Abstract
New neurons are continuously added throughout life to the dentate gyrus of the mammalian hippocampus. During embryonic and early postnatal development, the dentate gyrus is formed in an outside-in layering pattern that may extend through adulthood. In this work we aimed to systematically quantify the relative position of dentate granule cells generated at different ages. We used 5’-bromo-2’-deoxyuridine (BrdU) and retroviral methodologies to birth-date cells born in the embryonic, early postnatal and adult hippocampus and assessed their final position in the adult mouse granule cell layer. We also quantified both developmental and adult-born cohorts of neural progenitor cells that contribute to the pool of adult progenitor cells. Our data confirm that the outside-in layering of the dentate gyrus continues through adulthood and that early-born cells constitute most of the adult dentate gyrus. We also found that a substantial fraction of the dividing cells in the adult dentate gyrus were derived from early-dividing cells and retained BrdU, suggesting that a subpopulation of hippocampal progenitors divides infrequently from early development on.
Keywords: developmental neurogenesis, adult neurogenesis, dentate gyrus, neural stem cell, retrovirus, BrdU, cell layering
Introduction
The dentate gyrus (DG) of the hippocampus is one of the few mammalian brain structures that continue to generate new neurons through adulthood. Early birth-dating studies using tritiated thymidine established an outside-in developmental layering pattern in the granule cell layer (GCL). Specifically, cells born during embryonic development contribute preferentially to the outer GCL, and cells born postnatally remain closer to the hilus (Angevine, 1965; Bayer, 1980a; Crespo et al., 1986; Rakic and Nowakowski, 1981; Schlessinger et al., 1975).
These findings were further elaborated in the 1980s and 1990s, when the dividing cell populations involved in DG morphogenesis throughout development were characterized (Altman and Bayer, 1990; Bayer, 1980b; Bayer et al., 1982). During early embryonic development, cells lining the medial ventricles at the dentate notch, known as the primary dentate matrix, form the initial proliferative zone that will become the DG. Through a series of dentate migrations, the proliferative zone moves from the ventricles to the hilus as the rodent embryo approaches birth. The proliferative zone in the hilus from birth until approximately 3 weeks postnatal is known as the tertiary dentate matrix (Altman and Bayer, 1990; Bayer, 1980b; Bayer and Altman, 1974). This population contributes substantially to the adult GCL in both rats and mice (Angevine, 1965; Bayer, 1980a; Schlessinger et al., 1975). Gradually, the proliferative zone becomes restricted to a region known as the subgranular zone (SGZ), lying just beneath the GCL toward the hilus. Proliferation in the SGZ contributes to the addition of new neurons to the DG throughout the adult life of mammals. Studies examining only adult neurogenesis demonstrate that granule cells born in the adult are mostly positioned in the inner third of the GCL (Esposito et al., 2005; Kempermann et al., 2003). This developmental pattern is similar to that of the human DG (Eriksson et al., 1998; Humphrey, 1964; 1967), although in humans, completion of the period of rapid GCL neurogenesis occurs slightly earlier in postnatal development (Humphrey, 1967).
Altogether, these studies suggest that the outside-in DG layering may continue through adult neurogenesis. In other words, new neurons born in the adult DG may be positioned closer to the hilus compared to those born during postnatal and embryonic development. This positioning was suggested by Crespo et al. (1986) from an observation that the distance to the molecular layer of granule cells born at P10 in Sprague-Dawley rats remained constant but gradually increased in reference to the hilus as the rat aged. Furthermore, a recent study has extensively documented the layering of postnatal- and adult-born cells in the mouse DG (Muramatsu et al., 2007). However, no comprehensive study has examined the location and the numeric contribution of cells born from embryogenesis through adulthood in the DG. Moreover, it is not known whether adult progenitors derive from dividing cells during development or from quiescent cells that proliferate for a limited time and terminally differentiate once they are recruited into the cell cycle.
To assess the contributions of embryonic, early postnatal and adult neurogenesis to different layers of the dentate GCL, we labeled dividing cells of the embryonic, postnatal and adult DG and studied the distribution of their progenies at later stages, when the cells had reached their mature position in the GCL. We selected BrdU and retroviral labeling techniques to undertake this study, due to their distinctive strengths.
BrdU has two main advantages: its administration is non-invasive to the central nervous system, and it labels all cells that are replicating their DNA around the time of injection, allowing an accurate estimation of the fate of the progenitor cell population (Burns and Kuan, 2005; Cooper-Kuhn and Kuhn, 2002; Miller and Nowakowski, 1988). However, it may be diluted and become undetectable in neurons derived from progenitor cells undergoing multiple rounds of divisions (Dayer et al., 2003; Hayes and Nowakowski, 2002; Miller and Nowakowski, 1988). Therefore, after a long survival interval, it is unclear whether a decrease in BrdU(+) cells indicates cell death or dilution from repeated divisions. On the other hand, for cells that are BrdU(+) at the time of evaluation, we can be confident that they have divided only a few times since the time of BrdU administration (Dayer et al., 2003; Hayes and Nowakowski, 2002; Miller and Nowakowski, 1988).
In recent years, the retrovirus has become an important tool to birth-date newborn neurons because they only transduce cells that enter the M-phase of the cell cycle shortly after virus delivery (Coffin et al., 1997). Due to the limited diffusion of virus particles in vivo, retrovirus only labels cells that are in proximity to the site of virus delivery. Retroviral labeling permits direct visualization of the entire cell morphology, whereas BrdU immunohistochemistry only reveals the cell nucleus. In addition, two different retroviruses expressing different fluorescent markers allow the direct comparison of the cell layering from two developmental cohorts in the same mouse brain. We have therefore used both methods in the present study, so the results may be viewed in complement and directly contrasted.
