Abstract
Background
Cerebral amyloid angiopathy (CAA)-type 1 is characterized by amyloid β-protein (Aβ) deposition along cerebral capillaries and is accompanied by perivascular neuroinflammation and accumulation of phospho-tau protein. Tg-SwDI mice recapitulate capillary amyloid deposition and associated neuroinflammation but lack accmulation of perivascular phospho-tau protein.
Methods
Tg-SwDI mice were bred onto a nitric oxide synthase 2 gene knockout (NOS2−/−) background and aged for one year. Brains were harvested and analyzed using immunohistochemical and quantitative stereological methods to determine the extent of capillary amyloid deposition, perivascular activated microglia, and cell-specific accumulation of phospho-tau protein. Similar methods were also used to compare Tg-SwDI/NOS2−/− and human CAA-type 1 brain tissues.
Results
The absence of NOS2 had no effect on the regional pattern or frequency of capillary CAA or the numbers of perivascular activated microglia in Tg-SwDI mice. On the other hand, Tg-SwDI/NOS2−/− mice accumulated phospho-tau protein in perivascular neurons and activated microglia. Tg-SwDI/NOS2−/− mice exhibited a very similar distribution of capillary amyloid, activated microglia, and perivascular phospho-tau protein as seen in human CAA-type 1.
Conclusions
These findings indicate that Tg-SwDI/NOS2−/− mice more fully recapitulate the pathological changes observed with capillary amyloid in human CAA-type 1.
Keywords: capillary, cerebral amyloid angiopathy, nitric oxide synthase 2, phospho-tau, transgenic mice
Cerebral amyloid angiopathy (CAA) is a condition characterized by accumulation of fibrillar amyloid β-protein (Aβ) deposits in blood vessels of the brain of patients with Alzheimer’s disease and related disorders1. Aβ is a 4 kDa peptide that is derived from its parent protein, the amyloid β-protein precursor (AβPP), through the sequential processing by β- and γ-secretase enzymes2. CAA presents as two subtypes: the more common CAA type-2 affects arterioles and arteries of the cortex and leptomeninges whereas the less common CAA-type 1 affects cerebral capillaries with or without larger cerebral vessel involvement3. Capillary fibrillar amyloid deposition in CAA type-1 is accompanied by a localized robust neuroinflammatory response and perivascular phospho-tau protein accumulation that is not commonly associated with the affected larger vessels in CAA type-2. Previously, we generated a unique transgenic mouse, known as Tg-SwDI, that expresses in brain human AβPP harboring tandem Dutch (E22Q) and Iowa (D23N) Aβ mutations associated with familial forms of CAA4. Tg-SwDI mice were shown to develop early-onset and regionally extensive capillary amyloid with associated neuroinflammation reminiscent of CAA type-14,5.
Nitric oxide synthase 2 (NOS2), the inducible form of NOS, is one of three isoforms of NOS that are responsible for generating nitric oxide (NO)6. NO has been reported to possess both cytotoxic and cytoprotective activities depending on its concentration and cellular targets7. Recently, we showed that breeding Tg-SwDI mice onto a NOS2 deletion background (NOS2−/−) resulted in more severe capillary CAA related pathology including perivascular accumulation of phospho-tau protein, neuronal loss, and greater behavioral impairments8. In the present study we determined if the absence of NOS2 affected the frequency of capillary CAA and associated microglial activation in Tg-SwDI mice. We also evaluated what cell types exhibit perivascular phospho-tau accumulation in Tg-SwDI/NOS2−/− mice and how these pathologies directly compare with human capillary CAA type-1
Methods
Transgenic mice
All work with animals followed National Institutes of Health guidelines and was approved by the Stony Brook University Institutional Animal Care and Use Committee. The generation and characterization of Tg-SwDI and Tg-SwDI/NOS2−/− mice has been described4,5. For the experiments mice were aged to twelve months prior to euthanasia and collection of brain tissues for analysis.
Immunohistochemical analysis
Immunostainings of mouse and human brain tissues were performed on de-paraffined sections or fresh frozen sections as described9. The following antibodies were used: rabbit polyclonal antibody to collagen type IV for identification of blood vessels (1:100; Research Diagnostics Inc., Flanders, NJ); monoclonal antibody AT8 for detecting phospho-tau protein (1:250; Thermo Fisher Scientific); monoclonal antibody NeuN for identification of neurons (1:500; Chemicon); monoclonal antibody to glial fibrillary acidic protein (GFAP) for identification of astrocytes (1:1000, Chemicon); and monoclonal antibody to MHC class II for identification of activated microglia (1:200; BD Pharmingen, San Jose, CA). Thioflavin-S staining for fibrillar amyloid was performed as described9.
