Abstract
Arterial endothelial cells (EC) are attractive targets for gene therapy of atherosclerosis because they are accessible to hematogenous and catheter-based vector delivery and overlie atherosclerotic plaques. Vector-mediated expression—in EC—of proteins that mediate cholesterol transfer out of the artery wall and decrease inflammation could prevent and reverse atherosclerosis. However, clinical application of this strategy is limited by lack of a suitable gene-transfer vector. First-generation adenovirus (FGAd) is useful for EC gene transfer in proof-of-concept studies, but is unsuitable for atheroprotective human gene therapy because of limited duration of expression and proinflammatory effects. Moreover, others have reported detrimental effects of FGAd on critical aspects of EC physiology including proliferation, migration, and apoptosis. Here, we investigated whether helper-dependent adenovirus (HDAd) either alone or expressing an atheroprotective gene [apolipoprotein A-I (apoA-I)] could circumvent these limitations. In contrast to control FGAd, HDAd did not alter any of several critical EC physiologic functions (including proliferation, migration, apoptosis, metabolic activity, and nitric oxide (NO) production) and did not stimulate proinflammatory pathways [including expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and interleukin-6 (IL-6)]. Expression of apoA-I by HDAd reduced EC VCAM-1 expression. HDAd is a promising vector and apoA-I is a promising gene for atheroprotective human gene therapy delivered via EC.
Introduction
Atherosclerosis, a common human disease, is caused by the accumulation of blood-derived lipids and inflammatory cells in the artery wall.1 Dysfunctional arterial endothelial cells (EC) can contribute to the progression of atherosclerosis by attracting and retaining circulating leukocytes2 and by facilitating the influx of plasma lipids into the artery wall.3 When EC are lost from the arterial luminal surface (through denudation, apoptosis, or necrosis), exposure of thrombogenic subendothelial molecules such as collagen and tissue factor can trigger blood clot formation, leading to vessel occlusion, strokes, heart attacks, and death.4 In contrast, healthy EC are long-lived,5 resist leukocyte accumulation,6 regulate vascular tone via production of nitric oxide (NO),7 and migrate and divide as needed to maintain an EC-lined, nonthrombogenic vessel surface.
Because of the central role of EC in resisting the development of atherothrombotic disease, their accessibility to vector delivery, and their physical proximity to atherosclerotic plaques, several groups (including our own) have proposed gene-therapy strategies that rely on expression of therapeutic genes from EC. In general, these strategies aim to either block EC pathogenic activities (e.g., leukocyte adhesion and uptake of atherogenic lipoproteins)8,9 or enhance EC protective roles (e.g., prevention of thrombosis).10 None of these promising gene-therapy strategies has reached clinical application, most likely because of lack of a suitable vector to deliver therapeutic genes to EC in vivo.11
To deliver effective, EC-based gene therapy, a vector must efficiently transduce EC without affecting their normal, salutary physiologic functions. First-generation adenoviral (FGAd) vectors efficiently transduce EC both in vitro and in vivo.12,13 However, numerous in vitro studies show that FGAd can alter cellular proliferation, migration, and apoptosis, and increase expression of inflammatory cytokines and adhesion molecules.14,15,16,17,18,19,20,21,22 These limitations (as well as the transient nature of FGAd expression in vivo)13 must be overcome before adenovirus (Ad)-mediated EC gene transfer can be applied clinically. Helper-dependent adenoviral (HDAd) vectors, which lack all viral genes, have several advantages over FGAd, including larger cloning capacity, persistent transgene expression, and a diminished host immune response.23,24,25 HDAd express a transgene in EC in vivo for at least 8 weeks, and cause significantly less vascular inflammation than FGAd.26 However, HDAd could potentially stimulate EC inflammatory pathways through a capsid-initiated response,14 and the effect of HDAd on normal EC physiology is unknown.
Here, we report in vitro studies that test the hypothesis that HDAd—but not FGAd—can transduce EC effectively without either altering normal EC physiology or stimulating EC proinflammatory pathways. We simultaneously tested whether normal EC physiology is affected by HDAd-mediated overexpression of apolipoprotein A-1 (apoA-I). ApoA-I is an attractive candidate therapeutic gene for atherosclerosis because it can remove lipid from the artery wall and also has potent anti-inflammatory effects.27
Results
FGAdApoAI- and HDAdApoAI-transduced EC express functional rabbit apoA-I protein
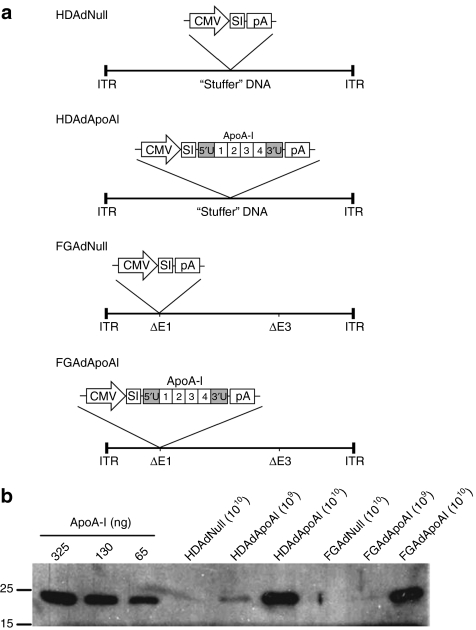
We first tested whether our FGAd and HDAd rabbit apoA-I vectors (FGAdApoAI and HDAdApoAI; Figure 1a) expressed rabbit apoA-I in cultured bovine aortic EC (BAEC). ApoA-I protein was present in medium of BAEC transduced with HDAdApoAI and FGAdApoAI, but not in medium of cells transduced with the control vectors HDAdNull and FGAdNull (Figure 1b). ApoA-I expression was easily detectable in cells transduced with 1010 viral particles/ml and was expressed at similar levels in HDAdApoAI- and FGAdApoAI-transduced cells (~50 µg/106 cells/24 hours). The transduction protocol used throughout this study yielded ~85% transduced EC, as determined with a green fluorescent protein (GFP)-expressing HDAd (Supplementary Figure S1).
Figure 1.
Expression of rabbit apoA-I in bovine aortic endothelial cells. (a) Viral constructs used in this study all contain a cytomegalovirus (CMV) promoter, a synthetic intron (SI), and an SV40 polyadenylation signal (pA). Exons 1–4 and the 5′ and 3′ untranslated regions (5′U and 3′U) of the rabbit apoA-I gene are indicated. E1 and E3 viral regions are deleted in FGAd (δE1; δ E3). All viral genes are deleted in HDAd, and are replaced with “stuffer” DNA. (b) Western analysis of apoA-I expression. Cells were incubated for 24 hours with one of the four Ad vectors at the concentrations indicated (viral particles/ml). Medium was removed and the cells incubated with serum-free DMEM for 24 hours. Conditioned media (20 µl/lane) were analyzed by western blot for apoA-I, using rabbit plasma as a quantitative control. Size markers are in kD. This experiment was repeated four times with similar results. apoA-I, apolipoprotein A-I; DMEM, Dulbecco's modified Eagle medium; FGAd, first-generation adenovirus; HDAd, helper-dependent adenovirus.
