Abstract
Background
Malignant transformation of hepatocellular adenomas (HCAs) into hepatocellular carcinomas (HCCs) has been reported repeatedly and is considered to be one of the main reasons for surgical treatment. However, its actual risk is currently unknown.
Objective
To provide an estimation of the frequency of malignant transformation of HCAs and to discuss its clinical implications.
Methods
A systematic literature search was conducted using the following databases: The Cochrane Hepatobiliary Group Controlled Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, MEDLINE and EMBASE.
Results
One hundred and fifty-seven relevant series and 17 case reports (a total of 1635 HCAs) were retrieved, reporting an overall frequency of malignant transformation of 4.2%. Only three cases (4.4%) of malignant alteration were reported in a tumour smaller than 5 cm in diameter.
Discussion
Malignant transformation of HCAs into HCCs remains a rare phenomenon with a reported frequency of 4.2%. A better selection of exactly those patients presenting with an HCA with an amplified risk of malignant degeneration is advocated in order to reduce the number of liver resections and thus reducing the operative risk for these predominantly young patients. The Bordeaux adenoma tumour markers are a promising method of identifying these high-risk adenomas.
Keywords: hepatocellular adenoma, liver adenoma, malignant transformation, hepatic adenoma, frequency, hepatocellular carcinomas
Introduction
Hepatocellular adenomas (HCAs) are uncommon and essentially benign tumours in the liver that occur predominantly, but not exclusively, in young women taking oral contraceptives (OCs).1,2 HCAs are caused by benign proliferation of hepatocytes with high glycogen and fat content but lack normal hepatic architecture. They usually present as a solitary nodule that may reach up to 30 cm in diameter.
Clinical manifestations range from asymptomatic presentation or abdominal pain localized to the epigastric region or right upper quadrant to a palpable liver mass or even life-threatening haemorrhage in the case of rupture.3,4 However, these tumours are most often encountered as an incidental finding during imaging for unrelated pathology.
Although the exact pathogenetic mechanism of the development of HCAs remains unknown, an association between formation of HCAs and the use of OCs or androgen-containing anabolic steroids is assumed.5–7 Studies from the past century suggest that long-term use of OCs increases the annual incidence of HCAs from 1 per million to 3 to 4 per 100 000.1,8 In addition, OCs and androgen-containing steroid anabolics have also been suggested to increase the number and size of these adenomas. Conversely, HCAs may shown signs of regression on discontinuance of OC use.9,10 HCAs are further reported to be associated with type I and type III glycogen storage disease (GSD) and, furthermore, are more likely to be multifocal or to become malignant in these patients.11
The most important complications of HCAs are haemorrhage and malignant transformation into hepatocellular carcinomas (HCCs). Thus, these are the two main reasons for surgical treatment. However, little is known about the true incidence, associated risk factors and the aetiology of malignant alteration of HCAs. The aim of the current systematic review is to provide an estimation of the frequency of this phenomenon by means of a systematic literature search and, moreover, to discuss the clinical implications.
Methods
Search strategy
A search of all literature up to February 2010 was performed independently by two investigators (J.H.M.B.S. and R.J.S.C.) employing all relevant databases including the MEDLINE, PubMed and EMBASE databases, The Cochrane Hepatobiliary Group Controlled Trials Register and The Cochrane Central Register of Controlled Trials (CENTRAL). Keywords were ‘hepatocellular adenoma’, ‘benign liver tumours’, ‘malignant transformation’ and ‘liver resection’. The search was limited to studies restricted to humans and articles published from 1970 onwards. This time period was chosen as the number of reports on hepatocellular adenomas began rising in the 1970s. All titles and abstracts were screened and relevant articles were selected.
Study selection criteria
Studies were included if they described a series of patients with HCAs undergoing surgery, embolization or other (conservative) treatment. Case reports and imaging studies of these benign lesions were also included. Only those studies containing a definite histological diagnosis of the tumours were included. Studies concerning HCAs in patients with GSD were excluded as these patients carry a higher risk of developing these lesions and, moreover, are thought to have an increased risk of malignant degeneration. Furthermore, patients with adenomatosis (more than 10 HCAs) were excluded, as this is considered as a different entity.12,13 No further formal quality assessment or selection criteria were employed.
Data extraction
The reference lists of retrieved articles were reviewed for potentially relevant studies and case reports were also reviewed. When the full text of an article was not available, an Inter Library Loan account was used to retrieve these articles from national libraries. All data of selected articles were screened for duplicate adenoma cases that had already been reported in prior studies. In the case of an overlapping series, only the most recent or complete publication was included. The corresponding author of relevant studies identified from the initial search, together with experts in the field, were contacted for any information on unpublished articles and in case of need for clarification.
Outcome measures and statistical analysis
The main outcome measure was the rate of malignant transformation. In addition, the numbers of (resected) HCAs, the number of females, mean age at presentation, mean diameter of the lesion, OC use and presence of haemorrhage were all assessed. All reported adenoma cases were listed in a table. All data were presented as mean or median values and percentages.
Results
A total of 3935 articles were identified through the electronic searches of PubMed (n = 120), The Cochrane Hepatobiliary Group Controlled Trials Register and CENTRAL in the Cochrane Library (n = 12) and a combined Ovid MEDLINE and EMBASE search (n = 3803). Through reading titles and abstracts 1196 duplicates as well as 2658 noticeably irrelevant articles and eight articles on GSD or adenomatosis were excluded. Furthermore, four articles were excluded as they contained a series of adenomas that had been previously reported. Altogether, 69 articles were selected for more detailed evaluation. From this analysis, a further 108 studies were included through cross-referencing and three studies were excluded because of a previously reported series of adenomas. In total, 174 articles (157 series and 17 case reports) on liver surgery and imaging, including case series of HCA, were retrieved (Fig. 1).
Figure 1.
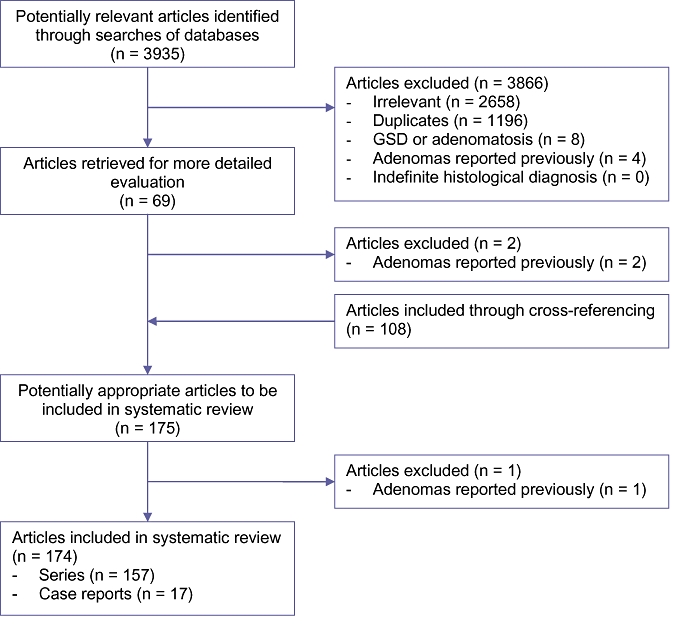
Flowchart literature search. GSD, glycogen storage disease
Hepatocellular adenoma series in the literature
The 157 series contained a total of 1617 HCAs worldwide, of which 1445 HCAs (89%) were resected (Table 1). Thirty-six patients with adenomatosis and 14 patients with GSD were excluded from a total of eight studies. There were 51 series that contained only a single patient with HCA. Most of these reports were published in the 1970s when individual cases of HCA in women using OCs were regularly reported. The three largest series contained 91,14 12415 and 12816 HCAs, respectively (patients having adenomatosis or GSD not included).
Table 1.
Overview of series of hepatocellular adenomas with or without malignant transformation into hepatocellular carcinomas
| Study | Year | HCA (n) | Resected (n) | HCC (n) | Female (n) | Mean age (y) | Mean diameter (cm) | OC use (n) | Haemorrhage (n) | Embolization (n) | Smallest diameter (cm)a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Malt et al.59 | 1970 | 4 | 3 | 0 | 3 | – | 15.8 | – | 1 | 0 | |
| Kay and Schatzki60 | 1971 | 1 | 0 | 0 | 1 | 26 | 10 | – | 0 | 0 | |
| Horvath et al.61 | 1972 | 1 | 1 | 0 | 1 | 28 | 15 | 1 | 0 | 0 | |
| Motsay and Gamble62 | 1972 | 5 | 5 | 0 | 5 | 30.2 | 9.6 | – | 1 | 0 | |
| Baum et al.63 | 1973 | 7 | 7 | 0 | 7 | – | – | 7 | 2 | 0 | |
| Contostavlos64 | 1973 | 1 | 0 | 0 | 1 | 37 | 15 | 1 | 1 | 0 | |
| Davis et al.65 | 1973 | 3 | 3 | 0 | 3 | 25 | – | – | 3 | 0 | |
| Hermann and David66 | 1973 | 1 | 1 | 0 | 1 | 20 | 12 | – | 1 | 0 | |
| Albritton et al.67 | 1974 | 4 | 4 | 0 | 4 | 32.8 | – | – | 0 | 0 | |
| Berg et al.68 | 1974 | 4 | 1 | 0 | 4 | 26.5 | – | 3 | 3 | 0 | |
| Kelso69 | 1974 | 1 | 1 | 0 | 1 | 36 | – | 1 | 1 | 0 | |
