Abstract
Background
Little is known about vitamin D status in breast cancer survivors. This issue is important since vitamin D influences pathways related to carcinogenesis.
Objective
The objective of this report is to describe and understand vitamin D status in a breast cancer survivor cohort.
Design
Data are from the HEAL (Health, Eating, Activity and Lifestyle) Study. Using a cross-sectional design, we examined serum concentrations of [25(OH)D] in 790 breast cancer survivors from western Washington, New Mexico and Los Angeles County. Cancer treatment data were obtained from SEER (Surveillance Epidemiology and End Results) registries and medical records. Fasting blood, anthropometry and lifestyle-habits were collected post-diagnosis and treatment. We examined distributions of [25(OH)D] by race/ethnicity, season, geography and clinical characteristics. Multivariate regression tested associations between [25(OH)D] and stage of disease.
Results
597 (75.6%) of women had low serum [25(OH)D] suggesting vitamin D insufficiency or frank deficiency. The overall mean (SD) was 24.8 (10.4) ng/ml, but lower for African-Americans [18.1 (8.7) ng/ml] and Hispanics [22.1 (9.2) ng/ml]. Women with localized (n=424) or regional (n=182) breast cancer had lower serum [25(OH)D] than women with in situ disease (n=184), (p = 0.03 and p = 0.02, respectively). Multivariate regression models controlled for age, BMI, race/ethnicity, geography, season, physical activity, diet and cancer treatments demonstrated that stage of disease independently predicted serum [25(OH)D] (p=0.02).
Conclusion
In these breast cancer survivors, the prevalence of vitamin D insufficiency was high. Clinicians might consider monitoring vitamin D status in breast cancer patients, together with appropriate treatments, if necessary.
Keywords: Vitamin D, breast cancer, 25(OH)D, vitamin D insufficiency, ethnicity
INTRODUCTION
Vitamin D is an essential nutrient for humans and has been primarily recognized for its role in prevention of bone diseases, such as vitamin D-related rickets, osteomalacia and age-related fractures (1). More recently, carcinogenesis-related functions have been identified for vitamin D including cellular differentiation, T cell-mediated immunity, cell cycle arrest and apoptosis (2-5). The Vitamin D Receptor (VDR), which is activated by 1,25-dihydroxyvitamin D [1,25(OH)D], has been found in nearly all tissues and organs in the human body and is responsible for the transcription of numerous genes related to cell cycle control, apoptosis and metastatic potential (6, 7). Thus, vitamin D is of considerable interest in relation to many cancers, including breast cancer. In addition to direct influences on the cell cycle and apoptosis, vitamin D may also exert its protective effect by influencing sex hormones and other crucial peptides (8-11). For example, while not completely understood, vitamin D analogues successfully inhibit the growth of mammary tumors (9, 11), and an estrogen-independent mechanism exists whereby vitamin D may reduce the proliferative effects of, IGF-I, which is a potent mitogen (8, 12, 13). One animal model has shown that a vitamin D analogue enhances the action of tamoxifen in mammary carcinoma (9).
Accumulating evidence from human observational studies suggests that both dietary and blood measures of vitamin D are inversely associated with incident breast cancer risk (14-17). A pooled analysis of serum 25-hydroxyvitamin D [25(OH)D] across two case control studies (701 cases and 724 controls in one study and 179 cases and controls in the other study) demonstrated that the pooled odds ratio for breast cancer was 0.50 (p, trend <0.001) for highest vs. lowest quintile of the biomarker (18). A comprehensive review evaluated publications on dietary and supplemental sources of vitamin D and related foods and nutrients, biomarkers of vitamin D and genetic variation in the VDR gene, all in relation to breast cancer risk (19). The review concluded that despite some inconsistencies, increasing vitamin D concentrations were associated with decreasing breast cancer risk (19). Although these important findings have implications for primary breast cancer prevention, little is known about vitamin D status among breast cancer survivors. Understanding vitamin D status among cancer patients is critical since vitamin D influences important cellular events related to prognosis and survival (e.g., apoptosis, cell cycle regulation) (4, 6, 19). Here we report on vitamin D status, assessed by circulating [25(OH)D], in a breast cancer survivor cohort. We also examine the relevance of breast cancer clinical characteristics and whether tamoxifen, which is used by many breast cancer patients, influences circulating concentrations of [25(OH)D] (9, 20).
METHODS
Study Design, Population and Recruitment
The Health, Eating, Activity and Lifestyle (HEAL) study is a population-based, multicenter, multiethnic prospective cohort study of 1,183 breast cancer patients investigating whether weight, physical activity, diet, hormones and other exposures affect breast cancer prognosis and survival. Details of the study design and procedures are published (21). Briefly, we utilized the National Cancer Institute’s Surveillance, Epidemiology, End Results (SEER) registries in New Mexico, Los Angeles County (CA), and western Washington state for study recruitment. In New Mexico, we recruited 615 women, aged ≥ 18 years, diagnosed with in situ to Stage IIIA breast cancer between July 1996 and March 1999. In western Washington, we recruited 202 women, aged 40-64 years, diagnosed with in situ to Stage IIIA breast cancer between September 1997 and September 1998. In Los Angeles County, we recruited 366 African-American women with stage 0 to IIIA primary breast cancer who had previously participated in the Los Angeles portion of the Women’s Contraceptive and Reproductive Experiences (CARE) or who had participated in a parallel case-control study of in situ breast cancer. Thus, the Los Angeles participants were a subset of women diagnosed with breast cancer between May 1995 and May 1998, were aged 35 to 64 years at diagnosis, were English speaking and born in the U.S. Procedures were approved by the Institutional Review Boards of the participating centers, in accord with an assurance filed with and approved by the U.S. Department of Health and Human Services; all participants gave written informed consent.
