Abstract
Bone marrow-derived dendritic cells (DCs) are cells of the immune system that have been used as a tool to boost, modulate, or dampen immune responses. In the context of autoimmunity, DCs can be modified to express immunoregulatory products encoded by transgenes, and used therapeutically in adoptive cellular therapy. DCs that were lentivirally transduced (lt) to express interleukin 4 (IL-4) can significantly delay or prevent the onset of autoimmune diabetes in nonobese diabetic (NOD) mice. However, modifying cells using viral vectors carries the dual risk of oncogenicity or immunogenicity. This study demonstrates that NOD DCs, electroporated with “translationally enhanced” IL-4 mRNA (eDC/IL-4), can be equally efficient therapeutically, despite the reduced amount and shorter duration of IL-4 secretion. Moreover, a single injection of eDC/IL-4 in NOD mice shortly after the onset of hyperglycemia was able to maintain stable glycemia for up to several months in a significant fraction of treated mice. Treatment with eDC/IL-4 boosted regulatory T (Tregs) cell functions and modulated T helper responses to reduce pathogenicity. Thus, treatment with DCs, electroporated with modified IL-4 mRNA to express IL-4 for up to 24 hours, constitutes a viable cellular therapy approach for the regulation of autoimmune diabetes, as a preferred alternative to the use of viral vectors.
Introduction
Type 1 diabetes (T1D) is a multifactorial autoimmune disease characterized by the progressive T cell-mediated destruction of β cells of the pancreatic islets. The nonobese diabetic (NOD) mouse model of T1D shares many similarities with human T1D and has been widely used to study the pathogenesis of disease and to test the efficacy of therapeutic approaches aimed at maintaining or restoring tolerance to and function of β cells.1,2 Among these approaches, dendritic cells (DCs) have received wide scrutiny because of their inherent ability to regulate immune responses. Depending on their type and maturation state, DCs can promote immune tolerance as well as trigger immune responses. Tolerogenic properties of DCs can be ascribed not only to their state of maturation (immature), but also to certain molecules expressed on their surface (e.g., programmed death ligand-1), intracellularly (e.g., indoleamine 2,3-dioxygenase) or secreted [e.g., interleukin 10 (IL-10)].3,4 Some other immunoregulatory products that are known to prevent or ameliorate autoimmune disease are either not expressed by DCs, or expressed in insufficient amounts. However, DCs remain vehicles of choice to express these immunomodulatory products because of their efficient migration to relevant lymphoid tissues and interaction with T cells. In mice, the pancreatic lymph nodes (PLNs) are among the few lymphoid tissues preferentially targeted by adoptively transferred bone marrow-derived DCs, which makes this strategy particularly relevant for the treatment of T1D.5
IL-4 is a pleiotropic cytokine whose contribution to immune regulation is well established. One of its most important roles is to support the differentiation and function of Th2 and B cells, while antagonizing the development and function of Th1 cells, implicated in numerous autoimmune diseases. IL-4 is not normally produced by DCs, and viral vectors have so far been the method of choice to express it ectopically. DCs modified to express IL-4 (DC/IL-4) have shown therapeutic efficacy in NOD mice,6,7 as well as in collagen-induced arthritis, a mouse model of rheumatoid arthritis.8,9 The approach of using viral vectors allows relatively stable expression of the transgene, but carries certain risks and disadvantages that are inherent to the type of viral vector used (most typically adenoviral and lentiviral), ranging from immunogenicity to oncogenicity. Nonviral approaches for ectopic expression of immunoregulatory products are not only safer, but should preserve the integrity of the DC, including its homing and T cell clustering capacity.
Modification of DCs by mRNA electroporation has been extensively studied for the treatment of cancer, where newly introduced mRNAs typically encode tumor antigens and other proteins that boost the immunogenicity of the DCs.10,11,12 However, this approach has not yet been successfully applied to autoimmune diseases. Here, we report the use of an IL-4 mRNA optimized for IL-4 protein expression to electroporate bone marrow-derived DCs and then treat NOD mice before or immediately after the onset of hyperglycemia. DCs electroporated with translationally enhanced IL-4 mRNA (eDC/IL-4), despite the short duration of IL-4 expression, had equivalent efficacy in preventing disease as did the previously described lentivirally transduced counterparts (ltDC/IL-4).7 In addition, eDC/IL-4 cells were able to induce a transient or sustained reversal of hyperglycemia in a significant fraction of mice treated after the onset of hyperglycemia. Treatment with eDC/IL-4 reinforced tolerance through several mechanisms, including induction of regulatory T cells (Tregs) and skewing of helper T cell populations. This study represents a proof of principle that electroporation of DCs with enhanced mRNA is a viable, safe, and effective substitute for viral vectors when using cellular gene therapy to treat autoimmune diseases such as T1D.
Results
Characterization of electroporated and transduced DCs
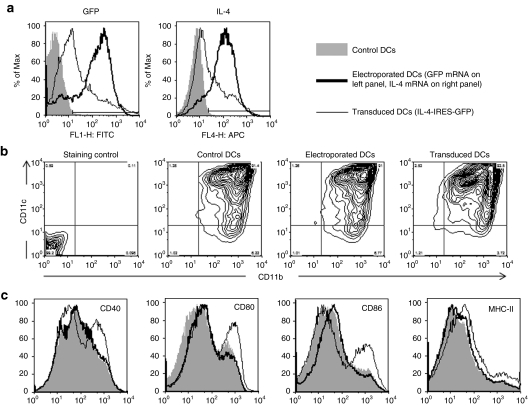
We compared mRNA electroporation (eDCs) and lentiviral transduction (ltDCs) for their efficiency of transgene transfer and expression and for their effect on the phenotype of DCs. While ltDCs were in contact with lentiviral particles for the final 2 days of a 7-day culture, eDCs were electroporated after the final harvest on day 7. Using our extensively optimized conditions, electroporation was carried out without significant loss of cells or change in viability (data not shown). Electroporation had the highest and most consistent transfection efficiency (80–85%), whereas lentiviral transduction efficiency was more variable (40–70%), but in general the transduced cells expressed higher levels of the transgene (Figure 1a and Supplementary Figure S1). Lentiviral transduction did not affect the differentiation of DCs (>90% CD11b+ CD11c+ at the end of the culture, Figure 1b), but increased their maturity, as seen by the expression of costimulatory molecules and class II major histocompatibility complex (Figure 1c).
Figure 1.
