Abstract
In this study we report on direct involvement of fluid shear stresses on the osteoblastic differentiation of marrow stromal cells. Rat bone marrow stromal cells were seeded in 3D porous titanium fiber mesh scaffolds and cultured for 16 days in a flow perfusion bioreactor with perfusing culture media of different viscosities while maintaining the fluid flow rate constant. This methodology allowed exposure of the cultured cells to increasing levels of mechanical stimulation, in the form of fluid shear stress, whereas chemotransport conditions for nutrient delivery and waste removal remained essentially constant. Under similar chemotransport for the cultured cells in the 3D porous scaffolds, increasing fluid shear forces led to increased mineral deposition, suggesting that the mechanical stimulation provided by fluid shear forces in 3D flow perfusion culture can indeed enhance the expression of the osteoblastic phenotype. Increased fluid shear forces also resulted in the generation of a better spatially distributed extracellular matrix inside the porosity of the 3D titanium fiber mesh scaffolds. The combined effect of fluid shear forces on the mineralized extracellular matrix production and distribution emphasizes the importance of mechanosensation on osteoblastic cell function in a 3D environment.
Out of all of the myriad of tissue types present in the body, bone is probably the type most associated with all things mechanical. Although the bones of the skeletal system serve other functions in diverse areas such as calcium metabolism and hematopoiesis, it is their role in skeletal integrity, support, and locomotion that is most prominent. Because of this role, bone, in addition to its structured extracellular matrix of inorganic and organic elements, contains a conglomeration of cell types that continually monitor and modify the bony structure in response to the ever-changing mechanical stressors (1). As would be expected, these same bone cells when cultured in vitro respond to a variety of mechanical signals including fluid flow, hydrostatic pressure, and substrate deformation.
Of these mechanical stressors, fluid flow has emerged as one of the strongest stimuli of bone cell behavior (2–5). The in vitro mechanical stimulation of bone cells by fluid flow has been implicated in the alteration of a variety of biochemical factors in cell behavior. Short-term exposure of osteoblastic cells to fluid shear induces a rapid increase in intracellular calcium (6, 7), a response that resembles the effect of parathyroid hormone on osteoblastic cells. Fluid flow-induced shear stress applied to osteoblastic cells for several hours has been shown to stimulate the release of nitric oxide (5, 8, 9), a short-lived radical and messenger implicated in several cellular functions, and prostaglandin (4, 5, 8, 10, 11) that may have an autocrine effect on osteoblastic cells. Fluid shear has been shown to up-regulate a variety of genes, including those of osteopontin and cyclooxygenase-2, and several other transcription factors and intracellular messenger systems like cAMP, mitogen-activated protein kinase, and G proteins (12–17). In addition, short-term fluid flow causes changes in the cell cytoskeleton by altering structural proteins and gap junctions (18–20). Long-term exposure of osteoblastic cells to moderate shear forces results in elevated alkaline phosphatase (AP) activity (21) (a marker of early osteoblastic differentiation) and increased mineralized matrix deposition (2, 22) (a marker of osteoblastic maturation). The signal transduction pathways of osteoblastic cells exposed to fluid flow for short periods (minutes to hours) or long periods (days to weeks) are still unknown, and they are not necessarily identical. Although the initiation of these biochemical changes by the relatively simple concept of flowing media over a sheet of cultured bone cells may at first seem artificial, the responsiveness of bone cells to the mechanical stimulation provided by fluid flow closely mimics the processes occurring in bone in vivo. It is thought that mechanical loading of the skeleton causes interstitial fluid flow throughout the lacunar and canalicular spaces in bone (23–29). The osteoblasts and osteocytes lining these spaces respond to this mechanostimulation provided by the fluid flow. The mechanotransduction of this response into alterations in biochemical behavior is thought to be the way in which bone is developed and remodeled in response to mechanical stressors (27–30). The regulatory pathways involved in the response of osteoblastic cells to fluid shear are not well understood and may converge on critical downstream regulatory pathways together with regulatory pathways responding to chemical agonists, cell–matrix interactions, and/or membrane ion channel-mediated signals. Understanding these processes better is of vital interest for a broad range of biological areas from basic bone biology to microgravity research, reflecting the concern over the loss of bone mass that occurs in extended space flight, to therapeutic fields like tissue engineering, which seek to develop treatment modalities with de novo tissue generation (31, 32).
