Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by motor neuron cell death in the cortex, brainstem, and spinal cord. Extensive efforts have been made to develop trophic factor-based therapies to enhance motor neuron survival; however, achievement of adequate therapeutic delivery to all regions of the corticospinal tract has remained a significant challenge. Here, we show that adeno-associated virus serotype 4 (AAV4)-mediated expression of insulin-like growth factor-1 (IGF-1) or vascular endothelial growth factor (VEGF)-165 in the cellular components of the ventricular system including the ependymal cell layer, choroid plexus [the primary cerebrospinal fluid (CSF)-producing cells of the central nervous system (CNS)] and spinal cord central canal leads to trophic factor delivery throughout the CNS, delayed motor decline and a significant extension of survival in SOD1G93A transgenic mice. Interestingly, when IGF-1- and VEGF-165-expressing AAV4 vectors were given in combination, no additional benefit in efficacy was observed suggesting that these trophic factors are acting on similar signaling pathways to modestly slow disease progression. Consistent with these findings, experiments conducted in a recently described in vitro cell culture model of ALS led to a similar result, with both IGF-1 and VEGF-165 providing significant motor neuron protection but in a nonadditive fashion. These findings support the continued investigation of trophic factor-based therapies that target the CNS as a potential treatment of ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder characterized by a selective loss of motor neurons in the motor cortex, brainstem, and spinal cord. Approximately 10% of cases of ALS are inherited with the most frequent cause of familial ALS being attributed to a mutation in the gene encoding cytosolic copper–zinc superoxide dismutase (SOD1).1 Similar to ALS patients, transgenic mice that express the mutant human SOD1 protein display progressive motor neuron degeneration, muscular atrophy, and a shortened lifespan.2 Although a number of pathological events including excitotoxicity, oxidative stress, mitochondrial dysfunction, aberrant axonal transport, cerebrovascular dysfunction, and inflammation have been implicated in ALS, the exact mechanisms responsible for initiation and progression of motor neuron degeneration remain unknown.3,4
A considerable effort has been vested in examining trophic factor-based therapies for the treatment of ALS due to their reported potent effects on motor neuron survival.5 However, ALS patients' response to recombinant trophic factor-based therapies has been minimally beneficial in most instances,6,7,8,9 suggesting that achieving adequate trophic factor delivery to the central nervous system (CNS) may be a critical prerequisite for clinical success. Recently, intramuscular,10,11,12 intracerebral,13,14 and intraspinal15 expression have all been used successfully to bypass the blood–brain barrier to deliver tropic factors to the CNS to slow disease progression in ALS mice. Although effective, each injection approach has limitations (e.g., achieving adequate distribution to all diseased regions, surgical implementation) that may make its translational benefit from ALS mice to patients challenging. Interestingly, recent work in a neonatal mouse model of spinal muscular atrophy has demonstrated the potential for adeno-associated virus (AAV) serotype 9 vectors to circumvent the blood–brain barrier to provide vast gene transduction to the spinal cord following intravascular injection.16,17 Whether or not AAV9 can be used to effectively deliver therapeutic genes in a motor neuron disease with adult onset such as ALS remains to be determined. Nevertheless, clearly there is a need to develop alternative delivery strategies that target all diseased regions and in a manner that is translatable from ALS mice to ALS patients.
AAV serotype 4 (AAV4) has recently been reported to have an affinity for cellular components of the ventricular system including the choroid plexus,18,19 which is responsible for producing a major proportion of cerebrospinal fluid (CSF) within the CNS. Increasing trophic factor concentration within CSF through AAV4-mediated gene transfer to the ventricular system may promote trophic factor delivery to multiple regions undergoing neurodegeneration in ALS. Two trophic factors that have shown the most promise thus far for ALS therapy are insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF); both slow disease progression in ALS animal models when administered using various delivery approaches.11,13,15,20,21,22,23 In the studies reported here, we monitored disease progression in symptomatic SOD1G93A mice following intracerebroventricular (ICV) injection of AAV4 vectors encoding either IGF-1 or VEGF. Moreover, because it is unknown if IGF-1 and VEGF are acting on similar or divergent pathways, we also examined the efficacy of these trophic factors in combination in ALS mice and in a recently developed in vitro cell culture model of ALS.13,24,25
Our findings demonstrate that ICV injection of IGF-1- or VEGF-expressing AAV4 vectors is a moderately effective approach at modifying disease progression in a mouse model of ALS. Interestingly, our in vitro and in vivo results showed that IGF-1 and VEGF are equally effective and when given in combination, no additional benefit was observed, suggesting that IGF-1 and VEGF likely activate a common signaling pathway that is therapeutically beneficial.