Quantitative analysis of regional capillary CAA and activated microglia
The percentage of thioflavin-S labeled blood vessels and immunostained activated microglia in the regions of the thalamus and fronto-temporal cortex was respectively quantified on the same set of systematically sampled thioflavin-S stained sections using the Stereologer software system (Systems Planning and Analysis) as described5,10.
Results
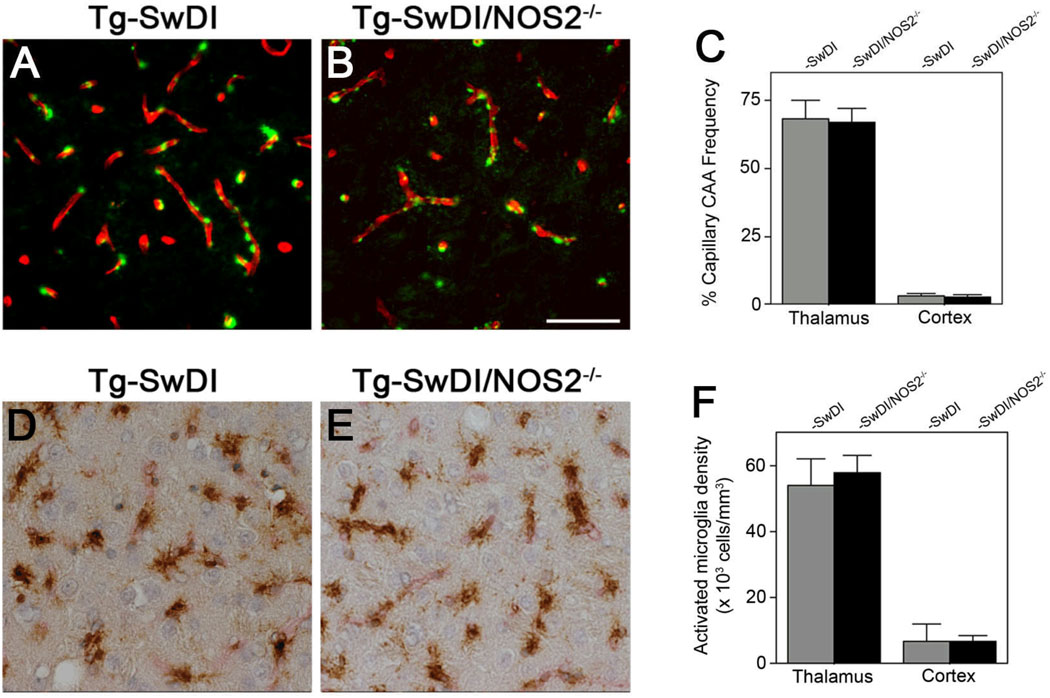
Immunohistochemical analysis to identify capillary fibrillar amyloid and the frequency of these deposits was measured using stereological methods. As shown in Figure 1, a similar pattern of capillary amyloid deposition in Tg-SwDI and Tg-SwDI/NOS2−/− mice was observed (Fig. 1A and 1B, respectively) with no quantitative difference in the regional frequency of affected capillaries (Fig. 1C). Similarly, a common pattern of capillary-associated activated microglia was observed in Tg-SwDI and Tg-SwDI/NOS2−/− mice (Fig. 1D and 1E, respectively) again with no difference in the regional densities of these neuroinflammatory cells (Fig. 1F). These findings indicate that the absence of NOS2 does not affect capillary CAA and associated activated microglia in Tg-SwDI mice.
Figure 1.
Quantitation of brain capillary amyloid frequency and associated activated microglia in Tg-SwDI mice and Tg-SwDI/NOS2−/− mice. Brain sections from twelve months old Tg-SwDI mice (A,D) or Tg-SwDI/NOS2−/− (B,E) mice were immunolabeled for collagen Type IV to identify capillaries (red) and stained with thioflavin S (green) to identify fibrillar amyloid (A,B) or immunolabeled for MHC II (brown) to identify activated microglia (D,E). Scale bar = 100µm. Quantitative stereological measurement of capillary amyloid frequency (C) and activated microglia densities (F) in thalamus and cortex of Tg-SwDI mice (gray bars) and Tg-SwDI/NOS2−/− mice (black bars). Data shown are the mean ± S.D. (n = 6 mice for each group).
