Abstract
Purpose of review
Recently, the prospective isolation and characterization of cancer stem cells (CSCs) from various human malignancies revealed that they are resistant to radiation and chemotherapies. Therefore, CSCs may be the “roots” and ideal target for therapeutic intervention. Here, we will focus on reviewing the historical perspective, recent literatures on bladder cancer stem cells and their clinical implications.
Recent findings
Cancer stem cells have been prospectively isolated from bladder cancer tissues from patient specimens, established cancer cell lines and xenografts, based on the expression of a combination of cell surface receptors, cytokeratin markers, drug transporters and the efficient efflux of the Hoechst 33342 dye (side population). Further, global gene expression profiling of CSCs revealed an activated gene-signature of CSCs similar to that of aggressive bladder cancer, supporting the concept that a tumor cell subpopulation is contributing to bladder cancer progression. Finally, our studies on the preclinical targeting of bladder CSCs in vitro and in xenografts using a blocking antibody for CD47 reveal promising efficacy.
Summary
Functionally distinct CSCs exist in human bladder cancer and can be prospectively isolated. Continuing research will be important to identify their cell of origin, programs balancing self-renewal and differentiation, and to identify additional therapeutic options to target bladder CSCs.
Keywords: Bladder cancer, cancer stem cells, basal cells, therapeutic targeting, CD47
Introduction
Cancer stem cells (CSCs) is best defined functionally, as a subpopulation of tumor cells that can enrich for tumorigenic property and can regenerate the heterogeneity of the original tumor in immunocompromised mice. The existence of CSCs was hypothesized in the 60’s, and experimentally isolated in the last decades, first in acute myeloid leukemia [1] and later in solid cancers such as breast cancer [1]. Importantly, CSCs were shown to be resistant to conventional therapies such as chemotherapy [2] and radiation [3]. Therefore, the prospective isolation, molecular characterization and therapeutic targeting of CSCs in bladder cancer will possibly mark major advances in understanding their pathogenesis.
Bladder Cancer Heterogeneity
Since the 70’s, it is well documented that intra-tumoral heterogeneity exists in human bladder cancer. This heterogeneity was observed histologically and functionally by biological assays defining proliferation [4,5], anchorage-dependent growth ability [6,7] and responses to therapies [8].
Early studies utilizing the soft-agar or methycellulose assay showed that only a small percentage (0.0004 – 1.7%) of bladder cancer cells but not normal urothelial cells was able to form colonies in this in vitro assay [4,6,7], which measured the anchorage-independent growth ability of transformed cells. It was found that bladder tumor cells able to form larger colonies in soft agar were restricted to a subpopulation of high-density small round cells, and tumor cells with intermediate-density could undergo several cell division but cannot form large colonies [4]. Studies using optical density, lectin-binding and flow cytometry clearly demonstrated three morphologically distinct cell types in the normal urothelium. These include small round cells of the basal layer, pyramidal cells of the intermediate layer and giant cells of the superficial layer [9,10]. Further attempts were made to generate monoclonal antibodies toward different layers of the bladder urothelium and to utilize these antibodies to define different histological subtypes of bladder TCCs [11]. It was demonstrated that a monoclonal antibody (MoAb21.48) that preferentially bind to the basal cell layer of normal urothelium identified papillary TCCs and showed diffused staining in poorly differentiated tumors. A monoclonal antibody (MoAb5.48) that preferentially bind to the superficial cell layers of normal urothelium usually showed binding in well differentiated TCCs and less binding in poorly differentiated TCCs [11]. Although cytokeratin and cell surface markers were not established during that time period to define the differentiation stages of the normal urothelium, these early data clearly implicated the unique biological properties of a basal cell-like bladder tumor cell subpopulation in their anchorage-independent growth ability and their association to poorly differentiated bladder cancer.
