Abstract
In a Children of Twins (COT) design, the environmental and genetic risk of a child is, in part, dependent upon the status of the father and the father’s cotwin. The logic of the COT method breaks down if the zygosity of the twin pair is confounded with the environment provided to the child (a version of the Equal Environment Assumption, EEA). If MZ twin fathers see each other more often than DZ twin fathers, and a child’s uncle is the affected twin in discordant pairs, this could increase the environmental risk of children of MZ over that of DZ discordant twins. The current study was designed to test the EEA in the COT design, specifically in children of alcohol and drug dependent fathers. Results indicated that MZ twins did have more contact than DZ twins. Regression analyses were conducted to predict child externalizing symptom counts from father’s zygosity group status, level of contact with father’s cotwin, and their interaction. Results found no significant interaction between father’s zygosity and the higher level of cotwin contact (seen in MZ twins) in predicting several measures of offspring externalizing risk. The results of this study suggested that the COT design does not confound zygosity with differences in environmental risk exposure, findings that support the validity of the EEA within this research context.
Keywords: Children of twins, Children of alcoholics, Equal environment assumption, Methodology
Introduction
Behavior genetic studies, which examine the influences of genes and environment on behavior, have sometimes been criticized for making assumptions about the environments that different people experience. Most notably, in twin research, the Equal Environments Assumption (EEA) assumes that the differences in the shared environments of fraternal (dizygotic, DZ) twin pairs is the same (in terms of influences relevant to the phenotype under study) as the differences in the environments of identical (monozygotic, MZ) twin pairs. This assumption is of great importance for the logic of the twin method. If MZ twins, who share all of their genes, are more similar to one another than are DZ twins, who share on average half of their (segregating) genes, researchers assume that this is a result of their greater genetic similarity. However, if MZ twins also have more similar environments than do DZ twins, the veracity of this conclusion is called into question and the impact of genetic influences could be overestimated. Previous research on the EEA, all of which has involved classic twin designs, has produced findings that are consistent with this assumption (e.g., Borkenau et al. 2002; Hettema et al. 1995; Lytton 1977; Morris-Yates et al. 1990; Plomin et al. 1976; Xian et al. 2000).
Another method used to examine genetic and environmental influences on behavior is the Children of Twins (COT) design, which can be especially important in evaluating the environmental impact of living with a disturbed parent (see D’Onofrio et al. 2003; Jacob et al. 2001; Silberg and Eaves 2004). Again, in any pair of MZ twins, both twins have the same genes, and thus the same chance of passing these genes onto their children. Given MZ twins who are discordant for a given phenotype, such as alcohol dependence (AD), only one member of the pair (the one with AD) will also contribute to a child’s environmental risk; that is, the children of only one of these twins will grow up in a family with an alcoholic father. In these families, the children who have an alcoholic uncle but an unaffected father have the same genetic predispositions to alcohol abuse as their cousins, but unlike their cousins, they do not experience the environmental impact of growing up with an alcoholic parent. Comparing the children of unaffected discordant twins to the children of affected twins and the children of control twins (where neither twin is affected) can disentangle genetic and environmental influences (controlling for the mother’s influences). Given the increasing use of the COT model, it is important to examine the assumptions that lie behind the comparisons that indicate genetic and environmental effects on behavior. The current study examines a version of the EEA that would affect conclusions made in COT studies.
The extant COT literature (e.g., Duncan et al. 2008; Haber et al. 2005; Jacob et al. 2003) has included examination of four basic risk groups: Group 1, father affected; Group 2, father unaffected but MZ cotwin affected; Group 3, father unaffected but DZ cotwin affected; and Group 4, controls with neither twin affected. Table 1 specifies the genetic and environmental risk levels for the groups in the COT design. Of interest to this examination are groups with low environmental risk (Groups 2, 3, and 4 who have an unaffected father), whose comparisons are informative regarding genetic effects on behavior because the groups only differ in level of genetic risk. Importantly, the comparisons between Groups 2, 3, and 4 assume equality of environments between children in each of these groups. However, past research has shown that MZ twins may have more contact with one another than DZ twins. To the extent that this is true, the children of unaffected MZ twins may see their affected uncle more than the children of unaffected DZ twins (based on the likely assumption that twin contact is associated with child–uncle contact). The former (MZ) group would then have increased genetic and environmental risk compared to the latter (DZ) group, leading to an inflated estimate of genetic effects. If the children of the MZ twins see their affected uncle/aunt more often, the critical question is whether this increased exposure influences the behavior of the child with respect to the outcomes of interest. It is important to examine both aspects of this logic in order to justify the conclusions made with COT designs.
Table 1.