Phenotype of electroporated and transduced DCs. (a) GFP and IL-4 expression by unmodified DCs (harvested on day 7), mRNA electroporated DCs (harvested and electroporated on day 7), and lentivirally transduced DCs (infected on day 5 and harvested on day 7). (b) CD11c and CD11b expression by unmodified, mRNA electroporated, and lentivirally transduced DCs. (c) CD40, CD80, CD86, and class II MHC (I-Ag7) expression by unmodified, mRNA electroporated and lentivirally transduced DCs. DC, dendritic cell; GFP, green fluorescent protein; IL, interleukin; MHC, major histocompatibility complex.
Function of modified DCs
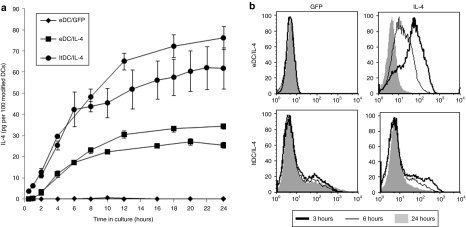
The function of electroporated and transduced DCs was assessed by the amount of IL-4 secreted using enzyme-linked immunosorbent assay (ELISA). We observed that eDC/IL-4 consistently secreted less than half the amount of IL-4 produced by ltDC/IL-4 within a culture period of 24 hours in vitro at 37 °C, whereas unmodified DCs produced no detectable levels of IL-4 (Figure 2a). IL-4 intracellular staining showed that eDC/IL-4 no longer produced IL-4 by 24 hours whereas ltDC/IL-4 maintained IL-4 production, suggesting that, as expected, eDC/IL-4 use up their capacity to make IL-4 faster than ltDC/IL-4 (Figure 2b).
Figure 2.
Function of electroporated and transduced DCs. (a) Time course of IL-4 secretion by unmodified DCs, eDC/IL-4, and ltDC/IL-4 in the first 24 hours after reculture at 37 °C in vitro. For better comparison between electroporation and lentiviral transduction, the values were normalized based on the efficiency of transfection/transduction, and shown as mean ± SD from different numbers of cell plated (normalized to 100 cells). Data from two independent experiments are shown. (b) GFP and intracellular IL-4 expression in eDC/IL-4 and ltDC/IL-4 after 3, 6, and 24 hours in culture. DC, dendritic cell; eDC, enhanced DC; GFP, green fluorescent protein; ltDC, lentivirally transduced DC; IL, interleukin; MHC, major histocompatibility complex.
Prevention of onset of hyperglycemia
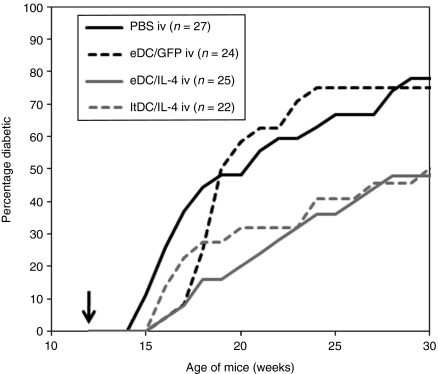
We previously published that ltDC/IL-4, injected into 12-week-old prediabetic NOD mice, can delay the time of disease onset and prevent disease in about 50% of treated mice.7 We asked whether eDC/IL-4 could work as a nonviral, potentially safer alternative to ltDC/IL-4. Cohorts of nondiabetic 12-week-old female NOD mice were treated with a single intravenous injection of phosphate-buffered saline (PBS), eDC/green fluorescent protein (GFP), eDC/IL-4, or ltDC/IL-4 (Figure 3). The control DCs (eDC/GFP) slightly delayed the onset of disease, but had no significant effect compared to PBS (P = 0.692). However, both eDC/IL-4 and ltDC/IL-4 significantly reduced the incidence of disease (P = 0.013 and 0.026, respectively). As previously shown with ltDC/IL-4,7 IL-4 expression by the DCs is required for the full therapeutic effect (eDC/GFP versus eDC/IL-4, P = 0.023). Finally, despite the reduced amount of IL-4 expressed by eDC/IL-4 compared to ltDC/IL-4, these two treatments were equivalent therapeutically (P = 0.967). It is noteworthy that some mice treated with eDC/IL-4 had modest increases in glycemia (150–200 mg/dl) with transient episodes >250 mg/dl. However, because 250 mg/dl was our cutoff threshold for hyperglycemia in a prevention setting, some treated mice, although initially considered diabetic, returned to <250 mg/dl and did not develop full blown hyperglycemia (>500 mg/dl) during the time they were monitored (Supplementary Figure S2). Thus, the therapeutic efficacy as shown in Figure 3 was slightly underestimated. Moreover, based on the amount of IL-4 produced in vitro, we estimated that every million of the eDC/IL-4 and ltDC/IL4 injected produce respectively 260 ± 30 and 440 ± 100 ng of IL-4 within 24 hours in vivo (the variability is dependent on the efficiency of transfection/transduction, which is more consistent with electroporation) (Supplementary Figure S3). No IL-4 was detected by ELISA in the serum of treated mice between 1 and 3 days after administration of eDC/IL-4 (data not shown).
Figure 3.
Incidence of diabetes after a single intravenous injection of PBS, eDC/GFP, eDC/IL-4, or ltDC/IL-4 in 12-week-old prediabetic NOD mice. The arrow indicates the time of treatment. Data are pooled from two independent experiments. Log rank test was used for statistical analysis. eDC, enhanced dendritic cell; GFP, green fluorescent protein; ltDC, lentivirally transduced DC; IL, interleukin; NOD, nonobese diabetic; PBS, phosphate-buffered saline.