Although other investigators have demonstrated the short-term effects (minutes to hours) of fluid flow in a planar environment on osteoblast messenger and chemomediator expression, previous work in our laboratory has shown that long-term exposure (days to weeks) to flow perfusion culture has significant effects on aspects of osteoblast differentiation and phenotypic expression, in particular, mineralized matrix production (2, 21, 22). The production of a mineralized matrix is the natural endpoint of the osteoblast phenotype and can readily be seen only in long-term cell culture (33). In addition, the use of biomaterial scaffolding for the cultured cells in a flow perfusion culture system permits the generation of a 3D, actively modeled, mineralized extracellular matrix and has demonstrated that flow perfusion also affects the architecture and 3D organization of the cellular constructs (2). This previous work showed that fluid flow dramatically increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner.
Different mechanisms can be postulated to explain these effects of flow perfusion culture on osteoblasts, including both fluid shear forces (14) and enhancement of chemotransport (34). The shear forces experienced by the cultured cells is directly proportional to the fluid flow rate of the culture media being perfused through the constructs, and the presumed greater mechanostimulatory effect of these higher shear forces may cause an enhancement of the expression of the osteoblast phenotype by the differentiating cells, resulting in increased mineralized matrix production. Of course, cultured cells depend on an adequate delivery of nutrients and sufficient removal of metabolic waste products for cell growth and function. This is especially important in the denser cell concentrations and structured organization present in 3D cultured cell constructs. The increased production of mineralized matrix seen with higher flow rates may reflect enhanced chemotransport with mitigation of diffusional limitations and increased nutrient delivery and metabolic waste removal occurring with flow perfusion.
Of these, flow-derived shear stress rather than chemotransport has been demonstrated in short-term experiments (up to 15 min) to be the most significant and the primary mediator of fluid flow mechanical stimulation at least as far as its impact on the chemical mediators nitric oxide and prostaglandin E2, long seen as markers of responsiveness of bone cells to mechanical stimulation (8). However, in longer-term experiments, chemotransport issues of nutrient delivery and waste removal may be more important, especially with the additional metabolic burden of osteoblast differentiation and matrix production and organization present. In addition, the multilayered cellular organization combined with the distribution of cells throughout a 3D scaffold can introduce diffusional limitations to chemotransport not present in planar culture. Understanding such aspects of 3D flow perfusion culture though is especially desirable as other research has shown that the organization of bone cells into 3D structures is crucial for ex vivo tissue formation and that the phenotypic behavior of cultured cells can greatly depend on the 3D matrix structure and organization (35, 36).
To elucidate the contributions of these two possible effectors, shear forces and chemotransport, in long-term 3D flow perfusion culture, differentiating marrow stromal osteoblasts were cultured on 3D titanium fiber mesh scaffolds with media of different viscosities and identical fluid flow rates, thereby maintaining similar levels of chemotransport, while experiencing increasingly higher rates of shear stress forces. In doing this, we wanted to disassociate the effects of chemotransport from the impact of increasing mechanical stress and stimulation on actively proliferating bone cells differentiating in a 3D scaffold and producing actively modeled mineralized matrix, to better understand the contribution of fluid shear forces in 3D flow perfusion culture systems. Titanium fiber meshes have been selected for this study because they are not degradable, so they will maintain constant material properties over the time of the experiment.
Materials and Methods
Cell Culture. Rat bone marrow stromal cells were obtained from the marrow of young adult male (6–8 weeks old) Wistar rats (Simonsen Laboratories, Gilroy, CA) using the method described by Maniatopoulos et al. (37) and Aubin (38). Cells were cultured for 6 days in α-MEM (Life Technologies, Grand Island, NY) supplemented with 10% FBS from selected lots, standard cell culture antibiotics, and the osteogenic supplements ascorbic acid (50 μg/ml), β-glycerophosphate (10 mM), and dexamethasone (10–8 M) (all from Sigma). The cells were enzymatically lifted from the T-75 flasks 6 days after harvesting by using 2 ml of trypsin/EDTA [0.25% (wt/vol) trypsin/0.02% EDTA, Sigma]. The cells were concentrated by centrifugation at 400 × g for 5 min, resuspended in a known amount of media, counted with a Coulter counter, and diluted to a concentration of 5 × 105 cells per 300 μl of media. A total of 5 × 105 cells were added to each one of the titanium meshes dropwise. Similar cell numbers have been used (2, 22) to seed marrow stromal osteoblasts on titanium meshes of comparable sizes.
The titanium fiber meshes (Bekaert, Zwevegem, Belgium) used had a volumetric porosity of 86% and a fiber diameter of 40 μm. The average pore size of the meshes was ≈250 μm. The meshes were disk-shaped with a diameter of 10 mm, a thickness of 0.8 mm, and a weight of 40 mg.