| Knapp and Ruebner70 | 1974 | 1 | 1 | 0 | 1 | 33 | 17 | 1 | 1 | 0 | |
| Tountas et al.71 | 1974 | 1 | 1 | 0 | 1 | 30 | – | 1 | 1 | 0 | |
| Ameriks et al.72 | 1975 | 8 | 8 | 0 | 8 | 34 | 15 | 8 | 3 | 0 | |
| Antoniades et al.73 | 1975 | 1 | 1 | 0 | 1 | 32 | 10.8 | 1 | 1 | 0 | |
| Antoniades and Brooks74 | 1975 | 1 | 1 | 0 | 1 | 30 | 6.5 | 1 | 1 | 0 | |
| Galloway et al.75 | 1975 | 4 | 1 | 0 | 4 | 41.5 | – | 1 | 2 | 0 | |
| Model et al.76 | 1975 | 1 | 1 | 0 | 1 | 31 | 2.5 | 1 | 1 | 0 | |
| Nissen and Kent77 | 1975 | 1 | 1 | 0 | 1 | 27 | – | 1 | 1 | 0 | |
| Stenwig and Solgaard78 | 1975 | 1 | 1 | 0 | 1 | 1 | 31 | 10 | 1 | 1 | |
| Ammentorp and Carson79 | 1976 | 4 | 4 | 0 | 4 | 28.8 | – | 4 | 0 | 0 | |
| Andersen and Packer80 | 1976 | 1 | 1 | 0 | 1 | 24 | 4.5 | 1 | 1 | 0 | |
| Baek et al.81 | 1976 | 1 | 1 | 0 | 1 | 31 | 18 | 1 | 1 | 0 | |
| Brander et al.82 | 1976 | 1 | 0 | 0 | 1 | 24 | – | 1 | 1 | 0 | |
| Edmondson et al.5 | 1976 | 42 | 41 | 0 | 42 | – | – | 34 | 29 | 0 | |
| Lansing et al.83 | 1976 | 3 | 3 | 0 | 2 | 33.7 | 8.7 | 1 | 1 | 0 | |
| Sears et al.84 | 1976 | 1 | 1 | 0 | 1 | 26 | 10 | 1 | 1 | 0 | |
| Chan and Detmer85 | 1977 | 4 | 4 | 0 | 4 | 35.3 | 11.3 | 4 | 2 | 0 | |
| Fechner86 | 1977 | 6 | 5 | 0 | 6 | 30.2 | – | 5 | 3 | 0 | |
| Fortner et al.87 | 1978 | 1 | 1 | 0 | – | – | – | – | – | 0 | |
| Gold et al.88 | 1978 | 12 | 7 | 0 | 7 | 30 | 6,8 | 6 | 1 | 0 | |
| Ramseur and Cooper89 | 1978 | 1 | 1 | 0 | 1 | 26 | 8 | 1 | 0 | 0 | |
| Bird et al.90 | 1979 | 1 | 1 | 0 | 1 | 39 | 15 | 1 | 1 | 0 | |
| Cady et al.91 | 1979 | 1 | 1 | 0 | – | – | – | – | – | 0 | |
| Catalano et al.92 | 1979 | 4 | 4 | 0 | 4 | 29 | – | 4 | 0 | 0 | |
| Mariani et al.41 | 1979 | 1 | 1 | 0 | 1 | 27 | 8.5 | 1 | 1 | 0 | |
| Wheeler et al.93 | 1979 | 1 | 0 | 0 | 1 | – | – | 1 | 0 | 1 | |
| Weil et al.94 | 1979 | 8 | 8 | 0 | 7 | 24 | – | 4 | 4 | 0 | |
| Kelly95 | 1980 | 2 | 1 | 0 | 2 | 30 | – | 2 | 1 | 0 | |
| Neuberger et al.96 | 1980 | 3 | 2 | 1 | 3 | 35 | – | 3 | 0 | 0 | – |
| Herczeg et al.97 | 1981 | 1 | 1 | 0 | 1 | 36 | – | 1 | 1 | 0 | |
| Isman et al.98 | 1981 | 2 | 2 | 0 | 0 | 41 | – | – | 2 | 0 | |
| Thompson and Little99 | 1981 | 1 | 1 | 0 | 1 | 30 | 9 | 1 | 0 | 0 | |
| Bühler et al.9 | 1982 | 3 | 0 | 0 | 3 | 30.3 | 6.7 | 3 | 1 | 0 | |
| Kerlin et al.100 | 1983 | 23 | 17 | 2 | 21 | 34 | 9 | 17 | 16 | 0 | – |
| Meensook and Sirisabya101 | 1983 | 1 | 1 | 0 | 1 | 25 | 16 | 1 | 1 | 0 | |
| Thompson et al.102 | 1983 | 5 | 5 | 0 | 5 | 32 | – | 2 | 2 | 0 | |
| Cassinelli103 | 1985 | 1 | 1 | 0 | 1 | 38 | – | 1 | 0 | 0 | |
| Gonzalez and Marks104 | 1985 | 12 | 12 | 0 | – | – | – | – | 2 | 0 | |
| Welch et al.105 | 1985 | 13 | 12 | 0 | 12 | 31 | 11 | 9 | 11 | 0 | |
| Mathieu et al.106 | 1986 | 27 | 27 | 0 | 27 | 34 | 7.5 | 26 | 5 | 0 | |
| Creagh et al.107 | 1988 | 1 | 1 | 0 | 0 | 27 | 4 | 1 | 1 | 0 | |
| Leese et al.2 | 1988 | 18 | 17 | 0 | 15 | 33 | 13 | 11 | 9 | 4 | |
| Marks et al.42 | 1988 | 3 | 3 | 0 | 3 | 35.7 | – | 3 | 1 | 0 | |
| Ringe et al.108 | 1989 | 5 | 4 | 0 | 4 | 34 | 10 | – | 1 | 0 | |
| Flowers et al.109 | 1990 | 6 | 6 | 0 | 5 | 28.8 | 7.3 | 5 | 6 | 0 | – |
| Iwatsuki et al.110 | 1990 | 25 | 25 | 0 | 20 | 31 | 12 | 12 | 4 | 0 | |
| Leborgne et al.111 | 1990 | 2 | 0 | 0 | 2 | 28 | 14 | 2 | 2 | 1 | |
| Tao47 | 1991 | 9 | 7 | 0 | 9 | 33.5 | – | 9 | 0 | 0 | – |
| Belghiti et al.112 | 1993 | 12 | 12 | 1 | 12 | 33 | 9.2 | 11 | 6 | 0 | – |
| Arrivéet al.113 | 1994 | 29 | 21 | 3 | 27 | 37.4 | 5.4 | 24 | 15 | 0 | – |
| Eckhauser et al.114 | 1994 | 8 | 8 | 0 | 8 | 31.5 | 9.5 | 7 | 0 | 0 | |
| Foster and Berman19 | 1994 | 13 | – | 1 | 12 | – | – | – | 1 | 0 | 12 |
| Golli et al.115 | 1994 | 8 | 8 | 0 | 7 | 30 | 6.8 | 6 | 1 | 0 | |
| Paineau et al.116 | 1994 | 1 | 1 | 0 | 0 | – | – | 0 | 0 | 0 | |
| Paulson et al.117 | 1994 | 9 | 6 | 0 | 8 | – | – | 6 | – | 0 | |
| Pertschy et al.118 | 1994 | 30 | 29 | 0 | – | – | – | – | 0 | 0 | |
| Cherqui119 | 1995 | 6 | 6 | 0 | 6 | 32 | 7.8 | 5 | 3 | 0 | |
| Chung et al.110 | 1995 | 16 | 15 | 0 | 14 | 34.6 | 5.4 | 5 | 12 | 0 | |
| Cuesta et al.120 | 1995 | 1 | 1 | 0 | 1 | 56 | 6 | – | 0 | 0 | |
| Ferzli et al.121 | 1995 | 1 | 1 | 0 | 1 | 43 | 9 | – | – | 0 | |
| Nagorney122 | 1995 | 24 | 19 | 1 | 22 | 35 | 9 | 9 | 4 | 0 | – |
| Ault et al.29 | 1996 | 11 | 4 | 3 | 10 | 37.6 | – | 9 | 4 | 4 | 5.5 |
| Azagra et al.123 | 1996 | 1 | 1 | 0 | 1 | 42 | 6 | – | 0 | 0 | |
| Cheng et al.124 | 1996 | 1 | 1 | 0 | 1 | 39 | 6.4 | 1 | 1 | 0 | |
| Kelly et al.125 | 1996 | 9 | 9 | 0 | – | – | – | – | 0 | 0 | |
| Vogl et al.126 | 1996 | 1 | 0 | 0 | 1 | 27 | – | – | 0 | 0 | |
| De Carlis et al.127 | 1997 | 19 | 19 | 2 | 19 | 31.8 | 7.9 | 17 | 5 | 0 | – |
| Krug et al.128 | 1997 | 8 | 8 | 0 | – | – | – | – | – | 0 | |
| Weimann et al.129 | 1997 | 41 | 36 | 2 | – | – | – | – | – | 0 | – |
| Croes et al.130 | 1998 | 8 | 8 | 0 | 8 | 30 | – | 6 | 8 | 0 | |
| Lezoche et al.131 | 1998 | 1 | 0 | 0 | 1 | – | 3 | – | – | 0 | |
| Meissner132 | 1998 | 1 | 1 | 0 | 1 | 41 | 4.5 | 1 | 1 | 0 | |
| Ott and Hohenberger133 | 1998 | 23 | 23 | 0 | – | – | – | – | 4 | 0 | |
| Katkhouda et al.134 | 1999 | 9 | 9 | 0 | – | – | – | 5 | 0 | 0 | |
| Closset et al.135 | 2000 | 16 | 16 | 1 | 16 | 35 | 8.1 | 10 | 7 | 0 | 15 |
| Herman et al.136 | 2000 | 10 | 10 | 0 | 10 | 29.2 | – | 9 | 0 | 0 | |
| Ichikawa et al.137 | 2000 | 24 | 13 | 2 | 20 | – | – | 12 | 10 | 0 | – |
| Mouiel et al.138 | 2000 | 1 | 1 | 0 | – | – | – | – | 0 | 0 | |
| Ji et al.139 | 2000 | 4 | 4 | 0 | 1 | 37 | – | 0 | – | 0 | |
| Aseni et al.39 | 2001 | 1 | 0 | 0 | 1 | 25 | 5.5 | 1 | 0 | 0 | |
| Charny et al.54 | 2001 | 12 | 8 | 1 | 10 | 34 | 8.6 | – | 0 | 0 | – |
| Heeringa and Sardi140 | 2001 | 1 | 1 | 0 | 1 | 27 | 5.5 | 1 | 1 | 0 | |
| Hung et al.141 | 2001 | 12 | 12 | 0 | 6 | 46.8 | 5.9 | 2 | 4 | 0 | |
| Kammula et al.142 | 2001 | 8 | 8 | 0 | – | – | – | – | – | – | |
| Mamada et al.143 | 2001 | 1 | 1 | 0 | 0 | 26 | 4.5 | 0 | 0 | 0 | |
| Reddy et al.144 | 2001 | 25 | 25 | 1 | 25 | 33 | 5.9 | 21 | 3 | 0 | 25 |
| Terkivatan et al.30 | 2001 | 33 | 19 | 0 | 29 | 36 | – | 27 | 12 | 0 | |
| Wilkens et al.145 | 2001 | 10 | 10 | 0 | 8 | 38.9 | 7.4 | – | – | 0 | |
| Antonetti et al.146 | 2002 | 1 | 1 | 0 | – | – | – | – | – | 0 | |
| Farges et al.147 | 2002 | 2 | 2 | 0 | – | – | – | – | – | 0 | |
| Marini et al.148 | 2002 | 7 | 7 | 1 | 7 | 37.4 | 13.5 | 0 | 7 | 3 | – |
| Croce et al.149 | 2003 | 2 | 2 | 0 | – | – | – | – | – | 0 | |
| Descottes et al.150 | 2003 | 17 | 17 | 0 | – | – | – | – | 0 | 0 | |
| Ho et al.151 | 2003 | 1 | 1 | 0 | – | – | 3.2 | – | – | 0 | |
| Morino et al.152 | 2003 | 5 | 5 | 0 | – | – | – | – | – | 0 | |
| Clarke et al.153 | 2004 | 8 | 8 | 0 | – | – | – | – | – | 0 | |
| Kim et al.154 | 2004 | 11 | 11 | 2 | – | – | – | – | 0 | 0 | – |
| Liu et al.155 | 2004 | 2 | 2 | 0 | – | – | – | – | – | 0 | |
| Ronald et al.156 | 2004 | 3 | 3 | 0 | 0 | 36 | 8.3 | 0 | 0 | 0 | |
| Atwell et al.57 | 2005 | 3 | 0 | 0 | 3 | 37 | 3.5 | 3 | 2 | 0 | |
| Dulucq et al.157 | 2005 | 3 | 3 | 0 | – | – | – | – | 0 | 0 | |
| Geller et al.158 | 2005 | 5 | 5 | 0 | – | – | – | – | – | 0 | |
| Giusti et al.159 | 2005 | 1 | 1 | 0 | 0 | 45 | 18 | 0 | 0 | 0 | |
| Psatha et al.160 | 2005 | 4 | 1 | 0 | 0 | 34.5 | 7 | 2 | 1 | 0 | |
| Socas et al.161 | 2005 | 2 | 0 | 0 | 0 | 29 | – | 2 | 1 | 0 | |
| Toso et al.162 | 2005 | 23 | 23 | 2 | – | – | – | – | 10 | 2 | 6.4 |
| Erdogan et al.58 | 2006 | 22 | 16 | 0 | 22 | 35.8 | 7.2 | 17 | 22 | 1 | |
| Learn et al.163 | 2006 | 1 | 1 | 0 | – | – | – | – | – | 0 | |
| Schemmer et al.164 | 2006 | 7 | 7 | 0 | – | – | – | – | – | 0 | |
| Tang et al.165 | 2006 | 1 | 1 | 0 | – | – | – | – | – | 0 | |
| Vibert et al.166 | 2006 | 3 | 3 | 0 | – | – | – | – | – | 0 | |
| Van der Windt et al.167 | 2006 | 48 | 16 | 0 | 48 | 36 | – | 45 | 13 | 3 | |
| Ardito et al.168 | 2007 | 7 | 7 | 0 | – | – | – | – | 0 | 0 | |
| Chaib et al.169 | 2007 | 28 | 28 | 0 | 24 | 36.3 | 8.0 | 22 | 3 | 0 | |
| Dagher et al.170 | 2007 | 6 | 6 | 0 | – | – | – | – | – | 0 | |
| Hompes et al.171 | 2007 | 2 | 2 | 0 | – | – | – | – | – | 0 | |
| Ibrahim et al.172 | 2007 | 5 | 5 | 0 | 1 | 37.2 | 8.1 | – | 0 | 0 | |
| Koffron et al.173 | 2007 | 47 | 47 | 0 | – | – | – | – | – | 0 | |
| Nissen et al.174 | 2007 | 2 | 2 | 0 | 2 | 46.5 | 5.3 | – | 0 | 0 | |
| Poultsides et al.175 | 2007 | 1 | 1 | 0 | 1 | 33 | 7 | – | 0 | 0 | |
| Reddy et al.176 | 2007 | 25b | 25 | 0 | 24 | 38 | 8.5 | 15 | – | 0 | |
| Stoot et al.3 | 2007 | 11 | 2 | 0 | 10 | 34 | 7 | 9 | 11 | 11 | |
| Teeuwen et al.177 | 2007 | 2 | 2 | 0 | 2 | 29.5 | – | 2 | 0 | 0 | |
| Balaa et al.178 | 2008 | 1 | 1 | 0 | – | – | – | – | – | 0 | |
| Buell et al.179 | 2008 | 25 | 25 | 0 | – | – | – | – | – | 0 | |
| Cho et al.28 | 2008 | 41 | 41 | 2 | 38 | 36 | – | 22 | 12 | 0 | 5.7 |
| Feng et al.180 | 2008 | 17 | 17 | 0 | – | – | – | – | – | 0 | |
| Machado et al.181 | 2008 | 3 | 3 | 0 | – | – | – | – | – | 0 | |