Reversal of hyperglycemia
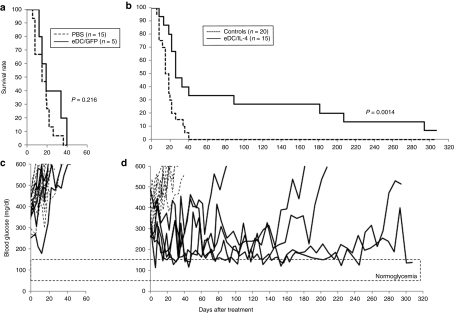
Next, we asked whether eDC/IL-4 had any beneficial effect when administered after the onset of hyperglycemia. A cohort of female NOD mice was monitored weekly for glycemia between the age of 11 and 30 weeks, and individual mice that became hyperglycemic (250–500 mg/dl) during the period of observation were treated with a single injection of PBS, or freshly prepared eDC/GFP or eDC/IL-4, and monitored twice weekly thereafter for blood glucose. Mice with a blood glucose >500 mg/dl over two consecutive measurements were sacrificed. Data in Figure 4a,b show the survival curve of control groups (PBS and eDC/GFP) and the eDC/IL-4-treated group. Mice treated with either PBS or eDC/GFP had to be sacrificed within 40 days, and there was no significant difference between the two controls (P = 0.216). However, one-third of mice treated with eDC-IL-4 survived well beyond 40 days, with a sustained period of reversal of hyperglycemia, compared to all controls (P = 0.0014). Blood glucose measurements on individual mice are reported in Figure 4c,d and demonstrate that the responding mice did not return to a state of normoglycemia, but instead to a state of controlled moderate hyperglycemia fluctuating between 150 and 300 mg/dl. Two factors can contribute to how well individual mice respond to the treatment: the severity of hyperglycemia and the age at the time of treatment; these parameters are plotted for both responding and nonresponding mice in Supplementary Figure S4. The average glycemia at time of treatment with eDC/IL-4 was 307 ± 37 and 354 ± 53 mg/dl for responding mice (n = 5) and nonresponding (n = 10), respectively. Although the data suggests better effectiveness in mice with less severe hyperglycemia, statistical significance was not reached (P = 0.071). The median age of treatment of controls and eDC/IL-4 treated mice (in weeks) was similar (18 and 19, respectively), but within eDC/IL-4 treated mice, the median age at the time of treatment was greater for responding mice (24) than for nonresponding mice (19).
Figure 4.
Reversal of hyperglycemia after treatment with eDC/IL-4. (a,b) Survival curve of mice treated with (a) PBS or eDC/GFP, and (b) of all control mice (PBS and eDC/GFP) or eDC/IL-4-treated mice. Mice were sacrificed when their blood glucose reached over 500 mg/dl after two consecutive measurements. Log rank test was applied for statistical analysis. (c,d) Blood glucose measurements in mice treated with (c) PBS (dashed line, n = 15) or eDC/GFP (solid lines, n = 5), and in mice treated with (d) eDC/IL-4 (n = 15). In d, responders were eDC/IL-4-treated mice that survived longer than control mice (n = 5, solid lines); nonresponders (n = 10) are represented in dashed lines. Initial blood glucose value (day 0) is the mean of two consecutive measurements (between 250 and 500 mg/dl) before treatment. eDC, enhanced dendritic cell; GFP, green fluorescent protein; IL, interleukin; NOD, nonobese diabetic; PBS, phosphate-buffered saline.
Changes in immune cell populations and cytokine profiles in targeted tissues
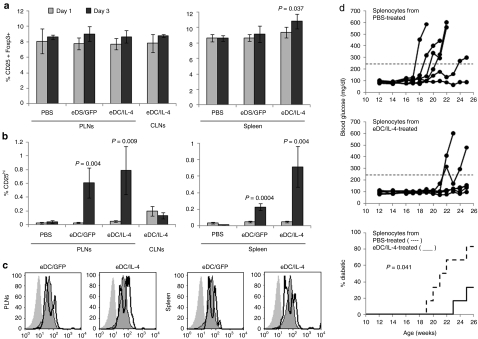
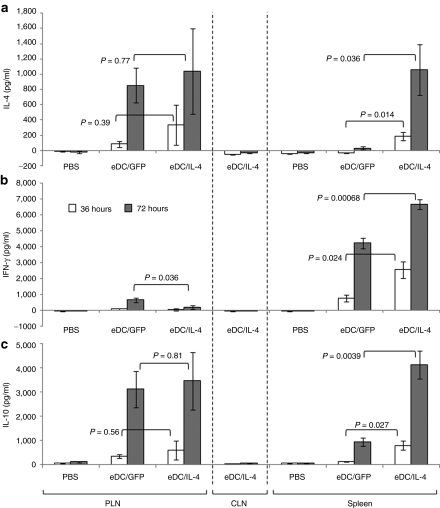
Targeted tissues (PLNs and spleen) and nontargeted tissue (cervical lymph nodes) were collected 1 and 3 days after intravenous injection of PBS, eDC/GFP, or eDC/IL-4. No major change in the percentage of the overall population of CD25+ Foxp3+ cells among CD4+ T cells was observed, except for a modest but significant increase in the spleen with eDC/IL-4 treatment (Figure 5a and Supplementary Figure S5a). However, a small population (up to 1% of CD4+ T cells) emerged with higher levels of CD25 and CD4 than the general CD4+ CD25+ population (Supplementary Figure S5b). This small population significantly increased between day 1 and 3 following treatment with both eDC/GFP and eDC/IL-4, but was further increased with eDC/IL-4 (Figure 5b). Moreover, these CD25hi cells were made up of two populations of Foxp3+ cells, one expressing higher levels of Foxp3 than the general CD25+ Foxp3+ population, and one expressing lower levels (Figure 5c). Adoptive transfer of splenocytes from eDC/IL-4-treated mice into NOD.SCID mice did not efficiently transfer disease when compared to splenocytes from PBS-treated mice (Figure 5d). Treatment with modified DCs did not significantly alter the population of activated (CD44+ CD69+) T cells (Supplementary Figure S6), nor did it lead to dramatic changes in different populations of splenic DCs (Supplementary Figure S7). Cells isolated from selected tissues were also cultured in vitro without stimulation to assess the relative levels of interferon γ (IFN-γ), IL-4, and IL-10 induced (Figure 6). No expression of these cytokines was seen from any tissue obtained at 1 day after treatment, from the tissues from PBS-treated mice or from control cervical lymph nodes from eDC/IL-4-treated mice. The cytokine profile on day 3 following treatment with control DCs (eDC/GFP) differed substantially between PLNs and spleen. eDC/GFP induced similar amounts of IFN-γ and IL-4 in the PLNs (IL-4/IFN-γ ratio = 1.26) but mostly IFN-γ in the spleen (IL-4/IFN-γ ratio = 0.007). The additional effects of IL-4 delivered by eDC/IL-4 led to different changes depending on the target tissue. In the PLNs, eDC/IL-4 reduced the expression of IFN-γ significantly, resulting in a more than sixfold increase of the IL-4/IFN-γ ratio to 7.8. In the spleen, eDC/IL-4 increased IL-4 and IL-10 more than it increased IFN-γ, resulting in a >14-fold increase of the IL-4/IFN-γ ratio to 0.1. The expression profile of IL-10 mimicked that of IL-4.
Figure 5.