Quantifications with both methods confirmed that the outside-in layering pattern continues through adulthood. In addition, we demonstrated such layering pattern in the same mouse brain by labeling two birth date-specific cohorts with retroviruses expressing different fluorescent reporters. Furthermore, we assessed the number of BrdU+ cells labeled at different stages that remained undifferentiated or in cell cycle. Our data suggest that at least a subpopulation of progenitors in the adult brain divides infrequently from early development on.
Materials and Methods
BrdU labeling
Female wild-type or Sox2-GFP transgenic mice in C57Bl/6 background were mated with Sox2-GFP C57Bl/6 transgenic males. Transgenic offspring did not survive well after developmental BrdU injection, so they were not used in this study. The date of plug was considered E0.5, and the date of birth was considered P0. Male and female offspring from these pregnancies were injected at 3 time points: embryonic, postnatal, and adult. For embryonic injections, pregnant females received 50 mg/kg of BrdU intraperitoneally (i.p.) on E15.5. Only one injection was possible at this time point, due to known teratogenic effects of BrdU on embryos (Kolb et al., 1999; Packard et al., 1974). For postnatal and adult injections, each mouse received 50 mg/kg BrdU twice daily, approximately 8h apart, on P5-7 (postnatal) or P35-37 (young adult). Animals were sacrificed at P63 by lethal overdose of a ketamine/xylazine mixture followed by perfusion transcardially with 4% paraformaldehyde. This date of sacrifice was chosen to be one month after the P35-37 BrdU injection time point so that labeled granule cells would have sufficient time to reach maturity [17].
Viral vectors
Murine Moloney leukemia virus-based CAG-GFP and CAG-RFP vectors used in this study have been previously described (Laplagne et al., 2006; Zhao et al., 2006). Retrovirus was synthesized in HEK293T cells, concentrated (108 pfu/ml), and collected by ultracentrifugation (Tashiro et al., 2006; van Praag et al., 2002; Zhao et al., 2006).
Retroviral injections
In utero
Timed-pregnant C57Bl/6 female mice received surgery when embryos reached E15.5. Each female was anesthetized with 10% Nembutal administered i.p. A longitudinal incision was made in the lower peritoneum, through which the intact uterus containing embryos was extracted, without disturbing its integrity or blood supply. The CAG-GFP retrovirus was mixed with 3% fast green dye at a 5:1 ratio and was injected into one lateral ventricle of each embryo via a micro glass capillary tube inserted through the uterine wall. The diffusion of the dye into the other ventricle confirmed proper injection placement. The uterus was placed back into the peritoneal cavity and the mothers were sutured and allowed to fully recover before they were put back with the colony.
Postnatal and adult
Adult mice were anesthetized using 80 μg ketamine/8 μg xylazine at 8 μl/g i.p. P7 pups were anesthetized by placing them on ice for 4 minutes. Stereotaxic injections were performed to deliver the CAG-GFP or CAG-RFP virus to the DG in P5-P7 and P42 mice, as previously described in detail (Laplagne et al., 2006; Zhao et al., 2006). P42 mice were housed with a running wheel starting 3 days before the injection. Both males and females were included in the analysis. All animal protocols were approved by the Salk Institutional Animal Care and Use Committee.
Immunohistochemistry
After transcardial perfusion, mouse brains were postfixed with 4% PFA overnight at 4°C, then transferred to 30% sucrose and stored at 4°C until sectioning. Brains utilized in BrdU experiments were hemisected immediately before sectioning. The right hemisphere was sectioned coronally and the left horizontally. Using a sliding microtome, all brains were cut through the extent of the hippocampus into 80-μm and 40-μm sections for postnatal and adult samples, respectively. Brains labeled with retrovirus were cut coronally in 40-μm thickness.
Sections were immunostained for primary antibodies against BrdU (rat anti-BrdU Accurate 1:250), NeuN (mouse monoclonal, Chemicon, 1:100), Ki67 (rabbit, Novacastra 1:500) and Sox2 (goat, Santa Cruz 1:250) (please see Table 1 for detailed information about primary antibodies). Secondary antibodies used were donkey anti-rat Cy3 (Jackson, 1:250), donkey anti-mouse Cy5 (Jackson, 1:250), and donkey anti-rabbit FITC (Jackson, 1:250). After immunostaining, sections were washed 6 times in TBS; DAPI 1:1000 was included in the second of these washes. Sections were mounted on double-subbed slides using DABCO mounting media. Immunostaining was visualized using the Bio-Rad R2100 confocal system with Nikon Eclipse TE300 or Olympus BX51 microscopes and a Zeiss Pascal Confocal microscope. Stereologic counts were performed using a MicroBrightField StereoInvestigator program optical fractionator (below).
Table 1.
A list of primary antibodies used in the study.
| Antigen | Immunogen | Manufacturer | Dilution used |
|---|---|---|---|
| BrdU | BrdU | Accurate (Westbury), rat monoclonal, clone BU1/75 (ICR1), OBT0030 | 1:250 |
| NeuN | Purified cell nuclei from mouse brain | Chemicon (Temecula), mouse monoclonal, clone A60, MAB377 | 1:100 |
| Ki67 | Prokaryotic recombinant fusion protein corresponding to a 1088 bp Ki67 motif-containing cDNA fragment | Novacastra (Newcastle upon Tyne, UK), rabbit polyclonal, NCL-Ki67p | 1:500 |
| Sox2 | Synthetic peptide of human Sox2 a.a. 249–265 | Chemicon (Temecula), rabbit polyclonal, AB5603 | 1:500 |
| Sox2 | human Sox2 a.a. 277–293 | Santa Cruz Biotechnology (Santa Cruz), goat polyclonal, sc-17320 | 1:500 |
Antibody Characterization
The list of primary antibodies used is provided in Table 1. BrdU antibody recognized cell nuclei in the proliferative zones in adult mouse brain sections. No signals were detected in brain sections that were not labeled by BrdU.