| Micchelli et al.31 | 2008 | 17 | 17 | 3 | – | – | – | c | – | 0 | 4 |
| Petri et al.182 | 2008 | 22 | 22 | 0 | 20 | 38.3 | 7.7 | – | – | 0 | |
| Popescu et al.183 | 2008 | 1 | 1 | 0 | 1 | 38 | 7.5 | – | – | 0 | |
| Pulitano et al.184 | 2008 | 7 | 7 | 0 | – | – | – | – | – | 0 | |
| Spencer et al.185 | 2008 | 1 | 1 | 0 | – | – | – | – | – | 0 | |
| Troisi et al.186 | 2008 | 11 | 11 | 0 | – | – | – | – | – | 0 | |
| Abu Hilal et al.187 | 2009 | 8 | 8 | 0 | – | – | – | – | – | 0 | |
| Al Awad-Jibara et al.188 | 2009 | 1 | 1 | 0 | 1 | 40 | 5.8 | 0 | 0 | 0 | |
| Bioulac-Sage et al.16 | 2009 | 128 | 128 | 5 | 116 | 41 | 7 | 106 | 23 | 0 | – |
| Bryant et al.189 | 2009 | 23 | 23 | 0 | – | – | – | – | – | 0 | |
| Deneve et al.15 | 2009 | 124 | 119 | 5 | 116 | 39 | – | 68 | 31 | 5 | – |
| Dokmak et al.14 | 2009 | 91 | 91 | 9 | 79 | – | – | 70 | 22 | – | 2–5 |
| Ito et al.190 | 2009 | 5 | 5 | 0 | – | – | – | – | – | 0 | |
| Machado et al.191 | 2009 | 3 | 3 | 0 | 3 | – | 11.6 | – | – | 0 | |
| Skalicky et al.192 | 2009 | 9 | 9 | 0 | – | – | – | – | – | 0 | |
| Tomus et al.193 | 2009 | 3 | 3 | 0 | – | – | – | – | – | 0 | |
| Watkins et al.194 | 2009 | 1 | 1 | 0 | 0 | 45 | 2.1 | 0 | 0 | 0 | |
| Total | 1617 | 1445 | 50 | 1075 | 787 | 400 | 35 | ||||
Smallest size of HCAs showing malignant transformation.
This study contained 2 groups, 25 patients without and 7 with glycogen storage disease (GSD) type Ia, of which the latter was excluded.
All cases with malignant transformation included OC use.
HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; Ocs, oral contraceptives; –, no data available or not reported.
Case reports on malignant transformation of hepatocellular adenomas
The literature search identified 17 case reports concerning malignant transformation (a total of 19 cases), which are presented in Table 2. The mean age of these patients at the time of surgery was 41 years (range, 19 to 70). Five of these patients (26%) were men, of whom one had a history of oral prednisolone use and another had a history of anabolic steroid use.17,18 Twelve of the 14 women reported to have malignant alteration of an HCA presented with a history of OC usage. The mean time elapsed between commencement of OC therapy and diagnosis of HCA was 14 years. Most cases of malignant transformation of HCAs were seen at the time of the diagnosis of HCA. Furthermore, only three cases (16%) among these 19 cases of malignant degeneration presented with multiple HCAs19–21 and six cases (32%) were complicated by haemorrhage.21–26
Table 2.
Overview of reported single cases of malignant transformation of hepatocellular adenomas into hepatocellular carcinomas
| Reference | Year | Age/gener | Use and duration of OCs or steroids (years) | Number of HCAs and size at time of diagnosis of HCC | Time to HCA diagnosis (years)a | Interval between diagnosis of HCA and HCC (years) | Haemorrhage | Resected | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Tesluk and Lawrie26 | 1981 | 34/F | Yes, 5 | Solitary, 16 cm | 5 | 3 | Yes | Yes | Postoperative death after one month |
| Gordon et al.22 | 1986 | 36/F | Yes, 14 | Solitary, 13 cm | 14 | 3 | Yes | Yes | Disease free |
| Gyorffy et al.20 | 1989 | 53/F | Yes, 19 | Multiple (3), 12 cm | 19 | 2 | No | No | Tumour-related death after 7 months |
| Korula et al.195 | 1991 | 40/F | Yes, 15 | Solitary, 6.5 cm | 21 | None | No | Yes | Disease free |
| Ferrell196 | 1993 | 29/F | Yes, 0.5 | Solitary, 18 cm | 5 | None | No | Yes | Disease free |
| Foster and Bermanb19 | 1994 | 56/F | Yes, 10 | Multiple (3–4) | 10 | 5 | No | No | Tumour-related death after 5 months |
| Herman et al.197 | 1994 | 30/F | Yes, 15 | – | 15 | None | No | Yes | n/a |
| Herman et al.197 | 1994 | 37/F | Yes, 20 | – | 20 | None | No | Yes | n/a |
| Perret et al.198 | 1996 | 24/F | Yes, 3 | Solitary, 14 cm | 3 | None | No | Yes | Disease free |
| Scott et al.199 | 1996 | 22/M | No | Solitary, 6 cm | – | None | No | Yes | Disease free |
| Ye et al.21 | 1999 | 42/F | Yes, 25 | Multiple (2), 9 cm | 25 | None | Yes | Yes | Disease free |
| Larson et al.25 | 2002 | 52/M | No | Solitary, 12 cm | – | 11 | Yes | Yes | Recurrence after 6 years |
| Chuang et al.17 | 2002 | 19/M | Yes, 15c | – | 15 | None | No | Yes | n/a |
| Chuang et al.17 | 2002 | 46/F | No | Solitary, 10 cm | – | None | No | Yes | n/a |
| Ito et al.23 | 2003 | 57/F | Yes, 1 month | Solitary, 10 cm | 30 | None | Yes | Yes | Disease free |
| Burri et al.200 | 2006 | 40/F | Yes, 4.5 | Solitary, 6 cm | 16 | None | No | Yes | Disease free |
| Colovic et al.27 | 2007 | 70/F | No | Solitary, 11.5 cm | – | None | No | Yes | Disease free |
| Gorayski et al.18 | 2008 | 35/M | Yes, 2 | Solitary, 9 cm | 10 | None | No | Yes | Disease free |
| Kim et al.24 | 2009 | 53/M | No | Solitary, 4.5 cm | – | None | Yes | Yes | Disease free |
Time to HCA diagnosis since start of oral contraceptive therapy (which may has been discontinued before diagnosis).
This case is also enlisted in Table 1.
Oral prednisolone.
HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; Ocs, oral contraceptives; –, no data available or not reported.
Although some authors noted that HCAs may regress on discontinuation of oral contraceptive use,9,10,22 three case reports suggested that even after discontinuation of OC use, HCC can still develop irrespective of the occurrence of regression of the HCA.20,22,26 Moreover, the reports by Chuang et al.17 and Colovic et al.27 showed that malignant transformation of HCAs can occur in patients without a history of OC use.
Frequency of malignant transformation of hepatocellular adenomas in the literature
Out of the 1617 HCAs listed in Table 1, 50 tumours (3.1%) underwent malignant transformation into HCC. By combining these data with the case reports aforementioned (Table 2), an estimation of the exact frequency of malignant alteration of HCAs could be made, as the data showed that 68 of a total of 1635 (4.2%) HCAs underwent malignant transformation. Moreover, of all resected HCAs (1462 in total), 4.5% contained focal malignancy. Although not the main scope of the current review, haemorrhage was found in 406 out of 1635 (25%) tumours, in keeping with literature data.13,14,28
Association between size of HCA and risk of malignant transformation
The size of HCA has, by current consensus, remained the main decision criterion in determining whether or not resection is indicated, based upon the observation that intratumoral bleeding only seldom takes place in lesions smaller than 5 cm.2,15,29 Concordant to this observation, most of the resected adenomas identified in the current search were resected after they reached a minimal size of 5 cm.4,30 However, limited data was hitherto reported concerning the possibility of an association between the size of HCAs and the risk of malignant transformation. Particularly, no specific studies on this subject have been conducted and, moreover, most series in the literature did not report precise measurements of the tumours. Nonetheless, some case reports and series of HCAs with particular diameters were reported. Deneve et al.15 analysed 124 patients with an HCA, of which five cases contained signs of malignant alteration. The mean size of these five tumours was 11.6 cm in largest diameter. No tumour smaller than 8 cm showed malignant transformation in this study. As shown in Table 2, the mean size of solitary HCAs with features of malignant alteration reported in the retrieved case reports was 10.5 cm (range 4.5 to 18 cm). Overall, the smallest size at which malignant transformation was reported in the literature was 4 cm. This malignant alteration occurred in a solitary tumour of a 23-year-old woman who had taken OCs for 8 years.31 In the series of 91 patients of Dokmak et al.,14 malignant alteration of either solitary or multifocal HCAs was seen in nine cases. In this study, only one case of malignant transformation was observed in an adenoma measuring less than 5 cm in diameter (2–5 cm, exact size not presented), concerning a male individual with a history of steroid intake. Overall, the current literature search showed that a total of three cases of malignant alteration (4.4% of all HCAs showing this phenomenon) occurred in a tumour measuring less than 5 cm in diameter.14,24,31
Discussion
This systematic review has focussed on the risk of malignant transformation of HCAs into HCCs. The present study shows that malignant alteration is a rare complication of these uncommon benign tumours. By performing a systematic search of studies reporting cases of this benign liver disease over the past 40 years and combining the data on these HCAs, a total of 1568 reported HCAs were found. The overall frequency of malignant transformation reported was 4.2% among all adenoma cases and 4.5% among all resected HCAs.