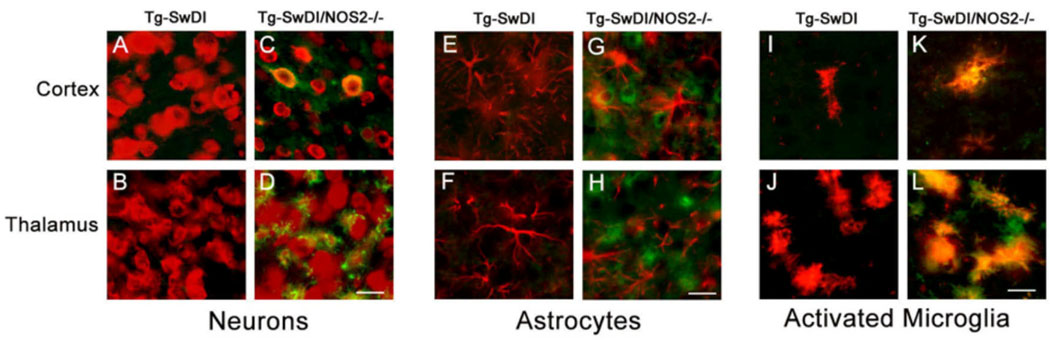
Previously we reported that perivascular AT8-positive phospho-tau protein accumulates in Tg-SwDI/NOS2−/− mice8. However, the specific cell types that accumulated phospho-tau were not thoroughly determined. Therefore, brain sections from Tg-SwDI and Tg-SwDI/NOS2−/− mice were double immunolabeled for AT8 phospho-tau and markers specific for neurons, astrocytes, or activated microglia. As shown in Fig. 2, AT8 phospho-tau labeling was only observed in Tg-SwDI/NOS2−/− mice. Co-localization of AT8 phospho-tau labeling was observed in cortical neurons and some thalamic neurons (Fig. 2C and 2D, respectively). In contrast, no co-localization of AT8 phospho-tau was observed with astrocytes (Fig. 2G and 2H). However, strong co-localization of AT8 phospho-tau was observed in some cortical but many thalamic activated microglia that are tightly associated with capillary amyloid deposits (Fig. 2K and 2L, respectively). These results indicate that AT8 phospho-tau is present in some perivascular neurons and many activated microglia in regions of capillary amyloid deposition in Tg-SwDI/NOS2−/− mice.
Figure 2.
Immunolabeling for hyperphosphorylated tau co-localizes with perivascular neurons and activated microglia in Tg-SwDI/NOS2−/− mice. Brain sections from twelve months old Tg-SwDI mice or Tg-SwDI/NOS2−/− mice were immunolabeled for hyperphosphorylated tau using AT8 antibody (green). Neurons were identified using NeuN antibody (A-D), astrocytes using GFAP antibody (E-H), and activated microglia using the MHC II antibody (I-L). Scale bars = 20 µm.
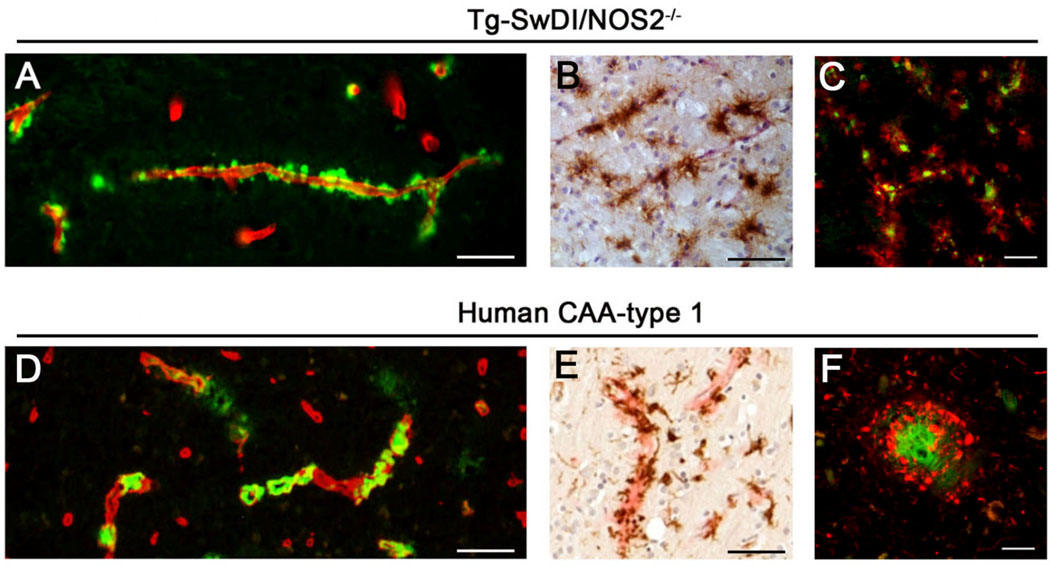
Capillary amyloid and associated pathology observed in Tg-SwDI/NOS2−/− mice were compared to human CAA type-1 brain tissues using immuno- and histo-chemical stainings. Thioflavin S staining revealed a very similar pattern of capillary amyloid accumulation in Tg-SwDI/NOS2−/− mice and human CAA type-1 (Fig. 3A and 3D, respectively). Furthermore, the capillary amyloid associated accumulation of perivascular activated microglia and perivascular phospho-tau in Tg-SwDI/NOS2−/− mouse brain (Fig. 3B and 3C, respectively) was highly reminiscent of that seen in human CAA type-1 brain (Fig. 3E and 3F, respectively). These findings show that Tg-SwDI/NOS2−/− mice recapitulate several key features of capillary amyloid and associated pathological changes observed in human CAA type-1.