HEAL participants completed extensive interviews within their first year after diagnosis (on average 7.5 months post-diagnosis) and 24 months later (within their third year after diagnosis; on average 31.5 months post-diagnosis). Of the 1,223 women initially enrolled in the study at baseline, 39 (3.2%) were later found to have had a prior breast cancer diagnosis (suggesting that the current diagnosis was either a recurrence or a second primary tumor) and one woman (< 1.0%) was found to have metastatic disease at initial diagnosis. Since the women with recurrent or metastatic disease no longer met the HEAL eligibility criteria, they were subsequently excluded. Of the remaining 1,183 women, 239 (20.2%) women did not return for the 24-month visit. Reasons for non-participation were death (n=44), illness (n=2), refusal (n=105), moved (n=16), unable to contact or locate (n=72). Nine-hundred forty-four women completed 24-month follow-up questionnaires, which included detailed questions on health, menopausal status, diet, physical activity, and alcohol and tobacco use. Staff also measured height and weight and collected a fasting blood specimen from all participants. We used the data and specimens collected at the 24-month interview for this report, but excluded those with no archived blood specimen (n=790 available for analysis).
Breast Cancer Stage of Disease and Cancer Treatment
Data on breast cancer stage of disease at diagnosis were obtained from the SEER registries. Participants were classified as having in situ, Stage I or Stage II-IIIA breast cancer based on AJCC stage of disease classification. Treatment data were obtained during a medical records review that provided more detailed information on chemotherapy, radiation and hormonal therapy than that maintained by the registries. Adjuvant treatment was categorized into four mutually exclusive groups: (i) surgery only; (ii) surgery and radiation; (iii) surgery and chemotherapy; and (iv) radiation and chemotherapy. Tamoxifen use was defined as self-reported current use at the 24-month interview/blood draw.
Blood Collection and 25-Hydroxyvitamin D Analysis
Fasting bloods were processed within three hours of collection and stored at −70° to −80° C until analysis. The biologically active form of vitamin D is [1,25(OH)D], but it is not a good biomarker due to its short half-life and tight homeostatic control (1, 22). Serum 25-hydroxyvitamin D [25(OH)D] is an excellent biomarker of vitamin D status, representing both cutaneous synthesis and dietary intake (1, 22). Serum [25(OH)D] was assayed using a radioimmunoabsorbant assay (RIA) (DiaSorin Inc., Sillwater, MN). We included blinded duplicates in each assay and the within and between assay coefficient of variation was 3.7%.
Dietary Assessment
Diet was assessed using a validated self-administered food frequency questionnaire (FFQ) that was designed for use in multiethnic postmenopausal women (23) This FFQ includes 122 line items, 19 adjustment questions and four summary questions. The database used to convert food information into nutrients is derived from the University of Minnesota’s Nutrition Data Systems for Research (NDS-R, version 2005) and includes recent analytic food values for vitamin D. Information on vitamin D-containing dietary supplements was obtained via: 1) close-ended questions on the use of specific single supplements, including vitamin D; and 2) open-ended questions (New Mexico and Washington only) on the use of any other dietary supplements used at least weekly. We defined vitamin D-containing supplements as either single supplements or combination supplements (e.g., vitamin D-calcium type mixtures). Multivitamins were not included because we had no information on specific brands or formulations. However, since a high proportion of women reported use of multivitamins (72.9%), additional adjustments would not likely provide meaningful information.
Anthropometry
Participants wore light clothing without shoes and weight was measured to the nearest 0.1 kg using a balance-beam laboratory scale (New Mexico and Washington) or portable scale (Los Angeles). Height was measured without shoes to the nearest 0.1 cm using a stadiometer or measuring tape affixed to a wall. All measurements were performed and recorded twice, then averaged for a final value. Body mass index (BMI) was computed as weight in kilograms divided by height in meters squared (kg/m2). We used the WHO-National Institutes of Health categorizations of normal weight and obesity based on BMI: normal = < 25.0 kg/m2, overweight = 25.0-29.9 kg/m2 and obese = ≥ 30.0 kg/m2 (24).
Other Data
Standardized information was collected on medical history, family history of breast and other cancers, smoking, physical activity and demographic data. Geographic locale serves as a surrogate of UV-B exposure since both latitude and altitude influence UV-B exposure (1, 25).
Statistical Analysis
We used descriptive statistics to characterize the study sample and to examine the distributions of serum concentrations of [25(OH)D] by race/ethnicity, geography and season. Multivariate linear regression determined the breast cancer clinical characteristics that independently predicted serum [25(OH)D] after adjustment for other variables. Covariates included in the models were selected from known or suspected a priori predictors of vitamin D from the published literature, such as BMI and physical activity (22, 26, 27). Variables were only retained in the multivariate models if the effect of the variable changed the serum [25(OH)D] by more than 10% (a standard analytic approach). Variables that were examined, but not retained in the final models included: smoking, menopausal status and alcohol intake. Since these variables were neither statistically significant, nor influential on the outcome of interest, they were not retained in the model. All analyses were completed with SAS (version 9.1, Cary, NC).