Children of twins design
| Group | Description | Genetic risk to child | Environmental risk to child |
|---|---|---|---|
| 1 | Affected twins | High | High |
| 2 | Non-affected MZ twins, with affected co-twin | High | Low |
| 3 | Non-affected DZ twins, with affected co-twin | Moderate | Low |
| 4 | Control twins | Low | Low |
The current study examined the assumptions of the COT design using twin contact as a proxy for the frequency/amount of contact between a child and his/her MZ or DZ uncle. The fathers’ group status was determined by examining lifetime alcohol dependence (AD) and drug dependence (DD) diagnoses of the twins. Several key questions were asked: (1) Do MZ twins have more contact than DZ twins? and (2) Do twins in Groups 2, 3, and 4 have different amounts of contact? (e.g., Do MZ twins with an AD/DD co-twin have more contact than DZ twins with an AD/DD co-twin, and does the amount of contact differ for these discordant pairs compared to the control twins?). If these two questions were answered in the affirmative, a third question of interest would be addressed: (3) Do these group differences in father/uncle contact affect children’s outcomes? If more frequent twin contact is associated with more problems in the children, and this contact differs by zygosity, the EEA would be violated and the conclusions of COT studies must be called into question. We hypothesized that neither the main effect of contact nor the interaction between twin contact and group status would have an effect on children’s outcomes, thereby supporting the validity of the COT design.
Methods
Participants and procedures
The participants in the study were middle-aged male twin veterans, their children, and the mothers of these children who were part of the Family Twin Studies (FTS) project that included the Children of Alcoholics study (COA; Jacob et al. 2003) and the Twins as Parents study (TAP, a children of drug dependent fathers study; Duncan et al. 2008; Scherrer et al. 2008). In total, the intake assessments for the two studies consisted of 1,295 COA twins and 725 TAP twins (1,774 twins total, since 246 families were in both studies, including 953 MZ twins and 821 DZ twins); 1,329 COA offspring and 839 TAP offspring (1,919 children total, with an average age of 21.4); and 904 COA mothers and 427 TAP mothers (1,202 mothers total).
All FTS twin participants were drawn from the Vietnam Era Twin Registry (VETR) (Eisen et al. 1987; Henderson et al. 1990), a registry where membership is not conditioned on having any medical or psychiatric disorder or any treatment seeking behavior, and which has been shown to be unbiased with regard to the demographic characteristics of the US population (Goldberg et al. 1987). All registry members were born between 1939 and 1957, and served in the US military between 1965 and 1975. Pairs for the FTS were selected according to a predetermined high-risk sampling plan, such that AD and DD fathers (and their co-twins) were oversampled. Further, selection was based on the following criteria: (1) both twins completed the 1987 VETR enrollment survey and the 1992 Harvard Drug Study (HDS; Tsuang et al. 1996) survey; (2) at least one twin reported that he had children born between 1974 and 1988; and (3) at least one twin met criteria for a lifetime diagnoses of DSM-III-R alcohol dependence (for COA) or drug dependence (for TAP) (as determined by the HDS; Tsuang et al. 1996), or both twins were part of a random sample of non-substance dependent control pairs. Response rates at data collection were quite similar across these two studies (Scherrer et al. 2004; Duncan et al. 2008). With the exception of the AD or DD selection criteria, both studies used equivalent selection procedures and assessment methods and batteries, thus supporting the planned merging of these data. The initial data collections were conducted in 2000–2001 for the COA study and in 2002–2004 for the TAP study. Data collection was independent of the investigators for both studies (as required by VETR policy) and once human subjects approvals and proper consents were obtained, fathers, mothers and offspring were interviewed by telephone using an automated Computer Assisted Telephone Interview (CATI) system which provided standardization of all interview questions and probes. More information about the samples and recruitment can be found in Jacob et al. (2003) and Duncan et al. (2008).
Comparison between high risk and normal control group means in the original HDS data and in the COA and TAP subsamples on selected sociodemographic variables were consistent and verified representative sampling. Additionally, sample attrition studies were conducted for the separate studies, and though some differences in non-participation rates were found, attrition was not associated with sample selection criteria (i.e., father’s affected status; see Scherrer et al. 2004; Duncan et al. 2008, for further information).
Of particular interest were differences observed in the families of fathers who met criteria for AD compared to DD. In Haber et al.’s (in press) report using this same sample, 70% of twin fathers meeting criteria for DD also met criteria for AD, thus demonstrating the high comorbidity of these disorders. As others have found (e.g., Dick et al. 2007), in our sample comorbid AD and DD diagnoses was related to issues of severity. In comparing sociodemographic characteristics, the families of DD fathers had significantly lower paternal full-time employment, lower household income, and higher rates of divorce compared to normal controls, findings not observed in families with paternal AD alone. Concerning psychopathology, both DD and AD fathers exhibited higher internalizing and externalizing disorders compared to normal controls, and DD father’s rates were consistently higher than AD father’s rates. Mother’s patterns were similar in these families but less severe. Thus, in this combined sample, a wide range of alcoholism severity was represented from uncomplicated AD to highly comorbid AD-DD. In the current study, this wider range of severity tended toward maximizing the impact of offspring contact with affected uncles as has been reported with MZ versus DZ twins, and therefore provided a more rigorous test of the EEA assumption.