Induction of functional Tregs. (a) Frequency of CD25+ Foxp3+ cells gated on CD4+ T cells in select lymphoid tissues 1 and 3 days after treatment. (b) Frequency of induced CD25hi cells among CD4+ T cells (gating shown in Supplementary Figure S5b). Mean ± SD is from n = 5 mice per group per time point. t-Test was performed for statistical analysis. P values shown on graph are between day 1 and day 3. Difference between eDC/GFP and eDC/IL-4 in spleen on day 3 was also significant (P = 0.035 on a; P = 0.01 on b). (c) Expression level of Foxp3 on CD4+ CD25− cells (shaded histogram, no line), CD4+ CD25+ cells (shaded histogram with line) and induced CD4+ CD25hi cells (thick line). Gating is shown in Supplementary Figure S5b. (d) Development of hyperglycemia in NOD.SCID mice injected at 10 weeks of age with 3 × 106 splenocytes from PBS-treated (upper panel) or eDC/IL-4-treated (middle panel) NOD mice (n = 6 mice per group). The incidence of diabetes is reported in the lower panel (mice were considered diabetic after two consecutive measurement of blood glucose over 250 mg/dl). Log rank test was used in statistical analysis. eDC, enhanced dendritic cell; GFP, green fluorescent protein; IL, interleukin; NOD, nonobese diabetic; PBS, phosphate-buffered saline.
Figure 6.
Local cytokine profile in targeted tissues. Cells from PLNs, CLNs, and spleen obtained 3 days after different treatments were cultured in vitro. (a) IL-4, (b) IFN-γ, and (c) IL-10 were measured by ELISA on supernatant collected 36 hours (white bars) or 72 hours (gray bars) after replating. Mean ± SD is from n = 5 mice per group. t-Test was performed for statistical analysis. P values shown on graph are between eDC/GFP and eDC/IL-4 for each time point. CLN, cervical lymph node; eDC, enhanced dendritic cell; GFP, green fluorescent protein; IFN-γ, interferon-γ IL, interleukin; NOD, nonobese diabetic; PBS, phosphate-buffered saline.
Discussion
DCs play a crucial role in regulating immune responses, and have attracted much attention as tools to manipulate the immune response toward a desired outcome (stronger immune responses against infections and tumors, versus dampened autoimmune or allergic responses). The use of DCs generated ex vivo to treat T1D has been proposed and tested by several groups.13,14,15,16,17 These approaches consist, for example, of rendering the DCs more tolerogenic by the use of particular cytokines in the culture, or introducing antisense oligonucleotides to downregulate costimulatory molecules. DCs can also be used as vehicles to express and deliver immunoregulatory products to sites of relevance in vivo. If such immunoregulatory products are not normally expressed by DCs, their respective genes must be introduced and adequately expressed. To date, only viral means of DC modification have been successfully applied to achieve prevention or treatment of autoimmune diseases in mouse models, but safety concerns have impeded the use of this approach in attempts at human therapy.
In the field of tumor immunology, researchers have achieved expression of certain tumor antigens using electroporation of autologous DCs with mRNA or translationally enhanced mRNA for vaccination strategies.10,11,12 This has led to several trials in humans, demonstrating the safety of mRNA electroporated DCs.18,19,20,21 In this present study, we explore and report for the first time the use of translationally enhanced mRNA electroporation as an alternative to viral means of modifying DCs to express immunomodulatory cytokines for adoptive cellular gene therapy of autoimmune diseases. Electroporation with mRNA was more efficient than lentiviral transduction in modifying a greater number of DCs, and did so with no apparent effect on their phenotype. Dullaers et al.22 have conducted a similar comparison and also noted that electroporation was more efficient, but that transduction led to higher levels of transgene expression, especially at later time points (beyond 24 hours). In agreement with this study, we found that certain proteins, like GFP, are still present in high amount in cells up to 3 days after electroporation. Expression of such proteins does not necessarily reflect the duration of mRNA expression if the expressed protein is very stable (slowly degraded). In the case of a secreted protein like IL-4, however, we did not detect expression beyond 24 hours, whereas ltDCs were still making IL-4 at this time. Unlike transduced cells, electroporated DCs bear a finite quantity of mRNA, most of which will in turn be translated into protein as long as the mRNA persists. In the case of cytokines like IL-4, most of the mature protein presumably gets secreted within 12 hours. Although eDC/IL-4 produced less IL-4 in a 24-hour period than ltDC/IL-4, they expressed higher levels on per cell basis within the first 6 hours after electroporation. This elevated initial level of expression, at the time of administration, may account for the biological effect of delayed disease progression which was comparable to that of ltDCs. Interestingly, electroporated DCs, in contrast to unmodified and transduced DCs, may become more refractory to producing IL-12 under certain maturation conditions.12,22 This phenomenon is worth further exploration, as impaired induction of IL-12 can enhance the effect of IL-4 expressed by therapeutic DCs.
Although various studies have reported an effect of DCs alone in preventing diabetes when injected into young NOD mice, DCs transduced by adenovirus or lentivirus to express IL-4, but not untransduced DCs, were able to significantly delay and/or prevent the onset of disease when injected in older prediabetic mice.6,7 One major concern with the electroporation method was the transient nature of IL-4 expression (limited to 24 hours). However, we have also reported that DCs are detected in the spleen within 4 hours and in the PLNs between 7 and 10 hours after injection,5 thus sufficiently early to expect IL-4 to still be produced in the tissue following transfer. We showed that eDC/IL-4 had a significant effect in preventing onset of disease, whereas control eDC/GFP did not, suggesting that IL-4, even transiently expressed, played an important role. Thanks to recent developments, T1D can be diagnosed earlier, allowing prevention strategies to be considered for patients with a high risk of disease (family history, permissive major histocompatibility complex, and the presence of relevant autoantibodies in the serum),23 and treatments that are still effective at a late stage before the onset of overt hyperglycemia deserve more attention. Currently, most efforts are still focused on treating recently diagnosed patients, and most successful therapies in the NOD mouse are only seriously considered if they are also able to reverse overt hyperglycemia. We showed that a single injection of eDC/IL-4 after onset of hyperglycemia had a significant effect, decreasing the severity of disease in a third of the mice for prolonged periods of time. The treatment was most effective in mice that had less severe hyperglycemia. In addition, older mice were more likely to respond to the treatment, possibly a reflection of late onset being the result of a more slowly progressing and less aggressive disease.