Although earlier series of HCAs had already shown one or two cases, Foster and Berman19 were the first to report an estimated risk of malignant transformation, as they found a frequency of 13%. Barthelmes and Tait,13 Cho et al.28 and Micchelli et al.31 used a similar approach for determining the incidence of malignant degeneration employed in the current study. However, these three studies identified a remarkably smaller number of case series than included in the current review, and, moreover, the latter study did not include cases of HCAs in which no malignant alteration was found.31 Additionally, most case series used in the frequency estimation comprised a limited sample size and reported only on resected adenomas. This could have led to an overestimation of the true risk, apart from the selection bias of reported studies and cases in general. The series of Dokmak et al.,14 Deneve et al.15 and Bioulac-Sage et al.16 seem more robust for estimating the frequency of malignant alteration, as these authors analysed a larger population containing 91, 124 and 128 patients, respectively (cases with adenomatosis or GSD not included). Hepatic adenomatosis is regarded as a different entity by many authors concerning presentation and size and number of the adenomas, as well as the different therapeutic options.12,13 Equally, several groups have recently reported germ line mutations of hepatocyte nuclear factor 1α inactivation in adenomatosis, and this has been suggested to be associated with maturity-onset diabetes of the young type 3.32–34 Also, these mutations may have implications on the risk of malignant degeneration. Therefore, and also to limit heterogeneity, liver adenomatosis was excluded in the present review. As previously mentioned, patients with known GSDs are at a higher risk of developing HCAs11,35 and were therefore not included in the current review.
Risk factors of malignant transformation of hepatocellular adenomas
Although the risk of malignant transformation seems small (4.2%), this is a serious complication which cannot be neglected. As HCAs are difficult to discriminate from HCCs, because of similar imaging characteristics and histopathological features, it is important to identify factors that increase the risk of malignant transformation. Unfortunately, in the current study a true risk analysis based on tumour size was difficult to perform as many studies only reported the mean size of the adenomas included. However, as three cases have been reported in which malignant transformation occurred in a tumour measuring less than 5 cm in diameter, the recommendation to only treat HCAs larger than 5 cm in diameter in order to prevent malignant transformation is open for debate.14,24,31
Upon reviewing the literature, several groups of patients were identified as having an increased risk of malignant alteration of these benign liver tumours. High-risk groups reported were those patients with a history of androgen or anabolic steroid intake,18 patients of male gender14,36 and, as previously stated, patients with GSD.11,37,38 Furthermore, as has been reported since the late 1970s, intake of OCs could potentially play a role in the enlargement of existing HCAs. To date, discontinuation of OC usage is still the advice given to patients that are diagnosed with an HCA,13 as several reports showed regression in size of the HCAs after cessation of OC intake.9,39,40 However, some case reports suggested that discontinuation of OCs will not abort the risk of malignant transformation.41,42 Therefore, even after discontinuation of OC use, long-term follow-up of patients with unresected HCAs remains necessary. Another proposed risk factor for malignant alteration is the presence of dysplasia in HCAs. This characteristic harbours a risk of progression to HCC.43–47
Several studies have identified mutations of the β-catenin gene in HCAs and reported that activated β-catenin mutations deregulate the β-catenin pathway. This pathway is part of the more complex Wnt signalling pathway which plays a major role in the proliferation of liver cells.36,48–50 These mutations may thus lead to hyperproliferation of liver cells and, consequently, malignancy. The Bordeaux group has performed genotype–phenotype analyses in HCAs and identified four different tumour subtypes with specific characteristics: (i) hepatocyte nuclear factor 1α (HNF1α) mutated (30%–50%), (ii) β-catenin-activated (10–15%), (iii) inflammatory (35%) and (iv) unclassified tumours (5%–10%).36,51,52 Hepatocellular carcinoma associated with adenoma was found in 46% of β-catenin-mutated tumours, whereas this has never been observed in inflammatory lesions and rarely found in HNF1α-mutated tumours. This suggests that HCAs with β-catenin activation carry a higher risk of malignant transformation. Furthermore, the Bordeaux group identified four immunohistochemical markers that characterize each of the four HCA subtypes with high specificity and sensitivity: liver-fatty acid binding protein (L-FABP), glutamine synthetase (GS), nuclear β-catenin and serum amyloid A (SAA).53 They found absence of L-FABP expression to indicate HNF1α mutation, whereas combined GS overexpression and nuclear β-catenin staining suggested β-catenin-activating mutations. Finally, the Bordeaux group noted that SAA staining and overexpression of C-reactive protein (CRP) predicted inflammatory HCA. These markers have proven to be a promising method to identify adenoma patients at risk of developing HCC. Table 3 provides an overview of HCA subtypes and their immunohistochemical markers.
Table 3.
Types of HCAs and their immunohistochemical markers
| HCA type | Frequency (%) | Malignant transformation | Markers |
|---|---|---|---|
| β-catenin activated | 10–15 | Yes | β-catenin+/GS+ |
| HNF1α inactivated | 30–50 | Rarely | LFABP− |
| Inflammatory | 35 | No | SAA+/CRP+ |
| Unclassified | 5–10 | No | None |
CRP, C-reactive protein; GS, glutamine synthetase; HCA, hepatocellular adenoma; HNF1α, hepatocyte nuclear factor 1α; LFABP, liver-fatty acid binding protein; SAA, serum amyloid A; +, positive; −, negative.
Future treatment perspectives
As for future treatment perspectives, more research is needed to investigate the mechanism of malignant degeneration. Only then can this group of predominantly young patients be withheld a potentially unnecessary liver resection, while this surgical treatment has still a reported morbidity and mortality of up to 27% and 3%, respectively.30,54–56 At present, for all patients presenting with one or more HCAs larger than 5 cm, resection is the treatment option of choice in accordance with the current guidelines. Only 4.2% of HCAs will have actual foci of HCC, and therefore a considerable number of resections will be performed in vain. However, this rate should preferably be seen as an upper limit of the true frequency as publication bias might have occurred. After all, men who have an anabolic steroid-induced adenoma containing foci of malignancy are more likely to be reported in the literature than those adenoma patients without malignant transformation. Nevertheless, over 95% of all patients presenting with HCAs measuring over 5 cm, will unnecessarily be exposed to a potentially hazardous surgical procedure. By identifying those patients who will derive most benefit from surgery as their HCA harbours an increased risk for malignant alteration, fewer patients will have to undergo this unnecessary surgery. The Bordeaux HCA markers are a promising risk prediction tool. However, biopsy could lead to haemorrhage, sample errors or tumour seeding, but these potential complications are rare in experienced centres. Also, the value of β-catenin staining needs to be studied more intensively. Recently, interest has turned towards less invasive procedures to treat patients that present with HCAs larger than 5 cm. There is preliminary evidence to suggest that developments in minimally invasive techniques such as (percutaneous) radiofrequency ablation (RFA) or microwave ablation may alter the treatment of HCA. The limited data so far available suggest that these techniques can be performed with low morbidity and zero mortality, but additional research is required to explore their exact role in adenoma treatment.57
Recently, two groups have reported the therapeutic effects of selective arterial embolization to stop haemorrhaging from ruptured adenomas.3,58 They also showed that embolizing ruptured adenomas prevented growth of these lesions. Subsequently, selective arterial embolization was utilized in a number of non-haemorrhaging adenomas.3 During follow-up, none of these adenomas grew and the majority even regressed in size. On examining the haemorrhaging and non-haemorrhaging adenomas separately, a statistically significant decrease in size was noted in both groups. It is this tumour regression, and its probable subsequent reduction of the risk of severe haemorrhaging and malignant transformation, that might propose selective arterial embolization as a novel treatment for large unruptured HCAs. As no significant complications from this treatment were reported arterial embolization of HCAs might be the direction for further future research.3,58 To the best of our knowledge, no studies have been performed to specifically investigate this treatment.
In conclusion, the current review shows that malignant transformation of HCAs into HCCs is a rare complication of these uncommon benign tumours. By pooling data of series and case reports, comprising more than 1600 reported HCAs, we found an overall frequency of malignant transformation of 4.2% for all adenomas and of 4.5% for all resected adenomas. A multicentre study with a large registry of HCAs is paramount for estimating the actual risk of malignant transformation. Further research should focus on the underlying mechanisms of malignant transformation of HCAs into HCCs, associated risk factors and the use of new tumour markers. By means of a better selection of precisely which patients with an HCA present with an increased risk of malignant degeneration – and who could therefore derive the greatest benefit from treatment – a reduction in unnecessary liver resections can be achieved. This would reduce the risks associated with surgery in these predominantly young patients.
Conflicts of interest
None declared.