The titanium meshes immediately after seeding were placed in 6-well plates, and 2–3 h after attachment 10 ml of complete media was added to each well. The next day, the seeded scaffolds were placed into the flow perfusion bioreactor and cultured for 4, 8, and 16 days. An equal number of scaffolds were placed into 6-well plates and cultured for 4, 8, and 16 days, serving as static controls.
Flow Perfusion Bioreactor. The flow perfusion bioreactor has been described (2, 39). Briefly, the flow perfusion bioreactor consists of six flow chambers. Each flow chamber contains a cassette in which the scaffold is press-fit. Each cassette is then sealed with two O-rings to ensure the flow path is confined to passage through the scaffold. Gas-permeable silicon tubing connects each flow chamber with a peristaltic pump and a medium reservoir. Each chamber is on its own an independent pumping circuit, but all circuits draw media from a common reservoir. The media in the perfusion bioreactor were completely changed every 2–3 days. For these experiments culture media were pumped continuously at a flow rate of 0.3 ml/min through each cell/scaffold construct and recirculated back to the reservoir.
In the flow perfusion bioreactor, the media used were supplemented with 0%, 3%, or 6% dextran (average MW 68,800, lot 90K1342, Sigma). The viscosity of the cell culture media was measured with a rheometer (AR 1000, TA Instruments, New Castle, DE). Complete media containing 3% dextran had a viscosity that was twice the viscosity of complete media without any dextran. Complete media containing 6% dextran had a viscosity that was three times higher the viscosity of complete media without any dextran. The bioreactor during its normal operation was placed in a cell culture incubator at 37°C with 5% CO2.
DNA Analysis. The cellularity of the cell/polymer constructs was determined by using a fluorometric double-stranded DNA quantification kit (PicoGreen, Molecular Probes). Cell/titanium constructs removed from the perfusion bioreactor or from the static culture at days 4, 8, and 16 were lysed by a freeze–thaw method in deionized distilled water. The DNA of the lysates was quantified by using the PicoGreen assay. The results were expressed as cells per scaffold by using a standard DNA curve created with double-stranded DNA standards and DNA extracted from known amounts of marrow stromal osteoblasts.
AP. Titanium meshes removed from the perfusion bioreactor or from the static culture at days 4, 8, and 16 were lysed by a freeze–thaw method in deionized distilled water. AP activity was measured by using a colorimetric endpoint assay that converts para-nitrophenylphosphate to para-nitrophenol (Sigma Assay Kit 104-LL).
Calcium Deposition Measurement. Calcium deposition in the titanium meshes was measured by using the ortho-cresolphtalein complexone method (Sigma Diagnostics, procedure no. 587). Briefly, scaffolds were removed from the perfusion bioreactor and the static culture at days 4, 8, and 16, washed with deionized distilled water, placed on an orbital shaker, and incubated overnight in the presence of 0.5 M acetic acid. A standard curve was generated by using serial dilutions of CaCl2 (0–400 μg/ml), and the calcium deposited in each scaffold was quantified and reported as mg Ca2+ equivalents.
Microscopy Analysis. Scanning electron microscopy (SEM) and light microscopy analysis were performed to evaluate the morphological appearance of the bone marrow cells and deposited matrix. Evaluation was done only for specimens that were cultured for 16 days. Samples for SEM were washed twice with PBS. Fixation was carried out for 30 min in 2% gluteraldehyde. Then substrates were washed twice with 0.1 M sodium-cacodylate buffer (pH 7.4), dehydrated in a graded series of ethanol, and dried by tetramethylsilane. The specimens were sputter-coated with gold, examined, and photographed with a Jeol 6310 SEM at an acceleration voltage of 20 kV.
The light microscopy samples were fixed in 10% formalin solution. After fixation, the samples were dehydrated in a graded series of ethanol and embedded in glycomethylmethacrylate. After polymerization, thin sections (10 μm) were prepared by using a cutting grinding technique (40). The sections were stained with methylene blue and basic fuchsin and examined with a light microscope.
Statistics. Multiple samples were collected in each measurement (n = 4–6 per group), and the results were expressed as means ± SD. Multiple pairwise comparisons have been performed by using the Tukey–Kramer procedure (41) at a significance level of 95%.
