The dentate gyrus (DG) of the mammalian hippocampus is hypothesized to mediate pattern separation—the formation of distinct and orthogonal representations of mnemonic information—and also undergoes neurogenesis throughout life. How neurogenesis contributes to hippocampal function is largely unknown. Using adult mice in which hippocampal neurogenesis was ablated we found specific impairments in spatial discrimination using two behavioral assays: a spatial navigation radial arm maze task and a spatial, but non-navigable, task in the mouse touch screen. Mice with ablated neurogenesis were impaired when stimuli were presented with little spatial separation, but not when stimuli were more widely separated in space. Thus, newborn neurons may be necessary for normal pattern separation function in the DG of adult mice.
The dentate gyrus (DG) is thought to contribute to spatial or episodic memory by functioning as a pattern separator (1-3). Pattern separation is the formation of distinct representations of similar inputs (4). At the cellular level, pattern separation is achieved through the dispersion of cortical inputs from the entorhinal cortex onto a greater number of dentate granule cells (DGCs) with small place fields. By virtue of low firing rates (5) and sparse connectivity between DGCs and CA3 pyramidal cells (6), DGCs are particularly adapted to maintain and transmit orthogonalized information. This ability to pattern separate, or differentially encode small or weak changes derived from increasingly similar or interfering inputs, is particularly important for the accuracy of memory encoding. Similarly, at the behavioral level, the ability to form and use memories derived from very similar stimuli that are closely presented in space and/or time depends upon the ability to pattern separate incoming, and often complex, information (1, 7, 8). Lesions of the complete DG circuitry result in impaired pattern separation dependent memory (7-9).
The DG is also one of two sites where neurogenesis is ongoing throughout life (10). Adult born neurons integrate into DG circuitry (11-13) and are thought to play a role in learning and memory (11, 14, 15), but their contribution to hippocampal function remains unclear, in part due to the limited availability of behavioral assays probing this question.
We used low dose X-irradiation (32) to focally ablate neurogenesis in the hippocampus of 8-week old adult female C57Bl/6 mice (16, 17) while sparing the rest of the brain, including the Subventricular Zone (SVZ) (Figs. 1A-C, 2A, 2G and H, S1A and B, S2-4). To confirm that newborn neurons had been persistently ablated as well as to examine the extent of inflammation in the hippocampus following a 2 month recovery period post irradiation, we analyzed the brains of IR and sham ‘test’ mice (n=5) that were killed the day behavioral testing commenced. IR ‘test’ mice did not show differences in microglia numbers or morphology compared to sham controls (Fig. S1C and D), but they did show a significant reduction in total numbers of both immature neurons and proliferating cells in the hippocampus (Fig. S2A-E).
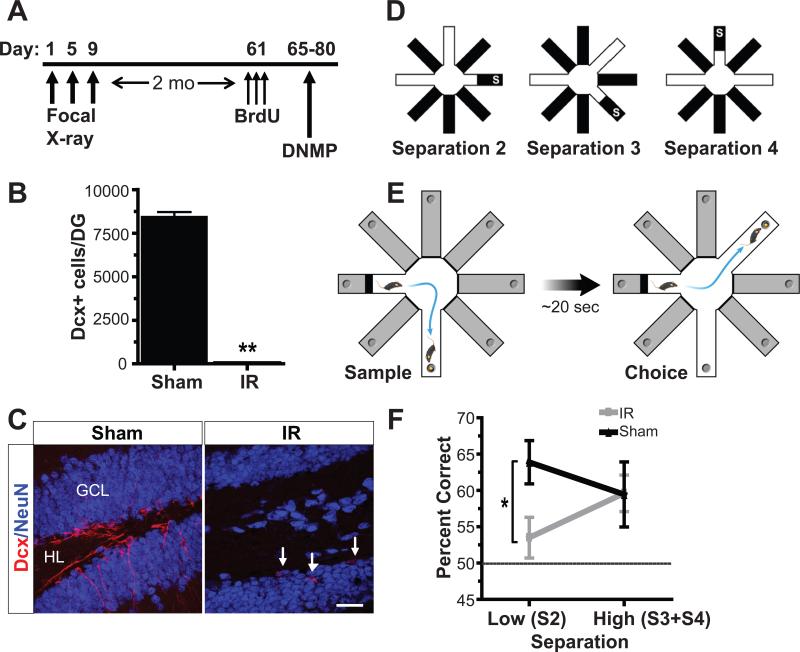
Figure 1. Mice with ablated neurogenesis due to focal X-irradiation show impaired spatial memory for similar, but not distinct, spatial locations in the radial arm maze.