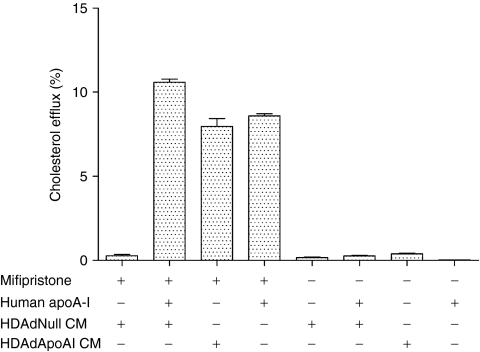
We used a cholesterol-efflux assay to test the biological activity of vector-expressed apoA-I. EC were transduced with either HDAdNull or HDAdApoAI, and conditioned medium collected over 24 hours. The conditioned medium was added to cholesterol-loaded baby hamster kidney (BHK) cells stably transfected with an inducible ATP-binding cassette A1 (ABCA1) transgene. In BHK cells expressing ABCA1, addition of conditioned medium from HDAdNull-transduced EC stimulated minimal cholesterol efflux (<1%; Figure 2). Addition of 5 µg/ml human apoA-I to this medium increased cholesterol efflux by 40-fold (11 ± 0.19 versus 0.28 ± 0.08%). Incubation of the BHK cells with conditioned medium from HDAdApoAI-transduced cells (containing ~7 µg/ml rabbit apoA-I) stimulated cholesterol efflux similarly (8.0 ± 0.47%). Addition of human apoA-I (5 µg/ml) alone had a similar effect. Control experiments performed without induction of ABCA1 confirmed that in all cases cholesterol efflux was ABCA1-dependent. Therefore, apoA-I from HDAd-transduced EC can stimulate the critical first step of reverse cholesterol transport: ABCA1-dependent cholesterol efflux.
Figure 2.
ApoA-I expressed by transduced endothelial cells increases cholesterol efflux. BHK cells were labeled with 3[H] cholesterol for 24 hours then for an additional 24 hours with or without mifipristone to induce ABCA1 expression. The cells were then incubated with one or more of: purified human apoA-I, medium conditioned by HDAdNull-transduced EC, or medium conditioned by HDAdApoAI-transduced EC. Cholesterol efflux was measured by counting 3[H] in medium and in cell extracts. Values are mean ± SEM for n = 3–6 for two independent experiments. apoA-I, apolipoprotein A-I; BHK, baby hamster kidney; CM, conditioned medium; EC, endothelial cells; FGAd, first-generation adenovirus; HDAd, helper-dependent adenovirus.
FGAd but not HDAd disrupts EC metabolic activity
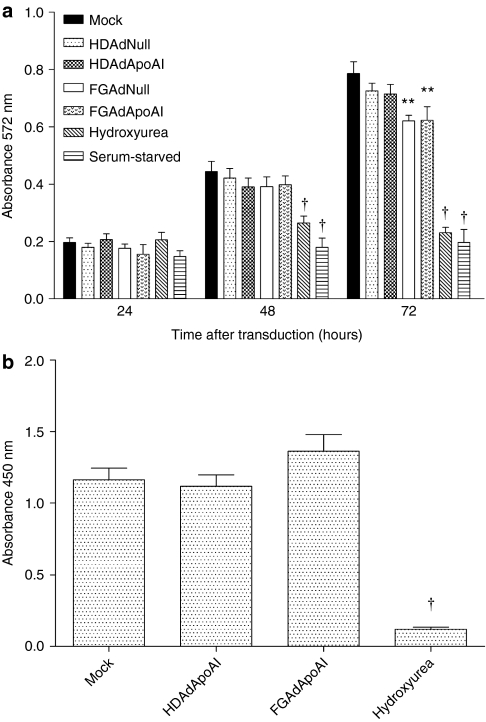
To test whether transduction with FGAd or HDAd—either with or without apoA-I expression—was toxic to EC, we used the MTT assay to measure metabolism of vector-transduced- and mock-transduced EC. Hydroxyurea treatment28 and serum-starvation were positive controls. After 24 hours, no difference in MTT reduction was observed among the groups of vector- and mock-transduced EC (Figure 3a). After 48 hours, only the hydroxyurea-treated and serum-starved EC were less metabolically active than mock-transduced EC. By 72 hours, the metabolic activity of both FGAdNull- and FGAdApoAI-transduced cells was significantly less than mock-transduced cells (20% decrease; P < 0.05).
Figure 3.
FGAd but not HDAd vectors disrupt endothelial cell metabolic activity. EC were transduced with the indicated vectors (1 × 1010 viral particles/ml). (a) Metabolic activity was measured with the MTT assay performed 24–72 hours after transduction. (b) Cell proliferation was measured with a BrdU incorporation assay performed 72 hours after transduction. Positive controls included serum starvation and hydroxyurea treatment. Values are spectrophotometer readings, and are presented as mean ± SEM with n = 14 from three independent experiments for (a), and n = 6 from one experiment for (b). The BrdU experiment was repeated with similar results (data not shown). **P < 0.01 versus mock-transduced cells. †P < 0.001 versus mock-transduced cells. BrdU, bromodeoxyuridine; EC, endothelial cells; FGAd, first-generation adenovirus; HDAd, helper-dependent adenovirus.
We considered whether decreased MTT reduction in FGAd-transduced EC at 72 hours could be due to an effect of FGAd on cell proliferation. We therefore used a bromodeoxyuridine (BrdU) incorporation assay to measure cell proliferation directly. There was no difference in BrdU incorporation among mock-, HDAdApoAI- and FGAdApoAI-transduced EC (Figure 3b).
Neither adenoviral transduction nor apoA-I expression alter EC migration or apoptosis
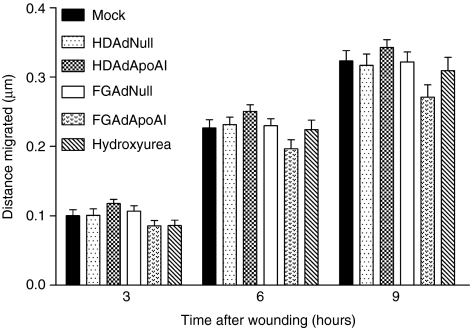
Migration of mock-, HDAd-, and FGAd-transduced cells was measured using a monolayer wound-healing assay performed 3, 6, and 9 hours after wounding. To exclude a contribution of cell proliferation to these results, we included a group of hydroxyurea-treated cells. As expected during this short time period, there was no evidence that cell proliferation contributed to wound closure (compare hydroxyurea-treated to mock-transduced cells; Figure 4). Cell migration was detected in all of the experimental groups, with no significant difference in migration among the groups at any of the time points. The FGAdApoAI-transduced cells tended to migrate less; however, this difference was not statistically significant.
Figure 4.