The NeuN antibody stained for nuclei of major types of neurons, as previously shown (Mullen et al., 1992).
The Ki67 antibody (NCL-Ki67p) recognized cell nuclei in the SGZ of the DG and the subventricular zone (SVZ) of the lateral ventricles. This staining pattern is consistent with the notion that the major germinal zones in the adult mammalian brain are located in the SGZ and the SVZ, and it is also consistent with previous reports (Seki et al., 2007).
The Sox2 antibodies recognized a band of ~34kDa on Western blot of rat hippocampal progenitor cell lysates (rabbit anti-Sox2, Chemicon) and of human and mouse embryonic stem cell lysates (goat anti-Sox2, Santa Cruz).
The rabbit anti-Sox2 (Chemicon) and goat anti-Sox2 (Santa Cruz) antibodies were raised against non-overlapping peptides from the C-terminus of the human Sox2 protein ( Poche et al., 2008, Wang et al., 2006 ) (see Table 1). They showed similar staining patterns in mouse brain sections and recognized cell nuclei in the germinal zones in the adult mouse brain. In addition, both antibodies specifically recognized cells that were transduced by a lentivirus that used Sox2 promoter to drive the expression of a GFP/cre fusion protein in the adult mouse hippocampus (Suh et al., 2007). Goat anti-Sox2 (Santa Cruz) antibodies were used in this study.
Cell quantification
Quantification of BrdU-labeled cells was performed stereologically (West et al., 1991). To determine the distribution of cells born at each time point within the DG, the GCL was partitioned into 3 “layers,” using the StereoInvestigator program (Microbrightfield, Inc.). The outer borders of the GCL were outlined at 20× using DAPI (4’,6-diamidino-2-phenylindole) stain at postnatal or NeuN stain at the adult sacrifice time points. Cursor size was then set such that points marked by the center of the cursor would be placed 20 μm in from the edge of the circular cursor (20-μm radius). The cursor edge was then repeatedly aligned with the edge of the GCL outline to delineate strips 20 μm into the GCL from the inner edge and 20 μm in from the outer edge of the GCL. In adult animals, this procedure resulted in 3 approximately 20-μm “layers” (one inner, one middle, one outer) through the GCL. In some cases (especially at different ages and at the edges of the dentate), the thickness of the middle layer was less than 20 μm; inner and outer layer widths were constant. The numbers of BrdU-positive cells in each layer were quantified. Cells overlapping more than 1 layer were assigned to the layer in which a greater portion of their volume resided.
A single optical fractionator run was performed over the entire GCL for each section; a different marker was used to count cells in each layer. Stereologic counts were performed using grid and counting frame sizes to approximate 10 or more sampling sites per section and 2–5 cells per counting frame and to achieve a sufficiently low coefficient of error (CE Gundersen) between sections. Grid and counting frame sizes for each group were as follows, respectively: postnatal sac P8 (supplement only) grid = 75 μm2, c.f. = 15μm2; embryonic sac P63 grid = 100 μm2, c.f. = 25 μm2; postnatal sac P63 grid = 100μm2, c.f. = 50 μm2; adult sac P63 grid = 100 μm2, c.f. = 100 μm2. Counting frame depth for all 40-μm sections was 10 μm, with a 3-μm estimated guard zone on top and bottom.
Quantification of retrovirally labeled cells was performed by counting all cells in every sixth coronal section displaying >30 labeled neurons throughout the septotemporal axis of the hippocampus. For each section, the GCL was divided in thirds and each retrovirally labeled neuron was assigned to the inner, middle or outer layers.
Colocalization was all performed using the Bio-Rad (Hercules, CA) Radiance 2100 laser scanning confocal system. Sequential 2 μm scanning was performed through the entire z-axis of every 12th DG-containing section with 40×oil immersion lens. Optical section thickness ranged from 16–26 μm. All Ki67(+) cells in each DG were identified using only that channel. Subsequently, each was evaluated in the other channels to determine whether it colocalized with BrdU. Evaluation was performed blind to BrdU treatment condition. Colocalization was defined as labeling in 2 channels that appeared to be in the same cell in multiple z planes. For Sox2(+) counts, all double-labeled Sox2(+)/BrdU(+) cells were counted using the confocal z-stack. The total numbers of BrdU(+) cells and of Sox2(+) cells were determined using the stereologic methodology described above (Sox2 grid = 150μm2, c.f. = 25μm2).
Presented images were processed using Adobe Photoshop 7.0. Images were adjusted only by cropping of the areas shown and with adjustments of brightness and contrast.