Measures
Twin contact, as a proxy for child–uncle contact, was assessed by telephone interview of the twins at the time of the COT studies, as was based on two items: “How often do you and your twin see each other?” and “How often do you and your twin contact each other by letter, email, fax, or phone?”. Answers were given on a 7-point scale: 7 = live together, 6 = almost every day, 5 = at least once a week, 4 = once or twice a month, 3 = a few times a year, 2 = less often, or 1 = not at all. Individuals who said they lived with their twin were not asked the ‘contact’ question, and were automatically given a score of 7. Seven individuals did not answer the contact questions. In the entire sample, scores from the two questions correlated .65 (N = 1,765), and an average of the two items was used in current analyses. If the twin and his cotwin were both in the data, they should, by definition, have reported the same score for the contact questions. Of the 739 full twin pairs, only 41 (5.5%) differed by more than one point on their average rating of see/contact (no difference: 44.2%; one point difference: 50.2%). MZ and DZ twins were about equally represented in the discrepant pairs (6.4 and 4.5%, respectively).
Father’s AD and DD status was determined by criteria from the Diagnostic and Statistical Manual for Mental Disorders IIIR (DSM-III-R; American Psychiatric Association 1987), as assessed by the 1992 Harvard Drug Study telephone interview (Tsuang et al. 1996). Drug dependence status was determined by collapsing diagnoses across 10 classes of illicit drugs.
For offspring outcomes, three major domains of externalizing behavior were selected for analyses: Alcohol Dependence (AD), Conduct Disorder (CD), and Nicotine Dependence (ND). Symptom counts of these variables were established via DSM-IV criteria (American Psychiatric Association 1994). Additionally, a total symptom count variable (AD + CD + ND) was calculated to index total externalizing problem behavior of the children.
The control variables of child gender, child age, father age, father’s years of education, mother’s AD symptoms, and a variable representing missing mother data were also included in the regression analyses. Mother information was missing for 90 of the children used in the study (that is, children in Groups 2, 3, and 4, see below). Rather than discarding the children with missing mother data from the analyses, the missing mother AD symptom data was scored as a zero, and an additional binary covariate identifying missing mother information (versus having mother information) was added to account for missingness. In this way, all data could be used but missing data patterns were also controlled.
Classification
The families were sorted into groups based on the AD and DD status of the father and his cotwin (from the Harvard Drug Study) in order to represent different levels of genetic and environmental risk associated with offspring outcomes in COT designs (see Table 1). For the combined dataset, families were classified into seven groups to account for both AD and DD diagnoses. In all instances, a drug dependence diagnosis took precedent over an alcohol dependence diagnosis: that is, men with a DD diagnosis were categorized as DD regardless of their alcohol status, while men categorized as AD did not have drug dependence. Group 1-AD and Group 1-DD consisted of families with a father affected with either AD or DD, respectively, and therefore offspring had high genetic and high environmental risk. Group 2-AD and Group 2-DD consisted of families with an MZ unaffected father but an AD or DD (respectively) affected cotwin uncle. These offspring had high genetic but low environmental risk. Group 3-AD and Group 3-DD consisted of families with a DZ unaffected father but an AD or DD (respectively) affected cotwin uncle. Group 3 children have moderate genetic and low environmental risk. Group 4 families were the control families, where fathers and cotwin uncles had neither an AD nor DD diagnosis and offspring had low genetic and low environmental risk. See Haber et al. (in press) for further description and explanation of this 7-group design. The focus of the current study was on the unaffected fathers who had an affected cotwin and the control fathers (Groups 2-AD, 2-DD, 3-AD, 3-DD, and 4), because these groups shared the same low environmental risk but differed in their level of genetic risk (high, moderate, low). Comparisons between the groups were thus informative about genetic influences. (Families classified into Groups 1-AD and 1-DD, who had high genetic and high environmental risk, were not used in the current report. The comparison of Group 1 to the other groups (especially Group 2) is most informative about environmental effects. Additionally, Group 1 is heterogeneous in that it includes families with cotwin uncles of various AD/DD statuses, and is thus not particularly informative with respect to the EEA.)
Analyses
The AD and DD distinctions in the classification scheme were necessary only in the presence of sample selection bias. The current dataset included two different studies that selected cases on the basis of two different diagnoses (COA selected for AD and TAP selected for DD). Given no selection bias between these AD and DD groups, they could be combined, and Groups 2 and 3 would include cotwins with either an AD or DD diagnosis. Tests for selection bias examined whether children with drug versus alcohol dependent uncles differed in the severity of their problem behavior for the outcomes of interest here. Within Group 2 and within Group 3, multivariate tests (MANOVAs) predicting the four child behavior outcomes (AD, CD, ND, and total problem symptom counts) from the covariates (child gender, child age, father age, father’s years of education, and mother’s AD symptoms) and a dichotomous variable representing the alcohol versus drug status of the cotwin were conducted in SPSS. As well, separate regression analyses examining selection bias for each child variable were conducted in SAS using PROC Mixed (Littell et al. 1996) to account for the correlated nature of the data. Conditional on these results, analyses were planned using combined Groups 2 and 3, as well as Group 4.
To assess the differences in levels of contact between MZ and DZ twins and between groups (Questions 1 and 2), mean differences were tested in SAS, using PROC Mixed (Littell et al. 1996) to account for the correlated nature of the data (twin pairs and multiple children per family).