Results
IGF-1 and VEGF-165 are neuroprotective of motor neurons in an in vitro model of ALS
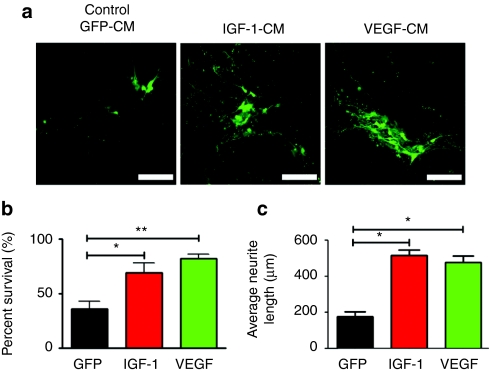
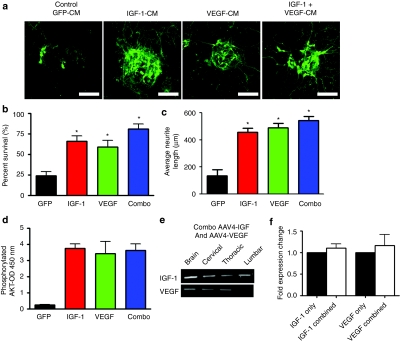
An in vitro-based model of familial ALS has been developed where mutant SOD1-expressing astrocytes confer toxicity toward cocultured motor neurons. We previously showed that supplementation of these cocultures with IGF-1 could protect motor neurons from aberrant G93A SOD1 astrocyte-mediated toxicity.13 Given that both IGF-1 and VEGF-165 have been shown to be neuroprotective in a number of animal studies, we directly compared IGF-1 versus VEGF-165 for motor neuron protection in this in vitro-based model. For these studies, mouse embryonic stem cell-derived motor neurons expressing green fluorescent protein (GFP) under the control of the motor neuron-specific Hb9 promoter were used. These motor neurons were cocultured with SOD1G93A mutant astrocytes in the presence of GFP, IGF-1, or VEGF-165 conditioned media. Both IGF-1- and VEGF-165-containing conditioned media were diluted to a final concentration of 1 µg/ml of recombinant protein as assessed by enzyme-linked immunosorbent assay of the conditioned medium. Control GFP-conditioned media did not appear to have secreted GFP as assessed by fluorescent microscopy and was diluted similarly as the IGF-1 and VEGF-165-containing conditioned media. Within 4 days of establishing the coculture, ~70% of motor neurons had perished in those cultures supplemented with the control GFP-conditioned media. In contrast, significant motor neuron protection was observed in those cultured in the presence of either IGF-1 and VEGF-165-containing conditioned media (Figure 1a,b). The VEGF-165 conditioned media-treated group showed a slight increase in percentage of surviving motor neurons when compared to that treated with IGF-1 [82 ± 2 (SEM) versus 69 ± 9 (SEM)] although this difference was not statistically different (P > 0.05) (Figure 1b). Both IGF-1- and VEGF-165-treated motor neurons displayed extensive neuritic extensions throughout the course of the experiment [516 ± 30 µm (SEM) and 479 ± 35 µm (SEM), respectively], compared to motor neurons treated with GFP-conditioned media where there was a significant loss of extensions (178 ± 28 µm (SEM) P < 0.05) (Figure 1a,c). In sum, these in vitro-based experiments demonstrated that both IGF-1 and VEGF-165 protein could protect motor neurons against mutant SOD1 astrocyte-mediated toxicity. Encouraged by these observations, we proceeded to evaluate the relative merits of these two trophic factors at prolonging survival in the high-expressing SOD1G93A mouse model of ALS.
Figure 1.
IGF-1 and VEGF-165 increases motor neuron survival in an in vitro coculture model of amyotrophic lateral sclerosis (ALS). (a) Mouse embryonic stem cell-derived Hb9:eGFP+ motor neurons cocultured with SOD1G93A astrocytes in the presence of IGF-1-CM or VEGF-CM were protective to motor neurons with increased neuritic extensions compared to control GFP-conditioned medium (GFP-CM). Original magnification ×20. Bar = 50 µm. (b) Quantification of Hb9:eGFP+ motor neuron survival after 4 days of coculture showed IGF-1-CM and VEGF-CM were neuroprotective (*P < 0.05). (c) Quantification of neurite lengths from Hb9-GFP+ motor neurons in the coculture assay exposed to GFP-CM, IGF-1-CM, or VEGF-CM demonstrating that IGF-1 and VEGF preserved neurite length versus control GFP-CM (*P < 0.05). CM, conditioned medium; eGFP, enhanced green fluorescent protein; IGF-1, insulin-like growth factor-1; VEGF, vascular endothelial growth factor.
AAV4 targets ependymal cells efficiently in the brain and spinal cord
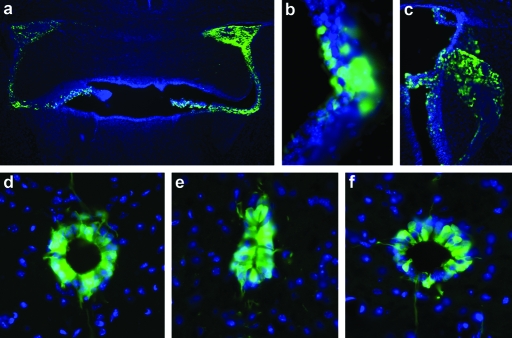
To facilitate broad distribution of IGF-1 and VEGF-165 in the CNS, recombinant AAV serotype 4 vectors that target ependymal cells residing within the CNS were utilized.18,19 This purported property of AAV4 vectors was confirmed by injecting a recombinant AAV4 vector encoding GFP into the lateral and fourth ventricles (2 × 1010 drps/ventricle) of SOD1G93A mice. Injecting into these two sites effectively targeted the ependymal cells lining the lateral (including the choroid plexus), 3rd and 4th ventricles (Figure 2a–c). ICV injection of AAV4-GFP also led to transduction and expression of GFP in the ependymal cells that compose the central canal of the cervical, thoracic, and lumbar regions of the spinal cord (Figure 2d–f). Very few neurons within the brain or spinal cord, were transduced as shown by the GFP fluorescence indicating that AAV4 may not effectively penetrate the ependymal cell layer to target cells widely within the CNS (data not shown). However, assuming the transduced ependymal cells would act like biological pumps to secrete high levels of expressed protein into the CSF, these findings suggest that ICV injection of an AAV4 vector encoding a therapeutic transgene such IGF-1 or VEGF-165 could result in widespread delivery throughout the brain and spinal cord. This gene delivery approach represents a more feasible delivery route than intraparenchymal injections, as it requires far fewer injections to effect broad targeting of the therapeutic to all the regions of the CNS affected in ALS.
Figure 2.
AAV4-GFP efficiently targets ependymal cells in the brain and spinal cord. (a) Microscopic images showing robust GFP expression in the ventricles and choroid plexus following intracerebroventricular delivery of AAV4-GFP. (b,c) Higher magnification of the ependymal cell layer and choroid plexus showing GFP expression. Examination within the cervical spinal cord showed efficient targeting of AAV4-GFP to (d) ependymal cells and to more caudal regions of the spinal cord such as the (e) thoracic and (f) lumbar regions. AAV4, adeno-associated virus serotype 4; GFP, green fluorescent protein.
