Abstract
The potential for increased sensitivity of tumor cells to oncolytic reovirus by altering the normal cell cycle using clinically available pharmacological agents was investigated. B16.F10 mouse melanoma cells were partially synchronized with hydroxyurea, thymidine, or by mitotic shake-off. Cell survival was determined using MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)- 2-(4-sulfophenyl)-2H-tetrazolium)] survival assay and virus yield in tumors by plaque assay. An enhanced sensitivity to reovirus was observed following the removal of either hydroxyurea or thymidine from the culture medium (P < 0.0001). The greatest survival difference compared to normal cycling cells was noted when the majority of cells were in S and G2/M phases, and was associated with increased viral replication. Cells collected by mitotic shake-off were nearly devoid of cells in S phase and were less susceptible to reovirus-induced cell kill than their nonsynchronized counterparts (P < 0.0001). In vivo combination of hydroxyurea followed by intratumoral reovirus resulted in reduced tumor growth and increased survival compared to monotherapy (P = 0.0041) at 15 days. Increased amounts of virus were retrieved from tumors from mice treated with sequential hydroxyurea/reovirus compared to concomitant treatment or reovirus monotherapy. These data justify clinical evaluation of this approach supported by the extensive experience, low cost, simple administration, and availability of hydroxyurea.
Introduction
There has been much interest in the clinical exploitation of viruses with oncolytic activity (reviewed in ref. 1). One such group of viruses are the ubiquitous and nonpathogenic reoviruses (respiratory enteric orphan viruses). Specifically, the type 3 Dearing strain has shown oncolytic activity in a wide range of human and mouse tumor cells2,3,4,5 and has been shown to cause tumor regression after intralesional injections in immunodeficient mice and after systemic administration in immunocompetent mice.6,7,8 The same T3D strain has been evaluated in a number of phase I clinical studies by intratumoral injection and systemic delivery with evidence of antitumor activity.9,10,11 There is a general consensus that the full potential of oncolytic viruses in the clinical arena will only be realized by combination with other treatments. Synergistic cell kill and improved survival, predominantly through enhanced apoptosis, have been demonstrated preclinically by combining reovirus with chemotherapy or radiotherapy, and clinical trials reflecting these approaches are now nearing completion.12,13,14 However, numerous reports in the literature have described an apparently altered susceptibility to viral infection, replication, and cell death at certain points in the cell cycle by a number of viruses and cell types.15,16,17 This is worthy of further evaluation as the implications with regard to enhanced antitumor effects have not been previously considered, i.e. administration of viral therapy at a time when an increased fraction of cells are susceptible to virus infection/replication could greatly increase oncolytic activity.
In this study, we investigated the effect of the cell cycle on susceptibility of normal cells and tumor cells to oncolytic reovirus at different time points following treatments (hydroxyurea and thymidine) that alter the normal cell cycle distribution. Our results indicate that the susceptibility of tumor cells both in culture and in vivo can be manipulated by agents known to affect cell cycling, resulting in enhanced sensitivity to reovirus. The effect appears to be due to increased viral replication within tumor cells. As the agents used for cell synchronization are in clinical practice, this approach may be a simple and cost-effective way of enhancing the effects of systemic reovirus in the clinical oncology arena.
Results
Reolysin does not alter cell cycle distribution of B16.F10 mouse melanoma cells
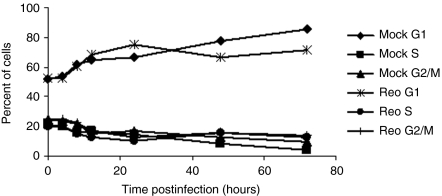
Reovirus-induced cell cycle arrest has been reported at both G1 (ref. 18) and G2/M (refs. 19,20) and shown to be virus strain, infectious dose, and cell line dependent.20 We wished to assess any direct effects of infection with reovirus T3D strain Reolysin on cell cycle distribution of B16.F10 cells at the doses we planned to use. Cells were either mock infected or infected with reovirus at a multiplicity of infection (MOI) of 10 and harvested at various time points postinfection. The samples were analyzed for DNA content using flow cytometry and the percentage of cells in G1, S, or G2/M phase of the cell cycle was determined (Figure 1). Infection of B16.F10 cells with this particular T3D strain at MOI 10 did not affect the cell cycle distribution.
Figure 1.
Reolysin does not alter cell cycle distribution of B16.F10 mouse melanoma cells. B16.F10 cells were seeded overnight and then either mock infected or infected with reovirus T3D strain Reolysin at a multiplicity of infection of 10. Cells were harvested at the time points indicated, stained with propidium iodide, and analyzed for DNA content using flow cytometry. Results are presented as the percentage of cells in G1, S, or G2/M phase of the cell cycle.
Susceptibility to reovirus-induced B16.F10 cell death is influenced by cell cycle
To determine the influence of cell cycle on susceptibility to reovirus-induced cell death, B16.F10 mouse melanoma cells were partially synchronized using hydroxyurea, thymidine, or mitotic shake-off and cell survival was measured following infection with reovirus at various time points after release from synchronization. Additional aliquots of cells were treated with cisplatin at the same time points and assessed for survival. Cisplatin is an alkylating agent, most active in the resting phase of the cell and therefore cell cycle nonspecific. Thus, any survival differences could be attributed to the direct effect of reovirus infection at specific points in the cell cycle and not a general effect of any cellular damage caused by the synchronization procedure.