(A) Mice were irradiated 2 months prior to behavioral testing. (B,C) Irradiation significantly reduced the total numbers of immature Dcx+ cells in irradiated (IR) mice (C, right, white arrows) compared to sham controls (C, left) (independent samples t-test, t(17)=29.82 , p<0.001). (D) Pattern separation was tested using a DNMP protocol in the RAM by varying the distance between sample and correct arms: S2/low, S3 and S4/high (S=start arm). (E) Each trial consisted of a sample phase (left) and a choice phase (right). The mouse had to non-match to the novel location. (F) IR mice were impaired at low (S2) but not high (S3+S4) separations in the DNMP task. Dashed line represents chance. Error bars represent SEM. Scale bars represent 25 μm. ** p<0.01, * p<0.05. GCL indicates granule cell layer; HL, hilus.
Figure 2. Mice with ablated neurogenesis due to focal X-irradiation show impaired spatial discrimination for similar, but not distinct, spatial locations but not impaired associative object in-place memory in the mouse touch screen.
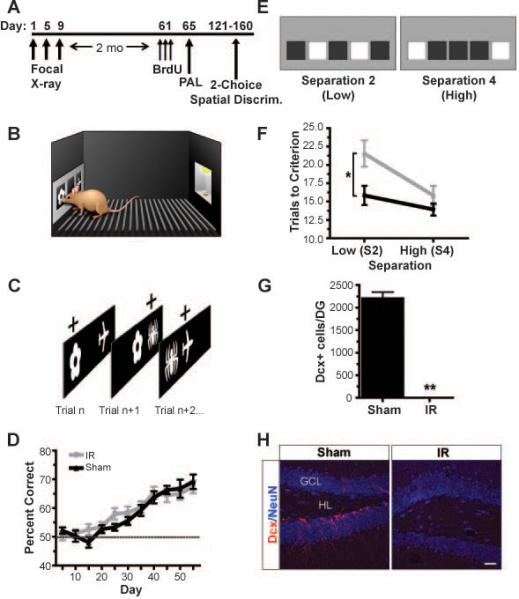
Mice were irradiated 2 months prior to behavioral testing as in (A). Following pre-training for 7-10 days in which mice learned to nose-touch stimuli on the infrared touch screen (B) to obtain a reward, mice were trained on an associative object-in-place task (PAL) (C). For example, as in the left panel of (C), mice had to choose flower-left as a correct association over the incorrect association of plane-right in order to obtain a reward. (D) Irradiated mice (IR) learned the PAL task at the same rate as sham controls (dashed line represents chance). (E) Mice were then tested on a two-choice spatial discrimination task in which mice had to respond to the correct location (e.g., left illuminated box of left screen, E) until a criterion of 7 of 8 consecutive correct touches was recorded before reversing to the previously incorrect location (e.g., right illuminated box of left screen, E). Mice were tested on either the low separation (S2; left screen) or the high separation (S4; right screen) as depicted in (E) during each testing day. (F) IR mice exhibited significantly impaired performance at low (S2) separations but not high (S4) separations during acquisition of this task, consistent with a pattern separation also deficit observed in the first experiment (Fig. 1). (G,H) Irradiation significantly reduced the total numbers of immature Dcx+ cells in IR mice (H, right) compared to sham controls (H, left) (independent samples t-test, t(17)=18.14, p<0.001). Error bars represent SEM. Scale bars represent 50 μm. **p<0.01, * p<0.05. GCL indicates granule cell layer; HL, hilus.