Standard clinical cutpoints of serum [25(OH)D] concentrations were used to define frank deficiency (< 10 ng/ml), insufficiency (10 to < 32 ng/ml) and sufficiency (≥ 32 ng/ml) (1, 22, 28). Season was classified as winter (December-February), spring (March-May), summer (June-August) or fall (September-November).
RESULTS
Demographic and breast cancer clinical characteristics of the 790 HEAL participants are presented in Table 1. The mean age was 57.3 years and 62.3% were overweight (BMI = 25.0-29.0) or obese (BMI ≥ 30). Over half of participants (59.4%) were non-Hispanic white, 26.5% were African-American and 11.4% were Hispanic. Participants were well-educated (73.7% with at least some college); 11.9% were smokers. There were no differences in age or smoking status between those who did vs. did not complete the 24 month interview. However, there were differences between those who did vs. did not return for the 24 month interview with regard to the proportion of patients from Los Angeles (African-American), income and education (p< 0.001) (data not shown).
Table 1.
Demographic and Medical Characteristics of Breast Cancer Survivors in the HEAL (Health, Eating, Activity and Lifestyle) Study1,2
| Characteristic | N=790 |
|---|---|
| Age (y), mean (SD) | 57.3 (10.5) |
| Body Mass Index, n (%) | |
| <25.0 (normal) | 298 (37.7) |
| 25.0 – 29.9 (overweight) | 255 (32.3) |
| ≥ 30.0 (obese) | 237 (30.0) |
| Study Site, n (%) | |
| Western Washington | 173 (21.9) |
| New Mexico | 409 (51.8) |
| Los Angeles | 208 (26.3) |
| Race/Ethnicity, n (%) | |
| Non-Hispanic White | 469 (59.4) |
| Hispanic | 90 (11.4) |
| African-American | 209 (26.5) |
| Other | 22 (2.8) |
| Income, n (%) | |
| <$20,000 per year | 154 (20.4) |
| $20,000 – $50,000 per year | 308 (40.9) |
| >$50,000 per year | 292 (38.7) |
| Education, n (%) | |
| High School or Less | 207 (26.4) |
| Some College/Technical School | 288 (36.5) |
| College Degree | 147 (18.6) |
| Graduate School/Degree | 147 (18.6) |
| Smoking Status, n (%) | |
| Never | 384 (48.5.6) |
| Former | 312 (39.5) |
| Current | 94 (11.9) |
| Vitamin D Dietary Intake (mcg/d), mean (SD) | 4.0 (3.3) |
| Vitamin D-Dietary Supplementsε, n (%) | |
| Yes | 210 (26.6) |
| No | 580 (73.4) |
| Serum [25(OH)D] (ng/ml) | |
| Total (n=790), mean (SD) 3 | 24.8 (10.4) |
| Non-Hispanic White (n=469) | |
| Mean (SD) | 28.4 (9.7) |
| 75th Percentile | 34.3 |
| 25th Percentile | 22.5 |
| African-American (n=209) | |
| Mean (SD) | 18.1 (8.7) |
| 75th Percentile | 22.5 |
| 25th Percentile | 11.8 |
| Hispanic (n=90) | |
| Mean (SD) | 22.1 (9.2) |
| 75th Percentile | 29.3 |
| 25th Percentile | 15.3 |
| Breast Cancer Stage of Disease, n (%) | |
| In Situ | 184 (23.3) |
| Localized | 424 (53.7) |
| Regional | 182 (23.0) |
| Estrogen Receptor Status, n (%) | |
| Positive | 441 (55.8) |
| Negative | 124 (15.7) |
| Unknown/Missing | 225 (28.5) |
| Breast Cancer Treatment, n (%) | |
| Surgery Alone | 247 (31.3) |
| Surgery + Radiation | 295 (37.3) |
| Surgery + Chemotherapy | 80 (10.1) |
| Radiation + Chemotherapy | 168 (21.3) |
| Tamoxifen Use (yes) , n(%) | 340 (43.0) |
Cell sizes may vary due to missing values.
Sample size for these analyses = 790; only HEAL participants with available blood from the 24 month interview were used in these analyses. Those who did not return for interview and blood draw (n = 393) were more likely to be African-American, live in Los Angeles, have an income and education in either the top or bottom group. Specific race/ethnic distributions not provided for women who did not report race/ethnicity or reported for mixed race/unknown.
Mixed race and other race not included in race/ethnicity distribution totals
Includes single supplements of vitamin D or vitamin D specific mixtures (i.e., calcium + vitamin D). Detailed data on ingredients in multivitamins not available.
The mean dietary vitamin D intake was 4.0 μg/day and 26.6% of women used single supplements of vitamin D or combination vitamin D-specific supplements (i.e., vitamin D plus calcium). Over half (55.8%) of the women had estrogen receptor-positive tumors and 43.0% reported use of tamoxifen. Over two-thirds of women received adjuvant breast cancer therapy. For the entire cohort, the mean (SD) concentration of serum [25(OH)D] was 24.8 (10.4) ng/ml.