Results
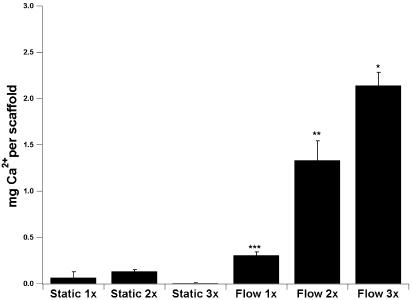
Mineralized Matrix Deposition. To determine the mineralized matrix deposition in the titanium meshes, which is an indication of the full maturation of the cultured osteoblastic cells, a calcium dissolution assay has been used. With all flow perfusion culture conditions the mineralized matrix deposition on the scaffolds cultured for 16 days was higher than statically cultured scaffolds (Fig. 1). Increasing cell culture media viscosity resulted in a progressive increase in mineralized matrix deposition (Fig. 1). By maintaining the same flow rate in the perfusion bioreactor and doubling the media viscosity the calcium deposition was increased 4-fold. Tripling the media viscosity in the perfusion bioreactor resulted in a 7-fold increase of the mineralized matrix deposition. Interestingly, the mineral deposition in titanium fiber meshes seeded and cultured in a perfusion bioreactor under identical conditions but with media lacking dextran (2) and a flow-rate of 1 ml/min was not statistically different with the mineral deposition observed in this study at a flow rate of 0.3 ml/min but with a viscosity that was three times higher (2.14 ± 0.14 with three times the normal viscosity at 0.3 ml/min vs. 1.965 ± 0.28 with normal viscosity at 1.0 ml/min). To confirm that calcium measurements represent active mineralization by the cultured cells, titanium scaffolds without cells were “cultured” under identical flow perfusion and static culture conditions for 16 days, and no detectable calcium deposition occurred.
Fig. 1.
Calcium deposition in titanium fiber meshes during the 16-day culture period represented as equivalents of Ca2+ based on CaCl2 standards used for the construction of the dose–response curve. Six cell culture conditions are reported: static and flow perfusion culture with dextran-free media (1x), media containing 3% dextran and twice the dextran-free media viscosity (2x), and media containing 6% dextran and three times the dextran-free media viscosity (3x). Pairwise comparisons were performed by using the Tukey–Kramer procedure with a significance level of 95%. *, highest calcium content; **, second highest calcium content; and ***, third highest calcium content.
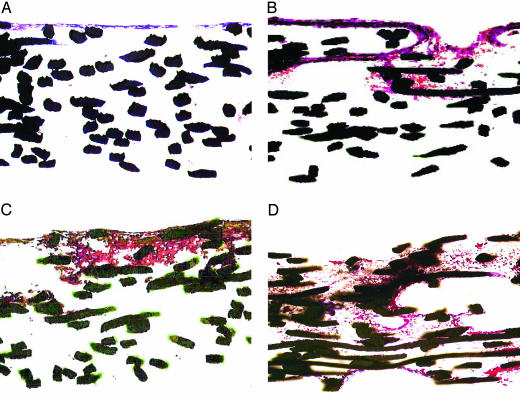
Cell and Extracellular Matrix Distribution. Histological sections of scaffolds cultured for 16 days under flow or static conditions and using cell culture media of varying viscosities demonstrated that flow perfusion had a positive effect in the deposition of extracellular matrix in the void space of the titanium meshes (Fig. 2) in agreement with previous studies that used titanium fiber meshes (2, 22) or collagen sponges (42). Static cultures developed a very thin layer of mineralized extracellular matrix, whereas flow perfusion allowed the generation of a better distributed extracellular matrix (Fig. 2). Increasing the viscosity of the cell culture media resulted in a dramatic improvement of the extracellular matrix distribution (Fig. 2). This trend was in good agreement with the increased calcium deposition observed when media with higher viscosity were used.
Fig. 2.
Representative histological sections of scaffolds cultured for 16 days with static culture and media having 0% dextran (A), flow perfusion and media having 0% dextran (B), flow perfusion and media having 3% dextran (C), and flow perfusion and media having 6% dextran (D). Images are of histological cross sections of the cultured scaffolds. For flow perfusion culture specimens, the flow direction was from the top of the image through the scaffold to the bottom. Sections have been stained with basic fuchsin and methylene blue and viewed at ×10 magnification. The fibers of the titanium meshes appear black.
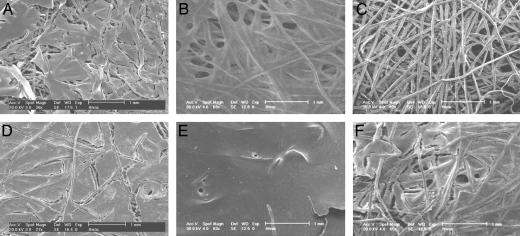
SEM analysis strengthened the observations of the histological analysis. Patches of extremely thin extracellular matrix appeared at the top layer of the disk-shaped titanium meshes after 16 days of culture under static conditions for all three viscosities used (Fig. 3). In contrast, all of the scaffolds cultured under flow perfusion demonstrated a continuous thick layer of extracellular matrix. Several pore structures appeared at the surface of all scaffolds cultured in the flow perfusion bioreactor with media having different viscosities (Fig. 3). Similar pore structures have been observed earlier at the surface of cell/titanium constructs cultured in a flow perfusion bioreactor (2).