Two months after irradiation, IR (n=10) and sham mice (n=9) were tested in a delayed non-matching to place (DNMP) radial arm maze (RAM) task that we developed to test spatial pattern separation dependent memory (Fig. 1). As we had hypothesized that deficits resulting from a knock-down of neurogenesis might be subtle, we purposely designed a challenging spatial task by using a large 8-arm RAM and ensuring the use of external spatial cues in forming spatial memories while eliminating odor as a facilitatory intramaze cue. The difficulty of this task was reflected in lower performance levels by sham mice compared to other RAM tasks (18). Mice were tested for the ability to select from a choice of two arms that had not been presented in a previous sample phase (DNMP) (Fig. 1E). During the sample phase all arms except a start arm and the sample arm were blocked off. The mouse was permitted to visit the sample arm and retrieve a food pellet reward. To eliminate the ability of mice to use odor as a facilitatory intramaze cue, the RAM apparatus was rotated on wheels between sample and choice presentations, such that the location of the start and sample arms, but not the arms themselves, were held constant during each trial. The rotation took approximately 20 seconds. During the choice phase, arms in the start and sample (unrewarded) locations and an additional correct (rewarded) location were open. Correct arms varied in distance from the sample arm by a spatial separation of 2, 3, or 4 arms (Fig. 1D). Mice that entered the correct (rewarded) arm were considered to have made correct choices. Mice that made incorrect choices (i.e., entered the sample/unrewarded arm) were allowed to self-correct. Mice received 4 trials (sample + choice phases) per day of pseudorandomly presented combinations of start+sample+correct arms for 15 consecutive days (60 trials total, 20 trials of each spatial separation) (32).
We analyzed pattern separation dependent memory by testing whether mice could differentiate between locations that were presented closely in space (S2) versus those that were more highly separated (S3 and S4). IR mice were selectively impaired at low separations (S2) but not at high separations (S3+S4) (significant interaction, repeated measures ANOVA: F(1,17)=4.57, p=0.047; Bonferroni corrected independent samples t-tests: S2: t(17)=2.55, p=0.021; S3+S4: t(17)=0.03, p=0.974) (Fig. 1F). These results suggest that adult hippocampal neurogenesis was not required to perform the task in which sample and correct arms were presented with a high degree of spatial separation (S3,S4) but was required to correctly discriminate between choice and sample arms when presented in close spatial proximity.
To further examine whether loss of adult hippocampal neurogenesis results in global hippocampal deficits or specific pattern separation memory deficits, we tested a naive cohort of IR (n=10) and sham (n=9) mice on a challenging hippocampus-dependent spatial learning task and a two-choice spatial discrimination (pattern separation) task in the mouse touch screen (Fig. 2) (32). The mouse touch screen is useful in that all trials are directed by the mouse through an initiation process and all testing is independent of the experimenter. The testing apparatus (Fig. 2B) consisted of a standard modular chamber fitted with an infrared touch screen, a pellet dispenser, and receptacle with light illumination and head entry detectors, and a tone generator. Mice were pre-trained through several iterative stages to nose-touch stimuli on the screen to obtain a reward (19). Mice were then trained on a paired associates learning (PAL) object-in-place task (20) that tests the ability to associate correctly three objects (flower, plane, spider) with their correct spatial locations on the screen (left, middle, right, respectively) (Fig. 2C). Mice were only rewarded when they identified the correct object in its correct location during a choice between two objects: one object in its correct spatial location and one object in one of two incorrect locations. Mice were given 36 trials/days plus correction trials over 55 days. Both IR and sham mice learned the task at the same rate (repeated measures ANOVA, F(53,742)=0.18, p=0.671) (Fig. 2D). The demonstration that the performance of IR mice was not different from that of sham mice on this hippocampus-dependent spatial task indicates that mice without neurogenesis are still capable of acquiring, at a normal rate, a complex task involving spatial information in the touch screen.