Transduction with HDAd or FGAd does not affect endothelial cell migration. EC were seeded in 24-well plates. After 24 hours, the cells were mock-transduced or incubated for 6 hours with one of the indicated vectors (1 × 1010 viral particles/ml). The EC monolayers were wounded with a pipette tip. The wounds were photographed immediately (time = 0) and again at 3, 6, and 9 hours and cell migration was calculated. Data are presented as mean ± SEM for n = 12–16 wells from four independent experiments. There were no significant differences between the mock-transduced cells and any of the other groups. EC, endothelial cells; FGAd, first-generation adenovirus; HDAd, helper-dependent adenovirus.
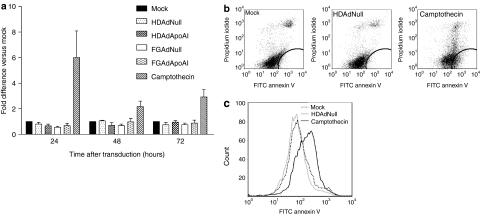
We measured apoptosis 24, 48, and 72 hours after mock-, HDAd-, or FGAd- transduction (Figure 5a). Only low levels of apoptosis were detected and these levels did not differ among the groups. In contrast, treatment with camptothecin, which induces apoptosis through cleavage of topoisomerase I,29 significantly increased apoptosis at all time points, as shown by an increase in annexin V-positive, propidium iodide-negative EC (Figure 5b,c).
Figure 5.
Transduction with HDAd or FGAd does not alter endothelial cell apoptosis. (a) Bovine aortic endothelial cells were seeded in 6-well plates. After 24 hours, the cells were mock-transduced or incubated for 6 hours with one of the indicated vectors (1 × 1010 viral particles/ml). Camptothecin-treated cells (untransduced, then treated with camptothecin for the last 24 hours before harvest) were a positive control for apoptosis at all time points. From 24–72 hours after transduction, cells were removed and analyzed for apoptosis by flow cytometry. Values are fold-increase in apoptosis versus mock-transduced cells (relative value = 1) at each time point and are presented as mean ± SEM for n = 3 independent experiments. None of the groups of vector-transduced cells differed significantly from the mock-transduced cells. (b) Flow cytometry measuring FITC-annexin V and propidium iodide fluorescence of mock-transduced, HDAdNull-transduced, and camptothecin-treated cells at 24 hours time point. (c) Plots of FITC-annexin V fluorescence from (b). EC, endothelial cells; FGAd, first-generation adenovirus; FITC, fluorescein isothiocyanate; HDAd, helper-dependent adenovirus.
Transduction with FGAd but not HDAd increases NO production
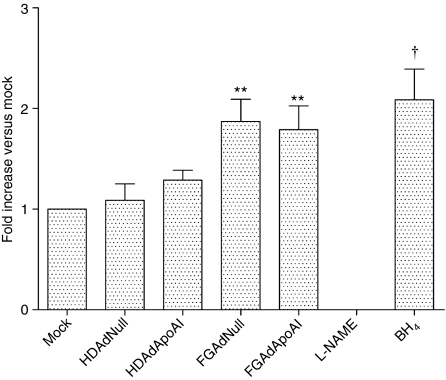
We used electron paramagnetic resonance (EPR) spin trapping to measure NO production 24 hours after mock-, HDAd-, or FGAd- transduction (Figure 6). Cells were treated with the NO synthase (NOS) inhibitor NG-nitro-ℓ-arginine methyl ester or the NOS cofactor tetrahydrobiopterin as positive controls for inhibition or stimulation of NO synthesis, respectively. Transduction with HDAdNull or HDAdApoAI did not significantly alter NO production. However, EC transduced with FGAdNull or FGAdApoAI produced significantly more NO than mock-transduced cells (80–90% more; P < 0.01 versus mock-transduced cells).
Figure 6.
FGAd but not HDAd vectors increase endothelial cell nitric oxide production. Bovine aortic EC were seeded in 100-mm dishes. After 24 hours, the cells were mock-transduced or incubated for 6 hours with one of the indicated vectors (1 × 1010 vp/ml). Nω-nitro-ℓ-arginine methyl ester (L-NAME) and tetrahydrobiopterin (BH4) were used as controls for inhibition and stimulation of nitric oxide synthase, respectively. Twenty-four hours after transduction or addition of the control reagents, cells were analyzed for NO by electron paramagnetic resonance spin-trapping. Values are expressed as a ratio to NO production by mock-transduced cells (relative value = 1) and are presented as mean ± SEM for n = 5 independent experiments. **P < 0.01 versus mock-transduced cells. †P < 0.05 versus mock-transduced cells. FGAd, first-generation adenovirus; HDAd, helper-dependent adenovirus.
FGAdApoAI increases ICAM-1 expression whereas HDAdApoAI decreases VCAM-1 expression
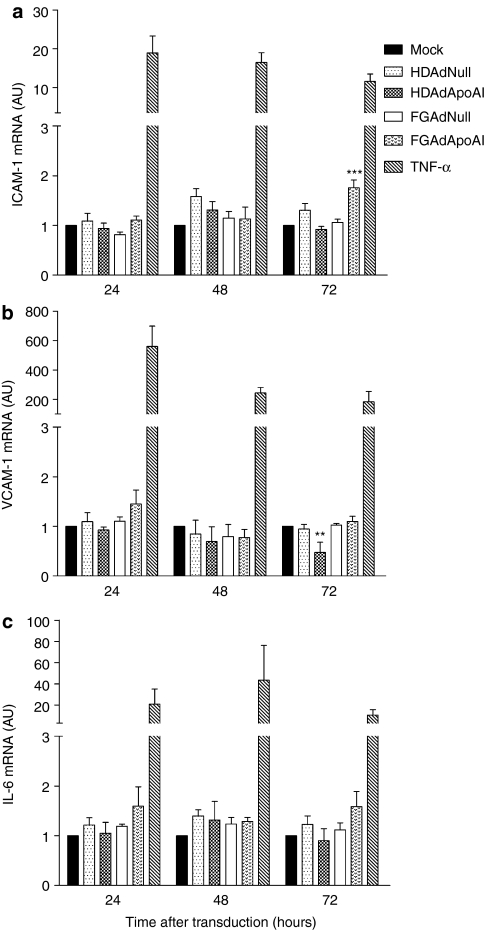
To test whether Ad transduction or apoA-I expression altered EC expression of adhesion molecules or interleukin-6 (IL-6), we measured mRNA encoding intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and IL-6. RNA from cells treated with tumor necrosis factor–α served as a positive control. Tumor necrosis factor–α dramatically increased expression of ICAM-1, VCAM-1, and IL-6 at all time points (24, 48, and 72 hours; Figure 7a–c). In contrast, transduction with FGAdNull, HDAdNull, FGAdApoAI, and HDAdApoAI did not increase ICAM-1, VCAM-1, or IL-6 expression versus mock-transduced cells at 24 or 48 hours postinfection. However, at 72 hours, cells transduced with FGAdApoAI had increased ICAM-1 expression (80% increase versus mock-transduced cells; P < 0.001) and cells transduced with HDAdApoAI had decreased VCAM-1 expression (50% decrease versus mock-transduced cells; P < 0.01). None of the vectors significantly altered IL-6 expression at 72 hours (Figure 7a–c).
Figure 7.