Fig. 3.
Representative SEM images of surfaces of scaffolds cultured for 16 days with static culture and 0% dextran (A), static culture and 3% dextran (B), static culture and 6% dextran (C), flow perfusion and 0% dextran (D), flow perfusion and 3% dextran (E), and flow perfusion and 6% dextran (F). Porous structures formed during flow perfusion culture are readily apparent in D–F.
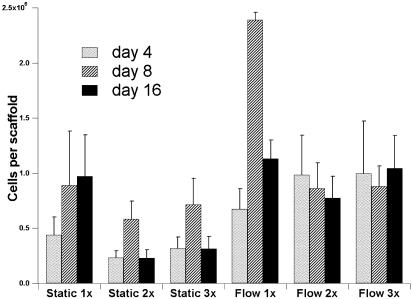
The cellularity of titanium meshes was measured on days 4, 8, and 16 (Fig. 4). Similar proliferation patterns have been observed in scaffolds cultured under flow or static conditions. The cell/titanium constructs reached their peak cellularities between the fourth and eighth day of culture, and after that point they either maintained a constant cellularity or demonstrated a decrease in the number of cells measured in each scaffold.
Fig. 4.
Cellularity of titanium fiber meshes during the 16-day culture period. Cell culture conditions for days 4, 8, and 16 are reported: static and flow perfusion culture with dextran-free media (1x), media containing 3% dextran and twice the dextran-free media viscosity (2x), and media containing 6% dextran and three times the dextran-free media viscosity (3x).
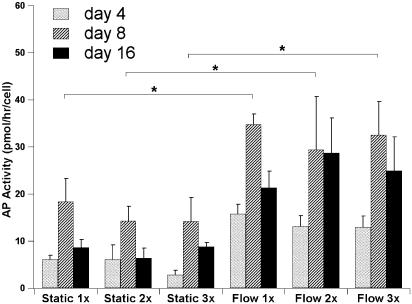
AP Activity. AP activity is a marker of early osteoblastic differentiation and commitment of marrow stromal cells toward the osteoblastic phenotype (43). The AP activity was determined for the fourth, eighth, and 16th day of culture, and it was normalized for the total number of cells per scaffold for each culture time point. For all three viscosities used and for both static and flow perfusion culture conditions a peak AP activity was observed on day 8 (Fig. 5). On day 8, the AP activity of cell/titanium constructs cultured under flow perfusion was higher than the AP activity of constructs cultured in static culture in agreement with previous studies (2, 22, 42). The viscosity of the culture media had no effect on the AP activity on day 8 (Fig. 5).
Fig. 5.
AP activity of titanium fiber meshes during the 16-day culture period. Cell culture conditions for days 4, 8, and 16 are reported: static and flow perfusion culture with dextran free media (1x), media containing 3% dextran and twice the dextran-free media viscosity (2x), and media containing 6% dextran and three times the dextran-free media viscosity (3x). Pairwise comparisons were performed by using the Tukey–Kramer procedure with a significance level of 95%.
Discussion
This study demonstrates that marrow stromal osteoblastic cells seeded on 3D fiber meshes and cultured for extended times respond to fluid flow shear forces. The addition of dextran in the cell culture media at different concentrations increased the media viscosity, and in the presence of continuous flow in a perfusion bioreactor the use of higher media viscosity resulted in increased mineralized matrix deposition. Fluid shear has been implicated in the up-regulation of mineralized matrix deposition in previous studies (2, 22) when titanium fiber meshes were cultured under similar conditions in a perfusion bioreactor with media containing no dextran and an increase of the fluid flow rate resulted in a dose-dependent increase of the deposited mineralized matrix. Similar conclusions have been drawn previously when osteoblastic cells have been cultured for very short times in 2D flow chambers when the exposure of the cells to increased shear forces resulted in increased cellular stimulation expressed by an increased production of cAMP (14) and nitric oxide release (44) but no direct link exists between these effects and the enhanced mineralization observed in this study.
