH receptor gene leading to an aberrant activation of hedgehog signaling pathway may promote the incidence of embryonal rhabdomyosarcoma, fetal rhabdomyoma, basal cell carcinoma, medulloblastoma and meningioma (Tiffin et al., 2003; Tostar et al., 2006). In the same pathway, the primitive neuroectodermal tumors (PNETs) including neuroblastoma, pheochromocytoma, ependymoblastoma and pineoblastoma, which are particularly manifest in pediatric population, may also originate during the embryonic development from neuroectodermal stem cells such as neural crest stem cells (MacDonald et al., 2003; Nakagawara and Ohira, 2004; Walton et al., 2004; Mannelli et al., 2007; Ross and Spengler, 2007). Although the embryonal origin of certain cancer types, the most types of human cancers appear rather to originate from a sequential and progressive accumulation of genetic abnormalities occurring in tissue-resident adult stem/progenitor cells concomitant with the changes in their microenvironments that lead to their malignant transformation in tumorigenic cancer stem/progenitor cells (Figs. 1, 2) (Mimeault and Batra, 2007a,b, 2008c; Mimeault et al., 2008a,b). These molecular transforming events in adult stem/progenitor cells may promote their sustained proliferation and aberrant differentiation, and thereby disrupt the normal mechanisms of tissue regeneration.
Numerous recent studies suggest that the cancer development may generally derive from the clonal expansion of cancer stem cells (CSCs) and/or their early progenies endowed with a high self-renewal capacity but aberrant differentiation potential that trigger the tumor growth (Mimeault and Batra, 2007a,b, 2008c; Mimeault et al., 2008a,b). In analogy with the normal tissue regeneration process mediated through tissue-resident adult stem/progenitor cells, CSCs can generate, through an asymmetric division, the daughter cells designated as transit amplifying (TA) cells with a malignant phenotype (Figs. 1,2). The malignant TA cells, in turn, can give rise to a heterogeneous population of poorly-, moderatelyand terminally-differentiated cells with aberrant functions (Mimeault and Batra, 2007a,b, 2008c; Mimeault et al., 2008a,b). Furthermore, the migration of malignant TA cells at distant sites within tissues from which they originate concomitant with the changes in their local microenvironment may result in a populational asymmetry and generation of malignant daughter cells endowed with different phenotypic properties including a self-renewal potential. In spite this importance advance, it will be important to determine the implication of tumorigenic CSCs versus their early progenies endowed with stem cell-like properties in development of specific cancer subsets, and more particularly whether different immature cancer cells may persist along cancer progression and drive tumor development.
In support with the critical functions of leukemic and tumorigenic cancer stem/progenitor cells in cancer development, recent investigations have led to the identification and isolation of different cancer-initiating cells in the most common human cancers and established cancer cell lines (Mimeault and Batra, 2007a,b, 2008c 2008a; Mimeault et al., 2008b). Among the cancer types harboring a subpopulation of leukemic or tumorigenic cancer stem/progenitor cells, there are leukemias, lymphomas, sarcomas, melanoma, brain tumors and a variety of epithelial cancers including skin, head and neck, thyroid, lung, cervical, renal, hepatic, esophageal, gastrointestinal, colon, bladder, pancreatic, prostate, mammary and ovarian cancers (Al-Hajj and Clarke, 2004; Hope et al., 2004; Jamieson et al., 2004; Matsui et al., 2004; Singh et al., 2004; Fang et al., 2005; Kim et al., 2005; Ponti et al., 2005; Maitland et al., 2006; Salmaggi et al., 2006; Ginestier et al., 2007; Hermann et al., 2007; Mimeault et al., 2007b; Prince et al., 2007; Ricci-Vitiani et al., 2007; Zhong et al., 2007; Eramo et al., 2008; Fillmore and Kuperwasser, 2008; Huang et al., 2008; Schatton et al., 2008; Shi et al., 2008; Wright et al., 2008; Yang et al., 2008a; Yu et al., 2008; Zhang et al., 2008a). It has been shown that the small subpopulations of isolated cancer stem/progenitor cells with stem cell-like properties displayed a greater clonogenic potential in vitro and generated leukemias or tumors with a higher incidence as compared to their differentiated progenies in animal model in vivo. In addition, the cancer progression is usually associated with the acquisition of a more malignant behavior by leukemic or tumorigenic stem/progenitor cells and their progenies.
Molecular transforming events in cancer stem/progenitor cells and their progenies associated with cancer initiation and progression
The transition from non-malignant hyperproliferative lesions to well established cancers has been associated with the occurrence of some oncogenic events in tissue-resident adult stem/progenitor cells and their microenvironment resulting into their acquisition of a malignant behavior (Li and Neaves, 2006; Mimeault and Batra, 2007a,b, 2008c; Das et al., 2008; Gumucio et al., 2008; Huang et al., 2008; Mimeault et al., 2008a, 2008b; Griffero et al., 2009). The transforming events include the stimulation of telomerase and inactivating mutations in numerous tumor suppressor gene products [p16INK4A, pRb, p53 and/or phosphatase and tensin homolog deleted on chromosome 10 (PTEN)]. Moreover, a constitutive activation of diverse growth factors and oncogenic signaling products [Ras, Myc, NF-κB, PI3K/Akt/mTOR, Bcl-2, survivin and/or fusion proteins resulting from chromosomal rearrangements] frequently occurs during cancer progression (Fig. 2) (Mimeault et al., 2007b; Sato et al., 2007; Sharpless and DePinho, 2007; Shiras et al., 2007; Tchernev and Orfanos, 2007; Vaish, 2007; Zhou et al., 2007; Dumont et al., 2008; Huang et al., 2008; Ma et al., 2008; Marusyk and DeGregori, 2008; Mimeault and Batra, 2009). In this regard, a growing body of evidence suggests that a progressive accumulation of different transforming events in tissue-resident adult stem/progenitor cells or pre-cancerous stem/progenitor cells with advancing age could be associated with an enhanced incidence of certain age-related cancer types including some epithelial cancers such as breast, prostate, pancreatic and colorectal cancers (Fig. 1) (Sharpless and DePinho, 2007; Griffero et al., 2009; Mimeault and Batra, 2009). Particularly, an activation of telomerase activity in the pre-cancerous cells could lead to their immortalization while the up-regulated expression and/or activity of diverse hormones, growth factors, cytokines and/or their cognate receptors in these immortalized cells could culminate to cancer development during chronological aging (Sharpless and DePinho, 2007; Griffero et al., 2009; Mimeault and Batra, 2009). In this regard, it has been proposed that the age-related increase in the number of cancer stem celllike markers such as CD44, CD166 and ESA as well as EGFR in macroscopically normal colonic mucosa could be associated with a predisposition to developing colorectal cancer with advancing age (Griffero et al., 2009). Further investigations are necessary to establish the phenotypic changes occurring in tissue-resident adult stem/progenitor cells versus their differentiated progenies during chronological aging that predispose to cancer formation in older individuals.