FGAdApoAI increases ICAM-1 expression and HDAdApoAI decreases VCAM-1 expression. Bovine aortic endothelial cells were seeded in 6-well plates. After 24 hours, the cells were mock-transduced or incubated for 24 hours with one of the indicated vectors (1 × 1010 vp/ml). Other cells were treated with tumor necrosis factor–α (TNF–α) to generate positive control RNA. Total RNA was extracted 24–72 hours after transduction or exposure to TNF-α for measurement by qRT-PCR of: (a) ICAM-1; (b) VCAM-1; and (c) IL-6. Values are expressed as a ratio to mRNA levels in mock-transduced cells (relative value = 1) and are presented as mean ± SEM for n = 3 (one well in each of three independent experiments). **P < 0.01 versus mock-transduced cells. ***P < 0.001 versus mock-transduced cells. FGAd, first-generation adenovirus; HDAd, helper-dependent adenovirus; ICAM-1, intercellular adhesion molecule-1; IL-6, interleukin-6; VCAM-1, vascular cell adhesion molecule-1.
Discussion
We tested the hypothesis that transduction of EC with HDAd—alone or expressing apoA-I—does not impair normal EC physiology or stimulate EC proinflammatory pathways. We also tested whether apoA-I would enhance NO production and downregulate adhesion molecule expression in EC, as reported by others.30,31 Our major findings were: (i) transduction of EC with HDAd alone did not alter cellular metabolic activity, proliferation, migration, apoptosis, or NO synthesis, and did not affect EC expression of ICAM-1, VCAM-1, or IL-6; (ii) transduction of EC with FGAd decreased cellular metabolic activity and increased NO synthesis; (iii) expression of apoA-I from HDAd—but not FGAd—decreased VCAM-1 expression; (iv) FGAd-driven expression of apoA-I increased ICAM-1 expression. Our findings suggest: neither transduction of EC with HDAd nor HDAd-mediated expression of apoA-I have significant detrimental effects on EC; HDAd-mediated expression of apoA-I has a modest anti-inflammatory effect; and HDAd appears to be a more suitable vector for EC gene transfer than FGAd.
EC must proliferate to replace neighboring EC that are lost due to normal turnover or during interventional procedures such as angioplasty and stenting. A useful gene-therapy vector should not impair EC metabolic activity or affect the ability of EC to proliferate. We are not aware that the MTT assay has been used previously to detect effects of Ad on EC health; however, others have reported significant effects of FGAd on proliferation of EC and other cell types, including both antiproliferative and mitogenic effects.15,16,17 Effects of HDAd on EC metabolic health and proliferation have not yet been reported. Here, we found that FGAd—but not HDAd—decreased EC metabolic activity, manifested as decreased MTT reduction 72 hours after transduction. However, neither FGAdApoAI nor HDAdApoAI altered EC proliferation at this time point.
A useful gene-therapy vector should also not prevent EC migration because EC must migrate laterally to replace neighboring EC. Others have reported both increased and decreased EC migration after exposure to FGAd. For example, human microvascular EC transduced with FGAd migrated more readily toward insulin-like growth factor-1.17 However, human umbilical vein endothelial cells exposed to FGAd migrated less rapidly toward stromal cell derived factor-1 in one study, but more rapidly toward VEGF-A in another study.18,19 Curiously, in both human umbilical vein endothelial cell studies the effects of FGAd on migration were attributed to the E4 region of the adenoviral genome. Here, we found no effect of either FGAd or HDAd on EC migration. The reason for the discrepancy between our FGAd results and others' is unclear; however, it could be because we used a lateral migration assay (essentially a wound-healing assay) whereas others used vertical migration assays (i.e., EC migration through a filter, typically used to study angiogenic activity). We chose the lateral migration assay because EC must migrate laterally, not vertically, to heal the surface of a denuded vessel.
We tested whether FGAd- or HDAd-affected EC apoptosis because enhanced EC apoptosis in vivo could expose thrombogenic subendothelium and would also lead to loss of transgene expression. Others have reported both pro- and antiapoptotic effects of FGAd.15,16,20 FGAd increased apoptosis of human airway cell lines and sensitized cells to tumor necrosis factor–α-induced apoptosis.15,20 Antiapoptotic effects of FGAd—specifically on EC—were attributed to proteins encoded by the E4 region and therefore should be absent in HDAd-transduced cells.16 Here, we found no effect of either FG- or HDAd on EC apoptosis. Inclusion of a positive control (camptothecin) and the absence of FGAd or HDAd-associated increases in either MTT reduction or BrdU incorporation (Figure 3) suggest that we would have detected both pro- and antiapoptotic effects of FGAd and HDAd if they were present. Our results do not directly conflict with others' because of differences in EC type, culture conditions, and transduction protocols.
In addition to its role in maintaining vascular tone, EC-derived NO can decrease adhesion molecule expression, and prevent platelet aggregation.32,33 Modest increases in NO production by EC are generally considered desirable and several groups have developed vascular gene therapies based on increasing EC NO synthesis by NOS gene transfer.34,35,36,37 We were surprised that transduction of EC with either FGAd increased NO bioavailability almost twofold. This degree of increased NO is similar to the doubling of NO activity in aorta of endothelial NOS-transgenic mice, in which arterial blood pressure is significantly reduced and arteries have a decreased response to NO-mediated vasodilators.38,39 Therefore, the magnitude of increased NO in FGAd-transduced EC appears to be physiologically significant and could be detrimental. Increased NO bioavailability in FGAd-transduced EC has not been reported by other groups developing NOS-based gene therapy; however, some have used Ad-β-galactosidase as a control vector instead of AdNull34,35,37 and others have relied on surrogate endpoints for detection of increased NO, such as western blotting for endothelial NOS.36 In contrast, we used the highly sensitive EPR spin-trapping technique to measure NO content. Stimulation of NO production by FGAd might be explained by FGAd activation of PI3-kinase/Akt signaling,19 which can activate NOS, leading to increased NO synthesis.40 Others have reported increased NOS activity in EC incubated with apoA-I protein;30 however, we found no effect of apoA-I on NO levels. Possible explanations include exposure to a lower concentration of apoA-I, use of a different type of EC, or use of a different method for measuring NO in the present study.
Several groups report activation of inflammatory pathways after exposure of cells to Ad. Experiments using UV-inactivated FGAd and HDAd suggest that a substantial part of this activation does not require Ad gene expression.41 Because enhanced inflammation—especially upregulation of adhesion molecules and atherogenic cytokines—would likely accelerate atherosclerosis, we tested whether FGAd and HDAd increased EC expression of ICAM-1, VCAM-1, and IL-6. We focused on these three molecules because others have reported upregulation of ICAM-1 and VCAM-1 in FGAd-transduced EC,21 and IL-6 is a marker of the early innate immune response to Ad.41 This early response is independent of Ad-mediated gene expression and could occur in both FGAd- and HDAd-transduced EC. We also tested whether apoA-I, which can block inflammatory pathways,27,31 would decrease expression of these inflammatory molecules.