To investigate the effects of the differences in contact on the children’s outcomes (Question 3), child outcomes were regressed on AD/DD group status (using two categorical variables to represent Groups 2, 3, and 4; with Group 4 as the reference group), twin contact, and the contact by group status interaction: Y = b0 + b1(AD/DDa) + b2(AD/DDb) + b3(Twin Contact) + b4(Contact × AD/DDa) + b5(Contact × AD/DDb). These analyses were conducted via SPSS. To approximate ANOVA results, group membership (AD/DDa and AD/DDb) was coded via weighted effect codes (because of unequal group sizes; West et al. 1996). In unweighted effect codes, the control/base group (in this case, Group 4) would be assigned a −1 for each dummy variable, with one of the other groups given a +1 code and the remaining group given a 0. Weighted effect codes work similarly, except that the values for the base group are weighted to reflect the size of each group [i.e., −n2/n4, or −245/440, was the value given to Group 4 (the base group) when Group 2 is the contrast group coded a 1]. The effect of twin contact was then interpreted as the weighted average of the slopes of the twin contact effect for the three groups. Regression analyses were completed in stages. First, all covariates were entered into the model, then group status, twin contact, and the contact by group status interaction were entered sequentially. For the group status variable and the interaction between twin contact and group status, this involved adding two variables to the regression equation [the two effect code variables that represent the three AD/DD groups for the former effect (regression coefficients b1 and b2) and the two interaction terms for the latter effect (regression coefficients b4 and b5)]. The overall significance of each effect (group status, twin contact, and their interaction), was tested by examining the change in R2. Any significant difference in child outcomes for each of the paired comparisons between the three AD/DD groups (Group 2v3, 2v4, and 3v4) were tested via dummy coding. All independent continuous variables were centered in order to facilitate interpretation of the different effects. Gender and missing mother information were coded via weighted effect codes in order to compute the regression line equations, so that the lines would be averaged across the all participants (coding in this way affected only the constant in the equation, not the coefficients for the various effects). Because SPSS does not account for the correlated nature of the data, additional regression analyses predicting child outcomes from group status, twin contact, and their interaction were completed using only one child per father to determine if multiple children per family influenced the results. Conclusions drawn from analyses with the full sample did not change, though with smaller sample sizes, p values increased.
Results
Classification/selection bias
As described in Analyses, an examination of possible selection bias (i.e., differential child behavior based on cotwin AD or DD status) was necessary to insure that differences in child outcomes were not systematically influenced by AD or DD selection in the study design. In Group 2, 42 (29.5%) families were classified as Group 2-DD and 100 (70.4%) as Group 2-AD. In Group 3, 46 (36.5%) families were classified as Group 3-DD and 80 (63.5%) as Group 3-AD. The MANOVAs predicting the four child behavior variables from the covariates and drug versus alcohol group status were not significant for Group 2 (F(3,230) = 1.3, p > .05) or Group 3 (F(3,204) = 1.7, p > .05). Separate regressions of each child behavior variable on the covariates and the drug/alcohol status variable in SAS also resulted in non-significant coefficients for the status variable in Groups 2 and 3 (p’s > .05). Therefore, the AD and DD groups could be combined in the current study to create AD/DD Group 2 and AD/DD Group 3 without concern for selection bias.
Thus, this discordant twin pair model consisted of pairs where one twin (the uncle) was diagnosed with either AD and/or DD while the other twin (the father) had no AD/DD diagnoses. In Group 2 (father unaffected, MZ cotwin AD/DD), there were 142 fathers and 245 children. In Group 3 (father unaffected, DZ cotwin AD/DD), there were 126 fathers and 229 children. In Group 4 (controls), there were 251 fathers (135 MZ twins and 116 DZ twins) and 440 children. Though not used in the current report, in Group 1 (father has AD and/or DD) there were 587 fathers (318 MZ twins and 269 DZ twins) with 1,003 children.
Sample descriptives and covariate characterization
The average age of the fathers was 51.9 years (SD = 2.9, range = 43–61), with an average years of education of 14.0 (SD = 1.8). Of the 268 discordant twin uncles 67.2% had AD only, 12.7% had DD only, 20.1% had comorbid AD and DD. The average age of the children was 21.4 years (SD = 4.3, range = 12–32), and about half of the children were female (51%). The number of AD symptoms for the mother ranged from 0 to 7, with 10.1% (N = 103) having three or more symptoms, 10.6% (N = 108) with two symptoms, 19.4% (N = 198) with 1 symptom, and 59.6% (N = 609) with no symptoms (with 4 mothers missing AD information).
Do MZs see each other more than DZs?
For the twin fathers (i.e., the twins who had children also enrolled in the study) in Groups 2, 3 and 4; the MZ twins had more contact (M = 4.08, SD = 1.13, N = 276) than the DZ twins (M = 3.55, SD = 1.11, N = 242), t(432) = 18.8, p < .001.1
Do twins in the four groups have different amounts of contact?
In the sample of fathers, the AD/DD group differences in twin contact were also significant, F(2,431) = 3.1, p < .05, with Group 2 (M = 3.9, SD = 1.1, N = 142) and Group 4 (M = 3.9, SD = 1.4, N = 250) having slightly higher levels of contact than Group 3 (M = 3.6, SD = 1.2, N = 126).2
Do these group differences affect offspring outcomes?