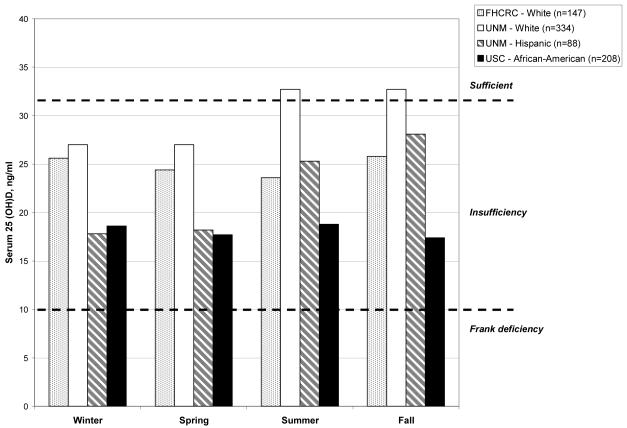
Very few of these breast cancer survivors had serum [25(OH)D] considered optimal for health (Figure 1) (1, 22) and concentrations differed across racial/ethnic groups by geography, but less so by season. The mean concentration of [25(OH)D] for African-American women in Los Angeles (n=208) was 18.1 ng/ml with little seasonal variation. For Hispanic women in New Mexico (n=88), the mean was 22.1 ng/ml, but ranged from 18.8 ng/ml in winter and spring to 27.0 ng/ml in summer and fall. The mean concentration of [25(OH)D] for all non-Hispanic white women was 28.4 ng/ml and ranged from 27.0 to 32.7 ng/ml across the seasons for women in New Mexico, while in Washington the low to high range was 23.6 ng/ml for summer and 25.8 ng/ml for autumn. Forty-nine women (6.2%) had frank [25(OH)D] deficiency (< 10.0 ng/ml) and 548 women (69.4%) had insufficient serum [25(OH)D] (10-32 ng/ml) (data not shown).
Figure 1. Vitamin D [25(OH)D] sufficiency, insufficiency and frank deficiency by race/ethnicity, geography and season in a cohort of breast cancer survivors.
·FHCRC = Fred Hutchinson Cancer Research Center, Seattle, WA; UNM = University of New Mexico, Albuquerque, New Mexico; USC = University of Southern California, Los Angeles County.
·Figure does not include 1 African-American and 2 Hispanics at the Seattle site since numbers would be too small for meaningful display by geographic locale.
We next investigated associations of serum [25(OH)D] with breast cancer stage of disease (Table 2). In analyses adjusted for age, BMI, race/ethnicity, geography, season of blood draw, physical activity, breast cancer treatment, and tamoxifen, women with localized or regional cancer had lower circulating concentrations of [25(OH)D] vs. those with in situ disease (p=0.05 and p=0.03, for local and regional disease, respectively). We then evaluated whether [25(OH)D] varied by tamoxifen use as shown in Table 3. In models adjusted for age, BMI, race/ethnicity, season of blood draw, vitamin-D specific dietary supplement use, study site, physical activity, breast cancer treatment and stage of disease, current tamoxifen use was associated with significantly higher concentrations of [25(OH)D] compared to no tamoxifen use (p=0.008). For ever tamoxifen use, the results were similar but the p-values slightly stronger. When these analyses considered hormone (estrogen) receptor status there were no differences in mean [25(OH)D] for those women with ER+ vs. ER− tumors.
Table 2.
Associations of Serum [25(OH)D] with Stage of Disease in a Multi-Ethnic Breast Cancer Survivor Cohort
| Stage of Disease | n | [25 (OH)D] (ng/ml) | |
|---|---|---|---|
| Unadjusted Mean1 (95% CI) | Adjusted Mean1,2 (95% CI) | ||
| In Situ | 184 | 25.3 (23.8, 27.8) | 25.6 (23.9, 27.4) |
| Localized | 424 | 25.3 (24.3, 26.3) | 24.0 (22.8, 25.2) |
| Regional | 182 | 23.2 (21.7, 24.7) | 23.1 (21.5, 24.6) |
Comparisons of means are least squared means from linear regression models.
Adjusted for Age, BMI, Race/Ethnicity, Geographical Site, Physical Activity, Season of Blood Draw, Treatment, and Tamoxifen use.
Unadjusted Comparisons:
In Situ vs. Regional, p=0.05
In Situ vs. Localized, p = 0.97
Localized vs. Regional, p=0.02
Adjusted Comparisons:
In Situ vs. Regional, p=0.03
In Situ vs. Localized, p=0.05
Localized vs. Regional, p=0.30
Table 3.