We found no effect of FGAdNull or HDAdNull on expression of ICAM-1, VCAM-1, or IL-6. However, FGAdApoAI increased ICAM-1 expression whereas HDAdApoAI reduced VCAM-1 expression. Because these two vectors express similar amounts of apoA-I protein (Figure 1) the effects of FGAdApoAI and HDAdApoAI on ICAM-1 and VCAM-1 cannot be attributed solely to apoA-I and must instead reflect an interaction of the EC responses to vector delivery and apoA-I. Vector–transgene interactions, also reported by others,22 are likely complex. The most significant aspects of this experiment are that HDAdNull did not activate EC and that HDAdApoAI had an anti-inflammatory effect (lowering VCAM-1 expression).
In summary, aortic EC can be transduced with HDAd without impairing normal EC physiology or upregulating expression of three important inflammatory markers. HDAd expression of apoA-I in EC has a modest anti-inflammatory effect. Our study has limitations because it was performed in vitro (in the absence of immune cells), and with only one concentration of virus. However, our in vitro transduction protocol would tend to accentuate the inflammatory effects of Ad because it includes a much longer exposure of EC to high-concentration Ad than occurs in vivo. Several issues remain to be addressed before HDAd is used clinically, including development of large-scale, standardized production methods and thorough examination of potential side effects of stuffer DNA. Nevertheless, when combined with our positive initial experience with HDAdNull in vivo in normal arteries,26 the present study supports the safety of HDAd for vascular gene transfer and provides a strong rationale for continuing to develop HDAdApoAI for vessel wall-targeted gene therapy that would prevent or reverse atherosclerosis.
Materials and Methods
Cell culture. BAEC (Cell Applications, San Diego, CA), human embryonic kidney 293 cells (American Type Culture Collection, Manassas, VA), 293-Cre cells (Microbix Biosystems, Toronto, ON, Canada),42 and BHK cells (American Type Culture Collection) were grown in high-glucose Dulbecco's modified Eagle medium (DMEM; GIBCO, Grand Island, NY) with 10% fetal bovine serum (Hyclone, Logan, UT), 100 IU/ml penicillin and 100 µg/ml streptomycin (GIBCO) at 37 °C in a 5% CO2 atmosphere. Culture medium was supplemented with geneticin (400 µg/ml final concentration) for growth of 293-Cre cells. For EPR spin-trapping assay, cells were cultured in low-glucose DMEM (GIBCO). BAEC were used between passages 4 and 6.
Adenoviral vectors. Four vectors were used in these experiments (Figure 1a): two FGAd (FGAdNull and FGAdApoAI), and two HDAd (HDAdNull and HDAdApoAI). HDAd were made with reagents available from Microbix. The construction of FGAdNull (contains a cytomegalovirus promoter-driven empty expression cassette) was described.26 To construct FGAdApoAI, a rabbit apoA-I complementary DNA (cDNA) was cloned by reverse transcription and PCR amplification of RNA from rabbit terminal ileum.43 Briefly, RNA was extracted with Trizol (Invitrogen, Carlsbad, CA), reverse-transcribed, and amplified using the Accuscript High-Fidelity RT-PCR kit (Stratagene, La Jolla, CA). The amplicon was digested with BamHI and ClaI, and ligated into pBluescript II (Stratagene). The resulting plasmid was digested with KpnI and SpeI to release the apoA-I cDNA, which was then ligated between the KpnI and NheI sites of pCI (Promega, Madison, WI), to yield pCIApoAI. Digestion of pCIApoAI with BglII and ClaI released an expression cassette comprising the apoA-I cDNA flanked by the cytomegalovirus promoter and SV40 polyadenylation signal. This cassette was ligated into the FGAd shuttle vector pdE1SP1A (Microbix). The shuttle vector plasmid was linearized by StuI digestion and FGAdApoAI virions produced.44
To construct HDAdApoAI, we first generated a shuttle vector to facilitate insertion of the apoA-I expression cassette into the HDAd backbone plasmid pC4HSU (Microbix). A 2,649 base pair EcoRI fragment containing an AscI site was removed from pC4HSU and ligated into pBluescript II, to generate “pBShuttle.” The apoA-I expression cassette was removed from pCIApoAI and linkers were used to add AscI sites to both ends of the cassette, which was then ligated into the AscI site of pBShuttle. This ligation placed the apoA-I expression cassette between fragments from pC4HSU, enabling insertion of the expression cassette into pC4HSU by homologous recombination. The expression cassette and flanking regions were released from pBShuttle by digestion with EcoRV and SacII. This fragment was electroporated into the recombination-permissive BJ5183 bacterial strain (Stratagene) along with AscI-digested pC4HSU. The bacteria were plated onto selective media and colonies screened by PCR for evidence of successful recombination. DNA was extracted from colonies showing evidence of expression cassette insertion, and used to transform DH10B cells (Invitrogen). Plasmid DNA from these cells was mapped by restriction digestion to verify integrity of the expression cassette, then used, along with 293-Cre cells and helper virus to generate and amplify HDAdApoAI.23,42 HDAdNull was produced similarly to HDAdApoAI, except that the apoA-I cDNA was not present in the expression cassette excised from pCI. HDAdGFP was produced with these same methods, using a pC4HSU-based plasmid containing a cytomegalovirus promoter-driven GFP transgene (Microbix).
Viral preparations. After purification of virions by CsCl ultracentrifugation and dialysis, virus concentration was measured by spectrophotometry at 260 nm.45 Viral preparations used here ranged from 5 × 1011 to 1.1 × 1013 viral particles/ml. For both HDAd and FGAd preps, DNA was extracted from purified virions using phenol chloroform and Phase Lock Gel tubes (Eppendorf, Hamburg, Germany), and E1A contamination determined by quantitative real-time PCR using ABsolute QPCR Low ROX Mix (Thermo Fisher Scientific, Waltham, MA) with primers and FAM/TAMRA-labeled probes (Applied Biosystems, Foster City, CA) for cytomegalovirus (to quantify total viral genomes) and E1A. Primers and probes used for real-time PCR are listed in Table 1. Only preparations with <1 E1A copy/106 viral genomes were used. For HDAd preparations, helper virus contamination was determined by quantitative PCR.
Table 1. Primer and probe sequences.
In vitro transduction. Cells were plated at various densities on 6, 24, and 96-well culture dishes and incubated for 24 hours before transduction. Virus was resuspended at 1 × 1010 viral particles/ml in cell culture medium and added to cells. After 6 hours, cells were washed twice in phosphate-buffered saline before fresh medium was applied. For experiments in which ICAM-1, VCAM-1, and IL-6 mRNA were measured, cells were exposed to virus for 24 hours, after which the infection medium was removed and cells washed. Viral vectors were resuspended in normal high-glucose growth medium for most transductions except those for which NO measurements were planned. In this case, low-glucose growth medium was used.
Transduction efficiency. EC were seeded at 2 × 105 cells/well in 6-well plates and either mock-transduced or transduced with HDAd. After 24 hours, cells were trypsinized and resuspended in 500 µl of phosphate-buffered saline. GFP expression was detected with a FACScan cytometer and CellQuest Pro acquisition software (Becton Dickinson, Franklin Lakes, NJ). Flow cytometry data were analyzed using FlowJo software (Tree Star, Ashland, OR). A gate was established using mock-transduced EC and then applied to analyze data from EC transduced with either HDAdNull or HDAdGFP. GFP-expressing EC were identified as positive for green fluorescence (x-axis; 530 nm filter) but negative for autofluorescence (y-axis; 575 nm filter). Ten thousand events were measured for each sample.