Results from the regression analyses are shown in Tables 2 and 3. As can be seen, neither the contact nor contact by group interaction effects were significant in predicting child outcomes. In contrast, group status was a significant predictor of offspring total problem symptoms, AD symptoms, and ND symptoms, with the Group 3 versus 2 difference significant for the former. Though not shown in the table, child age was a significant predictor of all four child outcomes, child gender was a significant predictor of child AD symptoms, CD symptoms, and total problem symptom counts, and mother AD symptoms was a significant predictor of child AD symptoms. That is, older children, males, and children with mothers with more AD symptoms had more behavior problems. Dropping the covariates from the model changed the analytic results only slightly: For all four child outcome variables, group status became more significant, and for the child total problem symptoms and alcohol symptoms, the contact by group interaction effect tended towards significance.
Table 2.
Regression coefficients and significance for alcohol/drug group, twin contact, and group × contact interaction effects on child problem behavior, with regression equation: Y = b0 + b1(AD/DDa) + b2(AD/DDb) + b3(Twin Contact) + b4(Contact × AD/DDa) + b5(Contact × AD/DDb)
| Effect | Child outcome variable |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Child AD symptoms |
Child ND symptoms |
Child CD symptoms |
Problem index |
|||||||||
| b (SE) | Beta | p | b(SE) | Beta | p | b(SE) | Beta | p | b(SE) | Beta | p | |
| MODEL 1 | ||||||||||||
| b1: AD/DD group 3 vs. 2a | −.21 (.14) | −.06 | .15 | −.21 (.15) | −.06 | .16 | −.34 (.14) | −.10 | .01 | −.76 (.33) | −.09 | .02 |
| b2: AD/DD group 4 vs. 2a | −.37 (.12) | −.12 | .002 | −.43 (.13) | −.13 | .001 | −.19 (.12) | −.06 | .11 | −1.0 (.28) | −.14 | < .001 |
| MODEL 2 | ||||||||||||
| b1: AD/DD group 3 vs. 2a | −.22 (.14) | −.06 | .12 | −.23 (.15) | −.06 | .13 | −.35 (.14) | −.10 | .01 | −.81 (.33) | −.09 | .01 |
| b2: AD/DD group 4 vs. 2a | −.37 (.12) | −.12 | .003 | −.43 (.13) | −.13 | .001 | −.18 (.12) | −.06 | .12 | −.99 (.28) | −.13 | < .001 |
| b3: Twin contact | −.05 (.05) | −.04 | .24 | −.07 (.05) | −.05 | .17 | −.06 (.04) | −.04 | .18 | −.18 (.10) | −.06 | .09 |
| MODEL 3 | ||||||||||||
| b1: AD/DD group 3 vs. 2a | −.20 (.14) | −.05 | .17 | −.22 (.15) | −.06 | .15 | −.34 (.14) | −.10 | .01 | −.76 (.33) | −.08 | .02 |
| b2: AD/DD group 4 vs. 2a | −.36 (.12) | −.11 | .004 | −.42 (.13) | −.13 | .001 | −.18 (.12) | −.06 | .14 | −.97 (.28) | −.13 | .001 |
| b3: Twin contactb | −.06 (.05) | −.04 | .21 | −.07 (.05) | −.05 | .16 | −.06 (.04) | −.05 | .17 | −.18 (.10) | −.06 | .08 |
| b4: Interaction 1c | .04 (.12) | .02 | .72 | −.02 (.13) | −.01 | .88 | .02 (.12) | .01 | .85 | .03 (.28) | .01 | .90 |
| b5: Interaction 2c | −.15 (.11) | −.07 | .18 | −.14 (.11) | −.06 | .24 | −.11 (.10) | −.06 | .29 | −.39 (.25) | −.08 | .11 |
AD alcohol dependence, DD drug dependence, ND nicotine dependence, CD conduct disorder. Child age, child gender, father age, father income, father education, mother’s AD symptoms, and missing mother data variables are also in the model
AD/DD group effects reported are for dummy coded variables, instead of weighted effect codes with Group 4 as the reference group, so that the Group 2 versus Group 3 effect size could be shown
The twin contact effect is a test of the weighted average of the contact slopes, from a regression equation with weighted effect codes for the alcohol/drug groups, instead of the dummy coded variables given in the table
Interaction 1 refers to the interaction between AD/DD group 3 versus 2 and Twin Contact, while Interaction 2 refers to the interaction between AD/DD group 4 versus 2 and Twin contact
Table 3.