We have previously shown that the homing of DCs to target tissues results in increased expression of CD25 and CD69 on CD4+ T cells.5 In this study, with a fivefold lower number of DCs used for therapy, we continued to observe induction of CD25hi cells that express Foxp3. This, combined with the lack of CD69 upregulation, suggests that induction of Tregs may contribute to the therapeutic effect of the DCs. The fact that splenocytes from eDC/IL-4-treated mice lose their ability to transfer disease in NOD.SCID mice is further suggestive that Tregs have been induced, but an enhanced deletion of autoreactive T cell within 3 days of treatment cannot be ruled out. Although CD25hi cells were induced by all electroporated DCs, the presence of IL-4 boosted their frequency, particularly in the spleen. Such beneficial effect of IL-4 on Tregs has been reported before.24,25 Another aspect of the therapeutic effect of eDC/IL-4 is a significant shift from Th1 to Th2 compared to eDC/GFP, characterized by a reduced Th1 response and/or an increased Th2 response, depending on the tissue analyzed, as well as significantly greater levels of IL-10 produced. The lack of response in the cervical lymph nodes of eDC/IL-4-treated mice confirms that therapeutic DCs only target limited and specific lymphoid tissues.5 Overall, it appears that the effect of eDC/IL-4 was more pronounced in the spleen than in the PLNs, possibly because DCs migrate faster to the spleen than to the PLNs5 and can therefore secrete more IL-4 before the mRNA get depleted and the expression shut down. In other published studies,7 we have determined that the efficacy of ltDC/IL-4 was more consistent using the intravenous route (which allows spleen homing) compared to the intraperitoneal route (which improves PLN homing, but prevents spleen homing), suggesting that homing to the spleen may be important in addition to homing to the PLNs. We have previously reported that certain changes in gene expression affect the PLNs very early in young NOD mice, but appear in the spleen only around 12 weeks of age.26 Because, we treated mice at 12 weeks of age, it is possible that homing to both the spleen and the PLNs is desired.
Although the therapeutic effect of eDC/IL-4 may not appear as effective as other therapies being investigated in NOD mice, including treatment with anti-CD3 antibodies or ex vivo amplified and stimulated Tregs, mRNA electroporation of DCs offers potential flexibility and room for improvement. First, different types of mRNA may be combined. For cancer immunotherapy, a combination of mRNA encoding both tumor antigens and factors enhancing DC maturity and stimulatory function has been used.13,27,28 In the case of autoimmune diseases, retaining DC immaturity and/or inducing tolerogenic properties is often preferable. In that respect, electroporation is a safer choice over transduction with viral vectors, which tends to increase DC maturity. Although IL-4 is an effective cytokine in the treatment of autoimmune diabetes, other molecules may be coexpressed to improve the effects of the DCs by, for example, preventing the maturation of the DCs, expressing inhibitory molecules (e.g., programmed death ligand-1, indoleamine 2,3-dioxygenase), enhancing trafficking to lymphoid or inflamed tissues (via certain chemokine receptors and adhesion molecules) or inducing more antigen-specific responses (expression of peptides recognized by self-reactive T cells). Secondly, the effect of both prophylactic and therapeutic treatments was achieved with a single injection of eDC/IL-4, but it is possible that several treatments with eDC/IL-4 at different intervals may increase the duration of the effect. To this end, cryopreservation of DCs will be required as adoptive cellular therapy with DCs represents a personalized medicine in which the cells cannot be continuously prepared. We have started to test cryopreserved eDC/IL-4 in therapy settings, and found that a similar proportion of mice responded to treatment, but with a more transient reversal of hyperglycemia. Although optimization will be required to improve recovery, viability, and function of cryopreserved murine bone marrow-derived eDC/IL-4, there is evidence that human (monocyte-derived) DCs retain excellent viability and function following recovery from cryopreservation.11,29
In conclusion, we have demonstrated that electroporation with translationally enhanced mRNA can be used to modify DCs to express immunoregulatory products for adoptive cellular gene therapy of autoimmune diseases. In the case of IL-4, which is secreted and not retained in the cells, the timeline of expression is short and lasts no longer than 24 hours. Nevertheless, this short “pulse” of IL-4 delivered in vivo was sufficient to induce therapeutic effects in NOD mice, both in prevention and treatment of disease, which were in part ascribed to Treg induction and helper T cell skewing. Modification by mRNA electroporation is a flexible method that allows for coexpression of multiple products that could be used to expand current therapeutic options.
Materials and Methods
Mice. Female NOD and NOD.SCID mice were purchased from the Jackson Laboratories (Bar Harbor, ME), and kept in our animal facility under specific pathogen free conditions. Bone marrow donors were 8–10 weeks old, while recipient mice were used as nondiabetic at 12 weeks of age (prevention experiments), or as recent onset hyperglycemic between 11 and 30 weeks of age. NOD.SCID mice were adoptively transferred at 10 weeks of age. All manipulations were approved by the Stanford Administrative Panel on Laboratory Animal Care.
Subcloning of mIL4Rot6 for RNA generation. mIL4 coding sequence was subcloned from pHR-IL4IG plasmid7 into pGem 4Z 64T plasmid using SmaI restriction sites. Translation enhancer element from the 3′ untranslated region of rotavirus gene 6 mRNA (rot 6 element)30 was PCR amplified from synthetically generated construct (Blue Heron Biotechnology, Bothell, WA) encoding the rot6 sequence using primers forward 5′-ACTGCTCGAGGACCAAGCTAACAACTTGG-3′ and reverse 5′-ACTGTTAATTAAGGTCACATCCTCTCACTATACC-3′ containing XhoI and PacI recognition sites indicated in italics, respectively. The amplified PCR fragment containing rot 6 element was subcloned downstream of stop codon of mIL4 in pGem4Z64T using XhoI and PacI restriction sites. The final construct was verified by sequencing through the coding region of mIL4 and junction to pGem4Z64T plasmid.