The stimulatory effects of fluid flow on the differentiation of osteoblastic cells seeded in 3D scaffolds can be attributed to the fluid shear forces that the cells are experiencing and the enhanced chemotransport provided by the continuously perfused media. When the fluid flow was maintained constant at 0.3 ml/min and the viscosity of the media was increased 2- and 3-fold, the shear forces that the cells were experiencing also increased without changing significantly the chemotransport characteristics. The shear forces experienced by the cells when dextran-free media was used did not exceed 0.1 dyne/cm2 as calculated by using a cylindrical pore model approximation for the geometry of the scaffold porosity (21). This estimation implies that even when media containing 6% dextran were used in the perfusion bioreactor the shear forces that the cells experienced at the beginning of the culture period had not exceeded 0.3 dyne/cm2. The shear forces presented earlier are only rough calculations because the cylindrical pore model approximation provides only order of magnitude estimates of the shear forces experienced by the cells, whereas in reality there is a wide spatial variability of shear forces inside the 3D fibrous scaffold. The values of the shear forces that have been demonstrated to be stimulatory for osteoblastic cells cultured in 2D flow chambers for short periods were at least 1 order of magnitude higher (2–20 dynes/cm2) (45). It has to be noted that those experiments were conducted in 2D planar environments for very short periods, generally not longer than several hours, whereas in this experiment extended culture periods allowed for the osteoblastic maturation, differentiation, and mineralized matrix deposition in a 3D environment that has been reported to be essential for the ex vivo formation of bone tissue (35). In addition, the generation of extracellular matrix at later times of the culture period resulted in the partial occupation of the void space of the scaffold porosity, resulting in a significant increase of the shear forces that the cells experienced at later culture times.
The lower shear forces that the cells experienced during the first stages of the culture period provide an explanation for the similar AP activity observed on day 8 in flow perfusion cultures using media with different viscosities. At that stage the mineralized extracellular matrix generated is minimal and the shear forces that the cells were experiencing were ranging between 0.1 and 0.3 dynes/cm2 (depending on the viscosity of the media), which may have caused significant differences only between nonsheared (static culture) and sheared (flow perfusion culture) cells. The extended restriction of the fluid flow path by day 16, clearly seen in the histological sections of the scaffolds that have been exposed to fluid flow (Fig. 2), may have resulted in an over an order of magnitude increase of the shear forces at some locations, an increase that can be further enhanced when media with higher viscosities were used. The AP activity decrease observed on day 16 (Fig. 5) is in good agreement with the temporal expression of AP activity of the osteoblastic phenotype that is characterized by a peak in the AP activity during the matrix maturation phase, when osteoblastic cells appear to be committed to differentiate, and a significant decrease in the AP activity at a later stage when osteoblastic cell mature and form mineralized extracellular matrix (33, 43).
Media with increased viscosities not only stimulated the enhancement of the mineralized matrix deposition but also generated mineralized extracellular matrix that was better distributed in the porosity of the 3D scaffolds (Fig. 2). The mechanism that caused the improved distribution of the deposited extracellular matrix is not clear. It is possible that increased shear stresses may have accelerated the downstream migration of the seeded osteoblastic cells. This observation is in good agreement with earlier studies where better extracellular matrix distribution was identified in scaffolds cultured under similar conditions with increased flow perfusion rates and dextran-free media (2). The elucidation of this phenomenon requires detailed studies on the effect of shear forces on the migration of osteoblastic cells in 3D environments at variable differentiation stages.
The cellularity of the titanium meshes did not significantly change (or even dropped) after day 8 (Fig. 4). Osteoblastic cells are not expected to proliferate significantly during the mineralization phase (33, 43). The continuous exposure of the cells to dexamethasone may also promote apoptosis to osteoblastic cells (46).
The appearance of pore-like structures at the surface of scaffolds cultured only under flow perfusion conditions (Fig. 3), which were also observed previously in perfused 3D cultures of osteoblastic cells (2, 22), denote that fluid shear forces may be implicated not only in the stimulation of osteoblastic cells but they also influence the microarchitecture of in vitro-generated extracellular matrices, a characteristic that can be used in tissue regeneration strategies. It also clearly demonstrates the importance of the cell culture environment in the differentiation of osteoblastic cells and the development of 3D tissues.
Conclusions
This study demonstrates not only that marrow stromal osteoblastic cells seeded in 3D in vitro cultures are sensitive to fluid flow shear forces, but also that increased shear forces (without significant changes in the chemotransport characteristics) result in enhanced mineralized extracellular matrix deposition (the hallmark of the complete osteoblastic differentiation) with improved spatial distribution. It can be concluded that fluid flow-induced shear forces are important biological stimuli of osteoblastic cells residing in 3D cellular networks, constituting bone-like tissue at different developmental stages.
Acknowledgments
This work was supported by National Institutes of Health Grant R01-AR42639.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AP, alkaline phosphatase; SEM, scanning electron microscopy.