Results
The GCL shows a distinct birth date-based, outside-in layering through adulthood
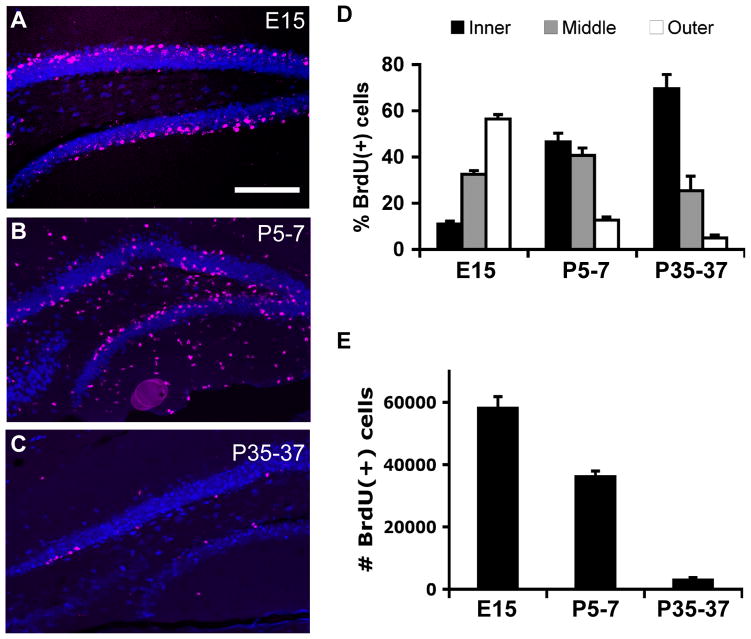
BrdU was injected in three groups of mice: embryonic day 15 (E15), postnatal day 5–7 (P5-7), and young adult (P35-37). All of these groups were assessed stereologically at P63 to evaluate the contribution of the BrdU+ cells to the adult DG (Figure 1A–C). Cells labeled in each successive group showed a distinctive outside-in chronological distribution along the adult GCL (Figure 1A–C). Cells born during embryogenesis formed the outermost layer of the GCL, closest to the molecular layer. Cells born on P5-7 localized more towards the middle and inner GCL. Cells born in young adult mice (P35-37) were restricted predominantly to the innermost layer, closest to the SGZ (Figure 1D; E n=10, P n=7; A n=4). A two-way ANOVA showed a significant (p<0.05) group (E15 vs. P5-7 vs. P35–57) by layer (in vs. mid vs. out) interaction. Post-hoc Bonferroni tests showed significant differences between all layers in all groups (p<0.05) except for the middle layer contributions of E15 vs. P5-7, E15 vs. P35-37, and the outer layer contribution of P5-7 vs. P35-37 (p>0.05). As previously documented, extensive proliferation of cells that remained in the hilus and molecular layer occurred postnatally (Figure 1B) (Bayer and Altman, 1974; Navarro-Quiroga et al., 2006).
Figure 1.
Quantitatively, embryonic dividing cells clearly made the largest numeric contribution to the adult DG, followed closely by postnatal dividing cells, with adult-born cells constituting only a small fraction (Figure 1D, E). The quantifications of BrdU(+) cell numbers presented here (Figure 1E) can only be compared at a qualitative level between developmental stages given 1) the differences in BrdU administration (single embryonic vs. 3d postnatal and adult injections) and 2) the variation in metabolism and placental/blood-brain permeability at these different developmental stages (Packard et al., 1973). Nevertheless, these data suggest that the P63 DG contains mainly embryonic and postnatally derived cells. In the adult mouse, there are approximately 300,000 granule cells per DG (Bonthius et al., 2004; Hong et al., 2007). Nearly 60,000 E15-labeled cells per DG remained visible in the P63 DG, and almost 40,000 cells were labeled when BrdU was injected at P5-7. By contrast, adult-dividing cells (P35-37) constituted only approximately 3,000 cells per DG at the same time point. The numbers of BrdU(+) cells were significantly different between all groups (p<0.05, one-way ANOVA and follow-up Tukey tests).
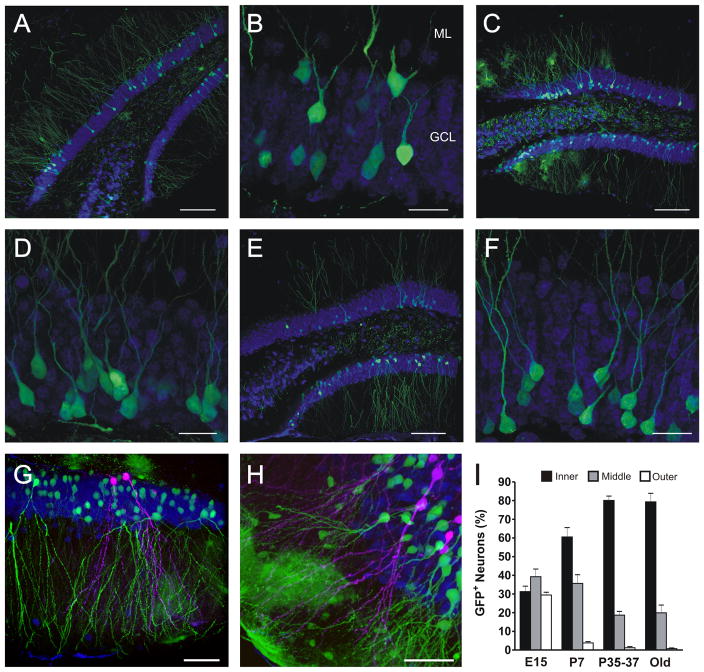
As a complementary method to assess DG layering, GFP-expressing retrovirus was injected into E15, P7 and P42 mice, and the distribution of GFP(+) neurons in the GCL was evaluated at P75 (10, 7 or 6 weeks after injection for respective groups) (Figure 2). Neurons born at E15 (Figure 2A, B) were evenly distributed throughout the inner, middle and outer layers of the GCL, whereas neurons generated at P7 (Figure 2C, D) and P42 (Figure 2E, F) were localized predominantly toward the inner and middle GCL, with a marked decrease in the contribution to the outer layer. About 80% of granule cells born in young adult mice were positioned in the inner layer and the remaining cells were scattered within the middle GCL (Figure 2I, “adult”). Layering of embryonic and adult (Figure 2G) or postnatal and adult (Figure 2H) cells was also visualized within the same brain, using RFP- and GFP-expressing retroviruses injected into the same animal’s DG at different time points.
Figure 2.