Generation of the GFP and mIL4Rot6 type I cap RNAs. Plasmid pGem4Z64TmIL4Rot6 was purified using QiaFilter maxiprep kit (Qiagen, Valencia, CA) and linearized with SpeI. Twenty-five microgram of purified linearized template was used to establish 1-ml transcription reaction using T7 Flash kit according to manufacturing instruction (Epicentre Biotechnologies, Madison, WI). The in vitro transcription was conducted for 2 hours at 37 °C. After digestion with added 50 µl of DNAseI for additional 30 minutes at 37 °C, the uncapped RNA was purified using RNeasy maxi columns (Qiagen). The purified uncapped RNA was capped in the post-transcriptional capping reaction for 1 hour at 37 °C using Script Cap Type I Capping kit scaled up to 3,340 µl reaction volume (Epicentre Biotechnologies). The polyadenylation reaction was carried out using A-plus PolyA Tailing kit (Epicentre Biotechnologies). The unpurified capping reaction was adjusted to 6,664 µl with water, 10× A-plus reaction buffer and ATP substrate according to manufacturer's instructions and incubated for 45 minutes at 37 °C swirling the reaction every 15 minutes. The final polyadenylated RNA modified with type I cap and ~200 polyA tail was purified using RNeasy maxi columns (Qiagen). The length of polyA tail was measured by comparing relative migration of unpolyadenylated and polyadenylated mIL4 RNAs on denaturing agarose gel followed by collecting image with Alphaimager and size analysis using ImageQuant Software (Alpha Innotech, San Leandro, CA). 2.2 mg of RNA eluted in water was adjusted to final concentration of 1 mg/ml and stored frozen in single-size aliquots in pyrogen-free RNAse-free Safe Lock tubes (Eppendorf, Hamburg, Germany). GFP RNA was generated from pGem4Z/GFP/A64 plasmid as described previously.31
Lentivirus generation, DC culture, transduction, and electroporation. Lentiviral particles expressing IL-4 and GFP were generated using the pHR-IL4IG vector as previously described.7 Bone marrow was harvested from the femurs, tibias, and pelvis of female mice. The bone marrow cells were depleted of CD3+, B220+, and Gr-1+ cells on AutoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany) using biotinylated antibodies (eBioscience, San Diego, CA) and antibiotin beads (Miltenyi Biotec), and then cultured at 37 °C in complete RPMI medium (10% fetal calf serum, 2 mmol/l -glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.1 mmol/l nonessential amino acids, 1 mmol/l sodium pyruvate, 14.3 µmol/l β-mercaptoethanol) in presence of recombinant mouse granulocyte-macrophage colony-stimulating factor and IL-4 (Peprotech, Rocky Hill, NJ) at 10 ng/ml each. On day 5, cells were infected with lentiviral particles (multiplicity of infection = 15–20) in presence of 10 µg/ml protamine sulfate (Sigma, St Louis, MO). After 16–24 hours incubation with virus, the medium was changed. Infected and noninfected DCs were collected on day 7. Noninfected DCs were washed three times in OptiMEM and electroporated in a 4 mm cuvette (5 × 106 DCs in 200 µl OptiMEM with 30 µg mRNA) on a Gene Pulser Xcell II electroporator (Bio-rad, Hercules, CA) using optimized parameters (300 V, 150 µF, 100 Ω).
Treatments and monitoring. 1 × 106 DCs (in 200 µl PBS) were injected intravenously into 12-week-old nondiabetic NOD mice (prevention) or 11–30-week-old NOD mice with recent onset hyperglycemia. In prevention studies, blood glucose was measured weekly and animals with levels >250 mg/dl over two consecutive bleedings were considered diabetic. In therapy studies, blood glucose was monitored weekly. Mice with high-blood glucose (250–500 mg/dl) were bled again 1 or 2 days later to confirm hyperglycemia and immediately treated. Treated mice were monitored twice weekly and animals with levels >500 mg/dl over two consecutive bleedings were sacrificed. In transfer experiments, splenocytes obtained 3 days after treatment were injected intravenously into 10-week-old NOD.SCID mice (3 × 106 cells per recipient). Recipient mice were followed every other week until the first onset of disease, and weekly thereafter. Mice were considered diabetic using the same criteria as in prevention studies.
Flow cytometric analysis. DCs were analyzed for GFP and surface markers expression (anti-CD11b, CD11c and I-Ak (also stains I-Ag7) were from BD Biosciences (San Jose, CA); anti-CD40, CD80, CD86, CD8a and SIRPα were from eBioscience). For intracellular staining of IL-4, transduced and electroporated DCs were recultured for 3–24 hours in complete medium without cytokines and with GolgiStop (BD Biosciences) added in the last 3–4 hours, processed with BD Fix/Perm kit (BD Biosciences) and stained using allophycocyanin-conjugated anti-IL-4 (eBioscience). T cells were stained for CD4, CD8a, CD25, CD44, CD69, and Foxp3 using antibodies from BD Biosciences and eBioscience. Fix/Perm kit from eBioscience was used for Foxp3 intracellular staining. All cells were analyzed on LSR1 (BD Biosciences) and data were computed using the FlowJo software (Tree Star, Ashland, OR).
ELISA. DCs were washed and replated at different numbers and several replicates in complete RPMI without granulocyte-macrophage colony-stimulating factor or IL-4. Supernatant was collected at various time points ranging from 30 minutes to 24 hours. ELISA was performed using standard protocol and reagents from BD Biosciences (anti-IL-4 antibody pair), Peprotech (IL-4 standard) and Sigma (ExtrAvidin peroxidase and TMB substrate). Data shown are mean ± SD from different number of live cells plated (5 × 103, 2.5 × 104, 1 × 105). The amount of IL-4 secreted was linearly correlated with the number of DCs (R2 > 0.98). For cytokine profile, splenocytes (1 × 106/well), PLN, or cervical lymph node cells (0.8 × 106/well) were replated in complete c-RPMI in four replicates. Supernatant was collected after 36 and 72 hours (one duplicate per time point). ELISA was performed as described above for IL-4, or using ELISA Max kits (Biolegend, San Diego, CA) for IFN-γ and IL-10.
SUPPLEMENTARY MATERIAL Figure S1. Efficiency of electroporation or transduction of DCs. GFP and intracellular IL-4 expression by DCs electroporated with GFP or IL-4 mRNA (4 hours after electroporation), or transduced with lentiviral particles expressing IL-4 and GFP. Figure S2. Evolution of blood glucose on a selection of mice treated with eDC/IL-4, showing occasional transient increase of glycemia over 250 mg/dl. Two of these mice showed such pattern over two consecutive weeks and were considered diabetic per our criteria. Figure S3. Consistency in the amount of IL-4 secreted by modified DCs in a 24 hours culture. Different bars represents independent experiments, in which eDC/GFP, eDC/IL-4 and ltDC/IL-4 were re-plated at different numbers (ranging from 103 to 105) in medium without cytokine for 24 hours. Mean ± SD from different cell numbers normalized to 106 total cells are shown. Figure S4. Factors influencing effectiveness of eDC/IL-4 treatment. Responders (open circles, n=5) and non-responders (filled circles, n=10) were plotted based on the age they became overtly hyperglycemic and treated, and on the level of hyperglycemia reached at that time. Figure S5. CD25+ Foxp3+ cells in the PLNs and spleen of treated mice. (a) Representative dot plot showing Foxp3 and CD25-expressing cells gated on CD4+ T cells. (b) Representative dot plot showing CD4+ CD25+ cells, as well as induced CD25hi cells. Figure S6. Activated CD4+ and CD8+ T cells in the PLNs and spleen of treated mice. (a) Representative dot plot showing CD44 and CD69-expressing cells gated on CD4+ or CD8+ T cells on day 3 after treatment. (b) Frequency of CD44+ CD69+ T cells; data show the mean ± SD from n=5 mice per group. Figure S7. Frequency of several DC subsets in the spleen of treated mice. (a) Representative dot plots showing four distinct DC subsets based on CD11b and CD11c expression, as well as expression of SIRPα and CD8α on each of these subsets. (b) Frequency of each DC subset among the total spleen cell population. (c) Frequency of SIRPαhi CD8α− within each DC subset. (d) Frequency of CD8α+ SIRPαlo within each DC subset. Data show the mean ± SD from n=5 mice per group. P values shown have been calculated using T-test.