Delivery of AAV4-IGF-1 or AAV4-VEGF-165 to the ventricles results in broad expression of the genes throughout the brain and spinal cord
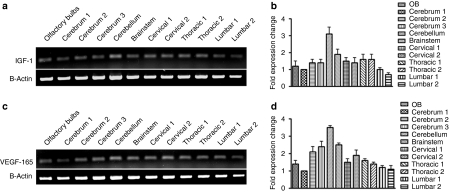
To confirm that the AAV4-IGF-1 and AAV4-VEGF-165 vectors performed similarly as AAV4-GFP vectors, the two trophic factor-expressing constructs were injected separately into the ventricles of ALS mice. Reverse transcriptase-PCR analysis of all sections of the brain and spinal cord showed expression of IGF-1 or VEGF-165, consistent with the results obtained using AAV4-GFP (Figure 3a–d). Quantitative PCR analysis was performed to assess the levels of gene expression in sections of the brain and spinal cord dissections. In the AAV4-IGF-1-injected animals, transcript levels of IGF-1 was highest in the cerebellum, however all regions demonstrated levels of expression comparable to the area of the brain that was injected (cerebrum section #1, Figure 3b). Expression levels of IGF-1 (Figure 3a) and VEGF-165 (Figure 3c) were similar in the brain and spinal cord suggesting that widespread and uniform distribution of the two transgenes were realized. The quantification of VEGF-165 transcript also showed a similar pattern as AAV4-IGF-1-injected animals, with levels highest in the cerebellum (Figure 3d). As expected, no IGF-1 or VEGF-165 transcripts were detected in the AAV4-GFP-treated animals (data not shown).
Figure 3.
Administration of AAV4-IGF-1 or AAV4-VEGF-165 leads to widespread expression of the respective transgenes throughout the brain and spinal cord of SOD1G93A mice. (a) Semiquantitative RT-PCR analysis showing IGF-1 transgene expression within the olfactory bulbs, cerebrum, brainstem, and spinal cord for AAV4-IGF-1-treated mice and (b) quantitative PCR comparing IGF-1 transgene expression throughout the CNS (relative to cerebrum section 1). (c) Semiquantitative PCR for AAV4-VEGF-165-treated mice with subsequent (d) quantitative PCR from each region. AAV4, adeno-associated virus serotype 4; CNS, central nervous system; IGF-1, insulin-like growth factor-1; RT-PCR, reverse transcription-PCR; VEGF, vascular endothelial growth factor.
ICV delivery of AAV4-IGF-1 or AAV4-VEGF-165 prolongs survival and improves motor function of SOD1G93A mice
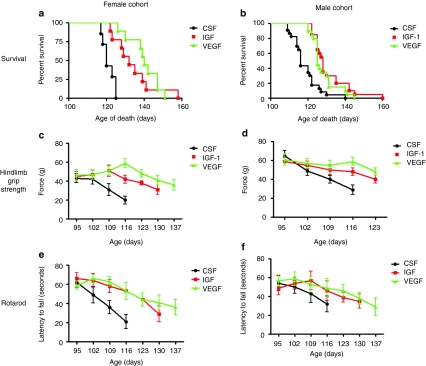
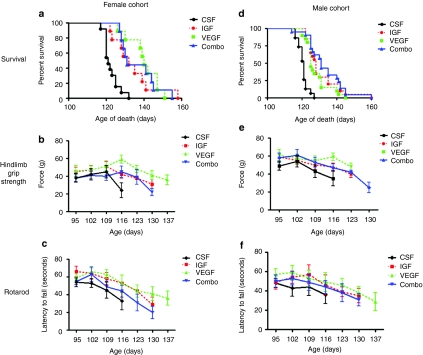
AAV4-IGF-1 or AAV4-VEGF-165 were administered by ICV injections into female and male SOD1G93A mice (n = 30–35 animals/group) at 85 days of age using a dose of 4 × 1010 viral particles/ventricle for a total injection of 8 × 1010 viral particles/mouse. Control mice received either ICV injection of a similar dose of AAV4-GFP or artificial CSF (aCSF). Impact of the different treatments on survival of SOD1G93A mice were analyzed by Kaplan–Meier survival curves and on motor functions using hindlimb grip strength and rotarod measurements. As male and female SOD1G93A mice present with slightly different onset and progression of disease, their motor performance, and survival were measured separately. Female SOD1G93A mice administered AAV4-IGF-1 showed a 12 day increase in median survival whereas those treated with AAV4-VEGF-165 showed a 20 day increase in median survival when compared with control mice (Figure 4a) (χ2 = 30.91, P = 0.0001). Although the median survival for the cohort of mice treated with AAV4-IGF-1 was lower than that with AAV4-VEGF-165, the difference was not significant (132 days versus 140 days, respectively, P > 0.05). However, the median lifespans of both of these treated groups were significantly different from that for the control animals, which exhibited a median lifespan of 120 days. There was no significant difference in longevity between female SOD1G93A mice treated with AAV4-GFP and those administered aCSF (data not shown). Male SOD1G93A mice treated with AAV4-IGF-1 showed a similar 12 day increase in median survival (128 days) whereas those administered AAV4-VEGF-165 exhibited a 9 day increase in median survival (125 days) when compared to control animals, who lived to a median lifespan of 116 days (Figure 4b) (χ2 = 34.33, P = 0.0001). As with the female mice, the difference in longevity observed between the AAV4-IGF-1 and AAV4-VEGF-165-treated animals was not statistically significantly (128 days versus 125 days, respectively, P > 0.05). There was also no statistical significance in longevity of male SOD1G93A animals treated with AAV4-GFP and aCSF (P > 0.05) (data not shown).
Figure 4.
AAV4-IGF-1 and AAV4-VEGF-165 significantly prolonged survival and motor function in sporadic amyotrophic lateral sclerosis mice. (a,b) Kaplan–Meier survival analysis of CSF, AAV4-IGF-1, and AAV4-VEGF-165-treated female and male SOD1G93A cohorts. (c,d) Hindlimb grip strength measurements for both female and male SOD1G93A cohorts. (e,f) Rotarod performance measurements for both female and male SOD1G93A cohorts. CSF-treated mice are indicated in black, IGF-1-treated mice are indicated in red, and VEGF-165-treated mice are indicated in green. AAV4, adeno-associated virus serotype 4; CSF, cerebrospinal fluid; IGF-1, insulin-like growth factor-1; RT-PCR, reverse transcription-PCR; VEGF, vascular endothelial growth factor.