These IR and sham mice were next tested for spatial discrimination ability in the touch screen using a hippocampus-dependent two-choice spatial discrimination paradigm (21) (Fig. 2E). Briefly, mice were required to choose the correct spatial location between 2 illuminated boxes in 2 of 5 possible locations until a criterion (7 of 8 consecutive touches) was reached. Once criterion was met, the correct and incorrect locations automatically switched. Similar to the DNMP task in the RAM, pattern separation was tested by varying the distance between choice locations. Lit choice boxes were either far apart, i.e., separated by 3 unlit ‘spaces’ (high separation, S4; Fig. 2E) or close together, i.e., separated by 1 unlit ‘space’ (low separation, S2; Fig. 2E). Spatial separations were held constant during each testing session/day but were varied across testing days.
In agreement with our findings using the RAM, IR mice were significantly impaired at low (S2) but not high (S4) separations during acquisition (significant interaction, repeated measures ANOVA, average trial to criterion: F(1,17)=6.04, p=0.025; Bonferroni corrected independent samples t-tests: S2: t(17)=2.54, p=0.020; S4: t(17)=0.63, p=0.540) (Fig. 2F). Ablating neurogenesis using focal X-irradiation induces impairments consistent with a deficit in pattern separation in two independent tasks carried out in two very different testing situations. This impairment appears to be specific, as IR mice were capable of learning difficult object-place associations (PAL) at the same rate and to the same performance level as sham mice. Furthermore, the spatial memory deficits observed were similar in both the navigable RAM and non-navigable touch screen.
Given concerns regarding potential off-target effects due to focal X-irradiation, despite the long recovery period, we employed a second independent method to knock down neurogenesis using lentiviral expression of dominant negative Wnt (dnWnt) protein and then tested these mice on the same RAM protocol (Figs. 3, S5A) used in the first experiment described above. We used the previously described lentiviral vectors expressing CMV-driven dnWnt followed by an internal ribosomal entry site (IRES)-green fluorescent protein (GFP) from the same vector or a control vector expressing only GFP (GFPcon) (22, 32).
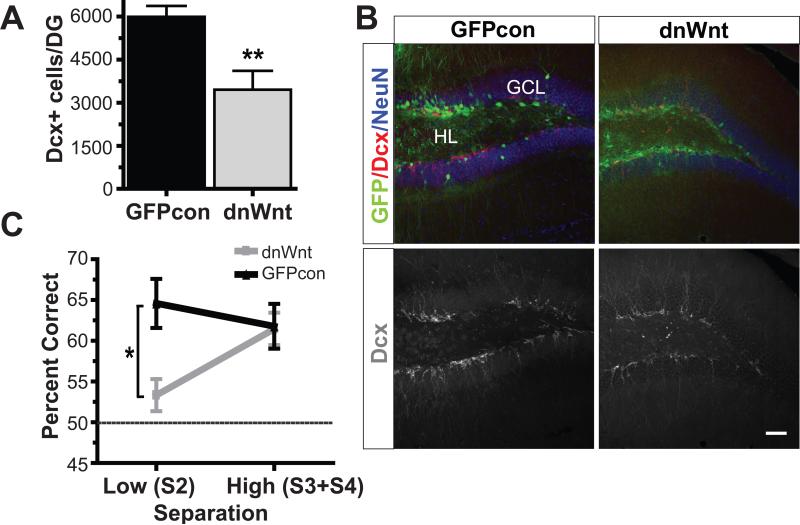
Figure 3. Mice with decreased neurogenesis due to targeted lentiviral expression of dominant negative Wnt show impaired spatial memory for similar, but not distinct, spatial locations in the radial arm maze in a similar pattern to that seen in irradiated mice.