We infer from the above results a birth date-based, outside-in layering to the DG. However, the perceived inner layering of adult-born cells might be due to an insufficient time post-injection for cells to finish migrating in the GCL. Embryonic-labeled cells, for instance, had 68 days to migrate from labeled division to assessment of their location, whereas adult-labeled cells had less than half that time. Therefore, we evaluated whether adult-born cells would change to a more outer layering if given more time to migrate after division. We labeled neurons born at P42 and analyzed their distribution 7–15 months later (Figure 2I, “old”). We observed no differences in the distribution of granule cells at this later time compared with that of younger cells (“P42” vs. “old”), in agreement with the notion that adult-born neurons reach their position in the GCL within the first few weeks of their development (Esposito et al., 2005; Kempermann et al., 2003). Hence, cell position in the DG depends on the developmental stage of the animal when a cell is born, and not on the age of the cell.
Cells dividing early and infrequently constitute much of the adult-dividing population
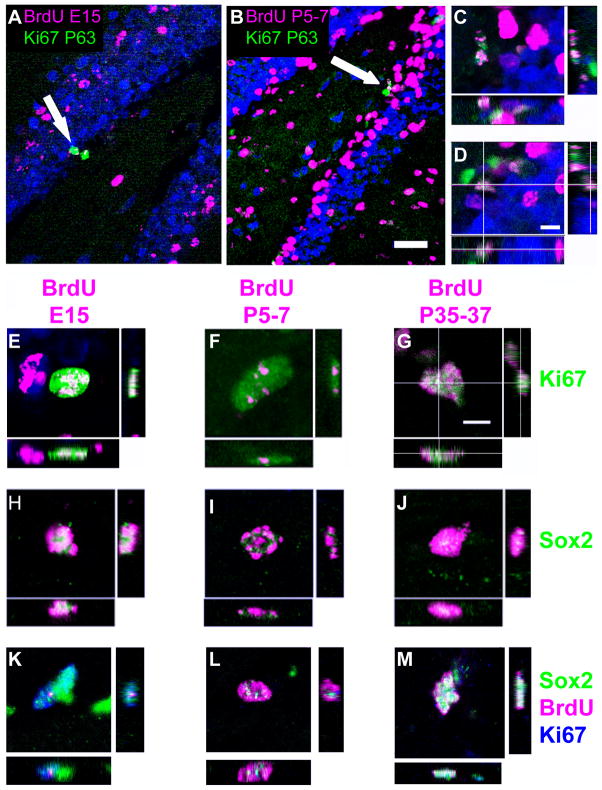
Using the same brain samples as in Figure 1, we asked to what extent cohorts of cells that divided in embryonic, early postnatal, or young adult stages contributed to the adult dividing population. Mice injected with BrdU on E15.5, or P5-7, or P35-37 were assessed at P63 for colocalization of the BrdU label with the mitotic cell marker Ki67 (Figure 3A–D) or a progenitor cell marker, Sox2. Although Sox2 expression alone is not sufficient to determine progenitor identity, the total number of Sox2(+) cells in the SGZ can be correlated with the number of progenitor cells.
Figure 3.
A small population of BrdU(+) cells in the GCL and SGZ from all injection time points was found to coexpress Ki67 and Sox2 (Figure 3E–M, Figure 4). In contrast to some previous findings (Dayer et al., 2003), this observation indicates that these double-labeled cells divided in the embryo, in the neonate, or in the adult animal and were still able to divide one month or more thereafter.
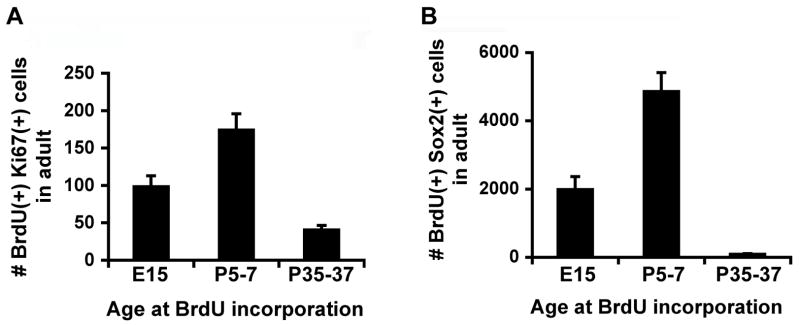
Figure 4.
In fact, cells that divided early in life made up a significant fraction of mitotic cells or Sox2(+) cells in the adult (Figure 4A, B, Table 2). One-way ANOVAs show that the numbers of BrdU and Ki67 co-labeled cells and the numbers of BrdU and Sox2 co-labeled cells were all significantly different (p<0.05) between injection time points. Post-hoc Tukey tests revealed significant (p<0.05) differences between all groups. By contrast, the total numbers of Ki67(+) cells and Sox2(+) cells in the adult brain did not differ significantly between groups (p>0.05), suggesting that BrdU administration at these different developmental stages did not have side effects on the SGZ progenitors. The GCL volume of embryonic BrdU-injected animals did differ statistically (p<0.05) from that of animals injected postnatally or in adulthood, which may be attributable to the teratogenic effects of BrdU (Kolb et al., 1999; Packard et al., 1974).
Table 2.