References
- 1.Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644–648. [PubMed] [Google Scholar]
- 2.Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12-year surgical experience from a specialist hepato-biliary unit. Ann Surg. 1988;208:558–564. doi: 10.1097/00000658-198811000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoot JH, van der Linden E, Terpstra OT, Schaapherder AF. Life-saving therapy for haemorrhaging liver adenomas using selective arterial embolization. Br J Surg. 2007;94:1249–1253. doi: 10.1002/bjs.5779. [DOI] [PubMed] [Google Scholar]
- 4.Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Tilanus HW, JN IJ. Treatment of ruptured hepatocellular adenoma. Br J Surg. 2001;88:207–209. doi: 10.1046/j.1365-2168.2001.01648.x. [DOI] [PubMed] [Google Scholar]
- 5.Edmondson HA, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470–472. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 6.Soe KL, Soe M, Gluud C. Liver pathology associated with the use of anabolic-androgenic steroids. Liver. 1992;12:73–79. doi: 10.1111/j.1600-0676.1992.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 7.Wanless IR, Medline A. Role of estrogens as promoters of hepatic neoplasia. Lab Invest. 1982;46:313–320. [PubMed] [Google Scholar]
- 8.Reddy KR, Schiff ER. Approach to a liver mass. Semin Liver Dis. 1993;13:423–435. doi: 10.1055/s-2007-1007370. [DOI] [PubMed] [Google Scholar]
- 9.Buhler H, Pirovino M, Akobiantz A, Altorfer J, Weitzel M, Maranta E, et al. Regression of liver cell adenoma. A follow-up study of three consecutive patients after discontinuation of oral contraceptive use. Gastroenterology. 1982;82:775–782. [PubMed] [Google Scholar]
- 10.Edmondson HA, Reynolds TB, Henderson B, Benton B. Regression of liver cell adenomas associated with oral contraceptives. Ann Intern Med. 1977;86:180–182. doi: 10.7326/0003-4819-86-2-180. [DOI] [PubMed] [Google Scholar]
- 11.Labrune P, Trioche P, Duvaltier I, Chevalier P, Odievre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276–279. doi: 10.1097/00005176-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Flejou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, et al. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology. 1985;89:1132–1138. [PubMed] [Google Scholar]
- 13.Barthelmes L, Tait IS. Liver cell adenoma and liver cell adenomatosis. HPB (Oxford) 2005;7:186–196. doi: 10.1080/13651820510028954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, et al. A Single Center Surgical Experience of 122 Patients with Single and Multiple Hepatocellular Adenomas. Gastroenterology. 2009;137:1698–1705. doi: 10.1053/j.gastro.2009.07.061. [DOI] [PubMed] [Google Scholar]
- 15.Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009;16:640–648. doi: 10.1245/s10434-008-0275-6. [DOI] [PubMed] [Google Scholar]
- 16.Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481–489. doi: 10.1002/hep.22995. [DOI] [PubMed] [Google Scholar]
- 17.Chuang WY, Chen TC, Hsu HL, Lee WC, Jeng LB, Huang SF. Liver cell adenoma with concomitant hepatocellular carcinoma: report of two cases. J Formos Med Assoc. 2002;101:798–802. [PubMed] [Google Scholar]
- 18.Gorayski P, Thompson CH, Subhash HS, Thomas AC. Hepatocellular carcinoma associated with recreational anabolic steroid use. Br J Sports Med. 2008;42:74–75. doi: 10.1136/bjsm.2007.03932. discussion 5. [DOI] [PubMed] [Google Scholar]
- 19.Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch Surg. 1994;129:712–717. doi: 10.1001/archsurg.1994.01420310044007. [DOI] [PubMed] [Google Scholar]
- 20.Gyorffy EJ, Bredfeldt JE, Black WC. Transformation of hepatic cell adenoma to hepatocellular carcinoma due to oral contraceptive use. Ann Intern Med. 1989;110:489–490. doi: 10.7326/0003-4819-110-6-489. [DOI] [PubMed] [Google Scholar]
- 21.Ye MQ, Suriawinata A, Ben Haim M, Parsons R, Schwartz ME. A 42-year-old woman with liver masses and long-term use of oral contraceptives. Semin Liver Dis. 1999;19:339–344. doi: 10.1055/s-2007-1007123. [DOI] [PubMed] [Google Scholar]
- 22.Gordon SC, Reddy KR, Livingstone AS, Jeffers LJ, Schiff ER. Resolution of a contraceptive-steroid-induced hepatic adenoma with subsequent evolution into hepatocellular carcinoma. Ann Intern Med. 1986;105:547–549. doi: 10.7326/0003-4819-105-4-547. [DOI] [PubMed] [Google Scholar]
- 23.Ito M, Sasaki M, Wen CY, Nakashima M, Ueki T, Ishibashi H, et al. Liver cell adenoma with malignant transformation: a case report. World J Gastroenterol. 2003;9:2379–2381. doi: 10.3748/wjg.v9.i10.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DH, Kim SU, Nam DH, Choi YJ, Park SM, Lee CK, et al. A case of hepatocellular carcinoma within hepatocellular adenoma in a non-cirrhotic male. Korean J Intern Med. 2009;24:147–152. doi: 10.3904/kjim.2009.24.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson KA, Weber SM, Fong Y, Blumgart LH. Malignant transformation of hepatic adenoma with recurrence after resection. HPB (Oxford) 2002;4:139–143. doi: 10.1080/136518202760388055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tesluk H, Lawrie J. Hepatocellular adenoma. Its transformation to carcinoma in a user of oral contraceptives. Arch Pathol Lab Med. 1981;105:296–299. [PubMed] [Google Scholar]
- 27.Colovic R, Grubor N, Micev M, Radak V. Hepatocellular adenoma with malignant alteration. Hepatogastroenterology. 2007;54:386–388. [PubMed] [Google Scholar]
- 28.Cho SW, Marsh JW, Steel J, Holloway SE, Heckman JT, Ochoa ER, et al. Surgical management of hepatocellular adenoma: take it or leave it? Ann Surg Oncol. 2008;15:2795–2803. doi: 10.1245/s10434-008-0090-0. [DOI] [PubMed] [Google Scholar]
- 29.Ault GT, Wren SM, Ralls PW, Reynolds TB, Stain SC. Selective management of hepatic adenomas. Am Surg. 1996;62:825–829. [PubMed] [Google Scholar]
- 30.Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Zondervan PE, Tilanus HW, et al. Indications and long-term outcome of treatment for benign hepatic tumors: a critical appraisal. Arch Surg. 2001;136:1033–1038. doi: 10.1001/archsurg.136.9.1033. [DOI] [PubMed] [Google Scholar]
- 31.Micchelli ST, Vivekanandan P, Boitnott JK, Pawlik TM, Choti MA, Torbenson M. Malignant transformation of hepatic adenomas. Mod Pathol. 2008;21:491–497. doi: 10.1038/modpathol.2008.8. [DOI] [PubMed] [Google Scholar]
- 32.Montano-Loza A, Rios-Vaca A, Remes-Troche JM, Meza-Junco J, Trinidad-Hernandez S. Hepatic adenomatosis in an Hispanic patient. A case report and review of the literature. Ann Hepatol. 2002;1:136–139. [PubMed] [Google Scholar]
- 33.Bacq Y, Jacquemin E, Balabaud C, Jeannot E, Scotto B, Branchereau S, et al. Familial liver adenomatosis associated with hepatocyte nuclear factor 1alpha inactivation. Gastroenterology. 2003;125:1470–1475. doi: 10.1016/j.gastro.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Greaves WO, Bhattacharya B. Hepatic adenomatosis. Arch Pathol Lab Med. 2008;132:1951–1955. doi: 10.5858/132.12.1951. [DOI] [PubMed] [Google Scholar]
- 35.Bioulac-Sage P, Laumonier H, Laurent C, Zucman-Rossi J, Balabaud C. Hepatocellular adenoma: what is new in 2008. Hepatol Int. 2008;2:316–321. doi: 10.1007/s12072-008-9075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 37.Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153–162. doi: 10.1007/s10545-005-7500-2. [DOI] [PubMed] [Google Scholar]
- 38.Velazquez I, Alter BP. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J Hematol. 2004;77:257–267. doi: 10.1002/ajh.20183. [DOI] [PubMed] [Google Scholar]
- 39.Aseni P, Sansalone CV, Sammartino C, Benedetto FD, Carrafiello G, Giacomoni A, et al. Rapid disappearance of hepatic adenoma after contraceptive withdrawal. J Clin Gastroenterol. 2001;33:234–236. doi: 10.1097/00004836-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Kawakatsu M, Vilgrain V, Erlinger S, Nahum H. Disappearance of liver cell adenoma: CT and MR imaging. Abdom Imaging. 1997;22:274–276. doi: 10.1007/s002619900188. [DOI] [PubMed] [Google Scholar]
- 41.Mariani AF, Livingstone AS, Pereiras RV, Jr, van Zuiden PE, Schiff ER. Progressive enlargement of an hepatic cell adenoma. Gastroenterology. 1979;77:1319–1325. [PubMed] [Google Scholar]
- 42.Marks WH, Thompson N, Appleman H. Failure of hepatic adenomas (HCA) to regress after discontinuance of oral contraceptives. An association with focal nodular hyperplasia (FNH) and uterine leiomyoma. Ann Surg. 1988;208:190–195. doi: 10.1097/00000658-198808000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anthony PP, Vogel CL, Barker LF. Liver cell dysplasia: a premalignant condition. J Clin Pathol. 1973;26:217–223. [PMC free article] [PubMed] [Google Scholar]
- 44.Ho JC, Wu PC, Mak TK. Liver cell dysplasia in association with hepatocellular carcinoma, cirrhosis and hepatitis B surface antigen in Hong Kong. Int J Cancer. 1981;28:571–574. doi: 10.1002/ijc.2910280507. [DOI] [PubMed] [Google Scholar]
- 45.Lee RG, Tsamandas AC, Demetris AJ. Large cell change (liver cell dysplasia) and hepatocellular carcinoma in cirrhosis: matched case-control study, pathological analysis, and pathogenetic hypothesis. Hepatology. 1997;26:1415–1422. doi: 10.1002/hep.510260607. [DOI] [PubMed] [Google Scholar]
- 46.Su Q, Benner A, Hofmann WJ, Otto G, Pichlmayr R, Bannasch P. Human hepatic preneoplasia: phenotypes and proliferation kinetics of foci and nodules of altered hepatocytes and their relationship to liver cell dysplasia. Virchows Arch. 1997;431:391–406. doi: 10.1007/s004280050116. [DOI] [PubMed] [Google Scholar]
- 47.Tao LC. Oral contraceptive-associated liver cell adenoma and hepatocellular carcinoma. Cytomorphology and mechanism of malignant transformation. Cancer. 1991;68:341–347. doi: 10.1002/1097-0142(19910715)68:2<341::aid-cncr2820680223>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 48.Chen YW, Jeng YM, Yeh SH, Chen PJ. P53 gene and Wnt signaling in benign neoplasms: beta-catenin mutations in hepatic adenoma but not in focal nodular hyperplasia. Hepatology. 2002;36:927–935. doi: 10.1053/jhep.2002.36126. [DOI] [PubMed] [Google Scholar]
- 49.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 51.Bioulac-Sage P, Blanc JF, Rebouissou S, Balabaud C, Zucman-Rossi J. Genotype phenotype classification of hepatocellular adenoma. World J Gastroenterol. 2007;13:2649–2654. doi: 10.3748/wjg.v13.i19.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bluteau O, Jeannot E, Bioulac-Sage P, Marques JM, Blanc JF, Bui H, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312–315. doi: 10.1038/ng1001. [DOI] [PubMed] [Google Scholar]