Prospective isolation of bladder cancer stem cells
Currently, the best model to identify cancer stem cells is to utilize primary or early passage tumor cells from patients, to examine their enriched ability to form xenografts in immunocompromised mice, and their ability to generate a heterogeneous population of tumor cells. This approach ensures that tumor cells are not pre-selected or adapted to a certain microenvironment after long period of passaging either in vitro or in vivo. Our laboratory has found that CD44, a cell surface marker primarily expressed in normal basal urothelial cells, is able to isolate tumor cells fulfilling the functional criteria of CSCs in several invasive human bladder cancer [12] (Table 1). The CD44+ CSCs are at least 10–200 fold enriched for tumorigenic properties in immunocompromised mice and were able to generate the heterogeneity of the original patient tumor [12]. Further characterization demonstrated that these CD44 expressing CSCs often co-localize with the basal cell marker cytokeratin 5, and is mutually exclusive with the differentiated/superficial cell marker cytokeratin 20 [12]. Independently, Yang et al have shown that in bladder cancer specimens, tumor cells expressing the variant isoform of CD44 (CD44v6) but negative for EMA enriches for CSCs [13] (Table 1). In established cell lines SW780 and T24, She et al and Ning et al were able to identify a tumor cell subpopulation that effectively efflux the Hoechst 33342 dye (commonly designated as side population). These SP cells were able to form colonies and xenograft tumors in nude mice more efficiently [14,15] (Table 1). Subsequently, He et al demonstrated that in xenografts formed from the SW780 cancer cell line, tumor cells with a strong expression of the 67-kDa laminin receptor (67LR) are at least 10-fold enriched for tumorigenic cells [16]. Additionally, they found in one early patient xenograft tumor that CEACAM6 (CD66C) low expressing cells (3%) are 70-fold enriched for tumorigenic potential. The authors also found that CK17, another cytokeratin marker specific to urothelial basal cells often co-localize with 67LR positive tumor cells and is mutually exclusive to CD66C [16] (Table 1). Although no combined positive/negative selection for both markers from the cell line or the xenograft tumor was shown, their data suggest a more basal compartment like phenotype for tumor-initiating cells in bladder cancer [16]. Recently, Su et al utilized aldehyde dehydrogenase 1 A1 (ALDH1A1) to isolate CSCs and showed that ALDH1A1 is inversely associated with cancer specific and overall survival [17] (Table 1). These data clearly support that a functionally distinct subpopulation of CSCs can be isolated from bladder cancer, although the relationship among these different CSC populations isolated using various markers or methods is unclear.
Table 1.
Phenotype of Bladder Cancer Stem Cells
Relationship to normal stem, progenitor and differentiated cells
The term “cancer stem cell” is often confusing, misleading many to believe that such cells indeed arise from normal adult stem cell. In fact, cancer stem cell currently is a functional definition that has no direct correlation to the cell of origin for CSCs. Based on the CD34+/CD38− immunophenotype of AML CSCs, Bonnet and Dick concluded that AML CSCs developed from hematopoietic stem cells HSCs that also express CD34+CD38− [1]. Subsequently our group showed that although the first important translocation occur in the HSCs, additional mutations occurred in downstream differentiated progenitor cell and is therefore the cell of origin [18]. The expression of basal cell markers such as CD44, CK5 and CK17 on CSCs from invasive bladder cancer specimens and xenografts formed from established cancer cell line suggest but did not directly show that CSCs from these specimens may arise from the basal cell compartment [12,16]. Moreover we observed in a bladder cancer tissue array with over 300 specimens that 60% of the tumors stained negative for CD44 [12]. Interestingly, CD44− primary patient tumors also engrafted when xenotransplanted into immunocompromised mice. So far no marker has been identified to subfractionate cells with enriched tumorogenic potential within CD44− tumors. We hypothesize that CSCs in these CD44− bladder cancers might arise from a different urothelial differentiation stage, possibly not from the basal compartment. The immunophenotypic profile of CSCs in bladder cancer might vary between different patient tumors. The common characteristics of normal and cancer-stem cells are their ability to (1) self-renew and (2) differentiate to give rise to all downstream cells, recapitulating the original cellular heterogeneity of normal and cancer tissue. In normal urothelium, Kurzrock et al has adapted the property of slow-cycling adult stem cells in other epithelial tissues with high tissue turnover rate (e.g. skin and intestine) and pulse-chased labeled rats with bromo-deoxyuridine (BrdU), which mark all dividing cells in the urothelium. With time, all proliferating cells will gradually lose the BrdU, and only slow-cycling stem cells will retain BrdU labeling. These so-called “label-retaining cells”(LRCs) were shown to localize in 9% of bladder basal cells in the rat [19]. These LRCs express beta4 integrin and were found to be highly clongenic in vitro [19]. The authors further characterized the three types of colonies formed from normal urothelium and designated a Type-3 cell seemed to harbor stem-like property, with relatively low proliferative and apoptotic index at first culture while they can be stimulated to proliferate rapidly upon serial passaging [20]. Zhang et al has recently described the isolation of a progenitor cell population from urine specimens that can by induced to differentiate multilineagely into urothelial, smooth muscle and even endothelial and interstitial cells [21]. Another study by Anumanthan et al has implicated the differentiation of bone marrow-derived mesenchymal stem cells into urothelial cells under the renal subcapsular space [22].