Western blotting. BAEC were seeded at 2 × 105 cells/well in 6-well plates and transduced with viral vectors. Twenty-four hours later, vector-containing medium was removed, cells were washed twice with phosphate-buffered saline and incubated with 600 µl of serum-free DMEM for an additional 24 hours. This conditioned medium (20 µl) was added to 7.7 µl of 4× loading buffer (50% glycerol, 125 mmol/l Tris-HCl, 4% sodium dodecyl sulfate, 0.08% bromophenol blue) and 3.2 µl of β-mercaptoethanol, and heated for 5 minutes at 95 °C. Samples were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to an Immobilon-P membrane (Millipore, Billerica, MA). Membranes were incubated with goat antihuman apoA-I primary antibody (Rockland, Gilbertsville, PA) and bound antibody detected with a peroxidase conjugated anti-goat antibody (Rockland) and the ECL Plus Western Blotting Detection Kit (GE Healthcare, Piscataway, NJ).
Cholesterol-efflux assay. EC were seeded at 1.5 × 106 cells/dish in 100-mm dishes and transduced with either HDAdNull or HDAdApoAI. Transduced EC were incubated with 4 ml of serum-free DMEM for 24 hours, which was then collected and analyzed by western blot. Medium from HDAdNull EC contained no detectable apoA-I; medium from HDAdApoAI cells contained ~7 µg/ml of rabbit apoA-I. Cholesterol efflux in BHK cells with inducible ABCA1 expression was measured as described.46 Briefly, BHK cells were labeled with 3[H] cholesterol (0.5 µCi/ml, 40–60 Ci/mmol; Amersham Pharmacia Biotech, Piscataway, NJ) for 24 hours, washed three times, then cultured for an additional 24 hours either with or without added mifipristone (10 nmol/l) to induce ABCA1 expression. BHK cells were then incubated with: conditioned medium from HDAdNull-transduced EC with or without added human apoA-I (5 µg/ml); conditioned medium from HDAdApoAI-transduced EC; human apoA-I (5 µg/ml) in DMEM with 1 mg/ml bovine serum albumin; or DMEM/bovine serum albumin alone for 2.5 hours at 37 °C. The medium was collected, centrifuged to remove detached cells, and counted for 3[H]. Cellular cholesterol was extracted using hexane/isopropanol and 3[H] counted. ApoA-I-mediated lipid efflux was calculated as the amount of 3[H] in the medium divided by total 3[H] (medium + cell extract) after subtraction of background cholesterol-efflux values from BHK cells incubated with DMEM/bovine serum albumin alone.
MTT assay. Addition of 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyltetrazolium bromide (MTT; American Type Culture Collection) to known numbers of EC in a control experiment revealed a linear relationship between cell number and absorbance (data not shown). EC were seeded at 2 × 103 cells/well in 96-well plates and transduced with viral vectors. As a positive control for an antiproliferative effect, some wells were treated with 2 mmol/l hydroxyurea instead of virus. MTT reagent (10 µl) was added at 24, 48, and 72 hours after transduction, and incubated at 37 °C for 3 hours. One hundred micro liters of detergent reagent was then added and the plates incubated in the dark for 3 hours at room temperature. Absorbance at 572 nm was measured using a spectrophotometer (Molecular Devices, Sunnyvale, CA).
BrdU incorporation. A BrdU incorporation assay (Calbiochem, Gibbstown, NJ) was used to measure cell proliferation at 72 hours after transduction. EC were seeded at 2 × 103 cells/well in 96-well plates and transduced with viral vectors. After 48 hours, cells were incubated with 50 µl of BrdU label (1:2,000 dilution in culture medium) for 24 hours, fixed, and incubated in anti-BrdU antibody (1:100 dilution) for 1 hour. Wells were washed three times with buffer and peroxidase-labeled goat anti-mouse immunoglobulin G (1:2,000 dilution) was added for 30 minutes. After three more washes, 100 µl of substrate solution was added, the reaction stopped after 15 minutes and absorbance in each well measured at 450 nm. Hydroxyurea was a positive control for antiproliferative effects.
Cell migration. Cell migration was measured with a monolayer wound-healing assay.47 BAEC were seeded at 6 × 104 cells/well in 24-well plates and transduced with viral vectors. Twenty-four hours after transduction, parallel wounds—1 cm apart—were made on cell monolayers using a pipette tip. Wounds were photographed immediately (t = 0) and again after 3, 6, and 9 hours. Using the photographs and computer-assisted planimetry, the total area of a 1 mm-long section of each wound was measured at each time point. To quantify cell migration, the area of the 1-mm long wound section at 3, 6, and 9 hours was subtracted from the area at t = 0 to give the average distance covered by migrating cells. To determine whether cell proliferation during this 9-hour period contributed to wound coverage, a control well of untransduced BAEC was treated with hydroxyurea to block proliferation (2 mmol/l final concentration; Sigma-Aldrich, St Louis, MO) and migration in this well was compared to migration in a well without hydroxyurea.48
Apoptosis. Apoptosis was measured with the Vybrant Apoptosis Kit #3 (Invitrogen). BAEC were seeded at 2 × 105 cells/well in 6-well plates and transduced with viral vectors. Cells were trypsinized at 24, 48, and 72 hours after transduction, washed in cold phosphate-buffered saline and resuspended in 500 µl of 1× annexin-binding buffer. Fluorescein isothiocyanate-annexin V (2.5 µl) and propidium iodide (1 µl of 100 µg/ml) were added, and the cell suspension incubated at room temperature for 15 minutes. An aliquot of cells treated with camptothecin (5 µmol/l final concentration) was used as a positive control. Flow cytometry and data analysis were performed using the equipment and software described above. Apoptotic cells were identified as annexin V-positive (519 nm filter) and propidium iodide-negative (617 nm filter). A gate to identify apoptotic cells was established using data from mock-transduced cells, and this gate was applied to all samples. For each sample, 10,000 events were recorded.
Measurement of NO. NO was measured by EPR spin trapping.38 BAEC were seeded at 1.5 × 106 cells/dish in 100 mm dishes and transduced with viral vectors. Twenty-four hours after transduction, the cells were washed with Krebs-HEPES buffer (99 mmol/l NaCl, 4.7 mmol/l KCL, 1.2 mmol/l KH2PO4, 1.9 mmol/l CaCl2, 25 mmol/l NaHCO3, 20 mmol/l HEPES) and incubated for 1 hour in a trap solution containing iron sulfate heptahydrate (Sigma-Aldrich) and DETC (Alexis, Lausen, Switzerland) (798 µmol/l C5H10NS2·Na·3H2O and 62 µmol/l FeSO4·7H2O dissolved in Krebs-HEPES buffer, previously deoxygenated using argon gas). Cells were washed in Krebs-HEPES buffer, removed with a cell scraper, and resuspended in 100 µl of buffer. The cell suspension was transferred to a 1 ml syringe and frozen in liquid nitrogen to mould a pellet, which was stored at −80 °C. EPR signals were measured with a Miniscope MS 200 EPR spectrometer (Magnettech, Berlin, Germany). Instrument settings were: Bo 3,275 G; modulation frequency 115 kHz; sweep-time 60 seconds; passes 10; microwave power 10 mW; Gain 9 E 1; modulation amplitude 7,000 mG. The resulting EPR signal amplitude was measured and normalized to protein content of each sample. Protein was measured with the DC Protein Assay (Bio-Rad, Hercules, CA).