- 53.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 54.Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, DeMatteo RP, Fong Y, et al. Management of 155 patients with benign liver tumours. Br J Surg. 2001;88:808–813. doi: 10.1046/j.0007-1323.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- 55.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion -7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. discussion -10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atwell TD, Brandhagen DJ, Charboneau JW, Nagorney DM, Callstrom MR, Farrell MA. Successful treatment of hepatocellular adenoma with percutaneous radiofrequency ablation. AJR Am J Roentgenol. 2005;184:828–831. doi: 10.2214/ajr.184.3.01840828. [DOI] [PubMed] [Google Scholar]
- 58.Erdogan D, Busch OR, van Delden OM, Ten Kate FJ, Gouma DJ, van Gulik TM. Management of spontaneous haemorrhage and rupture of hepatocellular adenomas. A single centre experience. Liver Int. 2006;26:433–438. doi: 10.1111/j.1478-3231.2006.01244.x. [DOI] [PubMed] [Google Scholar]
- 59.Malt RA, Hershberg RA, Miller WL. Experience with benign tumors of the liver. Surg Gynecol Obstet. 1970;130:285–291. [PubMed] [Google Scholar]
- 60.Kay S, Schatzki PF. Ultrastructure of a benign liver cell adenoma. Cancer. 1971;28:755–762. doi: 10.1002/1097-0142(197109)28:3<755::aid-cncr2820280334>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 61.Horvath E, Kovacs K, Ross RC. Ultrastructural findings in a well-differentiated hepatoma. Digestion. 1972;7:74–82. doi: 10.1159/000197263. [DOI] [PubMed] [Google Scholar]
- 62.Motsay GJ, Gamble WG. Clinical experience with hepatic adenomas. Surg Gynecol Obstet. 1972;134:415–418. [PubMed] [Google Scholar]
- 63.Baum JK, Bookstein JJ, Holtz F, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2:926–929. doi: 10.1016/s0140-6736(73)92594-4. [DOI] [PubMed] [Google Scholar]
- 64.Contostavlos DL. Letter: benign hepatomas and oral contraceptives. Lancet. 1973;2:1200. doi: 10.1016/s0140-6736(73)92956-5. [DOI] [PubMed] [Google Scholar]
- 65.Davis JB, Schrenken JR, Zimmerman O. Massive hemoperitoneum from rupture of benign hepatocellular adenoma. Surgery. 1973;73:181–184. [PubMed] [Google Scholar]
- 66.Hermann RE, David TE. Spontaneous rupture of the liver caused by hepatomas. Surgery. 1973;74:715–719. [PubMed] [Google Scholar]
- 67.Albritton DR, Tompkins RK, Longmire WP., Jr Hepatic cell adenoma: a report of four cases. Ann Surg. 1974;180:14–19. doi: 10.1097/00000658-197407000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berg JW, Ketalaar RJ, Rose EF, Vernon RG. Letter: hepatomas and oral contraceptives. Lancet. 1974;2:349–350. doi: 10.1016/s0140-6736(74)91725-5. [DOI] [PubMed] [Google Scholar]
- 69.Kelso DR. Letter: benign hepatomas and oral contraceptives. Lancet. 1974;1:315–316. doi: 10.1016/s0140-6736(74)92626-9. [DOI] [PubMed] [Google Scholar]
- 70.Knapp WA, Ruebner BH. Letter: hepatomas and oral contraceptives. Lancet. 1974;1:270–271. doi: 10.1016/s0140-6736(74)92580-x. [DOI] [PubMed] [Google Scholar]
- 71.Tountas C, Paraskevas G, Deligeorgi H. Letter: benign hepatoma and oral contraceptives. Lancet. 1974;1:1351–1352. doi: 10.1016/s0140-6736(74)90733-8. [DOI] [PubMed] [Google Scholar]
- 72.Ameriks JA, Thompson NW, Frey CF, Appelman HD, Walter JF. Hepatic cell adenomas, spontaneous liver rupture, and oral contraceptives. Arch Surg. 1975;110:548–557. doi: 10.1001/archsurg.1975.01360110094017. [DOI] [PubMed] [Google Scholar]
- 73.Antoniades K, Campbell WN, Hecksher RH, Kessler WB, McCarthy GE., Jr Liver cell adenoma and oral contraceptives. Double tumor development. JAMA. 1975;234:628–629. [PubMed] [Google Scholar]
- 74.Antoniades K, Brooks CE., Jr Hemoperitoneum from liver cell adenoma in a patient on oral contraceptives. Surgery. 1975;77:137–139. [PubMed] [Google Scholar]
- 75.Galloway SJ, Casarella WJ, Lattes R, Seamam WB. Minimal deviation hepatoma. A new entity. Am J Roentgenol Radium Ther Nucl Med. 1975;125:184–192. doi: 10.2214/ajr.125.1.184. [DOI] [PubMed] [Google Scholar]
- 76.Mdel DG, Fox JA, Jones RW. Letter: multiple hepatic adenomas associated with an oral contraceptive. Lancet. 1975;1:865. doi: 10.1016/s0140-6736(75)93051-2. [DOI] [PubMed] [Google Scholar]
- 77.Nissen ED, Kent DR. Liver tumors and oral contraceptives. Obstet Gynecol. 1975;46:460–467. [PubMed] [Google Scholar]
- 78.Stenwig AE, Solgaard T. Ruptured benign hepatoma associated with an oral contraceptive. A case report. Virchows Arch A Pathol Anat Histol. 1975;367:337–343. doi: 10.1007/BF01239340. [DOI] [PubMed] [Google Scholar]
- 79.Ammentorp PA, Carson RP. Hepatocellular adenoma and oral contraceptives. Ohio State Med J. 1976;72:283–286. [PubMed] [Google Scholar]
- 80.Andersen PH, Packer JT. Hepatic adenoma. Observations after estrogen withdrawal. Arch Surg. 1976;111:898–900. doi: 10.1001/archsurg.1976.01360260066018. [DOI] [PubMed] [Google Scholar]
- 81.Baek S, Sloane CE, Futterman SC. Benign liver cell adenoma associated with use of oral contraceptive agents. Ann Surg. 1976;183:239–242. doi: 10.1097/00000658-197603000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brander WL, Vosnides G, Ogg CS, West IE. Multiple hepatocellular tumours in a patient treated with oral contraceptives. Virchows Arch A Pathol Anat Histol. 1976;370:69–76. doi: 10.1007/BF00427311. [DOI] [PubMed] [Google Scholar]
- 83.Lansing PB, McQuitty JT, Bradburn DM. Benign liver tumors: what is their relationship to oral contraceptives? Am Surg. 1976;42:744–760. [PubMed] [Google Scholar]
- 84.Sears HF, Smith G, Powell RD. Hepatic adenoma associated with oral contraceptive use: an unusual clinical presentation. Arch Surg. 1976;111:1399–1403. doi: 10.1001/archsurg.1976.01360300089015. [DOI] [PubMed] [Google Scholar]
- 85.Chan CK, Detmer DE. Proper management of hepatic adenoma associated with oral contraceptives. Surg Gynecol Obstet. 1977;144:703–706. [PubMed] [Google Scholar]
- 86.Fechner RE. Benign hepatic lesions and orally administered contraceptives. A report of seven cases and a critical analysis of the literature. Hum Pathol. 1977;8:255–268. doi: 10.1016/s0046-8177(77)80022-1. [DOI] [PubMed] [Google Scholar]
- 87.Fortner JG, Kim DK, Maclean BJ, Barrett MK, Iwatsuki S, Turnbull AD, et al. Major hepatic resection for neoplasia: personal experience in 108 patients. Ann Surg. 1978;188:363–371. doi: 10.1097/00000658-197809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gold JH, Guzman IJ, Rosai J. Benign tumors of the liver. Pathologic examination of 45 cases. Am J Clin Pathol. 1978;70:6–17. doi: 10.1093/ajcp/70.1.6. [DOI] [PubMed] [Google Scholar]
- 89.Ramseur WL, Cooper MR. Asymptomatic liver cell adenomas. Another case of resolution after discontinuation of oral contraceptive use. JAMA. 1978;239:1647–1648. doi: 10.1001/jama.239.16.1647. [DOI] [PubMed] [Google Scholar]
- 90.Bird D, Vowles K, Anthony PP. Spontaneous rupture of a liver cell adenoma after long term methyltestosterone: report of a case successfully treated by emergency right hepatic lobectomy. Br J Surg. 1979;66:212–213. doi: 10.1002/bjs.1800660324. [DOI] [PubMed] [Google Scholar]
- 91.Cady B, Bonneval M, Fender HR., Jr Elective hepatic resection. Am J Surg. 1979;137:514–521. doi: 10.1016/0002-9610(79)90123-5. [DOI] [PubMed] [Google Scholar]
- 92.Catalano PW, Martin EW, Jr, Ellison C, Carey LC. Reasonable surgical treatment for tumors of the liver associated with the use of oral contraceptives. Surg Gynecol Obstet. 1979;148:759–763. [PubMed] [Google Scholar]
- 93.Wheeler PG, Melia W, Dubbins P, Jones B, Nunnerley H, Johnson P, et al. Non-operative arterial embolisation in primary liver tumours. Br Med J. 1979;2:242–244. doi: 10.1136/bmj.2.6184.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weil R, 3rd, Koep LJ, Starzl TE. Liver resection for hepatic adenoma. Arch Surg. 1979;114:178–180. doi: 10.1001/archsurg.1979.01370260068010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelly TR. Benign liver tumors: presenting profiles and treatment. Am Surg. 1980;46:398–402. [PubMed] [Google Scholar]
- 96.Neuberger J, Portmann B, Nunnerley HB, Laws JW, Davis M, Williams R. Oral-contraceptive-associated liver tumours: occurrence of malignancy and difficulties in diagnosis. Lancet. 1980;1:273–276. doi: 10.1016/s0140-6736(80)90776-x. [DOI] [PubMed] [Google Scholar]
- 97.Herczeg B, Magyar E, Petroczy G, Kovacs I, Ling L, Csitary F. [Oral contraceptives and liver tumors] Orv Hetil. 1981;122:1879–1883. [PubMed] [Google Scholar]
- 98.Isman H, Bourgeon R, Rampal P, Koch G, Mattei S. [Benign solid hepatic tumors: report on six cases. Diagnostic and therapeutic problems (author's transl)] Chirurgie. 1981;107:44–52. [PubMed] [Google Scholar]
- 99.Thompson JF, Little JM. Liver resection for neoplasm. Aust N Z J Surg. 1981;51:274–279. doi: 10.1111/j.1445-2197.1981.tb05956.x. [DOI] [PubMed] [Google Scholar]
- 100.Kerlin P, Davis GL, McGill DB, Weiland LH, Adson MA, Sheedy PF., 2nd Hepatic adenoma and focal nodular hyperplasia: clinical, pathologic, and radiologic features. Gastroenterology. 1983;84:994–1002. [PubMed] [Google Scholar]
- 101.Meensook C, Sirisabya N. Benign hepatic tumor and oral contraceptives. J Med Assoc Thai. 1983;66:299–302. [PubMed] [Google Scholar]
- 102.Thompson HH, Tompkins RK, Longmire WP., Jr Major hepatic resection. A 25-year experience. Ann Surg. 1983;197:375–388. doi: 10.1097/00000658-198304000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cassinelli GB. [Hepatic adenoma and oral contraceptives: personal case] Ann Ital Chir. 1985;57:101–107. [PubMed] [Google Scholar]
- 104.Gonzalez F, Marks C. Hepatic tumors and oral contraceptives: surgical management. J Surg Oncol. 1985;29:193–197. doi: 10.1002/jso.2930290313. [DOI] [PubMed] [Google Scholar]
- 105.Welch TJ, Sheedy PF, 2nd, Johnson CM, Stephens DH, Charboneau JW, Brown ML, et al. Focal nodular hyperplasia and hepatic adenoma: comparison of angiography, CT, US, and scintigraphy. Radiology. 1985;156:593–595. doi: 10.1148/radiology.156.3.3895291. [DOI] [PubMed] [Google Scholar]
- 106.Mathieu D, Bruneton JN, Drouillard J, Pointreau CC, Vasile N. Hepatic adenomas and focal nodular hyperplasia: dynamic CT study. Radiology. 1986;160:53–58. doi: 10.1148/radiology.160.1.3520655. [DOI] [PubMed] [Google Scholar]