In addition, the up-regulation of distinct oncogenic signaling pathways in tumorigenic cancer stem/ progenitor cells during cancer progression may also contribute to their sustained growth, survival and/or invasion (Bao et al., 2006; Haraguchi et al., 2006; Liu et al., 2006a; Onoue et al., 2006; Tso et al., 2006; Katoh, 2007; Mimeault and Batra, 2007a,b, 2008b,c, 2009; Mimeault et al., 2007b, 2008b; Nicolis, 2007; Sato et al., 2007; Glinsky, 2008; Huang et al., 2008; Moustakas and Heldin, 2008). These signaling pathways include hedgehog, epidermal growth factor (EGF)-EGFR system, Wnt/β-catenin, Notch, SCF/KIT, hyaluronan (HA)/CD44 receptor, interleukin-4 (IL-4)/IL-4Rα, stromal cell-derived factor-1 (SDF-1)/CXCR4, transforming growth factor-β (TGF-β) superfamily cytokines, and/or polycomb group (PcG) proteins. For instance, the stimulation of HA/CD44 signaling cascade may lead to the activation of multiple receptor tyrosine kinases (RTKs) such as EGFR, ErbB2, insulin growth factor 1 receptor-β (IGF1R-β), platelet-derived growth factor-β (PDGFR-β) and/or the c-MET receptor of hepatocyte growth factor (HGF) and up-regulation of distinct anti-apoptotic factors and ABC multidrug transporters (Misra et al., 2006; Gilg et al., 2008; Toole and Slomiany, 2008). Hence, these effects mediated through the stimulation of HA-CD44 axis may promote the survival, invasion, multidrug resistance and/or protection against DNA oxidative damages of cancer stem/progenitor cells and their progenies. In general, the cancer development is also accompanied by an enhanced glycolysis in cancer cells including cancer stem/progenitor cells (Das et al., 2008; Gerlee and Anderson, 2008; Kondoh, 2008; Olivotto and Dello, 2008). This phenomenon known as Warburg effect may contribute to the resistance of cancer cells to oxidative stress as well as their survival in intratumoral hypoxic conditions (Das et al., 2008; Kondoh, 2008; Gerlee and Anderson, 2008; Olivotto and Dello, 2008). Moreover, the EMT phenomenon, which occurs in embryonic stem cells (ESCs) through the tissue and organ morphogenesis and patterning during embryonic development as well as tissue regeneration and wound healing in adult, is also re-activated during the progression of numerous aggressive cancers (Figs. 1, 2). Among them, there are brain, skin, prostate, ovarian, mammary, hepatic, gastrointestinal, pancreatic and colorectal carcinomas (Bapat et al., 2005; Brabletz et al., 2005a; Zhou and Hung, 2005; Ratajczak et al., 2006; Thiery and Sleeman, 2006; Tso et al., 2006; Bailey et al., 2007; Hermann et al., 2007; Mimeault and Batra, 2007a,b, 2008b,c; Mimeault et al., 2007b, 2008a,b; Moustakas and Heldin, 2007; Shipitsin et al., 2007; Wang et al., 2007b; Das et al., 2008; Turley et al., 2008).
Molecular events in tumorigenic cancer stem/progenitor cells and their differentiated progenies during the EMT process associated with the formation of locally invasive and metastatic cancers
The acquisition of a more malignant behavior by tumor-initiating cells and their progenies in primary malignant neoplasms, including a migratory phenotype during EMT process, represents a determinant factor that may contribute to disease progression to locally invasive and metastatic cancer subtypes (Abraham et al., 2005; Bapat et al., 2005; Brabletz et al., 2005a; Zhou and Hung, 2005; Balic et al., 2006; Ratajczak et al., 2006; Thiery and Sleeman, 2006; Tso et al., 2006; Bailey et al., 2007; Hermann et al., 2007; Mimeault and Batra, 2007b; Moustakas and Heldin, 2007; Shah et al., 2007; Shipitsin et al., 2007; Spaderna et al., 2007; Wang et al., 2007b; Das et al., 2008; Mani et al., 2008; Morel et al., 2008; Storci et al., 2008; Turley et al., 2008). The occurrence of EMT program in cancer stem/progenitor cells and their progenies may lead to the changes in their morphology and differentiation including a loss of polarity and epithelial cell markers concomitant with a gain of mesenchymal phenotypes that promote their migratory ability. This process is generally associated with a disruption of cell-cell junctions, loss of contact inhibition and extensive reorganization of the actin cytoskeleton and remodeling of extracellular matrix (ECM) components that lead to an increase of the motile and invasive abilities of cancer cells (Fig. 2). A complex network of different oncogenic cascades initiated through activating mutations in signaling components such as Ras and persistent activation of different growth factor pathways may cooperate for inducing a more complete EMT program in cancer stem/progenitor cells and their progenies (Fig. 2) (Zhou and Hung, 2005; Ratajczak et al., 2006; Thiery and Sleeman, 2006; Tso et al., 2006; Mimeault and Batra, 2007b; Shah et al., 2007; Spaderna et al., 2007; Wang et al., 2007b; Das et al., 2008; Kong et al., 2008; Levina et al., 2008; Morel et al., 2008; Storci et al., 2008; Turley et al., 2008). Among them, there are diverse developmental pathways such as sonic hedgehog SHH/PTCH/SMO/GLIs, EGF/ EGFR/Ras/MAPKs, Wnt/β-catenin, Notch, bone morphogenic proteins (BMPs), fibroblast growth factor (FGF)/FGFR, TGF-β/TGFRβ and PDGF/PDGFR pathways (Fig. 2) (Zhou and Hung, 2005; Ratajczak et al., 2006; Thiery and Sleeman, 2006; Tso et al., 2006; Feldmann et al., 2007; Mimeault and Batra, 2007b; Moustakas and Heldin, 2007; Shah et al., 2007; Spaderna et al., 2007; Wang et al., 2007b; Das et al., 2008; Kong et al., 2008; Levina et al., 2008; Storci et al., 2008; Turley et al., 2008; DiMeo et al., 2009). The sustained stimulation of these growth factor pathways may result in an up-regulation of diverse gene products in cancer stem/progenitor cells and their differentiated progenies during the EMT program. The signaling elements that are frequently altered in cancer cells during the EMT process include a decreased expression of E-cadherin concomitant with an up-regulation of different signaling elements such as N-cadherin, vimentin, tenascin C, NF-κB, snail, slug, twist, β-catenin, CXCR4 and anti-apoptotic factors (Brabletz et., 2005a; Zhou and Hung, 2005; Onoue et al., 2006; Ratajczak et al., 2006; Thiery and Sleeman, 2006; Tso et al., 2006; Feldmann et al., 2007; Hermann et al., 2007; Mimeault and Batra, 2007b; Moustakas and Heldin, 2007; Shah et al., 2007; Spaderna et al., 2007; Wang et al., 2007b; Das et al., 2008; Dumont et al., 2008; Sarkar et al., 2008; Storci et al., 2008; Chen et al., 2009; DiMeo et al., 2009; Kurrey et al., 2009). These signaling elements may contribute to the invasive and metastatic phenotypes of cancer cells and enhanced resistance to radiation and chemotherapies.
Consistent with the critical functions of tumorigenic and migrating cancer stem/progenitor cells in invasion and metastases at distant sites, the cancer cells with the stem cell-like properties have been detected at invasive front in primary neoplasms, in blood and metastatic tissues from cancer patients (Brabletz et al., 2005a; Fang et al., 2005; Ponti et al., 2005; Balic et al., 2006; Hermann et al., 2007; Das et al., 2008; Fillmore and Kuperwasser, 2008; Schatton et al., 2008; Shi et al., 2008; Yang et al., 2008a). Particularly, the migrating cancer cells involved in several cancers such as melanoma, brain, breast, ovarian, prostate and pancreatic cancer express the CXCR4 receptor that may promote their invasion and migration to distant sites such as lymph nodes, bones, lungs and/or liver that are characterized by high expression levels of its ligand, SDF-1 (Geminder et al., 2001; Muller et al., 2001; Sun et al., 2003, 2005, 2007; Ratajczak et al., 2006; Hermann et al., 2007). The stimulation of SDF-1-CXCR4 signaling cascade may contribute to activate diverse signaling elements involved in survival, ECM degradation (metalloproteinases) and motility of cancer cells at the primary neoplasm. Moreover, the chemoattractant SDF-1 ligand gradient may promote the migration and adhesion of CXCR4+ circulating cancer cells including cancer stem/progenitor cells to endothelium and stromal ECM components at distant metastatic sites by modulating intracellular signaling pathways mediated by adhesion molecules and ECM/integrins (Ratajczak et al., 2004; Hartmann et al., 2005). For instance, the data from immunohistochemical analyses of primary pancreatic adenocarcinoma specimens from patients have revealed the presence of two different subpopulations of pancreatic cancer stem/progenitor cells, including tumorigenic CD133+/CXCR4− and migrating CD133+/CXCR4+ subsets localized in the bulk mass and invasive front of pancreatic tumor, respectively (Hermann et al., 2007). It has also been shown that CD133+ cells isolated from patient’s tumor tissues were able to form tumors after orthotopic injection in the pancreas of immunodeficient nude mice. Importantly, the depletion of CD133+/CXCR4+ migrating cancer stem/progenitor cells in the total cancer cell mass constituting highly metastatic human pancreatic cancer cell line L3.6 pl or inhibition of CXCR4 cascade effectively abrogated their metastatic capacity without altering their tumorigenic potential in animal models in vivo (Hermann et al., 2007). Hence, these observations suggest that the migrating CD133+/CXCR4+ cells may correspond to a more malignant cell subpopulation than tumorigenic CD133+/CXCR4− cells, which may have acquired a migratory phenotype during the EMT program, and thereby they may be involved in invasion and metastases to distant sites.