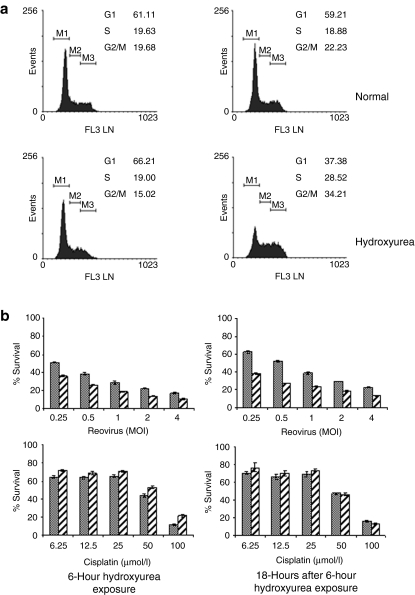
Hydroxyurea inhibits ribonucleotide reductase,21 blocking the de novo synthesis of DNA without interfering with synthesis of RNA or protein, and has been widely used for the in vitro induction of cell cycle arrest. Depending on the concentration of the drug, the duration of exposure, and the cells used, cell cycle arrest occurs at the G1/S boundary or in S phase.22 Cells were treated with 1 mmol/l hydroxyurea for 6 hours, washed in growth medium, and then incubated for a further 18 hours in fresh growth medium. At the end of the hydroxyurea exposure, cells exhibited a normal cell cycle profile; however, 18 hours later there was an increase in both S and G2/M populations and a corresponding decrease in G1 (Figure 2a). Cells at both time points were infected with reovirus or treated with cisplatin and survival as a percentage compared to normal cycling cells treated in an identical manner was determined (Figure 2b). Cells treated with hydroxyurea had consistently poorer survival at all MOI of reovirus at both time points (P < 0.0001). In contrast, hydroxyurea-treated cells subsequently exposed to cisplatin showed a small survival advantage immediately after removal of hydroxyurea (P < 0.0001) and no difference after 18 hours further incubation (P = 0.1786). We confirmed that this selectivity was not cell line–specific by evaluating the effects of reovirus on murine Renca cells using the same approach. Cells treated with hydroxyurea had consistently poorer survival between 0.1 and 1,000 MOI of reovirus at 24-hour time points (P = 0.0077). In contrast, hydroxyurea-treated cells subsequently exposed to cisplatin showed only small differences in survival at both time points (P = 0.82; Supplementary Figure S1).
Figure 2.
Synchronization of B16.F10 cells using hydroxyurea. B16.F10 cells were allowed to adhere overnight and then treated with 1 mmol/l hydroxyurea for 6 hours. At this time, the cells were either harvested for analysis or the medium was replaced with fresh culture medium without hydroxyurea and incubation continued for a further 18 hours. Untreated cells were used as normal cycling control populations. (a) Cells were harvested at the time points indicated, fixed, and stained with propidium iodide before fluorescence-activated cell-sorting analysis to determine DNA content. (b) At the end of the blocking period (6 hours) or 18 hours later, cells were harvested and aliquots of 7.5 × 103 cells were infected with reovirus at the MOI indicated or treated with cisplatin at the concentrations indicated (hatched bars). Cultures of normal cycling cells were prepared in parallel (solid bars). Cell survival after 48 hours incubation was estimated by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)- 2-(4-sulfophenyl)-2H-tetrazolium)] assay. Cells treated with hydroxyurea had consistently poorer survival at all MOI of reovirus at both time points (P < 0.0001). In contrast, hydroxyurea-treated cells subsequently exposed to cisplatin showed a small survival advantage immediately after removal of hydroxyurea (P < 0.0001) and no difference after 18 hours further incubation (P = 0.1786). Data shown are mean survival ± SEM. MOI, multiplicity of infection.
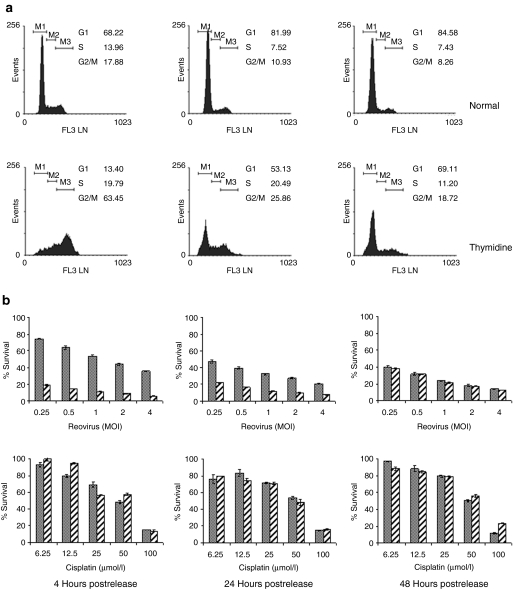
Another method widely used to induce cell cycle arrest and attain partial synchronization is double thymidine block (two periods of exposure to thymidine with a thymidine free interval). Treatment with thymidine blocks DNA replication and cells accumulate in S-phase. Four hours after release from the second thymidine block the majority of cells had progressed to G2/M phase (Figure 3a). Over the next 2 days, the cells progressed in to G1, assuming a nearly normal distribution by 48 hours postrelease. At 4 hours postrelease, the thymidine-synchronized cells proved to be extremely sensitive to reovirus-induced cell death (P < 0.0001). As the cells returned to normal distribution, this enhanced sensitivity diminished until at 48 hours postrelease there was no difference in survival (P = 0.5933). As with hydroxyurea-synchronized cells, there was a small survival advantage in thymidine-synchronized cells exposed to cisplatin 4 hours postrelease (P = 0.0040), but this also diminished over time (P = 0.01638 and P = 0.3563).
Figure 3.
Synchronization of B16.F10 cells by double thymidine block. Following overnight adherence, B16.F10 cells were cultured for 18 hours in medium supplemented with 2 mmol/l thymidine. After a 9-hour release period in nonsupplemented medium, the cells were cultured for a further 17 hours in 2 mmol/l thymidine-supplemented medium. Incubation continued in nonsupplemented medium for the duration indicated, at which point cells were harvested for analysis. Untreated cells were used as normal cycling control populations. (a) Cells were harvested at the time points indicated postrelease, fixed, and stained with propidium iodide before fluorescence-activated cell-sorting analysis to determine DNA content. (b) At the times indicated, aliquots of 7.5 × 103 treated (hatched bars) or normal cycling (solid bars) cells were infected with reovirus at the MOI indicated or cisplatin at the concentrations indicated. After 48 hours incubation, cell survival was estimated by MTS [3-(4,5-dimethylthiazol-2-yl)- 5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)] assay. At 4 hours postrelease, the thymidine-synchronized cells were more sensitive to reovirus-induced cell death than normal cycling cells (P < 0.0001), but as the cells returned to normal distribution, this effect was lost and there was no difference in survival by 48 hours (P = 0.5933). There was a small survival advantage in thymidine-synchronized cells exposed to cisplatin 4 hours postrelease (P = 0.0040), but this also diminished over time (P = 0.01638 and P = 0.3563). Data shown are mean survival ± SEM. MOI, multiplicity of infection.