Tests of female SOD1G93A mice treated with AAV4-IGF-1 or AAV4-VEGF-165 showed they exhibited significantly greater hindlimb grip strengths than those administered AAV4-GFP beginning at 109 days (P < 0.05) (Figure 4c). AAV4-IGF-1- or AAV4-VEGF-165-treated male SOD1G93A mice also showed statistically significant greater hindlimb grip strengths beginning at 116 days when compared to mice treated with AAV-GFP (P < 0.05) (Figure 4d). Analysis of treated female SOD1G93A mice on the rotarod showed a similar pattern as the hindlimb grip strength. Those treated with either AAV4-IGF-1 or AAV4-VEGF-165 displayed statistically significant improvements in performance starting at 109 days (P < 0.05) (Figure 4e). However, neither AAV4-IGF-1 nor AAV4-VEGF-165-treated male mice showed significant improvement over controls in this test (P > 0.05) (Figure 4f). In sum, it appeared that treatment with either AAV4-IGF-1 or AAV4-VEGF-165 could delay the precipitous decline in motor function seen in control mice.
Combination therapy with IGF-1 and VEGF-165 does not further improve neuroprotection of ALS neuronal cells in vitro
As statistically significant increases in both motor performance and survival were noted for both IGF-1- and VEGF-165-treated mice, we next asked whether combining these two trophic factors could provide enhanced motor neuron protection. To address this, condition media containing IGF-1, VEGF-165, or a combination of the two trophic factors were initially applied to the in vitro-based coculture model of familial ALS. Similar to previous experiments, GFP-containing conditioned media was used as a control. As noted earlier, both IGF-1 and VEGF-165 conditioned medias provided significant enhancement of motor neuron survival when compared to cocultures treated with the control-conditioned medium (Figure 5a,b). Cocultures treated with a combination of the two trophic factors also showed greater neurite extensions [544 ± 29 µm (SEM)] compared to control GFP-conditioned media [136 ± 45 µm (SEM)], but these improvements were not significantly greater than those treated with the IGF-1 [456 ± 31 µm (SEM) or VEGF-165 (490 ± 33 µm (SEM) individually (Figure 5c)]. Measurement of AKT phosphorylation levels using an enzyme-linked immunosorbent assay also showed those treated with IGF-1 or VEGF-165 were increased compared to GFP-conditioned media, the extent to which were not different from those treated with a combination of the two factors (Figure 5d). These findings suggest that IGF-1 and VEGF-1 might be acting on similar pathways to provide motor neuron protection.
Figure 5.
Combination therapy with both IGF-1 and VEGF-165 does not lead to additive motor neuron protection in vitro. (a) Treatment with IGF-1-conditioned media (CM), VEGF-CM, or IGF-1 and VEGF-CM rescued Hb9:eGFP-GFP+ motor neurons from SOD1G93A astrocyte-mediated toxicity. Original magnification ×20. Bar = 50 µm. (b) Surviving Hb9-GFP+ motor neurons were quantified after 4 days of coculture with SOD1G93A astrocytes. *P < 0.05. (c) Quantification of neurite lengths from Hb9-GFP+ motor neurons in the coculture assay exposed to conditioned medium demonstrating IGF-1 and VEGF and the combination preserved neurite length versus control GFP-CM (*P < 0.05). (d) Levels of phosphorylated Akt were measured in astrocytes by ELISA after 15 minutes of treatment with aCSF, IGF-1, VEGF, or a combination of IGF-1 and VEGF. (e) RT-PCR analysis for viral transgene expression in various regions of the brain and spinal cord after combined delivery of AAV4-IGF-1 and AAV4-VEGF-165. (f) Quantitative-RT-PCR for expression of IGF-1 or VEGF-165 following coadministration versus IGF-1 or VEGF-165 only. AAV4, adeno-associated virus serotype 4; CM, conditioned medium; CSF, cerebrospinal fluid; eGFP, enhanced green fluorescent protein; IGF-1, insulin-like growth factor-1; RT-PCR, reverse transcription-PCR; VEGF, vascular endothelial growth factor.
Combination of AAV4-IGF-1 and AAV4-VEGF-165 does not provide additional improvements in motor function or survival benefit to ALS mice
To confirm the findings from the in vitro studies, AAV4-IGF-1 and AAV4-VEGF-165 were administered into the ventricles of SOD1G93A mice. In these studies, 2 × 1010 drp of each viral vector was administered per ventricle for a total dose of 8 × 1010 drp/mouse (n = 25 animals/group). We first analyzed whether injecting two viruses in combination in vivo would result in transgene expression from both vectors. We found that both IGF-1 and VEGF-165 was expressed in the brain, along with the cervical, thoracic, and lumbar region of the spinal cord following coinjection (Figure 5e). We next tested whether the expression level of either of the transgenes was decreased compared to either factor injected individually. We found that the coinjection of the two viruses did not decrease transgene expression for IGF-1 or VEGF-165 compared to each factor delivered individually (Figure 5f). The effect of injecting the viral vectors on survival and motor function was compared with those administered aCSF. Dual injection of AAV4-IGF-1 and AAV4-VEGF-165 into female SOD1G93A resulted in a 10 day increase in median survival when compared to CSF-treated controls (131 versus 121 days) (χ2 = 29.98, P = 0.0001) (Figure 6a). Results of mice that had been treated individually with AAV4-IGF-1 or AAV4-VEGF-165 (from the first study) have been superimposed on the graph for comparative purposes. Measurements of hindlimb grip strength and performance on the rotarod demonstrated that the female SOD1G93A mice treated with the combination of trophic factors were significantly improved over the control mice. However, these improvements were not greater than noted with mice treated with the trophic factors individually (Figure 6b,c). Male SOD1G93A mice treated with the combination of AAV4-IGF-1 and AAV4-VEGF-165 vectors also showed a 10 day increase in median survival with animals living to a median of 131 days as opposed to 121 days for control, aCSF-treated mice (χ2 = 45.31, P = 0.0001) (Figure 6d). There were mild, but not statistically significant improvements in motor function in the treated male SOD1G93A mice as assessed using the hindlimb grip strength and rotarod tests at all of the time points analyzed (P > 0.05) (Figure 6e,f). These data indicated that both IGF-1 and VEGF-165 could confer a modest beneficial effect at delaying disease progression; however, the combination of the two did not yield additive or synergistic benefits.