Detection of gene expression by quantitative reverse-transcriptase-mediated PCR. Total RNA was extracted using Trizol (Invitrogen) and the RNeasy kit (Qiagen, Valencia, CA). RNA samples were adjusted to 50 ng/µl and genomic DNA removed by DNase I treatment (Fermentas, Glen Burnie, MD). Verso 1-Step qRT-PCR kit (Thermo Fisher Scientific) was used for both cDNA synthesis and amplification. ICAM-1 and VCAM-1 primers and probes were as described.49 ICAM-1, VCAM-1, and IL-6 mRNA levels were quantified by the comparative CT method using RNA from mock-transduced cells as the calibrator.50
Statistics. Data are expressed as mean ± SEM. One-way ANOVA with the Holm–Sidak correction for multiple comparisons was used to assess the significance of differences between the vector-transduced and mock-transduced groups. Student's t-test was used, as appropriate, to assess the significance of differences between the mock-transduced and the positive control groups. SigmaStat 3.1 (Systat, San Jose, CA) statistics software was used for all calculations.
SUPPLEMENTARY MATERIAL Figure S1. Transduction efficiency. EC were seeded at 2 × 105 cells/well in 6-well plates and transduced with either HDAdGFP or HDAdNull and green-fluorescent EC detected by flow cytometry. Transduction efficiency (%) is calculated as the number of green-fluorescent EC divided by the total number of EC analyzed. This result is representative of 3 independent experiments.
Acknowledgments
We thank Norma Rizzo for help with the Miniscope spectrometer, Anton McCourtie for help with viral construct preparation, AdVec, Inc. for permission to use the HDAd reagents, Kun Qian and Helén Dichek for assistance in measuring rabbit apoA-1, and Margo Weiss for administrative assistance. This work was supported by National Institutes of Health grant HL076226 and the John L. Locke, Jr. Charitable Trust. The authors declared no conflict of interest.
Supplementary Material
Transduction efficiency. EC were seeded at 2 × 105 cells/well in 6-well plates and transduced with either HDAdGFP or HDAdNull and green-fluorescent EC detected by flow cytometry. Transduction efficiency (%) is calculated as the number of green-fluorescent EC divided by the total number of EC analyzed. This result is representative of 3 independent experiments.
REFERENCES
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Carlos TM., and, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- Rong JX, Rangaswamy S, Shen L, Dave R, Chang YH, Peterson H, et al. Arterial injury by cholesterol oxidation products causes endothelial dysfunction and arterial wall cholesterol accumulation. Arterioscler Thromb Vasc Biol. 1998;18:1885–1894. doi: 10.1161/01.atv.18.12.1885. [DOI] [PubMed] [Google Scholar]
- Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- Hobson B., and, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984;49:405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cybulsky MI, Gimbrone MA., Jr, and, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Byrns RE, Buga GM., and, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Wilson JM., and, Muller DW. Adenovirus-mediated gene transfer of soluble vascular cell adhesion molecule to porcine interposition vein grafts. Circulation. 1994;89:1922–1928. doi: 10.1161/01.cir.89.5.1922. [DOI] [PubMed] [Google Scholar]
- Laukkanen J, Lehtolainen P, Gough PJ, Greaves DR, Gordon S., and, Ylä-Herttuala S. Adenovirus-mediated gene transfer of a secreted form of human macrophage scavenger receptor inhibits modified low-density lipoprotein degradation and foam-cell formation in macrophages. Circulation. 2000;101:1091–1096. doi: 10.1161/01.cir.101.10.1091. [DOI] [PubMed] [Google Scholar]
- Dichek DA, Anderson J, Kelly AB, Hanson SR., and, Harker LA. Enhanced in vivo antithrombotic effects of endothelial cells expressing recombinant plasminogen activators transduced with retroviral vectors. Circulation. 1996;93:301–309. doi: 10.1161/01.cir.93.2.301. [DOI] [PubMed] [Google Scholar]
- Le Brocq M, Leslie SJ, Milliken P., and, Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1631–1674. doi: 10.1089/ars.2007.2013. [DOI] [PubMed] [Google Scholar]
- Lemarchand P, Jaffe HA, Danel C, Cid MC, Kleinman HK, Stratford-Perricaudet LD, et al. Adenovirus-mediated transfer of a recombinant human α1-antitrypsin cDNA to human endothelial cells. Proc Natl Acad Sci USA. 1992;89:6482–6486. doi: 10.1073/pnas.89.14.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarchand P, Jones M, Yamada I., and, Crystal RG. In vivo gene transfer and expression in normal uninjured blood vessels using replication-deficient recombinant adenovirus vectors. Circ Res. 1993;72:1132–1138. doi: 10.1161/01.res.72.5.1132. [DOI] [PubMed] [Google Scholar]
- McCaffrey AP, Fawcett P, Nakai H, McCaffrey RL, Ehrhardt A, Pham TT, et al. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol Ther. 2008;16:931–941. doi: 10.1038/mt.2008.37. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Johnson LG, Huang W, Leigh MW., and, Boucher RC. Effect of adenoviral vector infection on cell proliferation in cultured primary human airway epithelial cells. Hum Gene Ther. 1995;6:1045–1053. doi: 10.1089/hum.1995.6.8-1045. [DOI] [PubMed] [Google Scholar]
- Ramalingam R, Rafii S, Worgall S, Brough DE., and, Crystal RG. E1(-)E4(+) adenoviral gene transfer vectors function as a “pro-life” signal to promote survival of primary human endothelial cells. Blood. 1999;93:2936–2944. [PubMed] [Google Scholar]
- Kornberg LJ., and, Grant MB. Adenoviruses increase endothelial cell proliferation, migration, and tube formation: partial reversal by the focal adhesion kinase inhibitor, FRNK. Microvasc Res. 2007;73:157–162. doi: 10.1016/j.mvr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam R, Worgall S, Rafii S., and, Crystal RG. Downregulation of CXCR4 gene expression in primary human endothelial cells following infection with E1(-)E4(+) adenovirus gene transfer vectors. Mol Ther. 2000;2:381–386. doi: 10.1006/mthe.2000.0131. [DOI] [PubMed] [Google Scholar]
- Zhang F, Cheng J, Hackett NR, Lam G, Shido K, Pergolizzi R, et al. Adenovirus E4 gene promotes selective endothelial cell survival and angiogenesis via activation of the vascular endothelial-cadherin/Akt signaling pathway. J Biol Chem. 2004;279:11760–11766. doi: 10.1074/jbc.M312221200. [DOI] [PubMed] [Google Scholar]
- Miller-Jensen K, Janes KA, Wong YL, Griffith LG., and, Lauffenburger DA. Adenoviral vector saturates Akt pro-survival signaling and blocks insulin-mediated rescue of tumor necrosis-factor-induced apoptosis. J Cell Sci. 2006;119 Pt 18:3788–3798. doi: 10.1242/jcs.03102. [DOI] [PubMed] [Google Scholar]