In addition, it has been shown that the occurrence of EMT may promote the metastatic dissemination of cancer stem/progenitor cells and their progenies (Abraham et al., 2005; Balic et al., 2006; Morel et al., 2008). For instance, the circulating tumor cells expressing the stem cell-like marker ALDH1 and EMT-associated molecules (twist, PI3Kα and Akt2) have been detected in blood samples from metastatic breast cancer patients (Aktas et al., 2009). Importantly, it has also been reported that the putative CD44+CD24−/low breast cancer stem cells were detected in all bone marrow patient’s specimens from early breast cancer patients expressing cytokeratin (CK+) with a high mean prevalence of 72% relative to a low prevalence inferior to 10% of cells in primary tumors (Balic et al., 2006). These data support the concept that the enhanced expression levels of specific gene products, and more particularly EMT-associated molecules, in cancer-initiating cells at primary neoplasms may promote their invasion and metastatic spread at definite distant sites.
Molecular events in the tumor stroma associated with the formation of locally invasive and metastatic cancers
The cancer progression is also accompanied by an extensive tumor stromal remodeling of ECM components and changes in gene expression patterns in activated tumor-associated stromal cells including myofibroblasts and/or stellate cells as well as infiltrating circulating endothelial progenitor cells (EPCs) and immune cells such as macrophages (Fig. 2) (Mimeault and Batra, 2007a,b, 2008d; Mimeault et al., 2007b, 2008b; Friedman, 2008; Kirkland, 2009). These changes in tumor stroma may contribute to the acquisition of stem cell-like phenotypes, a more malignant behavior and invasive ability by cancer cells. Especially, a variety of motomorphogens and chemoattractant factors may be secreted by stromal cells and promote the migration of cancer stem/progenitor cells and their progenies during the transition from localized cancers to invasive and metastatic disease stages. The soluble growth factors, cytokines and chemokines released by tumor stromal cells in reactive stroma include EGF, insulin-like growth factor (IGF), hepatocyte growth factor (HGF), TGF-β and SDF-1 as well as matrix metalloproteinases (MMPs) and urokinase plasminogen activator (uPA) (Brabletz et al., 2005a; Mimeault and Batra, 2006; Ratajczak et al., 2006; Kleeff et al., 2007; Mimeault and Batra, 2007a,b, 2008b; Mimeault et al., 2007b, 2008b; Spaderna et al., 2007). The release of these soluble factors in reactive stroma may promote, in a paracrine manner, the malignant transformation of cancer stem/progenitor cells and their differentiated progenies during the EMT process (Fig. 2). Moreover, the secretion of diverse angiogenic factors by tumor cells and myofibroblasts may stimulate the tissue-resident endothelial cells or EPCs as well as the recruitment of BM-derived hematopoietic cells including circulating EPCs at tumoral sites that can cooperate to induce the tumor neovascularization process. Hence, these molecular events may promote the invasion of tumorigenic and migrating cancer stem/progenitor cells into reactive stroma, dissemination through the near lymph nodes and peripheral circulation and metastasis at distant tissues and organs.
Importantly, the phenotypic changes of cancer stem/progenitor cells, including a re-differentiation towards the occurrence of mesenchymal-epithelial transition (MET) process, may also occur at certain metastatic tissues and organs including BM (Fig. 2) (Thiery and Sleeman, 2006; Spaderna et al., 2007). Moreover, the immature metastasis-initiating cells like adult stem/progenitor cells may exhibit a quiescent and less metabolically active state in their novel local microenvironment, niches prevalent at secondary anatomical sites. The adoption of a quiescent state by metastasis-initiating cells may explain, at least in part, the long-term dormancy phenomenon. The quiescence of these immature cancer cells may be associated with their persistence under form of micrometastases for a prolonged period of time without clinical or histopathologic signs of apparent macrometastases as well as their resistance to radiation or cytotoxic drugs targeting the proliferative cancer cells (Fig. 2) (Clezardin and Teti, 2007; Mimeault and Batra, 2007a; Mimeault et al., 2007b; Horak et al., 2008; ix-Panabieres et al., 2008; Mimeault et al., 2008a; Rak et al., 2008; Riethdorf et al., 2008; Trumpp and Wiestler, 2008; Vincent-Salomon et al., 2008). The changes in the local microenvironment of metastasis-initiating cells leading to the repression of metastasis suppressor genes and/or re-activation of mitogenic signaling pathways could however trigger their proliferation, secondary tumor growth and culminate to disease recurrence (Fig. 2) (Clezardin and Teti, 2007; Mimeault and Batra, 2007a; Mimeault et al., 2007b; Horak et al., 2008; ix-Panabieres et al., 2008; Rak et al., 2008; Riethdorf et al., 2008; Trumpp and Wiestler, 2008; Vincent-Salomon et al., 2008).
Collectively, these observations suggest that the tumorigenic and metastatic potentials of cancer stem/progenitor cells may be enhanced along disease progression through a sequential and progressive accumulation of oncogenic transforming events occurring during EMT process. Especially, the acquisition of a migratory phenotype and survival advantages by tumorigenic cancer stem/progenitor cells during the EMT process at primary neoplasm, concomitant with the changes in their local tumor microenvironment may result to their invasion and progression from organ-confined cancers to metastatic disease states (Fig. 2). Hence, the intrinsic and acquired resistance of tumor- and metastasis-initiating cells to current cancer therapies may lead to their persistence at primary and secondary neoplasms and disease relapse. In respect with this, we discussed accumulating lines of evidence suggesting that the acquisition of distinct phenotypes by tumor-initiating cells during cancer progression may lead to the formation of different cancer subtypes that differently respond to current therapeutic treatments.
Heterogeneity of cancers derived from distinct tumorigenic and migrating cancer stem/progenitor cells and their differential response to current clinical treatments
Numerous investigations revealed that the occurrence of different malignant transforming events in adult stem/progenitor cells during cancer initiation as well as an accumulation of distinct genetic and/or epigenetic alterations in cancer stem/progenitor cells along cancer progression may lead to the development of different cancer subtypes (Dontu et al., 2004; Brabletz et al., 2005a; Clarke et al., 2005; Teuliere et al., 2005; Anderson and Matsuno, 2006; Asselin-Labat et al., 2006; Buyse et al., 2006; Laakso et al., 2006; Sorlie et al., 2006; Tang et al., 2006; Tso et al., 2006; Dalerba et al., 2007; Fodde and Brabletz, 2007; Kelly et al., 2007; Kennedy et al., 2007; Langley and Fidler, 2007; Mimeault and Batra, 2007b; Zhou et al., 2007; Agelopoulos et al., 2008; Ben-Porath et al., 2008; Das et al., 2008; Huang et al., 2008; Quintana et al., 2008). This heterogeneity of cancers has important repercussion since these distinct cancer subtypes with variable degrees of differentiation and/or aggressivity may harbor distinct cancer-initiating cells exhibiting different phenotypic and functional properties, and differentially respond to current cancer therapies. More specifically, the expression of embryonic stem cell (ESC)-associated and mesenchymal genes and the acquisition of a migratory phenotype by poorly- or moderately-differentiated tumorigenic cancer stem/progenitor cells during the EMT program may result in the formation of highly invasive and metastatic cancer subtypes characterized by a poorly- to moderately-differentiated state (Abraham et al., 2005; Brabletz et al., 2005b; Anderson and Matsuno, 2006; Asselin-Labat et al., 2006; Balic et al., 2006; Buyse et al., 2006; Mimeault and Batra, 2007b; Ben-Porath et al., 2008; Aktas et al., 2009). In contrast, the tumorigenic cancer stem/progenitor cells that do not undertake the EMT transition could rather give rise to weakly invasive cancer subtypes (Mimeault and Batra, 2007b).