Figure 6.
Combined delivery of AAV-IGF-1 and AAV-VEGF does not lead to additive survival or functional benefits compared to treatment with either vector alone. (a,b) Kaplan–Meier survival analysis of (a) female and (b) male SOD1G93A mice treated with a combination of AAV4-IGF-1 and AAV4-VEGF-165 compared to aCSF-treated controls. (χ2 = 29.98, P = 0.0001 between combination treated and CSF groups). (c,d) Hindlimb grip strength was measured over time in (c) female and (d) male SOD1G93A mice treated with a combination of AAV4-IGF-1 and AAV4-VEGF-165 compared to aCSF-treated controls. No differences were found between those treated with a combination of AAV4-IGF-1 and AAV-VEGF-165 and those treated with either vector individually, P > 0.05. (e,f) Rotorod performance was measured over time in (e) female and (f) male SOD1G93A mice treated with a combination of AAV4-IGF-1 and AAV4-VEGF-165 and compared to aCSF-treated controls. No differences were found between those treated with a combination of AAV4-IGF-1 and AAV-VEGF-165 and those treated with either vector individually P > 0.05. AAV4, adeno-associated virus serotype 4; CM, conditioned medium; CSF, cerebrospinal fluid; eGFP, enhanced green fluorescent protein; IGF-1, insulin-like growth factor-1; RT-PCR, reverse transcription-PCR; VEGF, vascular endothelial growth factor.
Discussion
These experimental findings are the first to demonstrate that delivery of AAV4 vectors encoding IGF-1 and VEGF-165 into the ventricles with resultant transduction of the ependymal cells is an effective strategy for widespread delivery of the trophic factors within the CNS of ALS mice. Importantly, this strategy translated to a modest slowing in disease progression as measured by delayed decline in motor function and improved survival outcome. Moreover, evidence from both the in vitro and in vivo analysis suggests IGF-1 and VEGF-165 provides comparable efficacy and are likely acting on similar signaling pathways (i.e., AKT activation) to alleviate disease progression in ALS. These findings confirm the notion that targeting the CNS is important to the development of trophic factor-based therapies for the treatment of ALS.
Trophic factors have been widely reported to have potent effects on motor neuron survival;5 however, in the majority of instances trophic factor-based therapies have failed to provide meaningful benefit when administered either systemically or intrathecally into ALS patients.6,7,8,9,26,27 The poor response observed in ALS patients to trophic factor-based therapies may be due in part to inadequate trophic factor delivery to diseased regions of the CNS. In agreement with this supposition, a number of different delivery strategies that result in trophic factor delivery to the CNS have been shown to significantly slow disease progression in ALS mice.10,11,13,15 Although these approaches are effective in targeting most segments of the spinal cord, each delivery strategy has limited or no ability to deliver trophic factors to upper motor neurons within the brainstem and motor cortex. Recently, AAV serotype 4 (AAV4) has reported to have an affinity for cellular components of the ventricular system including the ependymal cell layer and the choroid plexus (i.e., the major CSF-producing cells of the CNS).18,19 Interestingly, AAV4-mediated expression of the lysosomal enzyme β-glucuronidase within the ventricular system of MPS VII mice (which have no endogenous β-glucuronidase activity) resulted in enzymatic activity throughout the brain, a reduction in neuropathology and improved functional outcome.19 In our current experiments, therefore, we wished to determine whether trophic factor gene transfer to the ventricular system could be exploited in a similar fashion to deliver IGF-1 and VEGF via the CSF throughout the CNS to effectively slow disease progression in ALS mice.
As reported previously, transgene expression both within the ependymal cell layer and choroid plexus was observed following ICV injection of a recombinat AAV4 vector encoding a reporter gene. Interestingly, we report here for the first time that ICV injection of an AAV4-serotype vector also led to transduction and transgene expression within the spinal cord central canal. Concomitant with AAV4-mediated expression of IGF-1 and VEGF-165 within the CNS, a significant delay in motor functional decline and significant extension in survival in SOD1G93A mice was observed. It is unclear whether this observed efficacy with IGF-1- and VEGF-165-expressing AAV4 vectors was dependent upon trophic factor gene transfer to the spinal cord central canal and whether this finding would be translatable to ALS patients. Given that the spinal cord central canal appears to become occluded with advancing age28 and the influence of ALS disease course on spinal cord central canal patency is unknown, the likelihood of making a similar observation in ALS patients seems low, but requires further investigation. Nevertheless, trophic factor delivery via the CSF from the intrathecal space would still likely occur and may be sufficient to achieve adequate trophic factor delivery to the spinal cord. For example, previous studies have shown that intrathecal infusion of IGF-1 slowed disease progression in ALS mice21 and delayed motor function decline in ALS patients as determined using the total Norris and limb Norris scales.29 However, it should be noted that in the same clinical study, intrathecal infusion of IGF-1 failed to alleviate motor decline on the bulbar Norris or vital capacity scales29 suggesting that targeting upper motor neurons may be critical for the development of trophic factor-based therapies that enhance overall patient survival.