Figure 3.
Brain capillary amyloid deposition and associated pathology in Tg-SwDI/NOS2−/− mice and human CAA Type 1. Brain sections from twelve months old Tg-SwDI/NOS2−/− mice (A,B) and a case of human CAA Type 1 (D,E) were immunolabeled for collagen Type IV to identify capillaries (red) and stained with thioflavin S (green) to identify fibrillar amyloid (A,D) or immunolabeled for MHC II (brown) to identify activated microglia (B,E). Scale bars = 50 µm. Similarly, brain sections from twelve months old Tg-SwDI/NOS2−/− mice (C) and a case of human CAA Type 1 (F) were immunolabeled for hyperphosphorylated tau using AT8 antibody (red) and stained with thioflavin S (green) to identify fibrillar amyloid. Scale bars = 20 µm.
Discussion
In the present study, we show that Tg-SwDI/NOS2−/− mice exhibit many of the pathological features of capillary amyloid found in human CAA type-1. The absence of NOS2 does not affect the regional distribution or frequency of capillary amyloid deposition nor the levels of associated activated microglia in Tg-SwDI mice. However, we previously reported that in the absence of NOS2 the Tg-SwDI mice show additional pathological features including perivascular phospho-tau, neuronal loss, and greater behavioral deficits8. Here we further investigated which types of perivascular cells exhibit AT8-positive phospho-tau accumulation. Although some cortical neurons showed labeling for phospho-tau we found strong labeling of perivascular activated microglia, particularly in the thalamic region that is affected with a high frequency of capillary amyloid. Although expression of phospho-tau is largely expected in neurons in neurodegenerative conditions, in mice activated microglia have been reported to express and accumulate tau protein following episodes of ischemic injury11. Whether this finding microglial phospho-tau presentation is restricted to Tg-SwDI/NOS2−/− mice or also observed in human cases of CAA type-1 remains to be further evaluated. In any case, the findings presented in this study suggest that Tg-SwDI/NOS2−/− mice recapitulate many of the pathological features of human CAA type 1 and provide a valuable in vivo model to further investigate this condition.
Acknowledgments
Sources of Funding
This work was supported in part by National Institutes of Health (NIH) grants RO1-NS55118 and RO1-AG23084.
Footnotes
Disclosures
None.
References
- 1.Rensink AA, de Waal RM, Kremer B, Verbeek MM. Pathogenesis of cerebral amyloid angiopathy. Brain Res Brain Res Rev. 2003;43:207–223. doi: 10.1016/j.brainresrev.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;8:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Del TK, Braak H. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61:282–293. doi: 10.1093/jnen/61.3.282. [DOI] [PubMed] [Google Scholar]
- 4.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, Van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of amyloid β-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid β-protein precursor. J Biol Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 5.Miao J, Xu F, Davis J, Otte-Holler I, Verbeek MM, Van Nostrand WE. Cerebral microvascular amyloid β protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid β precursor protein. Am J Pathol. 2005;167:505–515. doi: 10.1016/s0002-9440(10)62993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroncke KD, Fehsel CV, Kolb-Bachofen V. Implications of inducible nitric oxide synthase expression and enzyme activity. Antioxid Redox Signal. 2000;2:585–605. doi: 10.1089/15230860050192341. [DOI] [PubMed] [Google Scholar]
- 7.Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal. 2001;3:203–213. doi: 10.1089/152308601300185179. [DOI] [PubMed] [Google Scholar]
- 8.Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonareh N, Vitek MP, Colton CA. Progression of amyloid pathology to Alzheimer’s pathology in an APP transgenic mouse model by removal of NOS2. J Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson DW, Wertkin A, Mattiace LA, Fier E, Kress Y, Davies P, Yen SH. Ubiquitin immunoelectron microscopy of dystrophic neurites in cerebellar senile plaques of Alzheimer’s disease. Acta Neuropath. 1990;79:486–493. doi: 10.1007/BF00296107. [DOI] [PubMed] [Google Scholar]
- 10.Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological estimation of total microglia number in mouse hippocampus. J Neurosci Meth. 1998;84:101–108. doi: 10.1016/s0165-0270(98)00100-9. [DOI] [PubMed] [Google Scholar]
- 11.Uchihara T, Nakamura A, Arai T, Ikeda K, Tsuchiya K. Microglial tau undergoes phosphorylation-independent modification after ischemia. Glia. 2003;45:180–187. doi: 10.1002/glia.10318. [DOI] [PubMed] [Google Scholar]