(A,B) Dominant negative Wnt expression (B, right) significantly reduced the total numbers of immature Dcx+ cells in dnWnt mice (A) compared to GFP controls (B, left) (independent samples t-test: t(24)=3.47, p=0.002). Single channel images depicting Dcx+ cells are shown below triple channel images in (B). (C) Pattern separation was tested using a DNMP as in Fig.1. Dominant negative Wnt mice were impaired at low (S2) but not high (S3+S4) separations in the DNMP task. Error bars represent SEM. Scale bars represent 50 μm. ** p<0.01, * p<0.05. GCL indicates granule cell layer; HL, hilus.
Inhibition of Wnt signaling locally in the DG reduces the number of newborn neurons without affecting progenitor proliferation in other brain regions (22, 23). Eight-week old C57Bl/6 female mice received bilateral stereotaxic injections of 1 μl of either dnWntexpressing lentivirus (n=16) or GFPcon lentivirus (n=15) into the DG, resulting in a significant reduction in proliferating cells and neurogenesis (Fig. 3A and B, Fig. S5). Behavioral testing in the RAM commenced 2 months after viral injection. Similar to the pattern separation deficit observed in IR mice, dnWnt mice were impaired at low (S2) but not high (S3+S4) separations compared to GFPcon mice (significant interaction, repeated measures ANOVA: F(1,24)=4.51, p=0.044; Bonferroni corrected independent samples t-tests: S2: t(24)=3.02 , p=0.006; S3+S4: t(24)=0.46, p=0.926) (Fig. 3C). Thus, mice with decreased neurogenesis due to expression of the dnWnt protein are impaired at spatial pattern separation or the ability to correctly distinguish rewarded from nonrewarded spatial locations only when stimuli to be discriminated are presented closely in space.
This study provides experimental evidence of a role for newborn neurons in the adult DG in spatial discrimination, consistent with a role in spatial pattern separation. We used two independent strategies to ablate neurogenesis and the observed deficit was similar in two very distinct testing contexts. Importantly, mice with ablated neurogenesis showed a selective impairment specific to memory performance depending on pattern separation but were not impaired at the hippocampal dependent PAL task, indicating that mice were able to learn complex associations in which space was a component.
Previous studies involving rodent lesions of either the dorsal hippocampus (21) or the DG (7-9) suggest that regions outside of the DG are responsible for disambiguating memories derived from spatially distinct inputs (comparable to the large separations used in this study). In addition, it has been suggested that recruitment of independent cell populations in the CA3 alone, presumably via direct input from the entorhinal cortex (25), may be sufficient to disambiguate memories for more distinct spatial inputs (1, 26, 27) or make associations between objects and space (28). In this context it is interesting to note that the impairments following ablation of neurogenesis described here are parameter-sensitive (i.e., specific to conditions with a high premium on pattern separation), which may help to explain the variable and sometimes contradictory results from other neurogenesis-ablation studies in which this parameter was not explicitly considered (17, 23, 29, 30).
Interestingly, dnWNT mice with roughly a 50% decrease in neurogenesis had a similar pattern of impairment to that seen in the IR group in which neurogenesis was almost completely ablated, suggesting that there may be a critical threshold for the amount of neurogenesis that is behaviorally significant. A level-dependent requirement of adult neurogenesis for hippocampus-dependent learning has also been reported in rats (23). In addition, our RAM task may be sufficiently challenging that even partial manipulation of newborn neuron numbers is adequate to impair performance.
The DG has been shown to be important for pattern separation and our results show that adult neurogenesis appears to be important for the ability of the DG to perform that function optimally. It remains to be investigated whether immature neurons contribute to pattern separation directly or whether they contribute in more complex ways to a circuit necessary for normal DG function, as suggested by recent modeling studies (31), and whether the function of immature neurons is distinct from that of mature granule cells.