Effect (change in variance accounted for) of twin contact, alcohol/drug group, and the contact × group interaction on child outcomes
| Effect | Child outcome variable |
|||
|---|---|---|---|---|
| Child AD | Child ND | Child CD | Problem index | |
| Alcohol/drug groupa | ΔR2 = .01, p = .01 | ΔR2 = .01, p < .01 | ΔR2 = .01, p = .05 | ΔR2 = .01, p < .01 |
| Twin contactb | ΔR2 = .00, p = .24 | ΔR2 = .00, p = .17 | ΔR2 = .00, p = .18 | ΔR2 = .00, p = .09 |
| Contact × group interactiona | ΔR2 = .00, p = .16 | ΔR2 = .00, p = .42 | ΔR2 = .00, p = .38 | ΔR2 = .00, p = .14 |
| Total R2 of full model | .12 | .06 | .10 | .13 |
ΔR2 indicates the amount that the variance accounted for increased when the specified effect was added to the model The Alcohol Group effect was added to a base model that included the covariates
Two terms were added to the model for these effects (see equation and explanation in text)
Test of the weighted average of the contact slopes
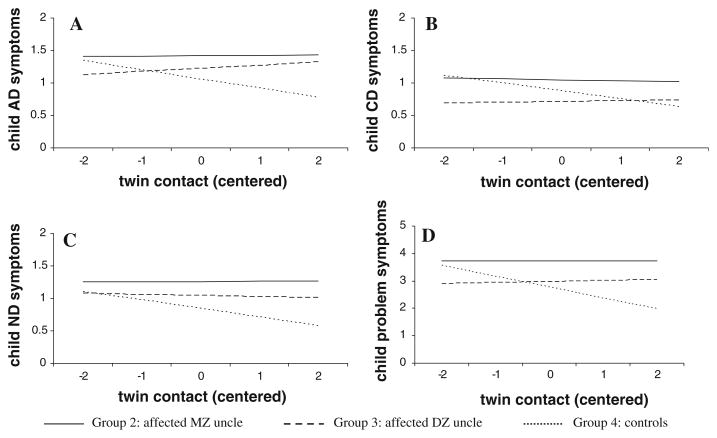
Though the effects of contact and contact’s interaction with group status were not significant, graphs were made of the regression lines for each group in order to visualize these effects. Figure 1 depicts the mean levels of child outcome (AD, ND, CD, and problem symptom counts in the four panels) for Groups 2 (MZ cotwin AD/DD), 3 (DZ cotwin AD/DD), and 4 (controls). Coefficients for the equations include control of the covariates (that is, assuming average child and father age, education, income, and mother symptoms and averaged across gender). Visual inspection of the lines suggested that Groups 2 and 3 are parallel with one another. Although the lines on the graphs were not parallel for Group 4 relative to Groups 2 and 3 (suggesting possible, though non-significant, interactions), there is no main effect of contact shown across the three groups. The level of problem behavior in children with control fathers decreased slightly with contact, while the level of child problem behavior in Groups 2 and 3 seemed to increase slightly with more contact.
Fig. 1.
Regression lines for each child outcome variable: Panel A, child alcohol dependence symptoms; Panel B, child conduct disorder symptoms; Panel C, child nicotine dependence symptoms; and Panel D, child problem symptoms. Lines are based on average values for the covariates. Note the different scale on the y-axis for problem symptoms. The actual range of twin contact was from −2.8 to 3.2
Discussion
The current study found that MZ twins had more contact with one another compared to DZ twins, but that degree of contact did not affect child outcomes in such a way that would invalidate the conclusions drawn from COT studies. One might expect that if a child has more contact with an affected, alcoholic or drug dependent uncle, this risky environment would confer higher risk of behavior problems on that child. This did not seem to be the case, since contact was not a significant predictor of child outcome. As well, the amount of contact between father and uncle did not significantly interact with alcohol/drug diagnosis group status to predict child outcomes. Interestingly, the largest change in child behavior with increasing levels of contact occurred in the control families (as seen in Fig. 1). Here, when fathers and uncles did not have AD/DD, more contact between the twins slightly reduced the amount of child problem behavior. This effect might represent better overall family functioning or family cohesion in non-affected families, as indicated by the increased contact. The typical concern with regard to COT designs, however, is a possible environmental difference between Groups 2 and 3; a difference that was not supported here. Though the current study was exploratory in nature and more needs to be done to thoroughly test the EEA, the results support the conclusion that the Children of Alcohol/Drug dependent Twins research design does not violate the EEA and that genetic effects are not confounded by environmental risk as might be conferred by an AD/DD MZ or DZ cotwin uncle. That is, MZ-DZ differences in the environmental exposure of a child to an AD/DD cotwin uncle are not confounded with genetic effects on the child’s outcomes.
With respect to how these results relate to genetic influence on child behavior, we do find an effect of group status on child behavior (the only significant effect in the regression analyses), as one would expect based on previous COT studies (e.g., Haber et al. 2005; Jacob et al. 2003; Slutske et al. 2008). For example, the parallel lines for Group 2 and Group 3 suggest a genetic effect on child outcome that does not change with increasing contact with the affected uncle. Though any interpretation of a contact by group status interaction (e.g., non-parallel lines) should be taken with caution since interaction effects were not significant, possibly interesting effects can be seen for child AD symptoms. Here, the smaller difference between the Group 2 and Group 3 lines at higher levels of contact may suggest smaller genetic effects on child behavior with more uncle contact, while the increasing difference between Group 4 and Groups 2 and 3 with increasing contact suggest stronger genetic influence on child behavior with more contact. The effect that family contact with an affected twin uncle may have on the heritability of child behavior should be investigated further. Clearly, past research has shown that child/adolescent behavior, including conduct problems and alcohol use, are genetically influenced (e.g., Haber et al. 2005; McGue et al. 2001; Saudino et al. 2005), and the current study supports this conclusion.