In addition, the changes in the local microenvironment of tumorigenic cancer stem/progenitor cells and their differentiated progenies including their localization within hypoxic zones of solid tumors may result in the expression of a different subset of oncogenic gene products during cancer development and be responsible, at least in part, for the intratumoral heterogeneity (Mimeault and Batra, 2007b; Das et al., 2008). This line of thought is well-supported by the observations indicating that certain invasive cancer types, such as mammary, ovarian, prostate, pancreatic, gastric, colorectal and squamous cell carcinomas, harbored an intratumoral heterogeneity (Galli et al., 2004; Brabletz et al., 2005a; Kalluri and Zeisberg, 2006; Hermann et al., 2007; Mimeault and Batra, 2007a,b, 2008c). These invasive cancer subtypes typically display distinct proliferating and differentiating regions, including a preferential localization of migrating cancer stem/progenitor cells at intratumoral hypoxic zones and invasive front (Fig. 2). In support with this model of heterogeneity of cancers, we are reporting in a more detailed manner, accumulating lines of experimental evidence obtained for breast and brain cancer subtypes underlining the complexity of clinical classification based on specific phenotypic features of cancer cells. The therapeutic significance of the heterogeneity of these cancer subtypes is also discussed.
Heterogeneity of breast cancers
At least five subtypes of breast cancer have been identified by comparing gene expression signatures of cancer cells and their invasive phenotype. The classification of breast cancer includes basal-like (estrogen receptor “ERα−”, progesterone receptor “PR−”, erbB2−/low, CK5/6+ and EGFR+); erbB2/HER2+ overexpressing (ERα− and PR−); luminal A (ERα+ and/or PR+ and erbB2−); luminal B (ERα+ and/or PR+ and erbB2+) and normal breast cancer subtype (high expression of normal epithelium genes and low expression of luminal epithelial gene products) (Clarke et al., 2005; Anderson and Matsuno, 2006; Asselin-Labat et al., 2006; Buyse et al., 2006; Calza et al., 2006; Carey et al., 2006b; Laakso et al., 2006; Lacroix, 2006; Sorlie et al., 2006; Tang et al., 2006; Agelopoulos et al., 2008). More recently, two other subtypes designated as metaplastic and claudinlow breast cancers, have also been characterized as triple negative for ER-α, PR and erbB2 (Fig. 2) (Hennessy et al., 2009). The triple-negative breast cancers (TNBCs), encompassing basal-like, metaplastic, claudinlow and normal breast cancer subtypes, represent one of the most aggressive and lethal breast cancer subgroup (Nielsen et al., 2004; Carey et al., 2006a; Haffty et al., 2006; Bauer et al., 2007; Rakha et al., 2007; Reis-Filho and Tutt, 2008; Schneider et al., 2008; Dawson et al., 2009; Jaspers et al., 2009). The patients with TNBC lacking ER-α, PR and erbB2 receptor expression are unresponsive to the treatments with anti-estrogen, hormonal therapies and/or specific inhibitors of erbB2 cascade such as trastuzumab (Schneider et al., 2008; Jaspers et al., 2009). Moreover, although TNBC patients initially respond to taxaneand/or anthracycline-based chemotherapies, the development of chemoresistance usually leads to disease recurrence and the death of patients (Dontu et al., 2004; Carey et al., 2006b; Sorlie et al., 2006; Rakha et al., 2007; Cheang et al., 2008; Li et al., 2008; Reis-Filho and Tutt, 2008; Dawson et al., 2009; Jaspers et al., 2009). In addition, the erbB2-overexpressing subtype has also been associated with a poorer prognosis and patient survival relative to differentiated ERα+ breast cancer subtypes despite patients are generally responsive to adjuvant treatment with trastuzumab (Carey et al., 2006b).
Collectively, these observations suggest that the malignant transformation of immature ERα−stem/progenitor cells in the basal compartment of breast epithelium may result in highly aggressive breast cancer subtypes that are less responsive to current clinical treatments. In support with this, it has been reported that the targeted expression of stabilized β-catenin in basal myoepithelial cells of mouse mammary epithelium resulted in an enhanced proliferation of basal-type celllike progenitors possessing an abnormal differentiation potential. This oncogenic event led to the development of invasive basal-type carcinomas (Teuliere et al., 2005). The ERα− breast cancer cells, which did not express the metastasis-associated gene 3 (MTA3) that inhibits snail transcriptional activity, also possessed a lower level of E-cadherin and displayed a higher migratory capacity than the ERα+ breast cancer cells (Fujita et al., 2003). Moreover, an increased expression of NF-κB in ERα−breast cancer cells may lead to an induction of EMT program throughout the stimulation of transcription factor RelB and enhanced expression of the anti-apoptotic protein, Bcl-2 that may contribute to treatment resistance (Wang et al., 2007b). In this matter, a differently expressed gene pattern, designated as invasiveness 186-gene signature (IGS), has also been detected in CD44+/CD24−/low tumorigenic breast cancer stem/progenitor cells relative to that of normal breast epithelial cells and associated with a poor overall survival of patients with breast cancer (Liu et al., 2007). Among the genes expressed in this very little population of CD44+/CD24−/low tumor-initiating cells, there are the gene products associated with the NF-κB and MAPK pathways, and epigenetic control of gene expression (Liu et al., 2007). In this regard, it noteworthy that the metaplastic and claudinlow breast cancer subtypes, which are usually enriched for the markers linked to EMT and tumorigenic CD44+/CD24− stem cell-like features and chemoresistance, frequent display an aberrant activation of PI3K/Akt pathway (Hennessy et al., 2009). Moreover, the results from another study have also revealed that the TGF-β pathway may be specifically activated in CD44+ breast cancer cells and its inhibition induced a more epithelial phenotype (Shipitsin et al., 2007). These data support the therapeutic interest of targeting NF-κB, MAPK, PI3K/Akt and/or TGF-β signaling elements to prevent the EMT process, eradicate breast cancer-initiating cells and improve the current clinical chemotherapies.
Heterogeneity of brain cancers
Among the brain cancer subtypes, the glioblastoma multiformes (GBMs), which represent a heterogeneous population of cancer cells, may arise from the malignant transformation of neural stem cells (NSCs) into brain tumor stem cells (BTSCs) (Galli et al., 2004; Yuan et al., 2004; Tso et al., 2006). BTSCs can acquire the mesenchymal properties like mesenchymal stem cells and give rise to further differentiated progenies. Primary GBMs, which are aggressive brain cancers that are frequently accompanied with EGFR overexpression, typically progress rapidly without evidence of a transitory step of lower-grade tumor. In this regard, it has been reported that CD133+ BTSCs found in three primary cell lines established from glioblastoma patients, expressed high mRNA levels of diverse stem cell-like markers (Liu et al., 2006a). These stemness gene products include CD44 and OCT-3/4 biomarkers, MGMT, BCRP/ABCG2 transporter, anti-apoptotic factors such as Bcl-2, survivin and inhibitor of apoptosis proteins (IAPs), hedgehog signaling elements SHH/PTCH/GLI and the transcriptional repressor of the E-cadherin, snail (Liu et al., 2006a). The isolated CD133+ glioblastoma cells were also more resistant to chemotherapeutic agents, such as temozolomide, carboplatin, etoposide and paclitaxel, as compared to the CD133− cell fraction (Liu et al., 2006a). Importantly, higher levels of CD133 stem cell-like surface marker, which may be associated with the presence of CD133+ BTSCs, were also detected in recurrent GBM tissues obtained from five patients relative to their respective newly diagnosed tumors (Liu et al., 2006a). It has also been reported that CD133+ glioma stem/progenitor cells from a recurrent tumor after malignancy progression displayed more aggressive and invasive phenotypes in vivo than CD133+ glioma stem/progenitor cells from the primary tumor of same patient (Huang et al., 2008). Together these observations suggest that the acquisition of a more malignant phenotype by CD133+ BTSCs during disease progression and their high chemoresistance may contribute, at least in part, to the recurrence of highly aggressive primary GBMs. On the opposite end, the secondary or progressive GBMs, which are often characterized by mutations in the p53 suppressor gene, appear to derive from low-grade tumors that did not show the changes in the gene expression pattern that are usually associated with the EMT program (Tso et al., 2006). Hence, the clinical management of these two different brain cancer subtypes with different aggressivity and phenotypic markers generally require distinct types of therapeutic treatments.