Preclinical investigation of IGF-1 in mutant SOD1 rodent models of ALS has yielded promising, but inconsistent results. A number of studies employing a variety of delivery strategies including intramuscular,20 intrathecal,21 and intraparenchymal13,15 have all reported that IGF-1 is effective is extending the lifespan of SOD1G93A mice. However, recent experiments employing similar delivery strategies have failed to confirm the beneficial effects of IGF-1 on SOD1G93A rodent lifespan.30,31,32 Moreover, it was also recently reported that overexpression of a specific isoform of IGF-1 within the CNS of SOD1G93A mice has no effect on disease course, however, this isoform was different from what we previously reported.20,30 Our current findings of delayed motor decline and improved survival following treatment with AAV4-IGF-1 support earlier work showing supplementation with IGF-1 is beneficial; however, it should also be noted that even in our experiments consistency was an issue as not all cohorts of mice responded equally to ICV injection of AAV4-IGF-1. For example, in our initial cohorts (regardless of sex) AAV4-IGF-1 treatment significantly extended survival by 20 days; however, after testing multiple cohorts the collective improvement ended up being 12 days, thus demonstrating the importance of including large enough numbers of animals for meaningful results.33 Inconsistent observations with IGF-1-based therapies may be related to a number of factors including colony genetic drift over time, disease variability that is inherent to the animal model,33 differences in IGF-1 concentration achieved within the CNS for a given delivery strategy, IGF-1 isoform(s) utilized,30 and husbandry (environmental) differences between labs. Thus, interpreting the results of various IGF-1 ALS studies may be difficult, particularly when multiple delivery platforms using various IGF-1 isoforms have been utilized in different laboratory settings. For example, the survival benefit generated in our present study with ICV injection of AAV4-IGF-1 in SOD1G93A mice is smaller (albeit not statistically significantly smaller) than what was previously reported with intramuscular injection of AAV2-IGF-1.20 This comparison, however, should be made with caution given that each delivery approach was not tested simultaneously in the same controlled experiment (i.e., in the same cohorts of mice). Furthermore, survival is an artificial end point with death being recorded when the animal can no longer right itself within 30 seconds. This makes it difficult to compare potential therapies that target muscle and regions of the spinal cord involved in righting versus therapeutic approaches that only target the CNS.
In contrast to IGF-1, preclinical studies examining the role of VEGF in modulating disease progression in ALS mice have yielded more consistent results. For example, deletion of the hypoxia response element in the VEGF promoter (i.e., VEGFδ/δ mice) in mice produced an ALS phenotype34 and SOD1G93A mice showed accelerated disease progression when crossed with VEGFδ/δ mutant mice.35 In addition, overexpression of VEGF delayed neurodegeneration and significantly extended survival in SOD1G93A mice.36 Furthermore, VEGF has been delivered in variety of ways (e.g., intravascular, instramuscular, and ICV) in a therapeutic manner to effectively slow disease progression in ALS rodent models.11,22,23 The results of our current experiments are in agreement with these previous findings, as ICV injection of AAV4-VEGF-165 also led to a significant extension of survival in SOD1G93A mice. However, it should be noted that as with our studies with AAV4-IGF-1, efficacy across different cohorts was also inconsistent following treatment with AAV4-VEGF-165. Initial cohorts of mice (regardless of sex) treated with AAV4-VEGF-165 showed a 20 day improvement in survival; whereas the collective median survival across multiple cohorts remained 20 days for female mice, but dropped to 9 days for male mice, once again indicating the importance of large and multiple cohorts for meaningful survival results in the rodent models of ALS.33
Most relevant to our current work is an experiment which demonstrated improved survival outcome in SOD1G93A rats following continuous ICV infusion of VEGF protein.22 Our findings confirm these initial reports and support the premise of enriching the CSF with VEGF as an approach to treat ALS. It is difficult, at least with the present data, to speculate which approach (protein or gene therapy) would provide the most meaningful clinical benefit in ALS patients. Clearly, if proven safe, continuous delivery of VEGF within the CSF to diseased regions of the CNS following a single injection of AAV4-VEGF would be a significant advantage over periodic infusions of protein via an indwelling catheter.
As both IGF-1 and VEGF have been shown to be effective in slowing disease progression in ALS rodent models, we sought to determine whether administering these trophic factors in combination would lead to additive or synergistic improvements in survival. In addition to testing this hypothesis in ALS mice, we also tested this concept in vitro using our recently developed cell culture model of ALS.13 Surprisingly, when AAV4-IGF-1 and AAV4-VEGF-165 were administered in combination into SOD1G93A mice, the observed efficacy was statistically comparable to what was found following treatment with each factor individually. Interestingly, similar results were found in vitro with each trophic factor providing equivalent motor neuron protection with no additive benefit when given together. Additional in vitro studies showed that IGF-1 and VEGF activated AKT in a similar fashion, and when given in combination, no additive activation was observed. Collectively, our in vivo and in vitro results suggest that IGF-1 and VEGF may be acting on similar signaling pathways to slow the disease course in ALS. The agreement between our in vivo and in vitro findings also supports the notion that in vitro models of ALS are useful for testing novel therapeutic targets for future therapy development.
In summary, we demonstrated that IGF-1 and VEGF gene transfer to cellular components of the ventricular system is an effective strategy for delivering trophic factors to multiple regions displaying neurodegeneration including the cortex, brainstem, and spinal cord to slow disease progression in ALS mice. Furthermore, our findings suggest that IGF-1 and VEGF-165 may be stimulating common signaling pathways to slow ALS disease course and confirm that trophic factor delivery to the CNS may be important for the future development of therapies for the treatment of ALS.
Materials and Methods
In vitro ALS models. Mouse embryonic stem cells that express GFP driven by the Hb9 promoter (HBG3 cells, gift from Tom Jessell, Columbia University, New York, NY) were cultured on primary mouse embryonic fibroblasts (Chemicon International, Temecula, CA) and differentiated into motor neurons as described earlier (Wichterle et al.). After 5 days of differentiation as embryoid bodies, ~50 embryoid bodies were infected with 2 × 109 viral particles of lentivirus-expressing human SOD1G93A or wild-type SOD1. Neural progenitors were harvested from the spinal cords of B6SJLTg (SOD1G93A) mice and wild-type B6SJL mice at 8 weeks of age by the Percoll density gradient centrifugation method as described earlier (Gage et al.). Spinal cord progenitors were cultured on poly-ornithine-/laminin-coated plates in fibroblast growth factor/endothelial growth factor/heparin-containing media as described. To induce astrocytic differentiation, fibroblast growth factor-2 and endothelial growth factor were removed and 10% fetal bovine serum was added to the media for 7 days. Twenty-four hours after infection of the motor neurons with lentivirus, motor neurons were plated on top of the astrocytes.