Several limitations of the current study deserve note. First, a measure of twin contact was used as a proxy for child-uncle contact, which was an indirect measure of environmental risk. It is unknown if twin contact actually leads to child-uncle contact. Additionally, twin contact was assessed when the children were age 12–32, and it is unknown whether the twins had more or less contact when the offspring were younger. For the older offspring in the sample, this could be especially problematic given the uncle contact variable is coded based on the twin father’s contact with the uncle but the offspring is no longer living at home with his/her father.
Second, it is acknowledged that confirming the null hypothesis does not provide the strongest approach to hypothesis testing (e.g., Cohen 2001). Given the finding that twin contact did not have a main effect on child behavior, it seems unlikely that twin contact is a strong environmental variable in these data. However, the results are also consistent with the conclusion that the environment truly does not effect child’s behavior.
Third, the COT design of this report used children of alcohol and drug dependent men. Other COT designs are also of interest (e.g., children of depressed individuals, smokers, divorce; D’Onofrio et al. 2007; Duncan et al. 2008; Sartor et al. 2008; Scherrer et al. 2008) where more work on environmental assumptions should be completed. An affected uncle/aunt with major depression or another mental health disorder might confer more or less environmental risk when a child has more (or less) contact with that relative.
Finally, the current study only assessed problem behavior classified as externalizing behaviors. Though it seems likely that the strongest effects of uncle alcohol use behavior would be within the externalizing domain, effects could be extended across domains into internalizing symptoms or personality.
Behavior genetic designs are informative about genetic and environmental influence on behavior, but the logic behind the designs only holds when certain assumptions are valid. The EEA is one of those key assumptions, where environmental similarity is assumed to be constant across certain pairs of individuals. In the COT design, it is assumed that children of fathers in Groups 2, 3, and 4 all have the same “low” environmental risk for disorder. The current study provides evidence that, in part, supports the validity of this assumption in COT designs; more investigations of this type are needed, however, to better establish the validity of the EEA in COT research designs.
Acknowledgments
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA-11667 and AA-11822), National Institute of Drug Addiction (DA-14364 and DA-020810), and with resources and facilities at the Veterans Affairs Palo Alto Health Care System, Palo Alto, CA. Additionally, the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Studies Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; National Institutes of Health; National Academy of Sciences; and the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Footnotes
Results did not differ when including Group 1 fathers or when including the “non-fathers” in the FTS sample. When including all FTS twins (all individuals in all four groups), the MZ twins’ mean level of contact was 4.1 (SD = 1.2, N = 945), which was significantly higher than the mean level of contact for DZ twins (M = 3.6, SD = 1.2, N = 820; t(1,022) = 53.8, p < .0001).
For the entire sample of twins (including “non-fathers”), Group 2 (MZ twins with an AD co-twin) and Group 4 (controls) had more contact than Group 3 (DZ twins with an AD co-twin), F(2,646) = 9.7, p < .001. The mean level of contact was 3.8 (SD = 1.1, N = 389) for Group 4, 4.0 (SD = 1.1, N = 228) for Group 2, and 3.5 (SD = 1.2, N = 204) for Group 3. Also, though not key to the current analyses, the mean level of contact for AD twins (Group 1) was 3.9 (SD = 1.2, N = 941) for the entire sample and 3.8 (SD = 1.2, N = 585) for the twins with children.
Contributor Information
Laura B. Koenig, Email: koenig@kutztown.edu, Family Research Center, Veterans Affairs Palo Alto Health Care System, Menlo Park, CA, USA. Kutztown University, Old Main 388-A, PO Box 730, Kutztown, PA 19530, USA
Theodore Jacob, Family Research Center, Veterans Affairs Palo Alto Health Care System, Menlo Park, CA, USA.
Jon Randolph Haber, Family Research Center, Veterans Affairs Palo Alto Health Care System, Menlo Park, CA, USA.