References
- 1.Buckwalter, J. A., Glimcher, M. J., Cooper, R. R. & Recker, R. (1995) J. Bone Joint Surg. 77, 1256–1289. [Google Scholar]
- 2.Bancroft, G. N., Sikavitsas, V. I., van den Dolder, J., Sheffield, T. L., Ambrose, C. A., Jansen, J. A. & Mikos, A. G. (2002) Proc. Natl. Acad. Sci. USA 99, 12600–12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein-Nulend, J., van der Plas, A., Semeins, C. M., Ajubi, N. E., Frangos, J. A., Nijweide, P. J. & Burger, E. H. (1995) FASEB J. 9, 441–445. [DOI] [PubMed] [Google Scholar]
- 4.Owan, I., Burr, D. B., Turner, C. H., Qiu, J., Tu, Y., Onyia, J. E. & Duncan, R. L. (1997) Am. J. Physiol. 273, C810–C815. [DOI] [PubMed] [Google Scholar]
- 5.Smalt, R., Mitchell, F. T., Howard, R. L. & Chambers, T. J. (1997) Am. J. Physiol. 273, E751–E758. [DOI] [PubMed] [Google Scholar]
- 6.Hung, C. T., Pollack, S. R., Reilly, T. M. & Brighton, C. T. (1995) Clin. Orthop. 313, 256–269. [PubMed] [Google Scholar]
- 7.Donahue, S. W., Donahue, H. J. & Jacobs, C. R. (2003) J. Biomech. 36, 35–43. [DOI] [PubMed] [Google Scholar]
- 8.Bakker, A. D., Soejima, K., Klein-Nulend, J. & Burger, E. H. (2001) J. Biomech. 34, 671–677. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, D. L., McAllister, T. N. & Frangos, J. A. (1996) Am. J. Physiol. 271, E205–E208. [DOI] [PubMed] [Google Scholar]
- 10.Ajubi, N. E., Klein-Nulend, J., Alblas, M. J., Burger, E. H. & Nijweide, P. J. (1999) Am. J. Physiol. 276, E171–E178. [DOI] [PubMed] [Google Scholar]
- 11.Saunders, M. M., You, J., Zhou, Z., Li, Z., Yellowley, C. E., Kunze, E. L., Jacobs, C. R. & Donahue, H. J. (2003) Bone 32, 350–356. [DOI] [PubMed] [Google Scholar]
- 12.You, J., Reilly, G. C., Zhen, X., Yellowley, C. E., Chen, Q., Donahue, H. J. & Jacobs, C. R. (2001) J. Biol. Chem. 276, 13365–13371. [DOI] [PubMed] [Google Scholar]
- 13.You, J., Yellowley, C. E., Donahue, H. J., Zhang, Y., Chen, Q. & Jacobs, C. R. (2000) J. Biomech. Eng. 122, 387–393. [DOI] [PubMed] [Google Scholar]
- 14.Reich, K. M., Gay, C. V. & Frangos, J. A. (1990) J. Cell. Physiol. 143, 100–104. [DOI] [PubMed] [Google Scholar]
- 15.Kurokouchi, K., Jacobs, C. R. & Donahue, H. J. (2001) J. Biol. Chem. 276, 13499–13504. [DOI] [PubMed] [Google Scholar]
- 16.Reich, K. M., McCallister, T. N., Gudi, S. & Frangos, J. A. (1997) Endocrinology 138, 1014–1018. [DOI] [PubMed] [Google Scholar]
- 17.Kapur, S., Baylink, D. J. & Lau, K. H. W. (2003) Bone 32, 241–251. [DOI] [PubMed] [Google Scholar]
- 18.Pavalko, F. M., Chen, N. X., Turner, C. H., Burr, D. B., Atkinson, S., Hsieh, Y. F., Qiu, J. & Duncan, R. L. (1998) Am. J. Physiol. 275, C1591–C1601. [PubMed] [Google Scholar]
- 19.Chen, N. X., Ryder, K. D., Pavalko, F. M., Turner, C. H., Burr, D. B., Qiu, J. & Duncan, R. L. (2000) Am. J. Physiol. 278, C989–C997. [DOI] [PubMed] [Google Scholar]
- 20.Thi, M. M., Kojima, T., Cowin, S. C., Weinbaum, S. & Spray, D. C. (2003) Am. J. Cell Physiol. 284, C389–C403. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein, A. S., Juarez, T. M., Helmke, C. D., Gustin, M. C. & Mikos, A. G. (2001) Biomaterials 11, 1279–1288. [DOI] [PubMed] [Google Scholar]
- 22.Van den Dolder, J., Bancroft, G. N., Sikavitsas, V. I., Spauwen, P. M. H., Jansen, J. A. & Mikos, A. G. (2003) J. Biomed. Mater. Res. 64, 235–241. [DOI] [PubMed] [Google Scholar]