Mean Concentrations of Serum [25(OH)D ]by Tamo xifen Use in aMultiethnic Cohort of Breast Cancer Survivors
| Tamoxifen Use (current use) |
n | [25 (OH)D] (ng/ml) | |
|---|---|---|---|
| Unadjusted Mean 1(95% CI) | Adjusted Mean 1,2(95% CI) | ||
| Yes | 340 | 25.9 (24.8, 27.0) | 25.1 (23.8, 26.3) |
| No | 450 | 24.0 (23.0, 24.9) | 23.2 (22.0, 24.5) |
| P-Value | p=0.009 | p=0.008 | |
|
Tamoxifen Use (ever use) |
|||
| Yes | 380 | 26.2 (25.1, 27.2) | 25.2 (23.9, 26.4) |
| No | 410 | 23.5 (22.5, 24.5) | 23.2 (22.0, 24.5) |
| p < 0.001 | p = 0.005 | ||
|
Estrogen Receptor
Status 3 |
|||
| Positive | 441 | 25.3 (24.3, 26.2) | 23.6 (21.7, 25.4) 4 |
| Negative | 124 | 21.6 (19.9, 23.4) | 23.0 (20.6, 25.3) 4 |
| p <0.001 | p = 0.57 | ||
Comparisons of means are least squared means from linear regression models.
Adjusted for Age, BMI, Race/Ethnicity, Geographical Site, Vitam in D-Specific Dietary Supplement Use, Physical Activity, Treatment, Season of Blood Draw, and Stage of Disease.
Excludes participants with unknown tumor horm one receptor status.
Includes additional adjustment for tamoxifen use.
Linear regression modeled the associations of well-known demographic and lifestyle predictors of serum [25(OH)D], plus those related to breast cancer disease status. We tested the independent contributions of breast cancer stage of disease at diagnosis, type of cancer treatment (i.e., surgery, radiation, chemotherapy), and use of tamoxifen in relation to vitamin D status (Table 4). Increasing BMI was inversely associated with serum [25(OH)D] (p<0.001), while physical activity was directly associated with serum [25(OH)D] (p<0.001). Race/ethnicity was a strong predictor of serum [25(OH)D]; compared to non-Hispanic Whites in Washington, those in New Mexico had higher circulating [25(OH)D] (p<0.001), while African-Americans and Hispanics had lower serum values (p< 0.001 and p<0.03, respectively). After controlling for these demographic and lifestyle variables, the variables for stage of disease and tamoxifen both remained significant predictors of serum [25(OH)D]. Compared to in situ disease, both localized (p=0.04) and regional (p=0.02) breast cancer were associated with lower serum [25(OH)D].
Table 4.
Results from Multivariate Model Predicting Serum [25(OH)D] in a Multiethnic Cohort of Breast Cancer Survivors (n=790)
| Serum [25 (OH)D] (ng/ml) |
Multivariate Model |
||
|---|---|---|---|
| Predictor Variables | β (SE ) | P-Value | Global P-Value1 |
| Age (years) | −0.01 (0.03) | 0.86 | 0.86 |
| BMI (kg/m2) | −0.34 (0.05) | <0.001 | <0.001 |
| Race (vs. Washington Non-Hispanic White) |
|||
| New Mexico Non-Hispanic White | 4.74 (0.96) | <0.001 | <0.001 |
| Hispanic | −2.61 (1.24) | 0.03 | |
| African-American | −5.58 (1.01) | <0.001 | |
| Other | −1.71 (1.98) | 0.39 | |
| Moderate-to-Vigorous Physical Activity | 0.04 (0.01) | <0.001 | <0.001 |
| Vitamin D Intake (mcg) | 0.11 (0.10) | 0.24 | 0.24 |
| Vitamin D Supplement Use (vs. None) | 2.65 (0.71) | <0.001 | <0.001 |
| Season (vs. winter) | |||
| Spring | −0.54 (0.92) | 0.56 | <0.001 |
| Summer | 2.49 (0.007) | 0.007 | |
| Fall | 2.79 (0.95) | 0.004 | |
| Treatment (vs. surgery only) | |||
| Surgery + Radiation | 0.37 (0.78) | 0.63 | 0.17 |
| Surgery + Chemotherapy | 2.63 (1.26) | 0.04 | |
| Chemotherapy + Radiation | 1.70 (1.07) | 0.11 | |
| Tamoxifen Use (vs. No) | 1.93 (0.67) | 0.004 | 0.004 |
| Stage of Disease (vs. In Situ) | |||
| Localized | −1.80 (0.86) | 0.04 | 0.02 |
| Regional | −2.85 (1.18) | 0.02 | |
| Time Since Diagnosis | −0.21 (0.14) | 0.13 | 0.13 |
Global p-values were calculated from the linear regression models and: (i) tested the categorical variables for overall significance (i.e., any difference by the variable), and (ii) tested each group vs. the reference).