Mitotic shake-off was used as a third method of producing a cell population with altered cell cycle distribution. Mitotic cells are less adherent and may be dislodged by tapping of the culture vessel and collected. This method requires no chemical intervention and may be considered nongenotoxic. Cells collected via this method were almost completely depleted of S-phase cells and consequently had elevated G1 and G2/M phase populations (Figure 4a). Eighteen hours later, some cells had entered S phase and although there were still fewer cells in S phase than in the normal cycling population, the profile was approaching normal distribution. Unlike the hydroxyurea- and thymidine-treated cells, which were more sensitive to reovirus-induced cell death, cells from mitotic shake-off time point T = 0 were more resistant (Figure 4b; P < 0.0001). Incubation for an additional 18 hours before infection reversed this profile and the mitotic shake-off population became more susceptible to reovirus-induced cell death (P < 0.0001). As before, there was little difference in survival between normal cycling and the mitotic shake-off cells when treated with cisplatin (P = 0.05939). After 18 hours of further incubation, the mitotic shake-off cells were slightly more susceptible to cisplatin treatment (P < 0.0001).
Figure 4.
Synchronization of B16.F10 cells by mitotic shake-off. Following overnight seeding, loosely adherent B16.F10 cells were dislodged from the culture flask by sharp tapping. Dislodged cells were collected and stored on ice until use. Fresh medium was added to the culture vessel and the process repeated very 20 minutes. Untreated cells were used as normal cycling control populations. Collected cells were either used immediately (T = 0) or replated for 18 hours before use. (a) At the time indicated, cells were fixed and stained with propidium iodide before fluorescence-activated cell-sorting analysis to determine DNA content. (b) At the times indicated, aliquots of 7.5 × 103 treated (hatched bars) or normal cycling (solid bars) cells were infected with reovirus at the MOI indicated or cisplatin at the concentrations indicated. After 48 hours incubation, cell survival was estimated by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)] assay. The mitotic shake-off cells were more resistant to reovirus-induced cell death than normal cycling cells (P < 0.0001). This profile reversed following a further 18-hour incubation with cells becoming more susceptible to reovirus-induced cell death (P < 0.0001). There was little difference in survival between normal cycling and the mitotic shake-off cells when treated with cisplatin (P = 0.05939), although after 18 hours further incubation, the mitotic shake-off cells were slightly more susceptible to cisplatin treatment (P < 0.0001). Data shown are mean survival ± SEM. MOI, multiplicity of infection.
Increased in vitro virus recovery from hydroxyurea-treated cells
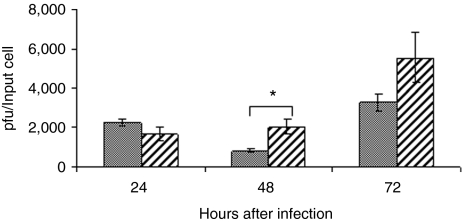
We next looked at virus yield from reovirus-infected hydroxyurea-treated cells compared to normal cycling cells. B16.F10 cells were treated with 1 mmol/l hydroxyurea and then incubated for a further 18 hours in fresh medium before infection with reovirus at an MOI of 10. Virus titer was determined by plaque assay at 24, 48, and 72 hours postinfection and compared to virus titer from normal cycling cells handled in an identical manner. A higher virus titer was recovered from hydroxyurea-treated cells at 48 (P = 0.038) and 72 hours (NS) postinfection (Figure 5). A similar result was observed with double thymidine blocked cells at 48 hours postinfection (data not shown) suggesting that the enhanced cell kill is due, at least in part, to an increase in virus production compared that of normal cycling cells.
Figure 5.
Increased virus recovery from hydroxyurea-treated cells. B16.F10 cells were treated with 1 mmol/l hydroxyurea for 6 hours and following a further 18-hour incubation in hydroxyurea-free medium were infected with reovirus at a multiplicity of infection of 10. Samples were collected at the time points indicated and assayed for virus titer by plaque assay (hatched bars) and compared to titers from cultures of normal cycling cells prepared in parallel (solid bars). Asterisk indicates statistical significance (P = 0.038). pfu, plaque-forming unit.
Sequential hydroxyurea/reovirus combination therapy reduces tumor growth and prolongs survival
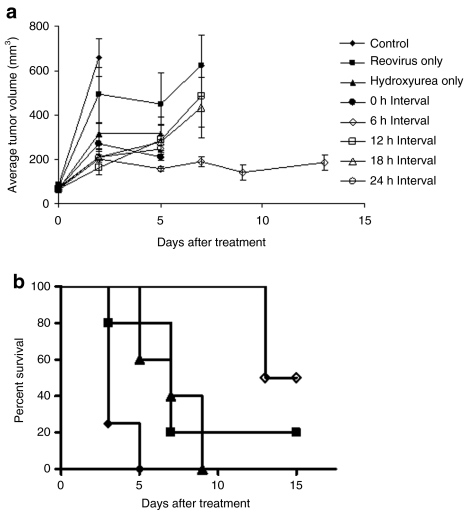
We wished to determine whether the enhanced cell death following reovirus infection we witnessed in vitro could be translated into an in vivo mouse model. C57Bl/6 mice bearing subcutaneous B16.F10 flank tumor were treated with 8 mg hydroxyurea intraperitoneally followed 0, 6, 12, 18, or 24 hours later by a single intratumoral injection of 3 × 108 plaque-forming unit (pfu) reovirus. Tumor volume was estimated and compared to control animals that received only hydroxyurea or reovirus, or Hanks Balanced Salt Solution (HBSS; Figure 6a). Mice treated with reovirus 6 hours after hydroxyurea treatment had significantly smaller tumors than reo-only-treated mice (P = 0.0075, day 7) and smaller tumors than mice in all other treatment groups. Animals were killed when tumors exceeded 15 mm in any one direction and survival was recorded on a Kaplan–Meier plot (Figure 6b). For clarity only data from the 6-hour interval group and the three control groups are shown. Mice treated with hydroxyurea and reovirus 6 hours apart had a significant survival advantage over the control groups (logrank test P = 0.0041).