- 107.Creagh TM, Rubin A, Evans DJ. Hepatic tumours induced by anabolic steroids in an athlete. J Clin Pathol. 1988;41:441–443. doi: 10.1136/jcp.41.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ringe B, Mauz S, Barg-Hock H, Kotzerke J, Pichlmayr R. [Surgery of benign liver tumors] Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1989:287–291. [PubMed] [Google Scholar]
- 109.Flowers BF, McBurney RP, Vera SR. Ruptured hepatic adenoma. A spectrum of presentation and treatment. Am Surg. 1990;56:380–383. [PubMed] [Google Scholar]
- 110.Iwatsuki S, Todo S, Starzl TE. Excisional therapy for benign hepatic lesions. Surg Gynecol Obstet. 1990;171:240–246. [PMC free article] [PubMed] [Google Scholar]
- 111.Leborgne J, Lehur PA, Horeau JM, Dupas B, Bourcheix LM, Petiot JM, et al. [Therapeutic problems caused by rupture of large hepatic adenoma with central location. Apropos of 3 cases] Chirurgie. 1990;116:454–460. [PubMed] [Google Scholar]
- 112.Belghiti J, Pateron D, Panis Y, Vilgrain V, Flejou JF, Benhamou JP, et al. Resection of presumed benign liver tumours. Br J Surg. 1993;80:380–383. doi: 10.1002/bjs.1800800340. [DOI] [PubMed] [Google Scholar]
- 113.Arrive L, Flejou JF, Vilgrain V, Belghiti J, Najmark D, Zins M, et al. Hepatic adenoma: MR findings in 51 pathologically proved lesions. Radiology. 1994;193:507–512. doi: 10.1148/radiology.193.2.7972769. [DOI] [PubMed] [Google Scholar]
- 114.Eckhauser FE, Knol JA, Raper SE, Thompson NW. Enucleation combined with hepatic vascular exclusion is a safe and effective alternative to hepatic resection for liver cell adenoma. Am Surg. 1994;60:466–471. discussion 72. [PubMed] [Google Scholar]
- 115.Golli M, Van Nhieu JT, Mathieu D, Zafrani ES, Cherqui D, Dhumeaux D, et al. Hepatocellular adenoma: color Doppler US and pathologic correlations. Radiology. 1994;190:741–744. doi: 10.1148/radiology.190.3.8115621. [DOI] [PubMed] [Google Scholar]
- 116.Paineau J, Hamy A, Visset J. [Our surgical experience in the resection of benign hepatic tumors. Apropos of 31 cases] J Chir (Paris) 1994;131:461–465. [PubMed] [Google Scholar]
- 117.Paulson EK, McClellan JS, Washington K, Spritzer CE, Meyers WC, Baker ME. Hepatic adenoma: MR characteristics and correlation with pathologic findings. AJR Am J Roentgenol. 1994;163:113–116. doi: 10.2214/ajr.163.1.8010195. [DOI] [PubMed] [Google Scholar]
- 118.Pertschy J, Ruckert JC, Manger T. [Diagnosis and surgical therapy of benign liver tumors] Zentralbl Chir. 1994;119:495–500. [PubMed] [Google Scholar]
- 119.Cherqui D, Rahmouni A, Charlotte F, Boulahdour H, Metreau JM, Meignan M, et al. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology. 1995;22:1674–1681. [PubMed] [Google Scholar]
- 120.Cuesta MA, Meijer S, Paul MA, de Brauw LM. Limited laparoscopic liver resection of benign tumors guided by laparoscopic ultrasonography: report of two cases. Surg Laparosc Endosc. 1995;5:396–401. [PubMed] [Google Scholar]
- 121.Ferzli G, David A, Kiel T. Laparoscopic resection of a large hepatic tumor. Surg Endosc. 1995;9:733–735. doi: 10.1007/BF00187953. [DOI] [PubMed] [Google Scholar]
- 122.Nagorney DM. Benign hepatic tumors: focal nodular hyperplasia and hepatocellular adenoma. World J Surg. 1995;19:13–18. doi: 10.1007/BF00316973. [DOI] [PubMed] [Google Scholar]
- 123.Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996;10:758–761. doi: 10.1007/BF00193052. [DOI] [PubMed] [Google Scholar]
- 124.Cheng PN, Shin JS, Lin XZ. Hepatic adenoma: an observation from asymptomatic stage to rupture. Hepatogastroenterology. 1996;43:245–248. [PubMed] [Google Scholar]
- 125.Kelly D, Emre S, Guy SR, Sheiner PA, Miller CM, Schwartz ME. Resection of benign hepatic lesions with selective use of total vascular isolation. J Am Coll Surg. 1996;183:113–116. [PubMed] [Google Scholar]
- 126.Vogl TJ, Hammerstingl R, Schwarz W, Mack MG, Muller PK, Pegios W, et al. Superparamagnetic iron oxide – enhanced versus gadolinium-enhanced MR imaging for differential diagnosis of focal liver lesions. Radiology. 1996;198:881–887. doi: 10.1148/radiology.198.3.8628887. [DOI] [PubMed] [Google Scholar]
- 127.De Carlis L, Pirotta V, Rondinara GF, Sansalone CV, Colella G, Maione G, et al. Hepatic adenoma and focal nodular hyperplasia: diagnosis and criteria for treatment. Liver Transpl Surg. 1997;3:160–165. doi: 10.1002/lt.500030209. [DOI] [PubMed] [Google Scholar]
- 128.Krug B, Zieren HU, Jung G, Hemme A, Heindel W, Krings F. Late results after resection of benign hepatic tumors: clinical and radiological findings. Eur Radiol. 1997;7:327–332. doi: 10.1007/s003300050160. [DOI] [PubMed] [Google Scholar]
- 129.Weimann A, Ringe B, Klempnauer J, Lamesch P, Gratz KF, Prokop M, et al. Benign liver tumors: differential diagnosis and indications for surgery. World J Surg. 1997;21:983–990. doi: 10.1007/s002689900337. discussion 90-1. [DOI] [PubMed] [Google Scholar]
- 130.Croes EA, van Gulik TM, Bosma A, de Wit LT, Gouma DJ. [Treatment of 8 patients with an acute hemorrhage in a hepatocellular adenoma at the Academic Medical Center, Amsterdam] Ned Tijdschr Geneeskd. 1998;142:2463–2468. [PubMed] [Google Scholar]
- 131.Lezoche E, Paganini AM, Feliciotti F, Guerrieri M, Lugnani F, Tamburini A. Ultrasound-guided laparoscopic cryoablation of hepatic tumors: preliminary report. World J Surg. 1998;22:829–835. doi: 10.1007/s002689900478. discussion 35-36. [DOI] [PubMed] [Google Scholar]
- 132.Meissner K. Hemorrhage caused by ruptured liver cell adenoma following long-term oral contraceptives: a case report. Hepatogastroenterology. 1998;45:224–225. [PubMed] [Google Scholar]
- 133.Ott R, Hohenberger W. [Focal nodular hyperplasia and liver cell adenoma: operation or observation?] Zentralbl Chir. 1998;123:145–153. [PubMed] [Google Scholar]
- 134.Katkhouda N, Hurwitz M, Gugenheim J, Mavor E, Mason RJ, Waldrep DJ, et al. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg. 1999;229:460–466. doi: 10.1097/00000658-199904000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Closset J, Veys I, Peny MO, Braude P, Van Gansbeke D, Lambilliotte JP, et al. Retrospective analysis of 29 patients surgically treated for hepatocellular adenoma or focal nodular hyperplasia. Hepatogastroenterology. 2000;47:1382–1384. [PubMed] [Google Scholar]
- 136.Herman P, Pugliese V, Machado MA, Montagnini AL, Salem MZ, Bacchella T, et al. Hepatic adenoma and focal nodular hyperplasia: differential diagnosis and treatment. World J Surg. 2000;24:372–376. doi: 10.1007/s002689910059. [DOI] [PubMed] [Google Scholar]
- 137.Ichikawa T, Federle MP, Grazioli L, Nalesnik M. Hepatocellular adenoma: multiphasic CT and histopathologic findings in 25 patients. Radiology. 2000;214:861–868. doi: 10.1148/radiology.214.3.r00mr28861. [DOI] [PubMed] [Google Scholar]
- 138.Mouiel J, Katkhouda N, Gugenheim J, Fabiani P. Possibilities of laparoscopic liver resection. J Hepatobiliary Pancreat Surg. 2000;7:1–8. doi: 10.1007/s005340050146. [DOI] [PubMed] [Google Scholar]
- 139.Ji Y, Zhu X, Sun H, Tan Y, Ma Z, Ye Q, et al. Hepatocellular adenoma and focal nodular hyperplasia: a series of 24 patients with clinicopathological and radiological correlation. Chin Med J (Engl) 2000;113:852–857. [PubMed] [Google Scholar]
- 140.Heeringa B, Sardi A. Bleeding hepatic adenoma: expectant treatment to limit the extent of liver resection. Am Surg. 2001;67:927–929. [PubMed] [Google Scholar]
- 141.Hung CH, Changchien CS, Lu SN, Eng HL, Wang JH, Lee CM, et al. Sonographic features of hepatic adenomas with pathologic correlation. Abdom Imaging. 2001;26:500–506. doi: 10.1007/s00261-001-0011-1. [DOI] [PubMed] [Google Scholar]
- 142.Kammula US, Buell JF, Labow DM, Rosen S, Millis JM, Posner MC. Surgical management of benign tumors of the liver. Int J Gastrointest Cancer. 2001;30:141–146. doi: 10.1385/IJGC:30:3:141. [DOI] [PubMed] [Google Scholar]
- 143.Mamada Y, Onda M, Tajiri T, Akimaru K, Yoshida H, Taniai N, et al. Liver cell adenoma in a 26-year-old man. J Nippon Med Sch. 2001;68:516–519. doi: 10.1272/jnms.68.516. [DOI] [PubMed] [Google Scholar]
- 144.Reddy KR, Kligerman S, Levi J, Livingstone A, Molina E, Franceschi D, et al. Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg. 2001;67:173–178. [PubMed] [Google Scholar]
- 145.Wilkens L, Bredt M, Flemming P, Becker T, Klempnauer J, Kreipe HH. Differentiation of liver cell adenomas from well-differentiated hepatocellular carcinomas by comparative genomic hybridization. J Pathol. 2001;193:476–482. doi: 10.1002/path.825. [DOI] [PubMed] [Google Scholar]
- 146.Antonetti MC, Killelea B, Orlando R., 3rd Hand-assisted laparoscopic liver surgery. Arch Surg. 2002;137:407–411. doi: 10.1001/archsurg.137.4.407. discussion 12. [DOI] [PubMed] [Google Scholar]
- 147.Farges O, Jagot P, Kirstetter P, Marty J, Belghiti J. Prospective assessment of the safety and benefit of laparoscopic liver resections. J Hepatobiliary Pancreat Surg. 2002;9:242–248. doi: 10.1007/s005340200026. [DOI] [PubMed] [Google Scholar]
- 148.Marini P, Vilgrain V, Belghiti J. Management of spontaneous rupture of liver tumours. Dig Surg. 2002;19:109–113. doi: 10.1159/000052022. [DOI] [PubMed] [Google Scholar]
- 149.Croce E, Olmi S, Bertolini A, Erba L, Magnone S. Laparoscopic liver resection with radiofrequency. Hepatogastroenterology. 2003;50:2088–2092. [PubMed] [Google Scholar]
- 150.Descottes B, Glineur D, Lachachi F, Valleix D, Paineau J, Hamy A, et al. Laparoscopic liver resection of benign liver tumors. Surg Endosc. 2003;17:23–30. doi: 10.1007/s00464-002-9047-8. [DOI] [PubMed] [Google Scholar]
- 151.Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213–221. [PubMed] [Google Scholar]
- 152.Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc. 2003;17:1914–1918. doi: 10.1007/s00464-003-9070-4. [DOI] [PubMed] [Google Scholar]