The coculture of motor neurons and astrocytes was cultured in conditioned media from human embryonic kidney carcinoma 293 cells transfected with a control GFP-expressing plasmid, an IGF-1-expressing plasmid or a VEGF-165-expressing plasmid and conditioned for 24 hours. The coculture media was replaced daily with fresh conditioned media. Cultures were fixed in 4% paraformaldehyde for Hb9-GFP quantification. All images were collected using a laser scanning confocal microscope while maintaining the same exposure time, magnification, and gain.
Animals. Transgenic male and female littermate mice that expressed the mutant SOD1G93A transgene at high-levels were divided equally among groups for these studies. SOD1 gene copy number and SOD1 protein expression were confirmed by PCR and western blot analysis. These mice display disease onset at 90 days and die ~30 days later.2 Animals were housed under light:dark (12:12 h) cycle and provided with food and water ad libitum. All procedures were performed using protocols approved by the Nationwide Children's Research Institutional Animal Care and Use Committee.
Stereotaxic surgery. After being anesthetized with isoflurane, mice (80–90 days of age) were injected into the lateral (i.e., A-P: 0.3 from bregma, M-L: −1.0 from bregma, D-V: −2.0 from dura, incisor bar: 0.0) and 4th ventricles (i.e., A-P: −5.90 from bregma, M-L: 0.0 from bregma, D-V: −2.9 from dura, incisor bar: 0.0) with one of the following AAV vectors: AAV4-GFP, AAV4-GFPl, AAV4-IGF-1, AAV4-VEGF-165, or a cocktail of both AAV4-IGF-1 and AAV4-VEGF-165. Vectors were delivered with a beveled 10 µl Hamilton syringe at a rate of 0.5 µl/min for a total of 2.0 × 1010 drp/injection site in experiment 1 (AAV4-IGF-1 versus AAV4-GFP) and experiment 2 (AAV4-VEGF versus AAV4-bgal). In experiment 3 (AAV4-GFP versus AAV4-IGF-1 versus AAV4-VEGF versus AAV4-IGF-1/AAV4-VEGF) a total of 4.0 × 1010 drps were injected per site. For the cocktail group each injection dose consisted of 2.0 × 1010 drps of each viral prep. The final injection volume for each vector was 10 µl/site as reported.19
Production of recombinant AAV4-IGF-1, AAV4-VEGF, and AAV4-GFP vectors. Recombinant AAV4 vectors were produced by triple transfection using calcium phosphate in human embryonic kidney carcinoma 293 cells as previously described.37 Briefly, a plasmid containing the rep gene from serotype 2 and a capsid gene from serotype 4 along with a helper adenoviral plasmid (Stratagene, Palo Alto, CA) was used. Virus was collected 72 hours post-transfection and processed on cesium chloride gradients as previously described.38 A contract manufacturing company (Virapur LLC, San Diego, CA) was used for some virus preparations. Titers were determined by quantitative PCR to be 3 × 1012 DNase resistant particles/ml. The complementary DNA for the human insulin-like growth factor-1 was generated as previously described. It encodes the class 1 IGF-1Ea with a portion of the 5′ untranslated region of IGF-1.20 The complementary DNA for VEGF-165 was obtained from Origene (Rockville, MD). The AAV-GFP construct was used as previously reported.20
Reverse transcriptase-PCR analysis of IGF-1 and VEGF mRNA. CNS tissue was harvested and dissected into specific regions (i.e., olfactory bulbs, cerebrum, brainstem, cerebellum, and spinal cord) for RNA analysis. The cerebrum was divided into 3 anterior posterior sections with cerebrum 1 being anterior and cerebrum 3 being posterior. Cerebrum section 1 contained the lateral ventricle injection site whereas the cerebellum section contained the 4th ventricle injection site. Each division (cervical, thoracic, and lumbar/sacral) of the spinal cord was also divided into two segments (e.g., cervical 1 and cervical 2). RNA was isolated using Trizol Reagent (Invitrogen, Temecula, CA) and reverse transcription was performed using the Superscript kit (Invitrogen) using oligo dT primers. No reverse transcriptase controls were performed to test for DNA contamination with no detectable products (data not shown). For specific cRNA amplification, primers against the 5′ untranslated region of the rAAV transcript; 5′ GTGGATCCTGAGAACTTCAG 3′ were used along with the 3′ primer; 5′ ATTGGGTTGGAAGACTGCTG 3′ which is homologous to IGF-1. Thirty cycles of PCR were performed, using a 2 minutes denaturation step at 95 °C, followed by cycles of 94 °C for 45 seconds, annealing at 60 °C for 30 seconds, and extension at 72 °C for 1 minute. Product was electrophoresed on 3% agarose gel. The amplified products were confirmed by sequencing to be specific for the human IGF-1 transcript. For quantitative PCR, IGF-1 primers were 5′TCTCTTCTACCTGGCGCTGT3′ and 5′CACGAACTGAAGAGCATCCA3′ and VEGF predeveloped assay (Applied Biosystems, Carlsbad, CA) primers with SYBR Green. GAPDH was used as an internal control in order to normalize expression levels.
Immunofluoresence. Frozen brain and spinal cord sections from 110-day-old mice treated with AAV4-GFP were analyzed for fluorescence using a Nikon Eclipse E800 fluorescent microscope, Melville, NY.
Rotarod, grip strength, and survival analysis. Testing of motor function using a rotarod device (Columbus Instruments, Columbus, OH) began at 60–70 days of age. Each weekly session consisted of three trials on the elevated accelerating rotarod beginning at 5 r.p.m./minute. The time each mouse remained on the rod was registered. Grip strength measurements for forelimb and hindlimb were tested weekly using a grip strength meter (Columbus Instruments). Each weekly session consisted of four tests per animal for each limb. A “death event” was entered when animals could no longer “right” themselves within 30 seconds after the animal was placed on its back or if the animals were found dead. “Death event” classification was performed by two individuals who were blinded to treatment at the time of assessment.