Figure 6.
Reduced tumor growth and prolonged survival following sequential hydroxyurea/reovirus combination therapy. C57Bl/6 mice bearing subcutaneous B16.F10 tumors were treated on day 0 with 8 mg hydroxyurea intraperitoneally and then 3 × 108 pfu reovirus intratumorally 0, 6, 12, 18, or 24 hours later. Control animals received either reovirus only hydroxyurea only, or an equivalent volume of Hanks Balanced Salt Solution, administered in an identical manner. (a) Tumors were measured on the days indicated and used to calculate tumor volume. Mice treated with hydroxyurea and reovirus 6 hours apart had significantly smaller tumors than reo-only-treated mice (P = 0.0075, day 7) and smaller tumors than mice in all other treatment groups. (b) These mice also exhibited a survival advantage compared to monotherapy and control-treated mice (logrank test P = 0.0041).
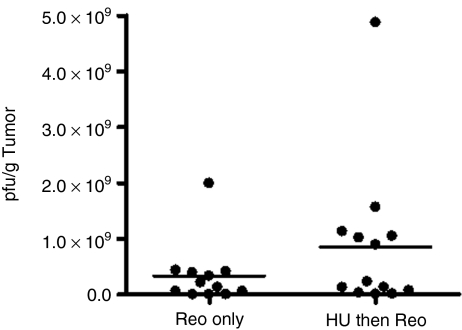
Elevated virus titer in tumors of sequentially treated mice
We next wished to determine whether, as suggested in vitro, an increase in virus production was a contributing factor to the reduced tumor size and increased survival advantage. Tumor material from B16.F10 tumors excised 72 hours after treatment with either reovirus alone, concomitantly or 6 hours after hydroxyurea treatment was assayed for plaque titer and data expressed as pfu/g tumor (Figure 7). Although not statistically significant (one-way analysis of variance; P > 0.05), there was a definite trend toward a higher viral titer in tumors of mice treated with sequential hydroxyurea/reovirus.
Figure 7.
Increased viral recovery from tumors of mice treated with sequential hydroxyurea/reovirus compared to reovirus monotherapy. C57Bl/6 mice bearing subcutaneous B16.F10 tumors were treated with 3 × 108 pfu reovirus intratumorally alone or 6 hours after 8 mg hydroxyurea in sterile Hanks Balanced Salt Solution intraperitoneally. Mice were killed 72 hours later and tumors excised. Plaque assays were performed on tumor material to quantify virus burden and results expressed as pfu/g tumor (unpaired two-tailed t-test; P = 0.2176). pfu, plaque-forming unit.
Effect of hydroxyurea treatment on susceptibility of relatively resistant cell lines to reovirus-induced cell death
Finally, we wished to determine whether pretreatment with hydroxyurea had any effect on the susceptibility of cell lines we previously found to be comparatively resistant to reovirus-induced cell death. The mouse fibroblast line NIH3T3, the human prostate cancer line Du-145, and the B16 derivative B16.ova were treated with hydroxyurea in the same manner as B16.F10 cells and then infected with tenfold dilutions of reovirus starting at MOI 1,000. Survival at 48 hours postinfection was compared to normal cycling cells. There was no difference in survival of any of the cell lines at 48 hours postinfection (Supplementary Figure S2), indicating that resistant cells cannot be made susceptible by accumulation of cells in S phase and G2/M phase using hydroxyurea.
Discussion
As a therapeutic agent, oncolytic reovirus has made the successful transition following early reports of tumor selective cell kill in ras-transformed fibroblasts, through to comprehensive clinical evaluation, as a single agent and in combination with chemotherapy and radiotherapy, across a wide range of common and rare cancers. The aim is to exploit potential synergy (in achieving enhanced cell kill) between reovirus and conventional cancer treatments. The underlying mechanisms of observed synergies in preclinical models are incompletely understood and may differ across cell lines according to the nature of the underlying molecular dysregulation the tumor cells harbor.
A number of oncolytic viruses have been combined with conventional cytotoxic agents in attempts to exploit particular properties of each individual agent, resulting in synergistic cell kill.23 We have determined whether it is possible to increase reovirus replication in tumor cells selectively pharmacologically by synchronizing a fraction of these cells using available drugs following numerous reports in the literature indicating that cell cycle phase may affect the ability of a virus to infect and replicate within a cell.15,16,17 There are few reports in the literature of deliberate cell cycle manipulation for increased viral oncolysis. In a non-small-cell lung cancer model, the combination of paclitaxel and vincristine suppressed adenovirus-induced S-phase arrest and caused G2/M arrest, which was accompanied by an increase in tumor apoptosis.24 Overexpression of survivin, especially within the nucleus, increases control over G(1)–S checkpoint via increased nuclear accumulation of cyclin D and cyclin-dependent kinase 4 and subsequent pRb phosphorylation. This observation was exploited by treating survivin-overexpressing cells with adenovirus dl922-947, which depends critically on an aberrant G(1)–S checkpoint. Nuclear expression of survivin-augmented-virus-induced S-phase induction and increased viral protein expression and overall viral replication, which led to an increase in antitumor activity both in vitro and in vivo.25
Because reovirus-induced cell cycle arrest has been reported at both G1 (ref. 18) and G2/M,19,20 we first investigated the effects of the T3D strain on our tumor model cells. We found no evidence of cell cycle perturbation at a dose of 10 pfu/cell over a 72-hour period. Poggioli et al. showed that G2/M arrest was determined by the reoviral S1 protein and that the ability to induce cell cycle arrest was determined by virus strain. The degree to which test cells were arrested, however, was dependent on both the cell line and the amount of reovirus used. In order to demonstrate appreciable cell cycle arrest in their system, an infectious dose of 100 pfu/cell was required. The systemic doses of reovirus used in current clinical trials would expose cells to much lower amounts of virus so it would therefore seem extremely unlikely that there would be any direct reovirus effect on cell cycle distribution.