Unlike the hematopoetic system [19], the skin [23] and mammary gland [24], no cell surface marker profile is established in the urothelium to distinguish stem, progenitor and terminally differentiated cells. However, cellular morphology, 2-D culture system and stratified organization clearly suggest that normal urothelial cells follow a lineage relationship and hierarchy with at least three distinctive cell types; Basal, intermediate and umbrella cells. It is very likely, that such cellular hierarchy with multiple differentiation steps exists in bladder cancer, that CD44+/K5+/K20− cells differentiate terminally into CD44−/K5−/K20+ cells (Figure 1). Within primary CD44+ patient xenograft tumors, we observed three different cell types with the morphology of basal, intermediate and differentiated (umbrella cells) urothelial cells. In early xenografts, CD44+ cells with the morphology of basal cells were mostly located at the edge of xenograft tumor-nodules while cells with intermediate and terminally differentiated morphology were predominately at the center. Our preliminary data suggest that bladder cancer cell heterogeneity is not random but the consequence of hierarchical differentiation from CSCs to terminally differentiated cells (unpublished data).
Figure 1.
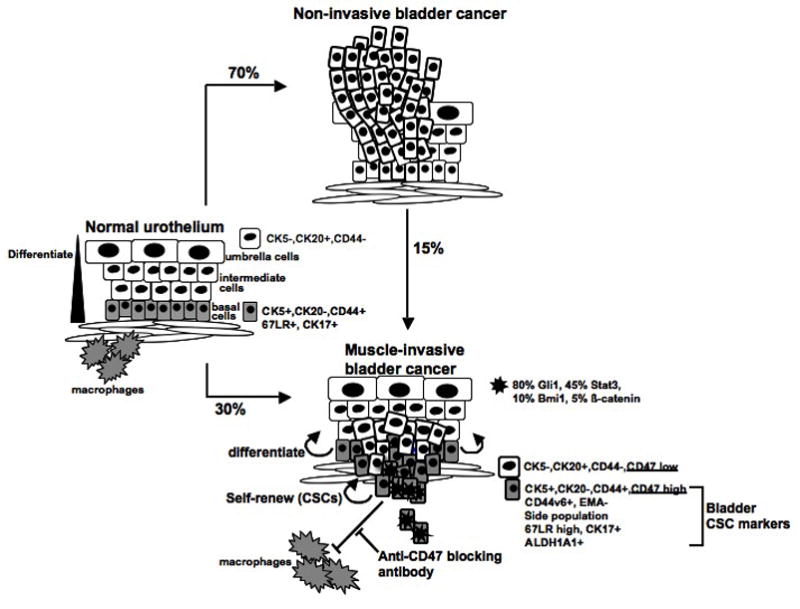
Schematics summarizing the significance of CSCs in human bladder cancer development.