Statistics. Survival analysis was performed by Kaplan−Meier analysis, which generates a χ2 value to test for significance. The Kaplan–Meier test was performed using the log-rank test equivalent to the Mantel–Haenszel test. In addition, two-tailed P values were calculated. When comparing survival curves, median survival times were calculated with a 95% confidence interval. All other statistical tests not involved in survival analysis were performed by multi-way analysis of variance followed by a Bonferroni post hoc analysis of means differences between groups (GraphPad Prism Software, San Diego, CA).
Acknowledgments
This work was funded in part by National Institute of Health Grant Nos. R01NS064492 and RC2 NS069476-01, Project A.L.S., and M.D.A to B.K.K.
REFERENCES
- Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM., and, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Cleveland DW., and, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Seeburger JL., and, Springer JE. Experimental rationale for the therapeutic use of neurotrophins in amyotrophic lateral sclerosis. Exp Neurol. 1993;124:64–72. doi: 10.1006/exnr.1993.1176. [DOI] [PubMed] [Google Scholar]
- Borasio GD, Robberecht W, Leigh PN, Emile J, Guiloff RJ, Jerusalem F, et al. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology. 1998;51:583–586. doi: 10.1212/wnl.51.2.583. [DOI] [PubMed] [Google Scholar]
- Kasarkis E. A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III) Neurology. 1999;52:1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- Miller RG, Petajan JH, Bryan WW, Armon C, Barohn RJ, Goodpasture JC, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann Neurol. 1996;39:256–260. doi: 10.1002/ana.410390215. [DOI] [PubMed] [Google Scholar]
- Lai EC, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, et al. Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group. Neurology. 1997;49:1621–1630. doi: 10.1212/wnl.49.6.1621. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Erickson D, Schaffer D, Hinh L, Gage FH., and, Peterson DA. Targeted retrograde gene delivery for neuronal protection. Mol Ther. 2002;5:50–56. doi: 10.1006/mthe.2001.0520. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, et al. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, Haidet AM, Yang W, Passini MA, Hester M, Clarke J, et al. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. 2008;16:1056–1064. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narai H, Nagano I, Ilieva H, Shiote M, Nagata T, Hayashi T, et al. Prevention of spinal motor neuron death by insulin-like growth factor-1 associating with the signal transduction systems in SODG93A transgenic mice. J Neurosci Res. 2005;82:452–457. doi: 10.1002/jnr.20668. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Haenggeli C, Gasmi M, Bishop KM, Bartus RT, Maragakis NJ, et al. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res. 2007;1185:256–265. doi: 10.1016/j.brainres.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM., and, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J., and, Levivier M. Recombinant AAV-mediated gene delivery to the central nervous system. J Gene Med. 2004;6 Suppl 1:S212–S222. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- Liu G, Martins I, Wemmie JA, Chiorini JA., and, Davidson BL. Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors. J Neurosci. 2005;25:9321–9327. doi: 10.1523/JNEUROSCI.2936-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar BK, Lladó J, Sherkat N, Rothstein JD., and, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Nagano I, Ilieva H, Shiote M, Murakami T, Yokoyama M, Shoji M, et al. Therapeutic benefit of intrathecal injection of insulin-like growth factor-1 in a mouse model of Amyotrophic Lateral Sclerosis. J Neurol Sci. 2005;235:61–68. doi: 10.1016/j.jns.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- Zheng C, Nennesmo I, Fadeel B., and, Henter JI. Vascular endothelial growth factor prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2004;56:564–567. doi: 10.1002/ana.20223. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T., and, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Bergstrom RA., and, Horazdovsky BF. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2009;73:1247; author reply 1247–1247; author reply 1248. doi: 10.1212/WNL.0b013e3181b26ae6. [DOI] [PubMed] [Google Scholar]
- Sorenson EJ, Windbank AJ, Mandrekar JN, Bamlet WR, Appel SH, Armon C, et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008;71:1770–1775. doi: 10.1212/01.wnl.0000335970.78664.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K, Hashizume Y, Yoshida M, Kameyama T., and, Sobue G. Age-related morphologic changes of the central canal of the human spinal cord. Acta Neuropathol. 1999;97:253–259. doi: 10.1007/s004010050982. [DOI] [PubMed] [Google Scholar]
- Nagano I, Shiote M, Murakami T, Kamada H, Hamakawa Y, Matsubara E, et al. Beneficial effects of intrathecal IGF-1 administration in patients with amyotrophic lateral sclerosis. Neurol Res. 2005;27:768–772. doi: 10.1179/016164105X39860. [DOI] [PubMed] [Google Scholar]
- Messi ML, Clark HM, Prevette DM, Oppenheim RW., and, Delbono O. The lack of effect of specific overexpression of IGF-1 in the central nervous system or skeletal muscle on pathophysiology in the G93A SOD-1 mouse model of ALS. Exp Neurol. 2007;207:52–63. doi: 10.1016/j.expneurol.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chian RJ, Li J, Ay I, Celia SA, Kashi BB, Tamrazian E, et al. IGF-1:tetanus toxin fragment C fusion protein improves delivery of IGF-1 to spinal cord but fails to prolong survival of ALS mice. Brain Res. 2009;1287:1–19. doi: 10.1016/j.brainres.2009.06.066. [DOI] [PubMed] [Google Scholar]
- Park S, Kim HT, Yun S, Kim IS, Lee J, Lee IS, et al. Growth factor-expressing human neural progenitor cell grafts protect motor neurons but do not ameliorate motor performance and survival in ALS mice. Exp Mol Med. 2009;41:487–500. doi: 10.3858/emm.2009.41.7.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9:4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, et al. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 2007;27:304–307. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Spratt SK, Lagarde C, Bohl D, Kaspar B, Sloan B, et al. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]