In this study, B16.F10 mouse melanoma cells were partially synchronized chemically in vitro using hydroxyurea and thymidine. Cell survival following infection with reovirus at various time points after release from synchronization was measured and compared with survival of normal cycling cells. We observed significant and impressive increases in reovirus susceptibility at various points after release from synchronization pressure compared to nonsynchronized cells in vitro as a result of increased viral replication. Exposure of cells to cisplatin, a pseudo-alkylating agent that causes double-stranded DNA adducts in a non-cell-cycle-specific manner did not result in altered sensitivity to reovirus. Similar differential sensitivity to reoviral oncolysis was also observed in Renca cells in hydroxyurea-synchronized versus nonsynchronized cells.
As it could be argued that the increased susceptibility was a reaction to cellular damage and not because of cell cycle manipulation, we also looked at a physical method of cell synchronization. Mitotic cells are less adherent and may be dislodged by tapping of the culture vessel and collected. This method requires no chemical intervention and may be considered nongenotoxic. At point of collection (T = 0), this loosely adherent population was almost completely depleted of S-phase cells and, in contrast to hydroxyurea- or thymidine-treated cells, was more resistant to reovirus-induced cell death. Like hydroxyurea- and thymidine-treated cells, the mitotic shake-off cells quickly reverted to normal cell distribution.
As hydroxyurea is currently utilized for the treatment of a variety of cancers and other disorders of cellular proliferation, it was logical to proceed with in vivo studies using this agent. We found that even one dose of intratumoral reovirus following hydroxyurea treatment was sufficient to affect tumor growth and overall survival. However, this synergistic effect was observed specifically at one time point where reovirus was administered 6 hours after hydroxyurea treatment, presumably as this provided a sufficient window for the hydroxyurea to have an effect on DNA synthesis and cell cycle. In humans, excretion of hydroxyurea is a nonlinear process occurring through two pathways. Approximately 50% of an administered dose is metabolized by the liver and is subsequently excreted in the urine as urea, while urinary excretion of intact drug in urine accounts for the balance of elimination. In adults with sickle cell anemia treated with hydroxyurea, mean cumulative urinary hydroxyurea excretion was 62% of the administered dose at 8 hours.26 Assuming similar kinetics in mice, maximum uptake and effect on cells would be expected within the first few hours of administration. At later time points the hydroxyurea is likely to have been extensively metabolized and excreted and the cells re-commenced DNA synthesis and progress through the cell cycle.
Mechanistically, in vitro, we found evidence of a higher virus titer in hydroxyurea-treated synchronized cells infected with reovirus compared to identically infected normal cycling cells. Although not statistically significant, there was a definite trend toward a higher viral titer in the tumors of mice treated with reovirus 6 hours after hydroxyurea than in tumors treated with reovirus alone or concomitantly with hydroxyurea.
Junction adhesion molecule-1 and sialic acid have been identified as cell surface reovirus binding sites,27,28 whereas disruption of the double-stranded RNA-activated protein kinase system and activation of the Ras pathway are important for viral replication and cytolysis of the host cell.29 As such, cells lacking these features should remain resistant to reovirus infection regardless of cell cycle. We wished to confirm this, as it has both therapeutic and safety implications. Our results indicate that perturbation of the cell cycle will not make resistant cells (including normal cells) susceptible to reovirus infection and thus the tumor specificity of reoviral therapy is retained. Normal healthy cells will remain unaffected and at no increased risk from the reovirus treatment; however, reovirus-resistant tumor cells will still remain resistant.
The extension of this approach to other oncolytic viruses in the clinic may be considered. In the case of DNA viruses such as herpes simplex virus, not surprisingly, hydroxyurea causes inhibition of herpes simplex virus DNA replication. However, this effect is reversible and upon removal of hydroxyurea there is early and renewed synthesis of virus DNA as determined by increased incorporation of 3H-thymidine into herpes simplex virus DNA.30,31 A formal comparison with our reovirus model is planned.
We have demonstrated in a mouse model that administration of a cell cycle active agent at a specific time before reovirus can greatly enhance the oncolytic effect. This approach is simple and cost-effective using a drug such as hydroxyurea, which is orally administered.