More adult proliferative cells are birth-dated embryonically and early postnatally than during adulthood
| BrdU (×104) | Ki67 | BrdU&Ki 67 | Sox2 (×104) | BrdU&Sox2 | GCL volume (×108 μm3) | %BK/B | %BS/B | |
|---|---|---|---|---|---|---|---|---|
| E15 | 5.79±0.673 | 1699.2±110 | 98.4±14.5 | 7.43±0.627 | 1983±380 | 2.78±0.104 | 0.171±0.0206 | 3.51±0.758 |
| P5-7 | 3.71±0.287 | 2338.8±236 | 193.2±31.1 | 5.67±0.702 | 4860±553 | 3.08±0.116 | 0.542±0.109 | 12.5±0.68 |
| P35-37 | 0.293±0.0939 | 1692.0±219 | 40.5±5.92 | 6.85±0.465 | 90±16.5 | 3.15±0.13 | 1.86±0.751 | 3.44±0.689 |
Data represent the mean ± standard error of data collected from n=4 or 5 brains. Total numbers of BrdU+, Ki67+, BrdU+Ki67+, Sox2+, BrdU+Sox2+ cells were stereologically estimated in the P63 unilateral GCL. Total numbers of BrdU+, BrdU+Ki67+ and BrdU+Sox2+ cells are significantly different between groups (p<0.05); total numbers of Ki67+ and Sox2+ cells are not different between groups (p>0.05). Note that a large proportion of the adult proliferating (Ki67+) or progenitor cells (Sox2) were labeled during postnatal and embryonic development. The embryonically injected group (E15) has a significantly smaller volume (p<0.05) in adulthood, perhaps due to the teratogenic effects of BrdU. The volumes of the postnatal and adult group GCLs do not differ significantly (P>0.05). The last two columns indicate the percentage of the total BrdU-labeled population that is actively dividing in the adult brain (Ki67+) or remain as progenitor cells (Sox2+). Note that early-dividing cells constitute a greater number, though a smaller percentage, of the adult dividing population compared with adult-dividing cells, even after a longer opportunity for BrdU dilution. For %BK/B, an ANOVA reveals a significant (p<0.05) difference between groups; post-hoc tests reveal p<0.05 only comparing groups E15 and P35-37. For %BS/B, all groups are significantly different (p<0.05) except for E15 vs. P35-37. Note that a greater percentage of the early-dividing population becomes adult progenitors.
These data likely underestimate the early contribution to the adult-dividing cell population, because BrdU can only be visualized in cells that have divided fewer than 9 times in vitro (Palmer et al., 2000) or 4 times in vivo (Dayer et al., 2003); after this, BrdU is diluted beyond the detectable limit of immunohistochemistry. Indeed, the proportion of BrdU+Ki67+ cells over total number of BrdU+ cells is the lowest when cells were labeled at E15 and the highest when cells were labeled at P35-37, consistent with the possibility that E15-labeled cells have divided more times than those labeled at P5-7 and P35-37. Even with the possibility of more label dilution, cells dividing at E15 and P5-7 contribute more to the proliferating population in the adult than those dividing at P35-37. These data suggest that progenitor cells in the dentate gyrus either decrease in number or divide less frequently when mice develop into early adulthood.
Our observation of BrdU labeling in any adult-dividing cells at all suggests that these BrdU(+) cells have divided only a limited number of times between early development and adulthood. For example, the BrdU+ cells that were labeled at E15 and detected at P63 must have divided less often than once in 7.84–17.25 days if we assume that BrdU labeling is diluted out within 4–9 cell cycles ((Dayer et al., 2003; Palmer et al., 2000) and that these cells divide at a steady but infrequent pace. Such limited division supports the existence of infrequently dividing “stem” cells within the SGZ of the DG.
Discussion
In this study, we used both BrdU and retrovirus birth-dating methods to assess the contribution of dividing cells at different developmental stages to the GCL in the adult DG, and we quantified their contribution to the proliferating cells and progenitors in the adult hippocampus. We confirmed that the “outside-in” layering pattern of the DG continues through adulthood and that cells born during early development make larger numeric contributions to both the total number of granule cells and the number of adult progenitors than those born in the adult. Our study also presented a within-subjects demonstration that cells that divided during early development can continue to divide in the adult. We also showed that a subpopulation of progenitors in the DG divides infrequently from early development on.
Consistent with earlier work (Angevine, 1965; Bayer, 1980b; Crespo et al., 1986; Muramatsu et al., 2007; Rakic and Nowakowski, 1981; Schlessinger et al., 1975), our experiments with both BrdU and retrovirus labeling demonstrated that a cell’s birth-date correlated with its subsequent location within the GCL. Early-born cells layered to the outside (closer to molecular layer) compared with later-born cells (closer to hilus). Retroviral data were also an important complement to the BrdU data, supporting the finding that the outside layering of BrdU+ labeled at E15 was not a consequence of BrdU cytotoxic effects that resulted in overall decreased DG volume. Using retrovirus, we were able to follow early-born cells without dilution of the label in the adult and to examine the layering of more than one proliferating population in the same brain using multiple fluorophores, thereby confirming the outside-in layering pattern of the GCL. Comparing the results from BrdU and retrovirus experiments, the percentage of labeled cells layered to the inside was considerably less after BrdU (Figure 1E) than after retroviral (Figure 2I) labeling in the embryonic and postnatally injected groups. We hypothesize that this difference is due to BrdU dilution in cells continuing to divide in inner layers; such dilution does not occur in retrovirus-labeled cells. On the other hand, we were not able to perform stereological quantifications on the total number of labeled cells or the number of proliferating/progenitor cells with retrovirus labeling, due to the highly variable labeling efficiency and possible silencing of retroviruses in neural stem cells (Ellis, 2005). It remains possible that our quantitative estimates of the embryonic contribution to the DG were low, due to BrdU teratogenic effects on the DG, altered BrdU distribution and metabolism through the placenta, and a single day of injection. We present estimates with this caveat.
Our findings yield strong support to previous anatomical findings (Angevine, 1965; Bayer, 1980b; Muramatsu et al., 2007; Schlessinger et al., 1975) that the GCL has an outside-in, birth date-based layering. The functional consequences of these anatomical differences are presently unclear. So far, only small differences have been shown between mature early- and adult-born cells, in terms of electrophysiologic and cell morphologic properties (Laplagne et al., 2006). However, abnormal layering of GCL has been associated with certain pathological conditions. For example, seizures induce changes in the reelin signaling pathway that may be responsible for the ectopic migration of granule cells (Gong et al., 2007).