In light of together these observations, it appears that different cancer subtypes may originate from distinct tumorigenic cancer stem/progenitor cells that acquire a specific oncogenic gene profile during cancer development and EMT process. Therefore, the management of these cancer subtypes may require different therapeutic strategies. In respect with this, we describe new targeting approaches that have been developed to eradicate the total cancer cell mass including tumor- and metastasis-initiating cells and their differentiated progenies.
Novel cancer therapies
The progression of organ-confined cancers to locally invasive and metastatic disease stages that are resistant to current anti-hormonal, radiation and/or chemotherapeutic treatments represents one of the major causes of disease recurrence and cancer-related deaths (Lacroix, 2006; Gray-Schopfer et al., 2007; Mimeault et al., 2007b, 2008a; Sathornsumetee et al., 2007; Sorscher, 2007; Mimeault and Batra, 2008c). Therefore, the molecular targeting of cancer- and metastasis-initiating cells that can contribute in a substantial manner to drive tumor growth and metastases at distant tissues and organs, resistance to current conventional therapies and disease relapse, constitute a promising approach to develop novel effective combination therapies against aggressive and recurrent cancers (Horak et al., 2008; Le Tourneau et al., 2008; Mimeault and Batra, 2008c,d; Mimeault et al., 2008a; Steeg and Theodorescu, 2008). Especially, the molecular targeting of the EMT- and multidrug resistance-associated molecules in cancer- and metastasis-initiating cells and their local microenvironment represent potential therapeutic strategies for overcoming treatment resistance and improving current cancer therapies (Wang et al., 2002; Horak et al., 2008; Steeg and Theodorescu, 2008). Consistently, numerous recent studies have revealed that molecular targeting of hedgehog, EGFR, Wnt/β-catenin, Notch, HA/CD44 and/or SDF-1/CXCR4 pathway as well as PI3K/Akt/mTOR, NF-κB, snail or twist signaling components and ABC multidrug transporters could be effective to eradicate the total cancer cell mass, including cancer- and metastasis-initiating cells, and thereby prevent disease relapse (Mimeault et al., 2007b, 2008b; Mimeault and Batra, 2008b,c,d, 2009; Chen et al., 2009; DiMeo et al., 2009).
Conclusions and perspectives
Recent advances in basic and clinical oncology have revealed that the tumorigenic and migrating cancer stem/progenitor cells can provide critical functions in tumor formation, metastases at distant sites, treatment resistance and disease relapse. Consequently, the molecular targeting of tumor- and metastasis-initiating cells and their microenvironment may represent a potential strategy for improving the efficacy of current cancer treatments. Future investigations are still essential to more precisely determine the specific biomarkers and altered gene products regulating the self-renewal, differentiation and/or treatment resistance of tumor- and metastasis-initiating cells during cancer initiation and progression to locally invasive and metastatic disease stages. Especially, a comparative analysis of gene expression profiles observed for tumor-initiating cells during primary cancer development and EMT process versus their respective normal tissue-resident stem/progenitor cells should shed light on the molecular transforming events occurring in these immature malignant cells and their pathological consequences. These studies should allow to more precisely definite the altered gene products that can contribute to the acquisition of a migratory phenotype by tumor-initiating cells during the EMT program and formation of invasive and metastatic cancer subtypes and help to develop new molecular targeting strategies that selectively eradicate these immature tumor-initiating cells.
In addition, a comparative analysis of signaling elements deregulated in tumorigenic and metastatic cancer stem/progenitor cell subpopulation relative to tumorigenic but not metastatic cancer stem/progenitor cell subset isolated from primary and metastatic patient tissues and well established cancer cell lines should permit to characterizing their specific phenotypic features. The determination of molecular events involved in the transcriptional up-regulation of SDF-1 and CXCR4 and downstream signaling effectors activated through SDF-1/CXCR4 axis in tumor- and metastatis-initating cells during cancer progression is also of particular therapeutic interest. Furthermore, the establishment of molecular mechanisms associated with the specific migration of metastasis-initiating cells to pre-determinate metastatic sites, dormancy phenomenon and re-activation of metastasis-initiating cells at distant sites after a long latency, is also essential to identify new potential therapeutic targets to counteract the metastasis formation and disease relapse. Additional investigations to establish the signaling elements deregulated in local microenvironment of tumor- and metastasis-initiating cells is also important to design novel adjuvant cancer treatments for reversing MDR phenotype and preventing disease recurrence. These studies should lead to identification of new biomarkers and molecular therapeutic targets that could be exploited to develop new diagnostic and prognostic methods and preventive and therapeutic approaches for treating and even curing the patients diagnosed with locally advanced, metastatic, recurrent and lethal cancers.
Acknowledgements
The authors on this work are supported by grants from the National Institutes of Health (CA78590, CA111294, CA133774 and CA131944). We thank Ms. Kristi L. Berger for editing the manuscript.