- 23.Piekarski, K. & Munro, M. (1977) Nature 269, 80–82. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery, R. J., Sutker, B. D., Bronk, J. T., Smith, S. R. & Kelly, P. J. (1988) Microvasc. Res. 35, 295–307. [DOI] [PubMed] [Google Scholar]
- 25.Cowin, S. C., Weinbaum, S. & Zeng, Y. (1995) J. Biomech. 28, 1281–1297. [DOI] [PubMed] [Google Scholar]
- 26.Otter, M. W., Bronk, J. T., Wu, D. D., Bieber, W. A., Kelly, P. J. & Cochran, G. V. B. (1996) Clin. Orthop. 324, 283–291. [DOI] [PubMed] [Google Scholar]
- 27.Cowin, S. C. & Weinbaum, S. (1998) Am. J. Med. Sci. 316, 184–188. [DOI] [PubMed] [Google Scholar]
- 28.Knothe-Tate, M. L., Knothe, U. & Niederer, P. (1998) Am. J. Med. Sci. 316, 189–195. [DOI] [PubMed] [Google Scholar]
- 29.Burger, E. H. & Klein-Nulend, J. (1999) FASEB J. 13, S101–S112. [PubMed] [Google Scholar]
- 30.Turner, C. H., Forwood, M. R. & Otter, M. W. (1994) FASEB J. 8, 875–878. [DOI] [PubMed] [Google Scholar]
- 31.Bancroft, G. N., Mikos, A. G. (2001) in Tissue Engineering for Therapeutic Use 5, eds. Ikada, Y. & Ohshima, N. (Elsevier, Amsterdam) pp. 151–163.
- 32.Sikavitsas, V. I., Temenoff, J. S. & Mikos, A. G. (2001) Biomaterials 22, 2581–2593. [DOI] [PubMed] [Google Scholar]
- 33.Lian, J. B. & Stein, G. S. (1992) Crit. Rev. Oral Biol. Med. 3, 269–305. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs, C. R., Yellowley, C. E., Davis, B. R., Zhou, Z., Cimbala, J. M. & Donahue, H. J. (1998) J. Biomech. 31, 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kale, S., Biermann, S., Edwards, C., Tarnowski, C., Morris, M. & Long, M. W. (2000) Nat. Biotechnol. 18, 954–958. [DOI] [PubMed] [Google Scholar]
- 36.Cukierman, E., Pankov, R., Stevens, D. R. & Yamada, K. M. (2001) Science 294, 1708–1712. [DOI] [PubMed] [Google Scholar]
- 37.Maniatopoulos, C., Sodek, J. & Melcher, A. H. (1988) Cell Tissue Res. 254, 317–330. [DOI] [PubMed] [Google Scholar]
- 38.Aubin, J. E. (1999) J. Cell Biochem. 72, 396–410. [PubMed] [Google Scholar]
- 39.Bancroft, G. N., Sikavitsas, V. I. & Mikos, A. G. (2003) Tissue Eng. 9, 549–554. [DOI] [PubMed] [Google Scholar]
- 40.Donath, K. (1988) Preparation of Histologic Sections by the Cutting-Grinding Technique for Hard Tissue and Other Material Not Suitable to Be Sectioned by Routine Methods (EXACT-Kulzer, Norderstedt, Germany).
- 41.Wadsworth, H. M. (1998) Handbook of Statistical Methods for Engineers and Scientists (McGraw–Hill, New York), 2nd Ed.
- 42.Glowacki, J., Mizuno, S. & Greenberger, J. S. (1998) Cell Transplant. 7, 319–326. [DOI] [PubMed] [Google Scholar]
- 43.Lian, J. B. & Stein, G. S. (1993) J. Oral Implant. 96, 95–105. [PubMed] [Google Scholar]
- 44.Klein-Nulend, J., Helfrich, M. H., Sterck, J. G. H., MacPherson, H., Joldersma, M., Ralston, S. H., Semeins, C. M. & Burger, E. H. (1998) Biochem. Biophys. Res. Commun. 250, 108–114. [DOI] [PubMed] [Google Scholar]
- 45.Hillsley, M. V. & Frangos, J. A. (1994) Biotechnol. Bioeng. 43, 573–581. [DOI] [PubMed] [Google Scholar]
- 46.Weinstein, R. S., Jilka, R. L., Parfitt, A. M. & Manolagas, S. C. (1998) J. Clin. Invest. 102, 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]