The functional significance of adult neurogenesis in the DG may not depend on the specific layering of adult-born cells but may rather on the unique process of newborn cells maturing and integrating into an existing circuit. Adult-born cells display distinct electrophysiological properties before they fully mature, and granule cells at immature stages are specifically required for certain learning tasks (Deng et al., 2009; Ge et al., 2007; Mongiat et al., 2009; Schmidt-Hieber et al., 2004). Therefore, it is possible that adult-born granule cells play a distinct role at immature stages but are functionally similar to early-born cells after they fully mature. On the other hand, although mature early- and adult-born cells are not significantly different from each other in general electrophysiological properties and connectivity, they may be recruited to different networks that represent the environment in which they matured (Aimone et al., 2009; Tashiro et al., 2007).
We show that early-proliferating, infrequently dividing cells constituted a considerable fraction of adult-dividing cells in the DG. Importantly, the lack of BrdU dilution in early-labeled, adult-dividing cells (Figure 3, 4) points to the existence of a population of infrequently dividing SGZ cells that continues to divide through adulthood. These cells must have divided fewer than 9 times (Palmer et al., 2000) between E15 and P63 – less often than once a week – and continued to divide in the adult. The number of such cells is not insignificant, as a single embryonic BrdU injection labels over 5% of the adult-dividing population (Table 2). These data certainly support the existence of a stem-like proliferative population that defies the previously proposed steady-state, 12–25h cell cycle model for the DG progenitors (Cameron and McKay, 2001; Hayes and Nowakowski, 2002; Nowakowski et al., 1989). In fact, two types of progenitor cells have been described in the adult hippocampus (Fukuda et al., 2003; Garcia et al., 2004; Seri et al., 2004; Suh et al., 2007). They are different in morphology, expression of molecular markers and mitotic features. Type 1 cells have radial processes that span the granule cell layer and ramify in the inner molecular layer, whereas type 2 cells do not have long processes. Although the lineage relationship between the two types of hippocampal progenitors awaits clarification, studies using different mouse models consistently showed that type 1 cells are relatively quiescent and type 2 cells are actively dividing (Fukuda et al., 2003; Seri et al., 2004; Suh et al., 2007). Our observation of BrdU retaining cells after a long survival interval clearly supports the contention that a subpopulation of hippocampal progenitors divides infrequently from early development on.
Comparing the three developmental time points, there is clearly a dramatic decrease in the proliferating population as the number BrdU+ cells labeled at P35-37 is only about 5–8% of those labeled at E15 and P5-7. This number is further decreased upon aging (Kempermann et al., 1998). The number of BrdU+Ki67+ cells also decreased significantly in young adult, which is only 20–40% of the number labeled at E15 and P5-7. Given the possibility of BrdU dilution over longer survival time intervals, we may be underestimating the changes in the BrdU+Ki67+ population. These data suggest that the progenitor population in the SGZ is either decreasing in number or becoming more quiescent when mice develop into early adulthood. Future studies will be necessary to examine how adult hippocampal progenitors are regulated and maintained throughout development.
It is clear that cells dividing during embryonic and early postnatal development contribute significantly to the progenitor population in the adult DG. Does that mean that a developmental insult to proliferation will damage future dividing populations more extensively, or more chronically, than a similar insult to the adult? Will adult neurogenesis be fully able to compensate for the effects of such a developmental insult? Current data concerning these questions are conflicting (Bayer and Altman, 1975; Bayer et al., 1973; Chen and Hillman, 1986; Ciaroni et al., 2002; Naylor et al., 2008; Zhang et al., 2009), and further exploration is needed. Addressing the roles of cells dividing during early development will help determine whether developmental neurogenesis figures distinctly in the form and function of the adult DG. It may also help to reveal which properties of adult-born neurons make lifelong neurogenesis useful and evolutionarily conserved.
Supplementary Material
Acknowledgments
We would like to thank Dr. Hoonkyo Suh for helpful suggestions to improve this manuscript, Eunice Mejia for technical assistance and advice, Soledad Espósito and Diego Laplagne for retroviral injections, Brad Aimone for discussions and Mary Lynn Gage for editorial comments. We also acknowledge the support of the UCSD Medical Scientist Training Program, The Salk Institute, The Lookout Fund, The Defense Advanced Research Projects Agency (DARPA), The U.S. National Institutes of Health (NS-05050217, NS-05052842 and MH090258), and the National Institutes of Aging (AG-020938), the James S McDonnell Foundation and the Mathers Foundation to F.H.G., and Agencia Nacional de Promoción Científica, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Howard Hughes Medical Institute to A.F.S. N.A.M. and V.C.P. were supported by fellowships from CONICET.