Materials and Methods
Cell lines. The mouse melanoma cell line B16.F10 was cultured in Dulbecco's modified Eagle's medium (Sigma, St Louis, MO) at 37 °C and 10% CO2. The comparatively reovirus-resistant B16 derivative, B16.ova, transduced with complementary DNA encoding the chicken ovalbumin (Ova) gene and expressing very low levels of the JAM-1 receptor was cultured in Dulbecco's modified Eagle's medium supplemented with 400 µg/ml G418 at 37 °C and 5% CO2. The mouse fibroblast lines L929 and NIH3T3 were cultured in Dulbecco's modified Eagle's medium at 37 °C and 5% CO2. The human prostate cancer cell line Du-145 was cultured in RPMI-1640 (Sigma) medium at 37 °C and 5% CO2. All media were supplemented with 2 mmol/l GlutaMAX-1 supplement (Invitrogen, Carlsbad, CA), 100 units/ml penicillin and 100 units/ml streptomycin (Sigma), and either 10% (vol/vol) fetal calf serum (Invitrogen) for routine passage or 2% (vol/vol) fetal calf serum for reovirus infection work. Renca, murine renal cancer, cells were generously provided by Onyvax, St Georges Hospital, London. The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U/ml of penicillin and 100 µg/ml of streptomycin, and incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Reovirus stock and chemotherapeutic agents. Reovirus type 3 Dearing strain (Reolysin) was obtained from Oncolytics Biotech (Calgary, Canada). Virus stock titer and virus stability was measured by standard plaque assay of serially diluted samples on L929 cells. Six-well plates were seeded with 1 × 106 L929 cells per well and infected with dilutions of viral stocks. After 3 hours incubation at 37 °C, the virus inoculum was removed and the wells were overlaid with a 1:1 mixture of 2% SeaPlaque agarose (Cambrex Bio Science Rockland, Rockland, ME) and 2× minimum essential medium (Invitrogen) supplemented to a final concentration of 5% (vol/vol) fetal calf serum, 100 units/ml penicillin/streptomycin, and 2 mmol/l GlutaMAX-1. Wells were stained with 500 µl 0.03% neutral red (Sigma) in phosphate-buffered saline (PBS) 72 hours postinfection and plaques were counted 3–4 hours later.
Cisplatin (cis diamminedichloroplatinum; Mayne Pharma, UK) was obtained from Royal Surrey County Hospital pharmacy.
Propidium iodide staining for FACS quantitation of DNA content to determine cell cycle phase. At various time points post-treatment, the cells were harvested by trypsinization and after washing once in cold PBS, aliquots were resuspended in 300 µl 50% fetal calf serum in PBS. The cells were fixed in 900 µl 70% ethanol in PBS added dropwise while vortexing and incubated for at least 24 hours at 4 °C. The fixed cells were washed once in cold PBS and resuspended in 5 µg/ml propidium iodide and 1 mg/ml Ribonuclease A in a total volume of 200 µl. The samples were incubated for 30 minutes at 37 °C before analysis by flow cytometry using a MACSQuant Analyser (Miltenyi Biotech, Bergisch Gladbach, Germany) and MACSQuantify software (Miltenyi Biotech).
Effect of reovirus infection on B16.F10 cell cycle distribution. B16.F10 cells were seeded overnight in 6-well plates before infection with reovirus at an MOI of 10. At various time points postinfection, the cells were harvested, fixed, and stained with propidium iodide as described above. Normal cycling control samples were collected at the same time from mock-infected cells and handled in the same manner. Cells were assessed using flow cytometry to determine DNA content and cell cycle phase.
Partial cell synchronization by exposure to hydroxyurea. B16.F10, B16.ova, NIH3T3, Renca, and Du-145 cells were seeded and allowed to adhere overnight. The next day the culture medium was replaced with fresh medium supplemented with 1 mmol/l hydroxyurea (Sigma) and incubation continued for 6 hours. The cells were either harvested for use at this point, or the hydroxyurea-containing medium was replaced with fresh growth medium containing no hydroxyurea and incubation continued for a further 18 hours at which point the cells were harvested.
Partial cell synchronization by double thymidine block. Following overnight adherence, the culture medium on B16.F10 cells was replaced with fresh medium supplemented with 2 mmol/l thymidine (Sigma) and incubation continued for 18 hours. The monolayers were then washed with PBS and incubated for 9 hours in fresh medium. At this point, the medium was again replaced with fresh medium containing 2 mmol/l thymidine and incubation continued for a further 17 hours. The cells were washed again with PBS, fresh medium was added, and the cells allowed to remain in culture until harvest at the time points indicated.
Partial cell synchronization by mitotic shake-off. Loosely adherent cells in mitotic phase were harvested from overnight cultures of B16.F10 cells by sharply tapping the flask several times. The cells dislodged into the medium were collected and stored on ice. The medium was replaced and incubation continued for a further 20 minutes. This procedure was repeated until sufficient cells had been collected.
In vitro survival assay. Cells, either synchronized or normal cycling, were seeded in 96-well plates at a density of 7.5 × 103 cells per well and then treated with known dilutions of reovirus or cisplatin. Control wells received an equivalent volume of assay medium. After 48 hours incubation, cell viability was determined using the CellTiter 96 AQueous One Solution Cell Proliferation Assay reagent MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; Promega, Madison, WI] according to manufacturer's instructions. Briefly, 20 µl of MTS reagent was added to each well and following incubation at 37 °C for 1–4 hours, absorbance was measured at 495 nm. Survival was calculated as a percent compared to untreated cells.
In vitro virus recovery. Synchronized B16.F10 cells were infected with reovirus at an MOI of 10. Samples were collected at 24, 48, and 72 hours postinfection and assessed for virus titer by plaque assay on L929 monolayers. Normal cycling B16.F10 cells were handled in an identical manner and used for comparative purposes.
In vivo studies. All procedures were approved by United Kingdom Home Office and institutional boards. C57Bl/6 mice were purchased from B and K Universal (Hull, UK) and all animal experiments were repeated at least three times. Subcutaneous tumors were established on the flank of each mouse by injecting 5 × 105 cells in a volume of 100 µl HBSS (Sigma). Animals were examined thrice weekly for tumor development. Three orthogonal tumor diameters (d1, d2, and d3) were measured using Vernier callipers and tumor volume was calculated from the formula V = π/6·d1·d2·d3. Animals were killed when tumor size exceeded 15 mm in any one dimension.
Once tumors were established (average tumor diameter 4.5–5.5 mm, ~10–12 days), mice were randomly assigned to treatment groups. Mice received 8 mg hydroxyurea in sterile HBSS intraperitoneally followed by 3 × 108 pfu reovirus in 100 µl volume intratumorally 0, 6, 12, 18, or 24 hours later. Control animals received either hydroxyurea or reovirus as monotherapies or an equivalent volume of HBSS alone, administered in an identical manner.