DISCUSSION
In this multi-ethnic breast cancer survivor cohort, few women had circulating concentrations of [25(OH)D] considered optimal for health (2, 22, 28). The high prevalence of insufficiency (69.4%) or frank deficiency (6.2%) is cause for concern. While data are sparse regarding whether vitamin D status predicts survival in breast cancer patients, some evidence suggests such an association for breast and other cancers. A Norwegian study examined associations of UV-induced vitamin D synthesis, assessed by season and latitude, with incidence and prognosis of breast, colon and prostate cancers (29). Breast cancer case-fatality was lowest for cancers diagnosed in summer and autumn, the seasons with the highest concentrations of [25(OH)D]. These analyses were controlled for age at diagnosis as well as stage of disease. However, the blood values used in the investigation were from population estimates rather than from study patients (29). Two reports from a study of early-stage non-small cell lung cancer suggest that both dietary intake of vitamin D and biomarker measures of [25(OH)D] were associated with improved survival (30, 31). The Health Professionals Follow-Up Study observed inverse associations between serum [25(OH)D] and total cancer incidence (RR= 0.83, 95% CI = 0.74-0.92) and cancer mortality (RR= 0.71, 95% CI=0.60-0.83) (32). While these recent studies (24-26) did not include breast cancer, the biological mechanisms may be similar across cancer sites. Vitamin D regulates cell growth, induces apoptosis, decreases proliferation and enhances the immune response in organs and tissues throughout the human body, including the breast (2, 28). In addition to vitamin D’s ability to directly affect pathways and mechanisms related to carcinogenesis, vitamin D enhances the immune response (2). Optimal vitamin D status is particularly critical for patients receiving chemotherapy due to their compromised immune function and susceptibility to infection; it has recently been shown that vitamin D plays a role in innate immunity (33). Schauber et al demonstrated that 1,25 (OH)D induces the expression of both CD14 and TLR2 and that multiple and diverse genes are up-regulated by vitamin D following an injury (33). The association of vitamin D with other diseases that have a potential immune-function or inflammation-type relationship has been reviewed by Bischoff-Ferrari et al (28). Whether the marginal to poor vitamin D status observed in HEAL is characteristic of other breast cancer survivors, or the extent to which marginal status influences factors directly or indirectly related to breast cancer outcomes is an important clinical issue. This matter may be particularly important for African-American women, as they had the lowest concentrations of [25(OH)D] in HEAL and they typically have poor breast cancer survival rates (34).
HEAL women with local or regional stage breast cancer had significantly lower circulating concentrations of [25(OH)D], compared to women with in situ disease. One explanation is that if a woman was vitamin D deficient prior to the diagnosis, the deficiency could have reduced vitamin D’s ability to suppress proliferation and metastasis. Under this scenario, early non-invasive lesions could possibly advance in the presence of a vitamin D deficiency. This mechanism is supported by in vitro (35) and experimental animal studies (36). However we are unable to directly test this mechanism in HEAL since enrollment in this survivor cohort occurred post-diagnosis. It is also possible that women with more advanced disease experience greater fatigue and malaise, which could limit outdoor physical activity and sun exposure, thus lowering serum [25(OH)D]. Similarly, more advanced disease patients may have poor dietary intake secondary to chemotherapy-related emesis. However, since this study assayed blood specimens collected, on average, 31.5 months post-diagnosis, it is unlikely that active treatment-related side-effects influenced these results. In addition, the table 4 parameter estimates for treatment were not statistically significant (p > 0.05 for all treatments), suggesting that completed treatment itself is not as influential as stage of disease at diagnosis. The possibility that stage of disease is independently associated with [25(OH)D] deserves further investigation, particularly if serum [25(OH)D] is confirmed as a reliable prognostic indicator for breast cancer, as has been suggested for other cancers (30, 32).
Our findings suggested that results differed slightly according to patient use of tamoxifen. We are aware of only one animal model study demonstrating that a vitamin D analogue enhanced the efficacy of tamoxifen in a mammary cancer model. The vitamin D analogue plus tamoxifen was 10-100 times more effective than the analogue alone (9).
Vitamin D therapy could be a useful, cost-effective treatment for breast cancer patients. Giovannucci predicted that every 25 nmol/L (10 ng/ml) increase in serum [25(OH)D] was associated with a 29% reduction in total cancer mortality in men (32). An increase in serum [25(OH)D] of this magnitude (10 ng/mL) can be achieved through dietary supplements or modest sun exposure (22, 28). Scientists and clinicians are currently considering how improvements in vitamin D status might enhance survivorship from colon, prostate and other cancers (37, 38). Our results suggest that breast cancer might be included in these discussions.
There are several strengths to this investigation. HEAL is a population-based prospective cohort study that includes breast cancer patients across varied race/ethnic groups and geographical locations. Because we conducted a medical records review, we have more detailed data on medical and hormonal treatments for breast cancer than would be obtained through SEER registries alone, or by self-report. We used standardized questions on diet and lifestyle factors and used measured height and weight. Our primary assessment of vitamin D status was a reliable biomarker, serum [25(OH)D] and the laboratory coefficients of variation from blinded duplicate samples was excellent (3.7%). Vitamin D metabolites are stable for long periods of time at −80°C and are unaffected by freeze-thaw cycles (39, 40). While the blood specimens were drawn 24-months after enrollment, breast cancer treatments (other than tamoxifen) were completed by the blood draw date, so confounding by treatment should be minimized.
There are also limitations. Firstly, while HEAL included African-American and Hispanic women, the study sample was still mostly non-Hispanic white women. Secondly, we did not collect data on sun exposure, sunscreen use or time spent out-of-doors, so we are unable to assess UV-B exposure; instead geographic location is a proxy for sunlight exposure (1, 25, 41). Thirdly, we had missing ER receptor status on approximately one-third of HEAL participants, so we had insufficient statistical power to test whether these tumor characteristics were associated with vitamin D status. Further, there are limitations to assessing dietary vitamin D in the diet due to the limited food sources, as well as to overall limitations in self-reported diet (42). Lastly, since we only have blood specimens collected post-breast cancer diagnosis, we are unable to definitively establish the temporal relationship between vitamin D status and any breast cancer clinical characteristics.