Supplementary Material
References and Notes
- 1.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Science. 2007 Feb 16;315:961. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 2.Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Science. 2008 Feb 29;319:1260. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- 3.Bakker A, Kirwan CB, Miller M, Stark CE. Science. 2008 Mar 21;319:1640. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marr D. Philos Trans R Soc Lond B Biol Sci. 1971 Jul 1;262:23. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 5.Jung MW, McNaughton BL. Hippocampus. 1993 Apr;3:165. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 6.Amaral DG, Ishizuka N, Claiborne B. Prog Brain Res. 1990;83:1. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert PE, Kesner RP, Lee I. Hippocampus. 2001;11:626. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert PE, Kesner RP, DeCoteau WE. J Neurosci. 1998 Jan 15;18:804. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunsaker MR, Kesner RP. Hippocampus. 2008;18:955. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao C, Deng W, Gage FH. Cell. 2008 Feb 22;132:645. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 11.van Praag H, et al. Nature. 2002 Feb 28;415:1030. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toni N, et al. Nat Neurosci. 2008 Aug;11:901. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jessberger S, Kempermann G. Eur J Neurosci. 2003 Nov;18:2707. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 14.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Proc Natl Acad Sci U S A. 1999 Nov 9;96:13427. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Nat Neurosci. 1999 Mar;2:260. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 16.Santarelli L, et al. Science. 2003 Aug 8;301:805. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 17.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. Neuroscience. 2005;130:843. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein D, et al. Pharmacol Biochem Behav. 1985 Feb;22:301. doi: 10.1016/0091-3057(85)90395-8. [DOI] [PubMed] [Google Scholar]
- 19.Morton AJ, Skillings E, Bussey TJ, Saksida LM. Nat Methods. 2006 Oct;3:767. doi: 10.1038/nmeth1006-767. [DOI] [PubMed] [Google Scholar]
- 20.Talpos JC, Winters BD, Dias R, Saksida LM, Bussey TJ. Psychopharmacology (Berl) 2009 Apr 9; doi: 10.1007/s00213-009-1526-3. [DOI] [PubMed] [Google Scholar]
- 21.McTighe S. MA, Romberg C, Bussey TJ, Saksida LM. Neuroreport. 2009 doi: 10.1097/WNR.0b013e32832c5eb2. In press. [DOI] [PubMed] [Google Scholar]
- 22.Lie DC, et al. Nature. 2005 Oct 27;437:1370. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 23.Jessberger S, et al. Learn Mem. 2009;16:147. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meshi D, et al. Nat Neurosci. 2006 Jun;9:729. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 25.Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Nature. 2007 Mar 8;446:190. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- 26.Leutgeb S, et al. Science. 2005 Jul 22;309:619. [Google Scholar]
- 27.Vazdarjanova A, Guzowski JF. J Neurosci. 2004 Jul 21;24:6489. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert PE, Kesner RP. Behav Neurosci. 2003 Dec;117:1385. doi: 10.1037/0735-7044.117.6.1385. [DOI] [PubMed] [Google Scholar]
- 29.Zhang CL, Zou Y, He W, Gage FH, Evans RM. Nature. 2008 Feb 21;451:1004. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 30.Wojtowicz JM, Askew ML, Winocur G. Eur J Neurosci. 2008 Mar;27:1494. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- 31.Aimone JB, Wiles J, Gage FH. Neuron. 2009 Jan 29;61:187. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Materials and methods are available as supporting material on Science Online.
- 33.We thank M.J. Armstrong, S. and J.B. Aimone for useful input and discussions, M.L. Gage for editorial comments, and J. Simon for assistance with figures. This work was funded in part by the James S. McDonnell, Lookout and Mather's Foundations, Kavli Institute for Brain and Mind, and the U.S. National Institutes of Health (NS-050217) (F.H.G.), and by generous donations from patients and families to the Huntington's and Parkinson's Disease Research Clinics at the BRC, University of Cambridge (R.A.B.). This work was also supported in part by MaxnetAging and NCCR Neural Plasticity and Repair (S.J.) and Marshall's and Jack Kent Cooke Foundation Scholarships (C.D.C.).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.