Viral recovery from tumors. Tumors were established in mice as described above. Following random assignment to treatment groups, mice received 3 × 108 pfu reovirus in 100 µl volume intratumorally alone, concomitantly or 6 hours after 8 mg hydroxyurea in sterile HBSS intraperitoneally. Mice were killed 72 hours later and tumors excised. Plaque assays were performed on tumor material to quantify virus burden and results expressed as pfu/g tumor.
Statistical analysis. Statistical analysis was performed using GraphPad Prism 4 (GraphPad Software, La Jolla, CA). Data from survival experiments were analyzed using analysis of variance models with reovirus or cisplatin dose and synchronization method (hydroxyurea, thymidine, mitotic shake-off, or none) as factors. The models were used to test for an overall effect of reovirus or cisplatin dose, synchronization method and the interaction between reovirus or cisplatin dose and synchronization method. Pair-wise comparisons were done using the t-test and in vivo survival data were analyzed using the logrank test.
All animal studies have been approved by the University of Surrey review board.
SUPPLEMENTARY MATERIAL Figure S1. Increased sensitivity of Renca cells to reovirus oncolysis following synchronization with hydroxyurea. Following overnight adherance, Renca cells were treated with 1mM hydroxyurea for 6 hours and then either harvested for analysis or the medium was replaced with fresh culture medium without hydroxyurea and incubation continued for a further 18 hours prior to harvest. At each time point, aliquots of 7.5 x 10E3 cells were infected with reovirus at the MOI indicated or treated with cisplatin at the concentrations indicated (hatched bars). Cultures of untreated cells were prepared in parallel (solid bars) and used as normal cycling control populations. Cell survival after 48 hours incubation was estimated by MTS assay. a. Cells infected with reovirus 24h after hydroxyurea exposure had consistently poorer survival compared to their untreated controls (p < 0.0001 at MOI's of 0.1, 1 and 10, asterisk). In contrast, b., hydroxyurea treated cells subsequently exposed to cisplatin, showed insignificant differences in survival compared to untreated controls at the same time points across a range of cisplatin doses. Representative figure from 3 repeat experiments. Data shown are mean survival + SEM. Figure S2. Hydroxyurea treatment does not affect susceptibility to reovirus induced cell death in relatively resistant cell lines. B16.ova (a), Du-145 (b) and NIH3T3 (c) cells were treated with 1 mM hydroxyurea for 6 hours and then cultured for a further 18h in fresh medium prior to infection with reovirus at the MOI indicated (hatched bars). Untreated cells (solid bars) were used as normal cycling control populations. Cell survival after 48 hours incubation was estimated by MTS assay. Pre-treatment with hydroxyurea did not affect cell survival in any of the cell lines tested (2 way ANOVA P>0.05). Data shown are mean survival ± SEM.1
Acknowledgments
This work was mainly conducted in Guildford, UK.
Supplementary Material
Increased sensitivity of Renca cells to reovirus oncolysis following synchronization with hydroxyurea. Following overnight adherance, Renca cells were treated with 1mM hydroxyurea for 6 hours and then either harvested for analysis or the medium was replaced with fresh culture medium without hydroxyurea and incubation continued for a further 18 hours prior to harvest. At each time point, aliquots of 7.5 x 10E3 cells were infected with reovirus at the MOI indicated or treated with cisplatin at the concentrations indicated (hatched bars). Cultures of untreated cells were prepared in parallel (solid bars) and used as normal cycling control populations. Cell survival after 48 hours incubation was estimated by MTS assay. a. Cells infected with reovirus 24h after hydroxyurea exposure had consistently poorer survival compared to their untreated controls (p<0.0001 at MOI's of 0.1, 1 and 10, asterisk). In contrast, b., hydroxyurea treated cells subsequently exposed to cisplatin, showed insignificant differences in survival compared to untreated controls at the same time points across a range of cisplatin doses. Representative figure from 3 repeat experiments. Data shown are mean survival + SEM.
Hydroxyurea treatment does not affect susceptibility to reovirus induced cell death in relatively resistant cell lines. B16.ova (a), Du-145 (b) and NIH3T3 (c) cells were treated with 1 mM hydroxyurea for 6 hours and then cultured for a further 18h in fresh medium prior to infection with reovirus at the MOI indicated (hatched bars). Untreated cells (solid bars) were used as normal cycling control populations. Cell survival after 48 hours incubation was estimated by MTS assay. Pre-treatment with hydroxyurea did not affect cell survival in any of the cell lines tested (2 way ANOVA P>0.05). Data shown are mean survival ± SEM.1
REFERENCES
- Sinkovics JG., and, Horvath JC. Natural and genetically engineered viral agents for oncolysis and gene therapy of human cancers. Arch Immunol Ther Exp (Warsz) 2008;56 Suppl 1:3s–59s. doi: 10.1007/s00005-008-0047-9. [DOI] [PubMed] [Google Scholar]
- Sei S, Mussio JK, Yang QE, Nagashima K, Parchment RE, Coffey MC, et al. Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells. Mol Cancer. 2009;8:47. doi: 10.1186/1476-4598-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Etoh T, Inomata M, Shiraishi N, Nishizono A., and, Kitano S. Efficacy of oncolytic reovirus against human breast cancer cells. Oncol Rep. 2008;19:1395–1398. [PubMed] [Google Scholar]
- Errington F, White CL, Twigger KR, Rose A, Scott K, Steele L, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15:1257–1270. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HJ, Koh SS, Cho IR, Srisuttee R, Park EH, Jhun BH, et al. Inhibition of GSK-3beta enhances reovirus-induced apoptosis in colon cancer cells. Int J Oncol. 2009;35:617–624. [PubMed] [Google Scholar]