In conclusion, in this multiethnic cohort of breast cancer survivors, most women had low serum concentrations of [25(OH)D], the primary biomarker used to assess vitamin D status. While insufficient vitamin D status is a concern in the general U.S. population (43, 44), particularly among the elderly (45) and African-Americans (43, 46), the findings from this study show that breast cancer survivors may be at particular risk for vitamin D deficiency. It is unknown whether vitamin D status predicts prognosis; further investigations are needed to examine this critical issue. In the meantime, clinicians may want to consider testing their breast cancer patients for serum [25(OH)D] and offer appropriate recommendations, if necessary, to improve vitamin D status.
Acknowledgements
The authors’ responsibilities were as follows – MLN designed the study and analyses and drafted the manuscript, BS was the study statistician and conducted all statistical analyses, BWH conducted the 25(OH)D assays and contributed to the interpretation of results and manuscript writing, AA, AMc, LB, FG, KB, RB secured funding, recruited participants and collected data, SW contributed to the drafting of the manuscript, RBB provided overall leadership to the HEAL study, including securing of funding and participation in all decisions related to analyses and manuscript preparation. All authors contributed to the interpretation of results and to the writing of the manuscript. All authors reviewed and accepted the final version.
Funding Sources: Funding for this work was provided by National Cancer Institute contracts N01-CN-75036-20, N01-CN-05228, N01-PC-67010/N01-PC-35139, N01-PC-67007/N01-PC-35138 and N01-PC-67009/N01-PC-35142, and National Institutes of Health training grant T32 CA09661. A portion of this work was conducted through the Clinical Research Center at the University of Washington and supported by National Institutes of Health grant M01-RR-00037. Data collection for the Women’s Contraceptive and Reproductive Experiences Study (CARE) at the University of Southern California was supported by the National Institute of Child Health and Human Development contract N01-HD-3-3175. Patient identification was supported in part by the California Department of Health Services grant 050Q-8709-S1528.
Footnotes
None of the authors had a conflict of interest.
References
- 1.Dietary Reference Intakes for Calcium, Phosphorous, Magnesium, Vitamin D and Fluoride. National Academy Press; Washington, DC: 1997. [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. American Journal of Clinical Nutrition. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 3.Jensen S, Madsen M, Lukas J. Inhibitory effects of 1,25-dihydroxyvitamin D2 on the G1-S phase controlling machinery. Molecular Endocrinology. 2001;15:1370–1380. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- 4.Colston KW, Berger U, Coombes CR. Possible role for vitamin D in controlling breast cancer cell proliferation. Lancet. 1989:188–91. doi: 10.1016/s0140-6736(89)91204-x. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GG, Wang M-H, Zhang M, Singh RJ, Siegal GP. 1a,25-dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiology Biomarkers & Prevention. 1997;6:727–32. [PubMed] [Google Scholar]
- 6.Trump D, Hershberger P, Bernardi R, et al. Anti-tumor activity of calcitriol:pre-clinical and clinical studies. Journal of Steroid Biochemistry & Molecular Biology. 2004;89:519–526. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 7.Pike JW, Meyers M, Watanuki M, et al. Perspectives on mechanisms of gene regulation by 1,25-dihydroxyvitamin D3 and its receptor. Journal of Steroid Biochemistry & Molecular Biology. 2007;103:389–95. doi: 10.1016/j.jsbmb.2006.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirianov G, Coulston K. Interaction of Vitamin D analogs with signaling pathways leading to active cell death in breast cancer cells. Steroids. 2001;66:309–318. doi: 10.1016/s0039-128x(00)00201-4. [DOI] [PubMed] [Google Scholar]
- 9.Anzano MA, Smith JM, Uskokovic MR, et al. 1-a,25-dihydroxy-16-ene-23-yne-26,27-hexaflourocholecalciferol, a new delanoid (vitamin D analog) for prevention of breast cancer in the rat. Cancer Research. 1994;54:1653–6. [PubMed] [Google Scholar]
- 10.Mizwicki MT, Bula CM, Bishop JE, Norman AW. A perspective on how the Vitamin D sterol/Vitamin D receptor (VDR) conformational ensemble model can potentially be used to understand the structure–function results of A-ring modified Vitamin D sterols. Journal of Steroid Biochemistry and Molecular Biology. 2005;97:69–82. doi: 10.1016/j.jsbmb.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RG, Moriarty RM, Mehta RR, et al. Prevention of preneoplastic mammary lesion development by a novel vitamin D analogue, 1 a-hydroxyvitamin D5. Journal of the National Cancer Institute. 1997;89:212–8. doi: 10.1093/jnci/89.3.212. [DOI] [PubMed] [Google Scholar]
- 12.Welsh J, Wietzke J. Impact of the vitamin D3 receptor on growth-regulatory pathways in mammary gland and breast cancer. Journal of Steroid Biochemistry & Molecular Biology. 2003;83:85–92. doi: 10.1016/s0960-0760(02)00277-7. [DOI] [PubMed] [Google Scholar]
- 13.Welsh J, Wietzke JA, Zinser GM, Byrne B, Smith K, Narvaez CJ. Vitamin D-3 receptor as a target for breast cancer prevention. Journal of Nutrition. 2003;133:2425S–2433S. doi: 10.1093/jn/133.7.2425S. [DOI] [PubMed] [Google Scholar]