- Rafii S, Dias S, Meeus S, Hattori K, Ramachandran R, Feuerback F, et al. Infection of endothelium with E1(-)E4(+), but not E1(-)E4(-), adenovirus gene transfer vectors enhances leukocyte adhesion and migration by modulation of ICAM-1, VCAM-1, CD34, and chemokine expression. Circ Res. 2001;88:903–910. doi: 10.1161/hh0901.089884. [DOI] [PubMed] [Google Scholar]
- Jornot L, Petersen H, Lusky M, Pavirani A, Moix I, Morris, et al. Effects of first generation E1E3-deleted and second generation E1E3E4-deleted/modified adenovirus vectors on human endothelial cell death. Endothelium. 2001;8:167–179. doi: 10.1080/10623320109051563. [DOI] [PubMed] [Google Scholar]
- Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA., and, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DJ., and, Ng P. Helper-dependent adenoviral vectors for gene therapy. Hum Gene Ther. 2005;16:1–16. doi: 10.1089/hum.2005.16.1. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Stapleton GE, Law M, Breinholt J, Palmer DJ, Zuo Y, et al. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Graf S, Massey PG., and, Dichek DA. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110:1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. [DOI] [PubMed] [Google Scholar]
- Tang C, Liu Y, Kessler PS, Vaughan AM., and, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakoff IH, Brown NC., and, Reichard P. Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res. 1968;28:1559–1565. [PubMed] [Google Scholar]
- Hsiang YH, Hertzberg R, Hecht S., and, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–14878. [PubMed] [Google Scholar]
- Drew BG, Fidge NH, Gallon-Beaumier G, Kemp BE., and, Kingwell BA. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc Natl Acad Sci USA. 2004;101:6999–7004. doi: 10.1073/pnas.0306266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik R, Bao S, Nobecourt E, Nicholls SJ, Dusting GJ, Barter PJ, et al. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis. 2008;196:240–247. doi: 10.1016/j.atherosclerosis.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Kubes P, Suzuki M., and, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P, Benjamin N., and, Collier J. The effect of endothelium-derived nitric oxide on ex vivo whole blood platelet aggregation in man. Eur J Clin Pharmacol. 1992;42:37–41. doi: 10.1007/BF00314917. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Mozes G, Schwartz RS, Gloviczki P, Crotty TB, Barber DA, et al. Adventitial gene transfer of recombinant endothelial nitric oxide synthase to rabbit carotid arteries alters vascular reactivity. Circulation. 1997;96:2254–2261. doi: 10.1161/01.cir.96.7.2254. [DOI] [PubMed] [Google Scholar]
- Channon KM, Qian H, Neplioueva V, Blazing MA, Olmez E, Shetty GA, et al. In vivo gene transfer of nitric oxide synthase enhances vasomotor function in carotid arteries from normal and cholesterol-Fed rabbits. Circulation. 1998;98:1905–1911. doi: 10.1161/01.cir.98.18.1905. [DOI] [PubMed] [Google Scholar]
- Janssens S, Flaherty D, Nong Z, Varenne O, van Pelt N, Haustermans C, et al. Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation. 1998;97:1274–1281. doi: 10.1161/01.cir.97.13.1274. [DOI] [PubMed] [Google Scholar]
- Ooboshi H, Toyoda K, Faraci FM, Lang MG., and, Heistad DD. Improvement of relaxation in an atherosclerotic artery by gene transfer of endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1998;18:1752–1758. doi: 10.1161/01.atv.18.11.1752. [DOI] [PubMed] [Google Scholar]
- Khoo JP, Alp NJ, Bendall JK, Kawashima S, Yokoyama M, Zhang YH, et al. EPR quantification of vascular nitric oxide production in genetically modified mouse models. Nitric Oxide. 2004;10:156–161. doi: 10.1016/j.niox.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, et al. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest. 1998;102:2061–2071. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., and, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–940. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- Chen L, Anton M., and, Graham FL. Production and characterization of human 293 cell lines expressing the site-specific recombinase Cre. Somat Cell Mol Genet. 1996;22:477–488. doi: 10.1007/BF02369439. [DOI] [PubMed] [Google Scholar]
- Schwab DA, Rea TJ, Hanselman JC, Bisgaier CL, Krause BR., and, Pape ME. Elevated hepatic apolipoprotein A-I transcription is associated with diet-induced hyper α lipoproteinemia in rabbits. Life Sci. 2000;66:1683–1694. doi: 10.1016/s0024-3205(00)00491-4. [DOI] [PubMed] [Google Scholar]
- Lee SW, Trapnell BC, Rade JJ, Virmani R., and, Dichek DA. In vivo adenoviral vector-mediated gene transfer into balloon-injured rat carotid arteries. Circ Res. 1993;73:797–807. doi: 10.1161/01.res.73.5.797. [DOI] [PubMed] [Google Scholar]
- Mittereder N, March KL., and, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram JF, Wolfbauer G, Vaughan AM, Tang C., and, Albers JJ. Phospholipid transfer protein interacts with and stabilizes ATP-binding cassette transporter A1 and enhances cholesterol efflux from cells. J Biol Chem. 2003;278:52379–52385. doi: 10.1074/jbc.M310695200. [DOI] [PubMed] [Google Scholar]
- Valster A, Tran NL, Nakada M, Berens ME, Chan AY., and, Symons M. Cell migration and invasion assays. Methods. 2005;37:208–215. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Lian J, Guoping C, Shapiro SS, Tran LP., and, Beacham DA. Glycoprotein Ibα can mediate endothelial cell migration on von Willebrand factor-containing substrata. Exp Cell Res. 1999;252:114–122. doi: 10.1006/excr.1999.4612. [DOI] [PubMed] [Google Scholar]
- Taubert A, Krüll M, Zahner H., and, Hermosilla C. Toxoplasma gondii and Neospora caninum infections of bovine endothelial cells induce endothelial adhesion molecule gene transcription and subsequent PMN adhesion. Vet Immunol Immunopathol. 2006;112:272–283. doi: 10.1016/j.vetimm.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Leutenegger CM, Alluwaimi AM, Smith WL, Perani L., and, Cullor JS. Quantitation of bovine cytokine mRNA in milk cells of healthy cattle by real-time TaqMan polymerase chain reaction. Vet Immunol Immunopathol. 2000;77:275–287. doi: 10.1016/s0165-2427(00)00243-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transduction efficiency. EC were seeded at 2 × 105 cells/well in 6-well plates and transduced with either HDAdGFP or HDAdNull and green-fluorescent EC detected by flow cytometry. Transduction efficiency (%) is calculated as the number of green-fluorescent EC divided by the total number of EC analyzed. This result is representative of 3 independent experiments.