Abbreviations
- ABC
ATP-binding cassette
- ALDH
aldehyde dehydrogenase
- BPH
benign prostatic hyperplasia
- BTSCs
brain tumor stem cells
- CDK
cyclin-dependent kinase
- COX-2
clyooxygenase 2
- CXCR4
chemokine receptor 4
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transition
- Fzd
Frizzeled receptor
- KIT
stem cell factor receptor
- HA
hyaluronan
- MAPKs
mitogen-activated protein kinase
- MEK
extracellular signal-related kinase kinase
- MET
mesenchymal-epithelial transition
- MDR
multidrug resistance
- MMPs
matrix metalloproteinases
- MGMT
O6-methylguanine DNA methyltransferase
- NF-κB
nuclear factor-kappa B
- NSCs
neural stem cells
- PI3K
phosphoinositide 3'-kinase
- PTCH
hedgehog patched receptor
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- Rb
retinoblastoma
- RTK
receptor tyrosine kinase
- SDF-1
stromal cell-derived factor-1
- SHH
sonic hedgehog ligand
- SMO
smoothened co-receptor
- TERT
telomerase reverse transcriptase
- TK
tyrosine kinase
- TR
telomere RNA component
- uPA
urokinase-type plasminogen activator
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor and Wnt, Wingless ligand
References
- Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin. Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- Agelopoulos K, Buerger H, Brandt B. Allelic imbalances of the egfr gene as key events in breast cancer progression--the concept of committed progenitor cells. Curr. Cancer Drug Targets. 2008;8:431–445. doi: 10.2174/156800908785133213. [DOI] [PubMed] [Google Scholar]
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- Anderson WF, Matsuno R. Breast cancer heterogeneity: a mixture of at least two main types? J. Natl. Cancer Inst. 2006;98:948–951. doi: 10.1093/jnci/djj295. [DOI] [PubMed] [Google Scholar]
- Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann. N.Y. Acad. Sci. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ. Steroid hormone receptor status of mouse mammary stem cells. J. Natl. Cancer Inst. 2006;98:1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- Aubert G, Lansdorp PM. Telomeres and aging. Physiol. Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J. Cell Biochem. 2007;102:829–839. doi: 10.1002/jcb.21509. [DOI] [PubMed] [Google Scholar]
- Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH, Cote RJ. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin. Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat. Rev. Cancer. 2005a;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005b;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am. J. Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyse M, Loi S, van't Veer L, Viale G, Delorenzi M, Glas AM, d'Assignies MS, Bergh J, Lidereau R, Ellis P, Harris A, Bogaerts J, Therasse P, Floore A, Amakrane M, Piette F, Rutgers E, Sotiriou C, Cardoso F, Piccart MJ. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J. Natl. Cancer Inst. 2006;98:1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- Calza S, Hall P, Auer G, Bjohle J, Klaar S, Kronenwett U, Liu ET, Miller L, Ploner A, Smeds J, Bergh J, Pawitan Y. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34. doi: 10.1186/bcr1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006a;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006b;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Catassi A, Servent D, Paleari L, Cesario A, Russo P. Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: implications on lung carcinogenesis. Mutat. Res. 2008;659:221–231. doi: 10.1016/j.mrrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin. Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, Ku HH, Chiou SH. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS ONE. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, Chen DT, Tai LK, Yung MC, Chang SC, Ku HH, Chiou SH, Lo WL. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem. Biophys. Res. Commun. 2009;385:307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- Chiba T, Miyagi S, Saraya A, Aoki R, Seki A, Morita Y, Yonemitsu Y, Yokosuka O, Taniguchi H, Nakauchi H, Iwama A. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 2008;68:7742–7749. doi: 10.1158/0008-5472.CAN-07-5882. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev. Biol. 2005;277:443–456. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Clezardin P, Teti A. Bone metastasis: pathogenesis and therapeutic implications. Clin. Exp. Metastasis. 2007;24:599–608. doi: 10.1007/s10585-007-9112-8. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu. Rev. Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–1830. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur. J. Cancer. 2009;45:27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S, Kuperwasser C. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol. Metab. 2004;15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc. Natl. Acad. Sci. USA. 2008;105:14867–14872. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death. Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, Gabrielson KL, Matsui W, Maitra A. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel AM, Sergent PA, Patnaude C, Szotek PP, Oliva E, Scadden DT, Seiden MV, Foster R, Rueda BR. Functional analyses of the cancer stem cell-like properties of human endometrial tumor initiating cells. Cell Cycle. 2008;7:242–249. doi: 10.4161/cc.7.2.5207. [DOI] [PubMed] [Google Scholar]
- Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Geminder H, Sagi-Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP, Ben-Baruchn A. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J. Immunol. 2001;167:4747–4757. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- Gerlee P, Anderson AR. A hybrid cellular automaton model of clonal evolution in cancer: the emergence of the glycolytic phenotype. J. Theor. Biol. 2008;250:705–722. doi: 10.1016/j.jtbi.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilg AG, Tye SL, Tolliver LB, Wheeler WG, Visconti RP, Duncan JD, Kostova FV, Bolds LN, Toole BP, Maria BL. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin. Cancer Res. 2008;14:1804–1813. doi: 10.1158/1078-0432.CCR-07-1228. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 Is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsky GV. "Stemness" genomics law governs clinical behavior of human cancer: implications for decision making in disease management. J. Clin. Oncol. 2008;26:2846–2853. doi: 10.1200/JCO.2008.17.0266. [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- Griffero F, Daga A, Marubbi D, Capra MC, Melotti A, Pattarozzi A, Gatti M, Bajetto A, Porcile C, Barbieri F, Favoni RE, Lo CM, Zona G, Spaziante R, Florio T, Corte G. Different response of human glioma tumor-initiating cells to EGFR kinase inhibitors. J. Biol. Chem. 2009;284:7138–7148. doi: 10.1074/jbc.M807111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio DL, Fagoonee S, Qiao XT, Liebert M, Merchant JL, Altruda F, Rizzetto M, Pellicano R. Tissue stem cells and cancer stem cells: potential implications for gastric cancer. Panminerva Med. 2008;50:65–71. [PubMed] [Google Scholar]
- Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506–513. doi: 10.1634/stemcells.2005-0282. [DOI] [PubMed] [Google Scholar]
- Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24:4462–4471. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, Liu W, Stivers D, Baggerly K, Carey M, Lluch A, Monteagudo C, He X, Weigman V, Fan C, Palazzo J, Hortobagyi GN, Nolden LK, Wang NJ, Valero V, Gray JW, Perou CM, Mills GB. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat. Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- Horak CE, Lee JH, Marshall JC, Shreeve SM, Steeg PS. The role of metastasis suppressor genes in metastatic dormancy. APMIS. 2008;116:586–601. doi: 10.1111/j.1600-0463.2008.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Gao Q, Guo L, Zhang C, Wei J, Li H, Jing WJ, Han X, Shi Y, Shih HL. Isolation and identification of cancerstem-like cells in esophageal carcinoma cell lines. Stem Cells Dev. 2009;18:465–473. doi: 10.1089/scd.2008.0033. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhang QB, Dong J, Wu YY, Shen YT, Zhao YD, Zhu YD, Diao Y, Wang AD, Lan Q. Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the primary tumor, and both can be maintained long-term in vitro. BMC Cancer. 2008;8:304. doi: 10.1186/1471-2407-8-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntly BJ, Shigematsu H, Deguchi K, Lee BH, Mizuno S, Duclos N, Rowan R, Amaral S, Curley D, Williams IR, Akashi K, Gilliland DG. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6:587–596. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- ix-Panabieres C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin. Cancer Res. 2008;14:5013–5021. doi: 10.1158/1078-0432.CCR-07-5125. [DOI] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Jaspers JE, Rottenberg S, Jonkers J. Therapeutic options for triple-negative breast cancers with defective homologous recombination. Biochim. Biophys. Acta. 2009;1796:266–280. doi: 10.1016/j.bbcan.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Johannessen TC, Bjerkvig R, Tysnes BB. DNA repair and cancer stem-like cells - Potential partners in glioma drug resistance? Cancer Treat. Rev. 2008;34:558–567. doi: 10.1016/j.ctrv.2008.03.125. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Katoh M. Networking of WNT, FGF, Notch, BMP, and Hedgehog signaling pathways during carcinogenesis. Stem Cell Rev. 2007;3:30–38. doi: 10.1007/s12015-007-0006-6. [DOI] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasserm A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Kennedy JA, Barabe F, Poeppl AG, Wang JC, Dick JE. Comment on "Tumor growth need not be driven by rare cancer stem cells". Science. 2007;318:1722. doi: 10.1126/science.1149590. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kirkland SC. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br. J. Cancer. 2009;101:320–326. doi: 10.1038/sj.bjc.6605143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Lohr JM, Friess H. Pancreatic cancer microenvironment. Int. J. Cancer. 2007;121:699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- Kondoh H. Cellular life span and the Warburg effect. Exp. Cell Res. 2008;314:1923–1928. doi: 10.1016/j.yexcr.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Kong D, Wang Z, Sarkar SH, Li Y, Banerjee S, Saliganan A, Kim HR, Cher ML, Sarkar FH. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26:1425–1435. doi: 10.1634/stemcells.2007-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- Laakso M, Tanner M, Nilsson J, Wiklund T, Erikstein B, Kellokumpu-Lehtinen P, Malmstrom P, Wilking N, Bergh J, Isola J. Basoluminal carcinoma: a new biologically and prognostically distinct entity between basal and luminal breast cancer. Clin. Cancer Res. 2006;12:4185–4191. doi: 10.1158/1078-0432.CCR-06-0353. [DOI] [PubMed] [Google Scholar]
- Lacroix M. Significance, detection and markers of disseminated breast cancer cells. Endocr. Relat. Cancer. 2006;13:1033–1067. doi: 10.1677/ERC-06-0001. [DOI] [PubMed] [Google Scholar]
- Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr. Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- Le Tourneau C, Faivre S, Raymond E. New developments in multitargeted therapy for patients with solid tumours. Cancer Treat. Rev. 2008;34:37–48. doi: 10.1016/j.ctrv.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS ONE. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl. Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer. 2006a;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N. Engl. J. Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Surim P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006b;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebinger MR, Giangreco A, Groot KR, Prichard L, Allen K, Simpson C, Bazley L, Navani N, Tibrewal S, Davies D, Janes SM. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. Br. J. Cancer. 2008;98:380–387. doi: 10.1038/sj.bjc.6604185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- MacDonald TJ, Rood BR, Santi MR, Vezina G, Bingaman K, Cogen PH, Packer RJ. Advances in the diagnosis, molecular genetics, and treatment of pediatric embryonal CNS tumors. Oncologist. 2003;8:174–186. doi: 10.1634/theoncologist.8-2-174. [DOI] [PubMed] [Google Scholar]
- Maitland NJ, Bryce SD, Stower MJ, Collins AT. Prostate cancer stem cells: a target for new therapies. Ernst. Schering. Found. Symp. Proc. 2006;5:155–179. doi: 10.1007/2789_2007_050. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinbergm RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli M, Simi L, Gagliano MS, Opocher G, Ercolino T, Becherini L, Parenti G. Genetics and biology of pheochromocytoma. Exp. Clin. Endocrinol. Diabetes. 2007;115:160–165. doi: 10.1055/s-2007-970407. [DOI] [PubMed] [Google Scholar]
- Marusyk A, DeGregori J. Declining cellular fitness with age promotes cancer initiation by selecting for adaptive oncogenic mutations. Biochim. Biophys. Acta. 2008;1785:1–11. doi: 10.1016/j.bbcan.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, McNiece I, Lin L, Ambinder RF, Peacock C, Watkins DN, Huff CA, Jones RJ. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–2345. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Functions of tumorigenic and migrating cancer progenitor cells in cancer progression and metastasis and their therapeutic implications. Cancer Metastasis Rev. 2007a;26:203–214. doi: 10.1007/s10555-007-9052-4. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial-mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann. Oncol. 2007b;18:1605–1619. doi: 10.1093/annonc/mdm070. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008a;4:27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Recent progress on normal and malignant pancreatic stem/progenitor cell research: therapeutic implications for the treatment of type 1 or 2 diabetes mellitus and aggressive pancreatic cancer. Gut. 2008b;57:1456–1468. doi: 10.1136/gut.2008.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Targeting of cancer stem/progenitor cells plus stem cell-based therapies: the ultimate hope for treating and curing aggressive and recurrent cancers. Panminerva Med. 2008c;50:3–18. [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Batra SK. Recent advances on the development of novel anti-cancer drugs targeting cancer stem/progenitor cells. Drug Develop. Res. 2008d;69:415–430. [Google Scholar]
- Mimeault M, Batra SK. Recent insights into the molecular mechanisms involved in aging and the malignant transformation of adult stem/progenitor cells and their therapeutic implications. Ageing Res. Rev. 2009;8:94–112. doi: 10.1016/j.arr.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Batra SK. Stem cells -- A revolution in therapeutics--Recent advances on the stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin. Pharmacol. Ther. 2007a;82:252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Mehta PP, Batra SK. Recent advances on cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J. Mol. Cell. Med. 2007b;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Batra SK. Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin. Pharmacol. Ther. 2008a;83:673–691. doi: 10.1038/sj.clpt.6100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Mehta PP, Hauke R, Batra SK. Functions of normal and malignant prostatic stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies. Endocr. Rev. 2008b;29:234–252. doi: 10.1210/er.2007-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J. Biol. Chem. 2006;281:34936–34941. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Dynamic control of TGF-beta signaling and its links to the cytoskeleton. FEBS Lett. 2008;582:2051–2065. doi: 10.1016/j.febslet.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Nakagawara A, Ohira M. Comprehensive genomics linking between neural development and cancer: neuroblastoma as a model. Cancer Lett. 2004;204:213–224. doi: 10.1016/S0304-3835(03)00457-9. [DOI] [PubMed] [Google Scholar]
- Nicolis SK. Cancer stem cells and "stemness" genes in neurooncology. Neurobiol. Dis. 2007;25:217–229. doi: 10.1016/j.nbd.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- Nijhof JG, van Pelt C, Mulder AA, Mitchell DL, Mullenders LH, de Gruijl FR. Epidermal stem and progenitor cells in murine epidermis accumulate UV damage despite NER proficiency. Carcinogenesis. 2007;28:792–800. doi: 10.1093/carcin/bgl213. [DOI] [PubMed] [Google Scholar]
- Olivotto M, Dello SP. Environmental restrictions within tumor ecosystems select for a convergent, hypoxia-resistant phenotype of cancer stem cells. Cell Cycle. 2008;7:176–187. doi: 10.4161/cc.7.2.5315. [DOI] [PubMed] [Google Scholar]
- Onoue T, Uchida D, Begum NM, Tomizuka Y, Yoshida H, Sato M. Epithelial-mesenchymal transition induced by the stromal cell-derived factor-1/CXCR4 system in oral squamous cell carcinoma cells. Int. J. Oncol. 2006;29:1133–1138. [PubMed] [Google Scholar]
- Park DM, Zhuang Z, Chen L, Szerlip N, Maric I, Li J, Sohn T, Kim SH, Lubensky IA, Vortmeyer AO, Rodgers GP, Oldfield EH, Lonser RR. von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med. 2007;4:e60. doi: 10.1371/journal.pmed.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: The CD44+{alpha}2{beta}1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak J, Milsom C, Yu J. Vascular determinants of cancer stem cell dormancy--do age and coagulation system play a role? APMIS. 2008;116:660–676. doi: 10.1111/j.1600-0463.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells 'hide out' in the bone marrow. Leukemia. 2004;18:29–40. doi: 10.1038/sj.leu.2403184. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67:8980–8984. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- Riethdorf S, Wikman H, Pantel K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int. J. Cancer. 2008;123:1991–2006. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]
- Rizo A, Vellenga E, de Haan G, Schuringa JJ. Signaling pathways in self-renewing hematopoietic and leukemic stem cells: do all stem cells need a niche? Hum. Mol. Genet. 2006;15:R210–R219. doi: 10.1093/hmg/ddl175. [DOI] [PubMed] [Google Scholar]
- Ross RA, Spengler BA. Human neuroblastoma stem cells. Semin. Cancer Biol. 2007;17:241–247. doi: 10.1016/j.semcancer.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Salmaggi A, Boiardi A, Gelati M, Russo A, Calatozzolo C, Ciusani E, Sciacca FL, Ottolina A, Parati EA, La Porta C, Alessandri G, Marras C, Croci D, De Rossi M. Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype. Glia. 2006;54:850–860. doi: 10.1002/glia.20414. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Li Y, Wang Z, Kong D. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int. Rev. Immunol. 2008;27:293–319. doi: 10.1080/08830180802276179. [DOI] [PubMed] [Google Scholar]