Acknowledgments
D.G.H., I.Y.T., and C.A.N. are employed by Argos Therapeutics. The authors also thank Melissa Adam and Aijing Starr, also employed by Argos Therapeutics, for preparation of optimized IL-4 cDNA template and for mRNA generation, respectively. This work was supported by grants from the National Institutes of Health (DK078123, to C.G.F.), the Juvenile Diabetes Research Foundation (3-2005-1019, to R.J.C.) and by a gift from Argos Therapeutics (to C.G.F.).
Supplementary Material
Efficiency of electroporation or transduction of DCs. GFP and intracellular IL-4 expression by DCs electroporated with GFP or IL-4 mRNA (4 hours after electroporation), or transduced with lentiviral particles expressing IL-4 and GFP.
Evolution of blood glucose on a selection of mice treated with eDC/IL-4, showing occasional transient increase of glycemia over 250 mg/dl. Two of these mice showed such pattern over two consecutive weeks and were considered diabetic per our criteria.
Consistency in the amount of IL-4 secreted by modified DCs in a 24 hours culture. Different bars represents independent experiments, in which eDC/GFP, eDC/IL-4 and ltDC/IL-4 were re-plated at different numbers (ranging from 103 to 105) in medium without cytokine for 24 hours. Mean ± SD from different cell numbers normalized to 106 total cells are shown.
Factors influencing effectiveness of eDC/IL-4 treatment. Responders (open circles, n=5) and non-responders (filled circles, n=10) were plotted based on the age they became overtly hyperglycemic and treated, and on the level of hyperglycemia reached at that time.
CD25+ Foxp3+ cells in the PLNs and spleen of treated mice. (a) Representative dot plot showing Foxp3 and CD25-expressing cells gated on CD4+ T cells. (b) Representative dot plot showing CD4+ CD25+ cells, as well as induced CD25hi cells.
Activated CD4+ and CD8+ T cells in the PLNs and spleen of treated mice. (a) Representative dot plot showing CD44 and CD69-expressing cells gated on CD4+ or CD8+ T cells on day 3 after treatment. (b) Frequency of CD44+ CD69+ T cells; data show the mean ± SD from n=5 mice per group.
Frequency of several DC subsets in the spleen of treated mice. (a) Representative dot plots showing four distinct DC subsets based on CD11b and CD11c expression, as well as expression of SIRPα and CD8α on each of these subsets. (b) Frequency of each DC subset among the total spleen cell population. (c) Frequency of SIRPαhi CD8α− within each DC subset. (d) Frequency of CD8α+ SIRPαlo within each DC subset. Data show the mean ± SD from n=5 mice per group. P values shown have been calculated using T-test.
REFERENCES
- Anderson MS., and, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Smits HH, de Jong EC, Wierenga EA., and, Kapsenberg ML. Different faces of regulatory DCs in homeostasis and immunity. Trends Immunol. 2005;26:123–129. doi: 10.1016/j.it.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Morelli AE., and, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- Creusot RJ, Yaghoubi SS, Chang P, Chia J, Contag CH, Gambhir SS, et al. Lymphoid-tissue-specific homing of bone-marrow-derived dendritic cells. Blood. 2009;113:6638–6647. doi: 10.1182/blood-2009-02-204321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feili-Hariri M, Falkner DH, Gambotto A, Papworth GD, Watkins SC, Robbins PD, et al. Dendritic cells transduced to express interleukin-4 prevent diabetes in nonobese diabetic mice with advanced insulitis. Hum Gene Ther. 2003;14:13–23. doi: 10.1089/10430340360464679. [DOI] [PubMed] [Google Scholar]
- Creusot RJ, Yaghoubi SS, Kodama K, Dang DN, Dang VH, Breckpot K, et al. Tissue-targeted therapy of autoimmune diabetes using dendritic cells transduced to express IL-4 in NOD mice. Clin Immunol. 2008;127:176–187. doi: 10.1016/j.clim.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim S, Evans CH, Ghivizzani SC, Oligino T., and, Robbins PD. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J Immunol. 2001;166:3499–3505. doi: 10.4049/jimmunol.166.5.3499. [DOI] [PubMed] [Google Scholar]
- Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, et al. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. J Clin Invest. 2001;107:1275–1284. doi: 10.1172/JCI11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels A, Tuyaerts S, Bonehill A, Corthals J, Breckpot K, Heirman C, et al. Electroporation of immature and mature dendritic cells: implications for dendritic cell-based vaccines. Gene Ther. 2005;12:772–782. doi: 10.1038/sj.gt.3302471. [DOI] [PubMed] [Google Scholar]
- Van Tendeloo VF, Ponsaerts P., and, Berneman ZN. mRNA-based gene transfer as a tool for gene and cell therapy. Curr Opin Mol Ther. 2007;9:423–431. [PubMed] [Google Scholar]
- Calderhead DM, DeBenedette MA, Ketteringham H, Gamble AH, Horvatinovich JM, Tcherepanova IY, et al. Cytokine maturation followed by CD40L mRNA electroporation results in a clinically relevant dendritic cell product capable of inducing a potent proinflammatory CTL response. J Immunother. 2008;31:731–741. doi: 10.1097/CJI.0b013e318183db02. [DOI] [PubMed] [Google Scholar]
- Feili-Hariri M, Flores RR, Vasquez AC., and, Morel PA. Dendritic cell immunotherapy for autoimmune diabetes. Immunol Res. 2006;36:167–173. doi: 10.1385/IR:36:1:167. [DOI] [PubMed] [Google Scholar]
- Lo J., and, Clare-Salzler MJ. Dendritic cell subsets and type I diabetes: focus upon DC-based therapy. Autoimmun Rev. 2006;5:419–423. doi: 10.1016/j.autrev.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Trucco M., and, Giannoukakis N. Immunoregulatory dendritic cells to prevent and reverse new-onset Type 1 diabetes mellitus. Expert Opin Biol Ther. 2007;7:951–963. doi: 10.1517/14712598.7.7.951. [DOI] [PubMed] [Google Scholar]