Molecular characterization and therapeutic targeting of bladder cancer stem cells
Our laboratory has analyzed the expression of genes implicated in the self-renewal of adult tissue stem cells by immunohistochemistry or in situ hybridization in over 300 patient specimens (e.g. Gli1, Bmi-1, Stat3, β-catenin, OCT-4 and Nanog) [12]. Our data revealed that bladder cancer is very heterogeneous in the signaling pathways involved in driving tumor progression (Figure 1). Using global gene expression profiling, we identified a gene-signature upregulated in patient CD44+ CSCs in comparison to CD44-tumor cells. This gene-signature was able to identify ~ 97% and 87% of invasive from non-invasive bladder in two separate published data sets [12]. And this gene-signature was able to identify a subset of non-invasive bladder cancer that recurred [12]. Our data support the notion that instead of the bulk tumor mass, bladder CSCs are important in driving invasive cancer progression. Independently, He et al examined the gene expression of 67LR bright and 67LR dim cells from xenografts generated from the SW780 cancer cell line. Their data revealed that the Wnt-signaling may be important in driving SW780 cancer progression [16]. However, our immunohistochemical analysis revealed that nuclear active form of β-catenin is only observed in less than 5% of all bladder cancer analyzed, the SW780 cells may be a unique model for β-catenin activated bladder cancer.
Emerging evidence in the literature support the notion that CSCs are more resistant to conventional therapies such as radiation [3] and chemotherapy [2]. Therefore, direct targeting of CSCs in combination to conventional therapies may provide better efficacies. Our recent studies revealed that the immunoglobulin-like transmembrane Integrin Associated Protein (IAP/CD47), an inhibitory signal for phagocytosis through macrophages, is expressed on all bladder cancer cells but significantly higher on bladder CSCs (CD44+) [12]. CD47 interacts with SIRPα, which is expressed on myeloid cells such as macrophages, granulocytes and dendritic cells [25]. This interaction negatively regulates phogocytosis [25]. Our group demonstrated that increased CD47 expression in human acute myeloid leukemia (AML) CSCs is an independent, poor prognostic factor [26]. Disruption of CD47-SIRPα with anti-CD47 antibody could deplete AML in a xenotransplantation mouse model, although CD47 blocking was insufficient to induce phagocytosis of normal HSCs [26]. Our results in bladder cancer revealed that CD47 expression positively correlated with the cancer cell engraftment in xenograft model (unpublished data). Blockade of CD47 with a monoclonal antibody (mAb) resulted in macrophage engulfment of human bladder cancer cells in vitro [12]. In vivo treatment with anti-CD47 mAb resulted in a significant reduction in the tumor volume of bladder cancer xenografts in a dose-dependent manner. Furthermore, anti-CD47 mAb blocked metastasis in vivo (unpublished data). Overall, CD47 is a desirable target to utilize the body’s self-defense mechanism to eradicate cancer. Since CD47 expression is specifically increased in the bladder CSCs population, it may be a promising target for bladder cancer therapy (Figure 1).
Conclusion
It is evident that functionally distinct CSCs exist in human bladder cancer and can be prospectively isolated. Continuing research will be important to identify their cell of origin (stage of differentiation), genetic and epigenetic programs that balance the self-renewal and differentiation of CSCs, and to identify additional therapeutic options to target bladder CSCs, e.g. inhibit self-renewal and drive differentiation of CSCs, antibody therapy or immune-based therapy.
Acknowledgments
We would like to acknowledge Ms Linda Quinn for her excellent administrative support, the Pride Family Fund and NCI CA129640-03 for funding support.
Contributor Information
Keith Syson Chan, Email: kc1@bcm.edu.
Jens-Peter Volkmer, Email: jvolkmer@bcm.edu.
Irving Weissman, Email: Irv@stanford.edu.