- Sathornsumetee S, Rich JN, Reardon DA. Diagnosis and treatment of high-grade astrocytoma. Neurol. Clin. 2007;25:1111–1139. doi: 10.1016/j.ncl.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Sato M, Shames DS, Gazdar AF, Minna JD. A translational view of the molecular pathogenesis of lung cancer. J. Thorac. Oncol. 2007;2:327–343. doi: 10.1097/01.JTO.0000263718.69320.4c. [DOI] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, Sledge GW, Carey LA. Triple-negative breast cancer: risk factors to potential targets. Clin. Cancer Res. 2008;14:8010–8018. doi: 10.1158/1078-0432.CCR-08-1208. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Banerjee D, Chandra S, Banerji SK, Ghosh R, Roy R, Banerjee S. Deregulation and cross talk among Sonic hedgehog, Wnt, Hox and Notch signaling in chronic myeloid leukemia progression. Leukemia. 2007;21:949–955. doi: 10.1038/sj.leu.2404657. [DOI] [PubMed] [Google Scholar]
- Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann. Surg. Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- She JJ, Zhang PG, Wang ZM, Gan WM, Che XM. Identification of side population cells from bladder cancer cells by dyecycle violet staining. Cancer Biol. Ther. 2008;7:1663–1668. doi: 10.4161/cbt.7.10.6637. [DOI] [PubMed] [Google Scholar]
- Shi GM, Xu Y, Fan J, Zhou J, Yang XR, Qiu SJ, Liao Y, Wu WZ, Ji Y, Ke AW, Ding ZB, He YZ, Wu B, Yang GH, Qin WZ, Zhang W, Zhu J, Min ZH, Wu ZQ. Identification of side population cells in human hepatocellular carcinoma cell lines with stepwise metastatic potentials. J. Cancer Res. Clin. Oncol. 2008;134:1155–1163. doi: 10.1007/s00432-008-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Shiras A, Chettiar ST, Shepal V, Rajendran G, Prasad GR, Shastry P. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25:1478–1489. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D'Angelica M, Kemeny N, Lyden D, Rafii S. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J. Clin. Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Wang Y, Xiao C, Johnsen H, Naume B, Samaha RR, Borresen-Dale AL. Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: gene expression analyses across three different platforms. BMC Genomics. 2006;7:127. doi: 10.1186/1471-2164-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorscher SM. Biological therapy update in colorectal cancer. Expert Opin. Biol. Ther. 2007;7:509–519. doi: 10.1517/14712598.7.4.509. [DOI] [PubMed] [Google Scholar]
- Spaderna S, Schmalhofer O, Hlubek F, Jung A, Kirchner T, Brabletz T. Epithelial-mesenchymal and mesenchymal-epithelial transitions during cancer progression. Verh. Dtsch. Ges. Pathol. 2007;91:21–28. [PubMed] [Google Scholar]
- Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat. Clin. Pract. Oncol. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storci G, Sansone P, Trere D, Tavolari S, Taffurelli M, Ceccarelli C, Guarnieri T, Paterini P, Pariali M, Montanaro L, Santini D, Chieco P, Bonafe M. The basal-like breast carcinoma phenotype is regulated by SLUG gene expression. J. Pathol. 2008;214:25–37. doi: 10.1002/path.2254. [DOI] [PubMed] [Google Scholar]
- Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha(v)beta(3) integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67:61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, Pienta KJ, Keller ET, McCauley LK, Taichman RS. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J. Bone Miner. Res. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell Biochem. 2003;89:462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- Sung JM, Cho HJ, Yi H, Lee CH, Kim HS, Kim DK, bd El-Aty AM, Kim JS, Landowski CP, Hediger MA, Shin HC. Characterization of a stem cell population in lung cancer A549 cells. Biochem. Biophys. Res. Commun. 2008;371:163–167. doi: 10.1016/j.bbrc.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Tang P, Wang X, Schiffhauer L, Wang J, Bourne P, Yang Q, Quinn A, Hajdu S. Expression patterns of ER-alpha, PR, HER-2/neu, and EGFR in different cell origin subtypes of high grade and non-high grade ductal carcinoma in situ. Ann. Clin. Lab. Sci. 2006;36:137–143. [PubMed] [Google Scholar]
- Tchernev G, Orfanos CE. Downregulation of cell cycle modulators p21, p27, p53, Rb and proapoptotic Bcl-2-related proteins Bax and Bak in cutaneous melanoma is associated with worse patient prognosis: preliminary findings. J. Cutan. Pathol. 2007;34:247–256. doi: 10.1111/j.1600-0560.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- Teuliere J, Faraldo MM, Deugnier MA, Shtutman M, Ben-Ze'ev A, Thiery JP, Glukhova MA. Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005;132:267–277. doi: 10.1242/dev.01583. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Tiffin N, Williams RD, Shipley J, Pritchard-Jones K. PAX7 expression in embryonal rhabdomyosarcoma suggests an origin in muscle satellite cells. Br. J. Cancer. 2003;89:327–332. doi: 10.1038/sj.bjc.6601040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema J-P, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin. Cancer Biol. 2008;18:244–250. doi: 10.1016/j.semcancer.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostar U, Malm CJ, Meis-Kindblom JM, Kindblom LG, Toftgard R, Unden AB. Deregulation of the hedgehog signalling pathway: a possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J. Pathol. 2006;208:17–25. doi: 10.1002/path.1882. [DOI] [PubMed] [Google Scholar]
- Trumpp A, Wiestler OD. Mechanisms of disease: cancer stem cells--targeting the evil twin. Nat. Clin. Pract. Oncol. 2008;5:337–347. doi: 10.1038/ncponc1110. [DOI] [PubMed] [Google Scholar]
- Tso CL, Shintaku P, Chen J, Liu Q, Liu J, Chen Z, Yoshimoto K, Mischel PS, Cloughesy TF, Liau LM, Nelson SF. Primary glioblastomas express mesenchymal stem-like properties. Mol. Cancer Res. 2006;4:607–619. doi: 10.1158/1541-7786.MCR-06-0005. [DOI] [PubMed] [Google Scholar]
- Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial-mesenchymal transition--does cellular plasticity fuel neoplastic progression? Nat. Clin. Pract. Oncol. 2008;5:280–290. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaish M. Mismatch repair deficiencies transforming stem cells into cancer stem cells and therapeutic implications. Mol. Cancer. 2007;6:26. doi: 10.1186/1476-4598-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent-Salomon A, Bidard FC, Pierga JY. Bone marrow micrometastasis in breast cancer: review of detection methods, prognostic impact and biological issues. J. Clin. Pathol. 2008;61:570–576. doi: 10.1136/jcp.2007.046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JD, Kattan DR, Thomas SK, Spengler BA, Guo HF, Biedler JL, Cheung NK, Ross RA. Characteristics of stem cells from human neuroblastoma cell lines and in tumors. Neoplasia. 2004;6:838–845. doi: 10.1593/neo.04310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007a;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- Wang X, Belguise K, Kersual N, Kirsch KH, Mineva ND, Galtier F, Chalbos D, Sonenshein GE. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat. Cell Biol. 2007b;9:470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jiang C, Lu J. Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol. Carcinog. 2002;34:113–120. doi: 10.1002/mc.10056. [DOI] [PubMed] [Google Scholar]
- Wei C, Guomin W, Yujun L, Ruizhe Q. Cancer stem-like cells in human prostate carcinoma cells DU145: The seeds of the cell line? Cancer Biol. Ther. 2007;6:763–768. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]
- Widera D, Kaus A, Kaltschmidt C, Kaltschmidt B. Neural stem cells, inflammation and NF-kappaB: basic principle of maintenance and repair or origin of brain tumours? J. Cell Mol. Med. 2008;12:459–470. doi: 10.1111/j.1582-4934.2007.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat. Rev. Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90(+) cancer stem cells in human liver cancer. Cancer Cell. 2008a;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau CK, Li ML, Tam KH, Lam CT, Poon RT, Fan ST. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008b;47:919–928. doi: 10.1002/hep.22082. [DOI] [PubMed] [Google Scholar]
- Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr. Opin. Hematol. 2008;15:162–168. doi: 10.1097/MOH.0b013e3282fa7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH, Yao XH, Gao L, Wang JM, Bian XW. Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett. 2008;265:124–134. doi: 10.1016/j.canlet.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008a;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Komaki R, Wang L, Fang B, Chang JY. Treatment of radioresistant stem-like esophageal cancer cells by an apoptotic gene-armed, telomerase-specific oncolytic adenovirus. Clin. Cancer Res. 2008b;14:2813–2823. doi: 10.1158/1078-0432.CCR-07-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhong X, Li Y, Peng F, Huang B, Lin J, Zhang W, Zheng J, Jiang R, Song G, Ge J. Identification of tumorigenic retinal stem-like cells in human solid retinoblastomas. Int. J. Cancer. 2007;121:2125–2131. doi: 10.1002/ijc.22880. [DOI] [PubMed] [Google Scholar]
- Zhou BP, Hung MC. Wnt, hedgehog and snail: sister pathways that control by GSK-3beta and beta-Trcp in the regulation of metastasis. Cell Cycle. 2005;4:772–776. doi: 10.4161/cc.4.6.1744. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Flesken-Nikitin A, Nikitin AY. Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic ducts. Cancer Res. 2007;67:5683–5690. doi: 10.1158/0008-5472.CAN-07-0768. [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]