- 14.Bertone-Johnson ER, Chen WY, Holick MF, et al. Plasma 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiology Biomarkers & Prevention. 2005;14:1991–7. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 15.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin d and breast cancer risk in women. Archives of Internal Medicine. 2007;167:1050–9. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 16.McCullough ML, Stevens VL, Diver WR, et al. Vitamin D pathway gene polymorphisms, diet, and risk of postmenopausal breast cancer: a nested case-control study. Breast Cancer Research. 2007;9:R9. doi: 10.1186/bcr1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiology Biomarkers & Prevention. 2007;16:422–9. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- 18.Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: Pooled analysis. Journal of Steroid Biochemistry & Molecular Biology. 2007;103:708–11. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiology Biomarkers & Prevention. 2006;15:1427–37. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 20.Adlercreutz H. Phytoestrogens and breast cancer. Journal of Steroid Biochemistry & Molecular Biology. 2002;83:113–8. doi: 10.1016/s0960-0760(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 21.McTiernan A, Rajan B, Tworoger S, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. Journal of Clinical Oncology. 2003;21:1961–6. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. Journal of Nutrition. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 23.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Annals of Epidemiology. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 24.Expert Panel on the Identification and Treatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. American Journal of Clinical Nutrition. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 25.Ramos J, Villa J, Ruiz A, Armstrong R, Matta J. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiology Biomarkers & Prevention. 2004;13:2006–2011. [PubMed] [Google Scholar]
- 26.Rock CL, Thornquist M, Kristal AR, et al. Demographic, dietary and lifestyle factors differentially explain variability in serum carotenoids and fat-soluble vitamins: baseline results from the Olestra Post-Marketing Surveillance Study. Journal of Nutrition. 1999;129:855–64. doi: 10.1093/jn/129.4.855. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. Vitamin D. In: Stipanuk MH, editor. Biochemical and Physiological Aspects of Human Nutrition. W.B. Saunders; Philadelphia, PA: 2000. pp. 624–636. [Google Scholar]
- 28.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. American Journal of Clinical Nutrition. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 29.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D 3 from sunlight may improve the prognosis of breast-, colon-and prostate cancer (Norway) Cancer Causes and Control. 2004;15:149–58. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 30.Zhou W, Heist RS, Liu G, et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small-cell lung cancer patients. Journal of Clinical Oncology. 2007;25:479–85. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 31.Zhou W, Suk R, Liu G, et al. Vitamin D is associated with improved survival in early-stage non-small cell lung cancer patients. Cancer Epidemiology Biomarkers & Prevention. 2005;14:2303–9. doi: 10.1158/1055-9965.EPI-05-0335. [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. Journal of the National Cancer Institute. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 33.Schauber J, Dorschner R, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. Journal of Clinical Investigation. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 35.Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Molecular Cancer Therapeutics. 2006;5:797–808. doi: 10.1158/1535-7163.MCT-05-0539. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T. 1 alpha, 25-dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis. 2005;26:429–40. doi: 10.1093/carcin/bgh332. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs ET, Alberts DS, Benuzillo J, Hollis BW, Thompson PA, Martinez ME. Serum 25(OH)D levels, dietary intake of vitamin D, and colorectal adenoma recurrence. Journal of Steroid Biochemistry & Molecular Biology. 2007;103:752–6. doi: 10.1016/j.jsbmb.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vijayakumar S, Mehta RR, Boerner PS, Packianathan S, Mehta R. Clinical trials involving vitamin D analogs in prostate cancer. Cancer Journal. 2005;11:362–73. doi: 10.1097/00130404-200509000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Berry JL, Selby PL, Davies M, Maritin J. Observations from the UK Supra-Regional Assay Service laboratory for the measurement of vitamin D metabolites. Journal of Steroid Biochemistry & Molecular Biology. 2007;103:477–9. doi: 10.1016/j.jsbmb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Antoniucci DM, Black DM, Sellmeyer DE. Serum 25-hydroxyvitamin D is unaffected by multiple freeze-thaw cycles. Clinical Chemistry. 2005;51:258–61. doi: 10.1373/clinchem.2004.041954. [DOI] [PubMed] [Google Scholar]
- 41.Solomon CC, White E, Kristal AR, Vaughan T. Melanoma and lifetime UV radiation. Cancer Causes & Control. 2004;15:893–902. doi: 10.1007/s10552-004-1142-9. [DOI] [PubMed] [Google Scholar]
- 42.Neuhouser ML, Tinker L, Schoeller DA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. American Journal of Epidemiology. 2008 doi: 10.1093/aje/kwn026. in press. [DOI] [PubMed] [Google Scholar]
- 43.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. American Journal of Clinical Nutrition. 2002;76:187–92. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 44.Holick MF. Vitamin D deficiency. New England Journal of Medicine. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 45.Visser M, Deeg DJH, Puts MTE, Seidell JC, Lips P. Low serum concentrations of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. American Journal of Clinical Nutrition. 2006;84:616–22. doi: 10.1093/ajcn/84.3.616. [DOI] [PubMed] [Google Scholar]
- 46.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. American Journal of Clinical Nutrition. 1998;67:1108–10. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]