- Coffey MC, Strong JE, Forsyth PA., and, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- Harrington KJ., and, Nutting CM. Interactions between ionizing radiation and drugs in head and neck cancer: how can we maximize the therapeutic index. Curr Opin Investig Drugs. 2002;3:807–811. [PubMed] [Google Scholar]
- Hirasawa K, Nishikawa SG, Norman KL, Coffey MC, Thompson BG, Yoon CS, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- Forsyth P, Roldán G, George D, Wallace C, Palmer CA, Morris D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigger K, Vidal L, White CL, De Bono JS, Bhide S, Coffey M, et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin Cancer Res. 2008;14:912–923. doi: 10.1158/1078-0432.CCR-07-1400. [DOI] [PubMed] [Google Scholar]
- Pandha HS, Heinemann L, Simpson GR, Melcher A, Prestwich R, Errington F, et al. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin Cancer Res. 2009;15:6158–6166. doi: 10.1158/1078-0432.CCR-09-0796. [DOI] [PubMed] [Google Scholar]
- Silver L., and, Anderson CW. Interaction of human adenovirus serotype 2 with human lymphoid cells. Virology. 1988;165:377–387. doi: 10.1016/0042-6822(88)90582-x. [DOI] [PubMed] [Google Scholar]
- Springett GM, Moen RC, Anderson S, Blaese RM., and, Anderson WF. Infection efficiency of T lymphocytes with amphotropic retroviral vectors is cell cycle dependent. J Virol. 1989;63:3865–3869. doi: 10.1128/jvi.63.9.3865-3869.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson B, Koch T., and, Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987;61:972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragovi HU, Rebai N, Di Guglielmo GM, Macleod R, Sheng J, Rubin DH, et al. A G1 cell cycle arrest induced by ligands of the reovirus type 3 receptor is secondary to inactivation of p21ras and mitogen-activated protein kinase. DNA Cell Biol. 1999;18:763–770. doi: 10.1089/104454999314908. [DOI] [PubMed] [Google Scholar]
- Poggioli GJ, Dermody TS., and, Tyler KL. Reovirus-induced sigma1s-dependent G(2)/M phase cell cycle arrest is associated with inhibition of p34(cdc2) J Virol. 2001;75:7429–7434. doi: 10.1128/JVI.75.16.7429-7434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli GJ, Keefer C, Connolly JL, Dermody TS., and, Tyler KL. Reovirus-induced G(2)/M cell cycle arrest requires sigma1s and occurs in the absence of apoptosis. J Virol. 2000;74:9562–9570. doi: 10.1128/jvi.74.20.9562-9570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timson J. Hydroxyurea. Mutat Res. 1975;32:115–132. doi: 10.1016/0165-1110(75)90002-0. [DOI] [PubMed] [Google Scholar]
- Ashihara T., and, Baserga R. Cell synchronization. Meth Enzymol. 1979;58:248–262. doi: 10.1016/s0076-6879(79)58141-5. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gao L, Yeagy B., and, Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10:371–379. [PubMed] [Google Scholar]
- AbouEl Hassan MA, Braam SR., and, Kruyt FA. Paclitaxel and vincristine potentiate adenoviral oncolysis that is associated with cell cycle and apoptosis modulation, whereas they differentially affect the viral life cycle in non-small-cell lung cancer cells. Cancer Gene Ther. 2006;13:1105–1114. doi: 10.1038/sj.cgt.7700984. [DOI] [PubMed] [Google Scholar]
- Connell CM, Wheatley SP., and, McNeish IA. Nuclear survivin abrogates multiple cell cycle checkpoints and enhances viral oncolysis. Cancer Res. 2008;68:7923–7931. doi: 10.1158/0008-5472.CAN-08-0817. [DOI] [PubMed] [Google Scholar]
- Charache S, Dover GJ, Moore RD, Eckert S, Ballas SK, Koshy M, et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79:2555–2565. [PubMed] [Google Scholar]
- Barton ES, Connolly JL, Forrest JC, Chappell JD., and, Dermody TS. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J Biol Chem. 2001;276:2200–2211. doi: 10.1074/jbc.M004680200. [DOI] [PubMed] [Google Scholar]
- Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, et al. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- Strong JE., and, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612–616. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong JE., and, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612–616. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai J., and, Becker Y. Replication of herpes simplex virus DNA after removal of hydroxyurea block from infected cells. J Gen Virol. 1977;37:429–433. doi: 10.1099/0022-1317-37-2-429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Increased sensitivity of Renca cells to reovirus oncolysis following synchronization with hydroxyurea. Following overnight adherance, Renca cells were treated with 1mM hydroxyurea for 6 hours and then either harvested for analysis or the medium was replaced with fresh culture medium without hydroxyurea and incubation continued for a further 18 hours prior to harvest. At each time point, aliquots of 7.5 x 10E3 cells were infected with reovirus at the MOI indicated or treated with cisplatin at the concentrations indicated (hatched bars). Cultures of untreated cells were prepared in parallel (solid bars) and used as normal cycling control populations. Cell survival after 48 hours incubation was estimated by MTS assay. a. Cells infected with reovirus 24h after hydroxyurea exposure had consistently poorer survival compared to their untreated controls (p<0.0001 at MOI's of 0.1, 1 and 10, asterisk). In contrast, b., hydroxyurea treated cells subsequently exposed to cisplatin, showed insignificant differences in survival compared to untreated controls at the same time points across a range of cisplatin doses. Representative figure from 3 repeat experiments. Data shown are mean survival + SEM.
Hydroxyurea treatment does not affect susceptibility to reovirus induced cell death in relatively resistant cell lines. B16.ova (a), Du-145 (b) and NIH3T3 (c) cells were treated with 1 mM hydroxyurea for 6 hours and then cultured for a further 18h in fresh medium prior to infection with reovirus at the MOI indicated (hatched bars). Untreated cells (solid bars) were used as normal cycling control populations. Cell survival after 48 hours incubation was estimated by MTS assay. Pre-treatment with hydroxyurea did not affect cell survival in any of the cell lines tested (2 way ANOVA P>0.05). Data shown are mean survival ± SEM.1