- Besin G, Gaudreau S, Ménard M, Guindi C, Dupuis G., and, Amrani A. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes. 2008;57:2107–2117. doi: 10.2337/db08-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoukakis N, Phillips B., and, Trucco M. Toward a cure for type 1 diabetes mellitus: diabetes-suppressive dendritic cells and beyond. Pediatr Diabetes. 2008;9 3 Pt 2:4–13. doi: 10.1111/j.1399-5448.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- Kyte JA, Mu L, Aamdal S, Kvalheim G, Dueland S, Hauser M, et al. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther. 2006;13:905–918. doi: 10.1038/sj.cgt.7700961. [DOI] [PubMed] [Google Scholar]
- Van Driessche A, Van de Velde AL, Nijs G, Braeckman T, Stein B, De Vries JM, et al. Clinical-grade manufacturing of autologous mature mRNA-electroporated dendritic cells and safety testing in acute myeloid leukemia patients in a phase I dose-escalation clinical trial. Cytotherapy. 2009;11:653–668. doi: 10.1080/14653240902960411. [DOI] [PubMed] [Google Scholar]
- Schuurhuis DH, Verdijk P, Schreibelt G, Aarntzen EH, Scharenborg N, de Boer A, et al. In situ expression of tumor antigens by messenger RNA-electroporated dendritic cells in lymph nodes of melanoma patients. Cancer Res. 2009;69:2927–2934. doi: 10.1158/0008-5472.CAN-08-3920. [DOI] [PubMed] [Google Scholar]
- Routy JP, Boulassel MR, Yassine-Diab B, Nicolette C, Healey D, Jain R, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol. 2010;134:140–147. doi: 10.1016/j.clim.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dullaers M, Breckpot K, Van Meirvenne S, Bonehill A, Tuyaerts S, Michiels A, et al. Side-by-side comparison of lentivirally transduced and mRNA-electroporated dendritic cells: implications for cancer immunotherapy protocols. Mol Ther. 2004;10:768–779. doi: 10.1016/j.ymthe.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Sherr J, Sosenko J, Skyler JS., and, Herold KC. Prevention of type 1 diabetes: the time has come. Nat Clin Pract Endocrinol Metab. 2008;4:334–343. doi: 10.1038/ncpendmet0832. [DOI] [PubMed] [Google Scholar]
- Skapenko A, Kalden JR, Lipsky PE., and, Schulze-Koops H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25-CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- Pillai AB, George TI, Dutt S., and, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113:4458–4467. doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama K, Butte AJ, Creusot RJ, Su L, Sheng D, Hartnett M, et al. Tissue- and age-specific changes in gene expression during disease induction and progression in NOD mice. Clin Immunol. 2008;129:195–201. doi: 10.1016/j.clim.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels A, Breckpot K, Corthals J, Tuyaerts S, Bonehill A, Heirman C, et al. Induction of antigen-specific CD8+ cytotoxic T cells by dendritic cells co-electroporated with a dsRNA analogue and tumor antigen mRNA. Gene Ther. 2006;13:1027–1036. doi: 10.1038/sj.gt.3302750. [DOI] [PubMed] [Google Scholar]
- Bonehill A, Tuyaerts S, Van Nuffel AM, Heirman C, Bos TJ, Fostier K, et al. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther. 2008;16:1170–1180. doi: 10.1038/mt.2008.77. [DOI] [PubMed] [Google Scholar]
- Ponsaerts P, Van Tendeloo VF, Cools N, Van Driessche A, Lardon F, Nijs G, et al. mRNA-electroporated mature dendritic cells retain transgene expression, phenotypical properties and stimulatory capacity after cryopreservation. Leukemia. 2002;16:1324–1330. doi: 10.1038/sj.leu.2402511. [DOI] [PubMed] [Google Scholar]
- Yang AD, Barro M, Gorziglia MI., and, Patton JT. Translation enhancer in the 3'-untranslated region of rotavirus gene 6 mRNA promotes expression of the major capsid protein VP6. Arch Virol. 2004;149:303–321. doi: 10.1007/s00705-003-0211-9. [DOI] [PubMed] [Google Scholar]
- Boczkowski D, Nair SK, Nam JH, Lyerly HK., and, Gilboa E. Induction of tumor immunity and cytotoxic T lymphocyte responses using dendritic cells transfected with messenger RNA amplified from tumor cells. Cancer Res. 2000;60:1028–1034. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Efficiency of electroporation or transduction of DCs. GFP and intracellular IL-4 expression by DCs electroporated with GFP or IL-4 mRNA (4 hours after electroporation), or transduced with lentiviral particles expressing IL-4 and GFP.
Evolution of blood glucose on a selection of mice treated with eDC/IL-4, showing occasional transient increase of glycemia over 250 mg/dl. Two of these mice showed such pattern over two consecutive weeks and were considered diabetic per our criteria.
Consistency in the amount of IL-4 secreted by modified DCs in a 24 hours culture. Different bars represents independent experiments, in which eDC/GFP, eDC/IL-4 and ltDC/IL-4 were re-plated at different numbers (ranging from 103 to 105) in medium without cytokine for 24 hours. Mean ± SD from different cell numbers normalized to 106 total cells are shown.
Factors influencing effectiveness of eDC/IL-4 treatment. Responders (open circles, n=5) and non-responders (filled circles, n=10) were plotted based on the age they became overtly hyperglycemic and treated, and on the level of hyperglycemia reached at that time.
CD25+ Foxp3+ cells in the PLNs and spleen of treated mice. (a) Representative dot plot showing Foxp3 and CD25-expressing cells gated on CD4+ T cells. (b) Representative dot plot showing CD4+ CD25+ cells, as well as induced CD25hi cells.
Activated CD4+ and CD8+ T cells in the PLNs and spleen of treated mice. (a) Representative dot plot showing CD44 and CD69-expressing cells gated on CD4+ or CD8+ T cells on day 3 after treatment. (b) Frequency of CD44+ CD69+ T cells; data show the mean ± SD from n=5 mice per group.
Frequency of several DC subsets in the spleen of treated mice. (a) Representative dot plots showing four distinct DC subsets based on CD11b and CD11c expression, as well as expression of SIRPα and CD8α on each of these subsets. (b) Frequency of each DC subset among the total spleen cell population. (c) Frequency of SIRPαhi CD8α− within each DC subset. (d) Frequency of CD8α+ SIRPαlo within each DC subset. Data show the mean ± SD from n=5 mice per group. P values shown have been calculated using T-test.