Supported by:
The Lookout Fund
The McDonnell Foundation
The Mathers Foundation
The U.S. National Institutes of Health (NS-05050217, NS-05052842 and MH090258)
National Institutes of Aging (AG-020938)
The Argentine Agency for the Promotion of Science and Technology (ANPCyT)
The Argentine Council for Scientific and Technical Research (CONICET)
Howard Hughes Medical Institute, USA
Literature Cited
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61(2):187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. The Journal of comparative neurology. 1990;301(3):365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Angevine JB., Jr Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp Neurol Suppl:Suppl. 1965;2:1–70. [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. The Journal of comparative neurology. 1980a;190(1):87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Development of the hippocampal region in the rat. II. Morphogenesis during embryonic and early postnatal life. The Journal of comparative neurology. 1980b;190(1):115–134. doi: 10.1002/cne.901900108. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. The Journal of comparative neurology. 1974;158(1):55–79. doi: 10.1002/cne.901580105. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. The effects of X-irradiation on the postnatally-forming granule cell populations in the olfactory bulb, hippocampus, and cerebellum of the rat. Exp Neurol. 1975;48(1):167–174. doi: 10.1016/0014-4886(75)90231-9. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Brunner RL, Hine R, Altman J. Behavioural effects of interference with the postnatal acquisition of hippocampal granule cells. Nat New Biol. 1973;242(120):222–224. doi: 10.1038/newbio242222a0. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216(4548):890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, McKim R, Koele L, Harb H, Karacay B, Mahoney J, Pantazis NJ. Use of frozen sections to determine neuronal number in the murine hippocampus and neocortex using the optical disector and optical fractionator. Brain Res Brain Res Protoc. 2004;14(1):45–57. doi: 10.1016/j.brainresprot.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Burns KA, Kuan CY. Low doses of bromo- and iododeoxyuridine produce near-saturation labeling of adult proliferative populations in the dentate gyrus. Eur J Neurosci. 2005;21(3):803–807. doi: 10.1111/j.1460-9568.2005.03907.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. The Journal of comparative neurology. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chen S, Hillman DE. Selective ablation of neurons by methylazoxymethanol during pre- and postnatal brain development. Exp Neurol. 1986;94(1):103–119. doi: 10.1016/0014-4886(86)90275-x. [DOI] [PubMed] [Google Scholar]
- Ciaroni S, Cecchini T, Ferri P, Ambrogini P, Cuppini R, Lombardelli G, Peruzzi G, Del Grande P. Postnatal development of rat dentate gyrus: effects of methylazoxymethanol administration. Mech Ageing Dev. 2002;123(5):499–509. doi: 10.1016/s0047-6374(01)00359-1. [DOI] [PubMed] [Google Scholar]
- Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002;134(1–2):13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- Crespo D, Stanfield BB, Cowan WM. Evidence that late-generated granule cells do not simply replace earlier formed neurons in the rat dentate gyrus. Exp Brain Res. 1986;62(3):541–548. doi: 10.1007/BF00236032. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. The Journal of comparative neurology. 2003;460(4):563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29(43):13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16(11):1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25(44):10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23(28):9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nature neuroscience. 2004;7(11):1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27(8):1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134(1–2):77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Hong SM, Liu Z, Fan Y, Neumann M, Won SJ, Lac D, Lum X, Weinstein PR, Liu J. Reduced hippocampal neurogenesis and skill reaching performance in adult Emx1 mutant mice. Exp Neurol. 2007;206(1):24–32. doi: 10.1016/j.expneurol.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Humphrey T. Some observations on the development of the human hippocampal formation. Trans Am Neurol Assoc. 1964;89:207–209. [PubMed] [Google Scholar]
- Humphrey T. The development of the human hippocampal fissure. J Anat. 1967;101(Pt 4):655–676. [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18(9):3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Pedersen B, Ballermann M, Gibb R, Whishaw IQ. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J Neurosci. 1999;19(6):2337–2346. doi: 10.1523/JNEUROSCI.19-06-02337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4(12):e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457(1):44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Mongiat LA, Esposito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4(4):e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116(1):201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Muramatsu R, Ikegaya Y, Matsuki N, Koyama R. Neonatally born granule cells numerically dominate adult mice dentate gyrus. Neuroscience. 2007;148(3):593–598. doi: 10.1016/j.neuroscience.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Navarro-Quiroga I, Hernandez-Valdes M, Lin SL, Naegele JR. Postnatal cellular contributions of the hippocampus subventricular zone to the dentate gyrus, corpus callosum, fimbria, and cerebral cortex. The Journal of comparative neurology. 2006;497(5):833–845. doi: 10.1002/cne.21037. [DOI] [PubMed] [Google Scholar]
- Naylor AS, Bull C, Nilsson MK, Zhu C, Bjork-Eriksson T, Eriksson PS, Blomgren K, Kuhn HG. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105(38):14632–14637. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18(3):311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Packard DS, Jr, Menzies RA, Skalko RG. Incorportaiton of thymidine and its analogue, bromodeoxyuridine, into embryos and maternal tissues of the mouse. Differentiation. 1973;1(6):397–404. doi: 10.1111/j.1432-0436.1973.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Packard DS, Jr, Skalko RG, Menzies RA. Growth retardation and cell death in mouse embryos following exposure to the teratogen bromodeoxyuridine. Exp Mol Pathol. 1974;21(3):351–362. doi: 10.1016/0014-4800(74)90101-4. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. The Journal of comparative neurology. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Poche RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. The Journal of comparative neurology. 2008;510(3):237–250. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Nowakowski RS. The time of origin of neurons in the hippocampal region of the rhesus monkey. The Journal of comparative neurology. 1981;196(1):99–128. doi: 10.1002/cne.901960109. [DOI] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. The Journal of comparative neurology. 1975;159(2):149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Seki T, Namba T, Mochizuki H, Onodera M. Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. The Journal of comparative neurology. 2007;502(2):275–290. doi: 10.1002/cne.21301. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. The Journal of comparative neurology. 2004;478(4):359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell stem cell. 2007;1(5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27(12):3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Zhao C, Gage FH. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat Protoc. 2006;1(6):3049–3055. doi: 10.1038/nprot.2006.473. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Stromberg GP, Whitney JT, Brower NW, Klymkowsky MW, Parent JM. Sox3 expression identifies neural progenitors in persistent neonatal and adult mouse forebrain germinative zones. The Journal of comparative neurology. 2006;497(1):88–100. doi: 10.1002/cne.20984. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zhang L, Blomgren K, Kuhn HG, Cooper-Kuhn CM. Effects of postnatal thyroid hormone deficiency on neurogenesis in the juvenile and adult rat. Neurobiol Dis. 2009;34(2):366–374. doi: 10.1016/j.nbd.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.