- 153.Clarke D, Currie E, Madhavan K, Parks R, Garden O. Hepatic resection for benign non-cystic liver lesions. HPB (Oxford) 2004;6:115–119. doi: 10.1080/13651820410026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim J, Ahmad SA, Lowy AM, Buell JF, Pennington LJ, Moulton JS, et al. An algorithm for the accurate identification of benign liver lesions. Am J Surg. 2004;187:274–279. doi: 10.1016/j.amjsurg.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 155.Liu CL, Fan ST, Lo CM, Chan SC, Tso WK, Ng IO, et al. Hepatic resection for incidentaloma. J Gastrointest Surg. 2004;8:785–793. doi: 10.1016/j.gassur.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 156.Ronald M, Woodfield J, McCall J, Koea J. Hepatic adenomas in male patients. HPB (Oxford) 2004;6:25–27. doi: 10.1080/13651820310020846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dulucq JL, Wintringer P, Stabilini C, Berticelli J, Mahajna A. Laparoscopic liver resections: a single center experience. Surg Endosc. 2005;19:886–891. doi: 10.1007/s00464-004-2044-3. [DOI] [PubMed] [Google Scholar]
- 158.Geller DA, Tsung A, Maheshwari V, Rutstein LA, Fung JJ, Wallis Marsh J. Hepatic resection in 170 patients using saline-cooled radiofrequency coagulation. HPB (Oxford) 2005;7:208–213. doi: 10.1080/13651820510028945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Giusti S, Donati F, Paolicchi A, Bartolozzi C. Hepatocellular adenoma: imaging findings and pathological correlation. Dig Liver Dis. 2005;37:200–205. doi: 10.1016/j.dld.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 160.Psatha EA, Semelka RC, Armao D, Woosley JT, Firat Z, Schneider G. Hepatocellular adenomas in men: MRI findings in four patients. J Magn Reson Imaging. 2005;22:258–264. doi: 10.1002/jmri.20375. [DOI] [PubMed] [Google Scholar]
- 161.Socas L, Zumbado M, Perez-Luzardo O, Ramos A, Perez C, Hernandez JR, et al. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br J Sports Med. 2005;39:e27. doi: 10.1136/bjsm.2004.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Toso C, Majno P, Andres A, Rubbia-Brandt L, Berney T, Buhler L, et al. Management of hepatocellular adenoma: solitary-uncomplicated, multiple and ruptured tumors. World J Gastroenterol. 2005;11:5691–5695. doi: 10.3748/wjg.v11.i36.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Learn PA, Bowers SP, Watkins KT. Laparoscopic hepatic resection using saline-enhanced electrocautery permits short hospital stays. J Gastrointest Surg. 2006;10:422–427. doi: 10.1016/j.gassur.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 164.Schemmer P, Friess H, Hinz U, Mehrabi A, Kraus TW, Z'Graggen K, et al. Stapler hepatectomy is a safe dissection technique: analysis of 300 patients. World J Surg. 2006;30:419–430. doi: 10.1007/s00268-005-0192-9. [DOI] [PubMed] [Google Scholar]
- 165.Tang CN, Tsui KK, Ha JP, Yang GP, Li MK. A single-centre experience of 40 laparoscopic liver resections. Hong Kong Med J. 2006;12:419–425. [PubMed] [Google Scholar]
- 166.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 167.van der Windt DJ, Kok NF, Hussain SM, Zondervan PE, Alwayn IP, de Man RA, et al. Case-orientated approach to the management of hepatocellular adenoma. Br J Surg. 2006;93:1495–1502. doi: 10.1002/bjs.5511. [DOI] [PubMed] [Google Scholar]
- 168.Ardito F, Tayar C, Laurent A, Karoui M, Loriau J, Cherqui D. Laparoscopic liver resection for benign disease. Arch Surg. 2007;142:1188–1193. doi: 10.1001/archsurg.142.12.1188. discussion 93. [DOI] [PubMed] [Google Scholar]
- 169.Chaib E, Gama-Rodrigues J, Ribeiro MA, Jr, Herman P, Saad WA. Hepatic adenoma. Timing for surgery. Hepatogastroenterology. 2007;54:1382–1387. [PubMed] [Google Scholar]
- 170.Dagher I, Proske JM, Carloni A, Richa H, Tranchart H, Franco D. Laparoscopic liver resection: results for 70 patients. Surg Endosc. 2007;21:619–624. doi: 10.1007/s00464-006-9137-0. [DOI] [PubMed] [Google Scholar]
- 171.Hompes D, Aerts R, Penninckx F, Topal B. Laparoscopic liver resection using radiofrequency coagulation. Surg Endosc. 2007;21:175–180. doi: 10.1007/s00464-005-0846-6. [DOI] [PubMed] [Google Scholar]
- 172.Ibrahim S, Chen CL, Wang SH, Lin CC, Yang CH, Yong CC, et al. Liver resection for benign liver tumors: indications and outcome. Am J Surg. 2007;193:5–9. doi: 10.1016/j.amjsurg.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 173.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–392. doi: 10.1097/SLA.0b013e318146996c. discussion 92-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nissen NN, Grewal N, Lee J, Nawabi A, Korman J. Completely laparoscopic nonanatomic hepatic resection using saline-cooled cautery and hydrodissection. Am Surg. 2007;73:987–990. [PubMed] [Google Scholar]
- 175.Poultsides G, Brown M, Orlando R., 3rd Hand-assisted laparoscopic management of liver tumors. Surg Endosc. 2007;21:1275–1279. doi: 10.1007/s00464-006-9174-8. [DOI] [PubMed] [Google Scholar]
- 176.Reddy SK, Kishnani PS, Sullivan JA, Koeberl DD, Desai DM, Skinner MA, et al. Resection of hepatocellular adenoma in patients with glycogen storage disease type Ia. J Hepatol. 2007;47:658–663. doi: 10.1016/j.jhep.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 177.Teeuwen PH, Ruers TJ, Wobbes T. [Hepatocellular adenoma, a tumour particularly seen in mostly young women] Ned Tijdschr Geneeskd. 2007;151:1321–1324. [PubMed] [Google Scholar]
- 178.Balaa FK, Gamblin TC, Tsung A, Marsh JW, Geller DA. Right hepatic lobectomy using the staple technique in 101 patients. J Gastrointest Surg. 2008;12:338–343. doi: 10.1007/s11605-007-0236-6. [DOI] [PubMed] [Google Scholar]
- 179.Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248:475–486. doi: 10.1097/SLA.0b013e318185e647. [DOI] [PubMed] [Google Scholar]
- 180.Feng ZQ, Huang ZQ, Xu LN, Liu R, Zhang AQ, Huang XQ, et al. Liver resection for benign hepatic lesions: a retrospective analysis of 827 consecutive cases. World J Gastroenterol. 2008;14:7247–7251. doi: 10.3748/wjg.14.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Machado MA, Makdissi FF, Galvao FH, Machado MC. Intrahepatic Glissonian approach for laparoscopic right segmental liver resections. Am J Surg. 2008;196:e38–e42. doi: 10.1016/j.amjsurg.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 182.Petri A, Hohn J, Kokai EL, Savanya GK, Lazar G. Surgery of benign liver tumors: indications for treatment: twenty years' experience. Hepatogastroenterology. 2008;55:592–595. [PubMed] [Google Scholar]
- 183.Popescu I, Ciurea S, Romanescu D, Boros M. Isolated resection of the caudate lobe: indications, technique and results. Hepatogastroenterology. 2008;55:831–835. [PubMed] [Google Scholar]
- 184.Pulitano C, Catena M, Arru M, Guzzetti E, Comotti L, Ferla G, et al. Laparoscopic liver resection without portal clamping: a prospective evaluation. Surg Endosc. 2008;22:2196–2200. doi: 10.1007/s00464-008-0022-x. [DOI] [PubMed] [Google Scholar]
- 185.Spencer L, Metcalfe MS, Strickland AD, Elsey EJ, Robertson GS, Lloyd DM. Lessons from laparoscopic liver surgery: a nine-year case series. HPB Surg. 2008;2008:458137. doi: 10.1155/2008/458137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Troisi R, Montalti R, Smeets P, Van Huysse J, Van Vlierberghe H, Colle I, et al. The value of laparoscopic liver surgery for solid benign hepatic tumors. Surg Endosc. 2008;22:38–44. doi: 10.1007/s00464-007-9527-y. [DOI] [PubMed] [Google Scholar]
- 187.Abu Hilal M, Underwood T, Taylor MG, Hamdan K, Elberm H, Pearce NW. Bleeding and hemostasis in laparoscopic liver surgery. Surg Endosc. 2010;24:572–577. doi: 10.1007/s00464-009-0597-x. [DOI] [PubMed] [Google Scholar]
- 188.Al Awad-Jibara A, Chirinos Vargas JR, Baena SJ, Pirela-Finol CA, Brea-Andrade AF, Yajure-Perozo ME. [Laparoscopic hepatectomy in benign solid tumor: case report] Cir Cir. 2009;77:223–227. [PubMed] [Google Scholar]
- 189.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–111. doi: 10.1097/SLA.0b013e3181ad6660. [DOI] [PubMed] [Google Scholar]
- 190.Ito K, Ito H, Are C, Allen PJ, Fong Y, Dematteo RP, et al. Laparoscopic versus open liver resection: a matched-pair case control study. J Gastrointest Surg. 2009;13:2276–2283. doi: 10.1007/s11605-009-0993-5. [DOI] [PubMed] [Google Scholar]
- 191.Machado MA, Makdissi FF, Surjan RC, Herman P, Teixeira AR, MC CM. Laparoscopic resection of left liver segments using the intrahepatic Glissonian approach. Surg Endosc. 2009;23:2615–2619. doi: 10.1007/s00464-009-0423-5. [DOI] [PubMed] [Google Scholar]
- 192.Skalicky T, Treska V, Sutnar A, Liska V, Molacek J, Mirka H, et al. [Surgical treatment of benign liver tumours – indications and results] Zentralbl Chir. 2009;134:141–144. doi: 10.1055/s-2008-1076871. [DOI] [PubMed] [Google Scholar]
- 193.Tomus C, Iancu C, Bala O, Graur F, Furcea L, Zaharie F, et al. [Liver resection for benign hepatic lesion: mortality, morbidity and risk factors for postoperative complications] Chirurgia (Bucur) 2009;104:275–280. [PubMed] [Google Scholar]
- 194.Watkins J, Balabaud C, Bioulac-Sage P, Sharma D, Dhillon A. Hepatocellular adenoma in advanced-stage fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:932–936. doi: 10.1097/MEG.0b013e328311a67c. [DOI] [PubMed] [Google Scholar]
- 195.Korula J, Yellin A, Kanel G, Campofiori G, Nichols P. Hepatocellular carcinoma coexisting with hepatic adenoma. Incidental discovery after long-term oral contraceptive use. West J Med. 1991;155:416–418. [PMC free article] [PubMed] [Google Scholar]
- 196.Ferrell LD. Hepatocellular carcinoma arising in a focus of multilobular adenoma. A case report. Am J Surg Pathol. 1993;17:525–529. doi: 10.1097/00000478-199305000-00013. [DOI] [PubMed] [Google Scholar]
- 197.Herman P, Machado MA, Volpe P, Pugliese V, Vianna MR, Bacchella T, et al. [Transformation of hepatic adenoma into hepatocellular carcinoma in patients with prolonged use of oral contraceptives] Rev Hosp Clin Fac Med Sao Paulo. 1994;49:30–33. [PubMed] [Google Scholar]
- 198.Perret AG, Mosnier JF, Porcheron J, Cuilleron M, Berthoux P, Boucheron S, et al. Role of oral contraceptives in the growth of a multilobular adenoma associated with a hepatocellular carcinoma in a young woman. J Hepatol. 1996;25:976–979. doi: 10.1016/s0168-8278(96)80305-9. [DOI] [PubMed] [Google Scholar]
- 199.Scott FR, el-Refaie A, More L, Scheuer PJ, Dhillon AP. Hepatocellular carcinoma arising in an adenoma: value of QBend 10 immunostaining in diagnosis of liver cell carcinoma. Histopathology. 1996;28:472–474. doi: 10.1046/j.1365-2559.1996.t01-3-297345.x. [DOI] [PubMed] [Google Scholar]
- 200.Burri E, Steuerwald M, Cathomas G, Mentha G, Majno P, Rubbia-Brandt L, et al. Hepatocellular carcinoma in a liver-cell adenoma within a non-cirrhotic liver. Eur J Gastroenterol Hepatol. 2006;18:437–441. doi: 10.1097/00042737-200604000-00020. [DOI] [PubMed] [Google Scholar]