Hong Xian, Research Services, St. Louis Veterans Affairs Medical Center, St. Louis, MO, USA. Department of Medicine, Division of General Medical Sciences, Washington University School of Medicine, St. Louis, MO, USA.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 3rd edn, revised (DSM-III-R) American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 1994. (DSM-IV) [Google Scholar]
- Borkenau P, Riemann R, Angleitner A, Spinath FM. Similarity of childhood experiences and personality resemblance in monozygotic and dizygotic twins: a test of the equal environments assumption. Pers Individ Differ. 2002;33:261–269. [Google Scholar]
- Cohen BH. Explaining psychological statistics. 2. Wiley; New York: 2001. [Google Scholar]
- D’Onofrio BM, Turkheimer E, Eaves LJ, Corey LA, Berg K, Solaas MH, et al. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. J Child Psychol Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer E, Emery RE, Maes HH, Silberg J, Eaves LJ. A children of twins study of parental divorce and offspring psychopathology. J Child Psychol Psychiatry. 2007;48:667–675. doi: 10.1111/j.1469-7610.2007.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Wang JC, Hinrichs A, Bertelsen S, Bucholz KK, Schuckit M, et al. Alcohol dependence with comorbid drug dependence: genetic and phenotypic associations suggest a more sever form of the disorder with stronger genetic contribution to risk. Addiction. 2007;102:1131–1139. doi: 10.1111/j.1360-0443.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- Duncan AE, Sartor CE, Scherrer JF, Grant JD, Heath AC, Nelson EC, et al. The association between cannabis abuse and dependence and childhood physical and sexual abuse: evidence from an offspring of twins design. Addiction. 2008;103:990–997. doi: 10.1111/j.1360-0443.2008.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen SA, True WR, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet Med Gemellol. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Goldberg J, True W, Eisen S, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: ascertainment bias. Acta Geneticae Medicae et Gemellogogiae. 1987;36:67–78. doi: 10.1017/s0001566000004608. [DOI] [PubMed] [Google Scholar]
- Haber JR, Jacob T, Heath AC. Paternal alcoholism and offspring conduct disorder: evidence for the ‘common genes’ hypothesis. Twin Res. 2005;8:120–131. doi: 10.1375/1832427053738782. [DOI] [PubMed] [Google Scholar]
- Haber JR, Bucholz KK, Jacob T, Grant JD, Scherrer JF, Sartor CE, et al. Effect of paternal alcohol and drug dependence on offspring conduct disorder: gene-environment interplay. J Stud Alcohol Drugs. doi: 10.15288/jsad.2010.71.652. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. Physical similarity and the equal-environments assumption in twin studies of psychiatric disorders. Behav Genet. 1995;25:327–335. doi: 10.1007/BF02197281. [DOI] [PubMed] [Google Scholar]
- Jacob T, Sher KJ, Bucholz KK, True WR, Sirevaag EJ, Rohrbaugh J, et al. An integrative approach for studying the etiology of alcoholism and other addictions. Twin Res. 2001;4:103–118. doi: 10.1375/1369052012218. [DOI] [PubMed] [Google Scholar]
- Jacob T, Waterman B, Heath A, True WR, Bucholz KK, Haber JR, et al. Genetic and environmental effects on offspring alcoholism: new insights using a children-of-twins design. Arch Gen Psychiatry. 2003;60:1265–1272. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. SAS Institute, Inc; Cary: 1996. [Google Scholar]
- Lytton H. Do parents create, or respond to, differences in twins? Dev Psychol. 1977;13:456–459. [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001;25:1166–1173. [PubMed] [Google Scholar]
- Morris-Yates A, Andrews G, Howie P, Henderson S. Twins: a test of the equal environments assumption. Acta Psychiatr Scand. 1990;81:322–326. doi: 10.1111/j.1600-0447.1990.tb05457.x. [DOI] [PubMed] [Google Scholar]
- Plomin R, Willerman L, Loehlin JC. Resemblance in appearance and the equal environments assumption in twin studies of personality traits. Behav Genet. 1976;6:43–52. doi: 10.1007/BF01065677. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Xian H, Scherrer JF, Lynskey MT, Duncan AE, Haber JR, et al. Psychiatric and familial predictors of transition times between smoking stages: results from an offspring-of-twins study. Addict Behav. 2008;33:235–251. doi: 10.1016/j.addbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino KJ, Angelica R, Plomin R. The etiology of behavior problems in 7-year-old twins: substantial genetic influence and negligible shared environmental influence for parent ratings and ratings by same and different teachers. J Abnorm Child Psychol. 2005;33:113–130. doi: 10.1007/s10802-005-0939-7. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, Waterman BM, Heath AC, Bucholz KK, True WR, Jacob T. Are substance use, abuse and dependence associated with study participation? Predictors of offspring nonparticipation in a twin-family study. J Stud Alcohol. 2004;65:140–144. doi: 10.15288/jsa.2004.65.140. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, Grant JD, Duncan AE, Pan H, Waterman B, Jacob T, et al. Measured environmental contributions to cannabis abuse/dependence in an offspring of twins design. Addict Behav. 2008;33:1255–1266. doi: 10.1016/j.addbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg J, Eaves LJ. Analysing the contribution of genes and parent–child interaction to childhood behavioural and emotional problems: a model for the children of twins. Psychol Med. 2004;34:347–356. doi: 10.1017/s0033291703008948. [DOI] [PubMed] [Google Scholar]
- Slutske WS, D’Onofrio BM, Turkheimer E, Emery RE, Harden KP, Heath AC. Searching for an environmental effect of parental alcoholism on offspring alcohol use disorder: a genetically informed study of children of alcoholics. J Abnorm Psychol. 2008;117:534–551. doi: 10.1037/a0012907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Lyong MJ, Eisen S, Goldberg J, True WR, Lin N, et al. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3, 372 twin pairs. Am J Med Genet. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- West SG, Aiken LS, Krull JL. Experimental personality designs: analyzing categorical by continuous variable interactions. J Pers. 1996;64:1–48. doi: 10.1111/j.1467-6494.1996.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Xian H, Scherrer JF, Eisen SA, True WR, Heath AC, Goldberg J, et al. Self-reported zygosity and the equal-environments assumption for psychiatric disorders in the Vietnam Era Twin Registry. Behav Genet. 2000;30:303–310. doi: 10.1023/a:1026549417364. [DOI] [PubMed] [Google Scholar]