References
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Mackillop WJ, Bizarri JP, Ward GK. Cellular heterogeneity in normal and neoplastic human urothelium. Cancer Res. 1985;45:4360–4365. [PubMed] [Google Scholar]
- 5.Lipponen PK, Eskelinen MJ, Nordling S. Intratumoral heterogeneity of DNA indexes in transitional cell bladder cancer: relation to tumor histology. Eur Urol. 1991;20:311–314. doi: 10.1159/000471723. [DOI] [PubMed] [Google Scholar]
- 6.Buick RN, Stanisic TH, Fry SE, Salmon SE, Trent JM, Krasovich P. Development of an agar-methyl cellulose clonogenic assay for cells in transitional cell carcinoma of the human bladder. Cancer Res. 1979;39:5051–5056. [PubMed] [Google Scholar]
- 7.Kovnat A, Buick RN, Connolly JG, Jewett MA, Keresteci AG, Tannock IF. Comparison of growth of human bladder cancer in tissue culture or as xenografts with clinical and pathological characteristics. Cancer Res. 1984;44:2530–2533. [PubMed] [Google Scholar]
- 8.Anderson KC, Simpson WG, Ballou RJ, Harty JI, Tseng MT. In vitro chemosensitivity of J-82 human bladder cancer cells. Urol Res. 1986;14:141–144. doi: 10.1007/BF00255833. [DOI] [PubMed] [Google Scholar]
- 9.Ward GK, Stewart SS, Price GB, Mackillop WJ. Cellular heterogeneity in normal human urothelium: an analysis of optical properties and lectin binding. J Histochem Cytochem. 1986;34:841–846. doi: 10.1177/34.7.3754881. [DOI] [PubMed] [Google Scholar]
- 10.Ward GK, Stewart SS, Price GB, Mackillop WJ. Cellular heterogeneity in normal human urothelium: quantitative studies of lectin binding. Histochem J. 1987;19:337–344. doi: 10.1007/BF01680450. [DOI] [PubMed] [Google Scholar]
- 11.Dotsikas MG, Konowalchuk T, Major PP, Kovac PE, Ward GK, Stewart SS, Price GB, Elhilali MM, Mackillop WJ. Cellular heterogeneity in normal and neoplastic human urothelium: a study using murine monoclonal antibodies. Br J Cancer. 1987;56:439–444. doi: 10.1038/bjc.1987.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J, Jr, Chang HY, van de Rijn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. A comprehensive study using patient bladder cancer specimens and archived tissue for the prospective isolation and molecular characterization of bladder CSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YM, Chang JW. Bladder cancer initiating cells (BCICs) are among EMA-CD44v6+ subset: novel methods for isolating undetermined cancer stem (initiating) cells. Cancer Invest. 2008;26:725–733. doi: 10.1080/07357900801941845. [DOI] [PubMed] [Google Scholar]
- 14.She JJ, Zhang PG, Wang ZM, Gan WM, Che XM. Identification of side population cells from bladder cancer cells by DyeCycle Violet staining. Cancer Biol Ther. 2008;7:1663–1668. doi: 10.4161/cbt.7.10.6637. [DOI] [PubMed] [Google Scholar]
- 15.Ning ZF, Huang YJ, Lin TX, Zhou YX, Jiang C, Xu KW, Huang H, Yin XB, Huang J. Subpopulations of stem-like cells in side population cells from the human bladder transitional cell cancer cell line T24. J Int Med Res. 2009;37:621–630. doi: 10.1177/147323000903700304. [DOI] [PubMed] [Google Scholar]
- 16.He X, Marchionni L, Hansel DE, Yu W, Sood A, Yang J, Parmigiani G, Matsui W, Berman DM. Differentiation of a highly tumorigenic basal cell compartment in urothelial carcinoma. Stem Cells. 2009;27:1487–1495. doi: 10.1002/stem.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass SA, Jiang F. Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomarkers Prev. 19:327–337. doi: 10.1158/1055-9965.EPI-09-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci U S A. 2000;97:7521–7526. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Label-retaining cells of the bladder: candidate urothelial stem cells. Am J Physiol Renal Physiol. 2008;294:F1415–1421. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- *20.Thangappan R, Kurzrock EA. Three clonal types of urothelium with different capacities for replication. Cell Prolif. 2009;42:770–779. doi: 10.1111/j.1365-2184.2009.00647.x. A study revealing the possible existence of normal progenitor cells in bladder urothelium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, Atala A. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–2233. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Anumanthan G, Makari JH, Honea L, Thomas JC, Wills ML, Bhowmick NA, Adams MC, Hayward SW, Matusik RJ, Brock JW, 3rd, et al. Directed differentiation of bone marrow derived mesenchymal stem cells into bladder urothelium. J Urol. 2008;180:1778–1783. doi: 10.1016/j.juro.2008.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 26.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


