Abstract
Objectives
Although infrequent, Grade C postoperative pancreatic fistulae (POPF) following pancreaticoduodenectomy (PD) are morbid and potentially lethal. Traditional management of a disrupted pancreaticojejunostomy (PJ) anastomosis consists of either wide external drainage or completion pancreatectomy. The aim of this study is to describe an alternative management approach to PJ dehiscence after PD.
Methods
A bridge stent technique is employed in the setting of a disrupted PJ anastomosis. Upon re-exploration, a 5-Fr or 8-Fr silastic feeding tube stent is placed across a gap between the jejunal enterotomy and the pancreatic duct, and secured with an absorbable suture at both ends. Depending upon the degree of local inflammation, this may be externalized by coursing the stent downstream through the pancreaticobiliary drainage limb in a Witzel fashion.
Results
Over 8 years and 357 PDs with duct-to-mucosa PJ reconstruction, seven ISGPF (International Study Group on Pancreatic Fistula) Grade C fistulae occurred (2%). Two patients ultimately died secondary to POPF (neither anastomosis was dehisced). The described technique was used in the other five patients, all of whom had evidence of a dehisced PJ anastomosis. All originally had at least two or three recognized risk factors for POPF development (high-risk pathology, soft gland, duct diameter ≤3 mm, estimated blood loss ≥1000 ml). All patients survived this complication and were discharged from hospital. There have been no longterm external fistulae, nor any recognized PJ strictures or remnant atrophy (median follow-up: 10.7 months).
Conclusions
In the context of a dehisced pancreaticojejunal anastomosis, the bridge stent technique is a safe and effective method of management that contributes to diminished mortality and helps to salvage pancreatic function.
Keywords: pancreatic resection, outcomes, pancreatic neoplasia
Introduction
Despite overall improvements in morbidity and mortality following pancreaticoduodenectomy (PD), the clinically relevant pancreatic fistula remains problematic in terms of both management and resource utilization. Pancreatic fistulae are now clearly defined and graded according to the International Study Group on Pancreatic Fistula (ISGPF) system1. The deleterious clinical and economic impacts of Grade C postoperative pancreatic fistulae (POPF), which are responsible for significant increases in duration of hospitalization, use of services and total costs, have been specifically demonstrated.2
The traditional mode of management of the overtly dehisced pancreaticojejunostomy (PJ) involves surgical re-exploration with such options as: wide external drainage; revision of the initial pancreaticojejunal anastomosis; conversion to an alternative pancreaticoenteric anastomosis; intentional pancreatic ductal occlusion, and even completion pancreatectomy.3–6 The last of these has previously been advocated as the operation of choice when the degree of inflammation precludes the safe revision of the original anastomosis.3–6 However, it comes at a price of absolute endocrine and exocrine insufficiency and still has a significant mortality rate.3,5,6 Alternatively, although it is initially safer and seemingly more efficient, wide external drainage can be an incomplete solution that requires more interventions and prolonged management.4 The aim of this study is to present another option for the management of the dehisced pancreaticojejunal anastomosis, which may be more practical and definitive in the setting of marked inflammation and/or an unstable patient.
Materials and methods
With the approval of the institutional review board, a pancreatic resection database was used to identify and review all patients who underwent PDs with duct-to-mucosa PJ anastomosis our institution during 2001–2009. Of note, a two-layer duct-to-mucosa PJ anastomosis is routinely employed, most often without stent placement. Pancreatogastrostomy reconstruction is rarely utilized. Patients who suffered a Grade C POPF were included for further analysis. The initial operation with respect to POPF risk factors, re-operative findings and clinical outcomes were annotated, as were the outcomes of salvage procedures.
Establishment of the diagnosis
The preoperative diagnosis of PJ anastomotic dehiscence was based on clinical decompensation with evidence of sepsis in conjunction with radiographic findings by axial computed tomography (CT) (Fig. 1) demonstrating peripancreatic gas with an associated ‘gap’ between the jejunum and the pancreatic neck transaction margin. In patients in whom an internalized pancreatic anastomotic stent was employed at the construction of the original anastomosis, this stent may, on occasion, be found to have obviously migrated into the peritoneal cavity (Fig. 2). Sepsis was defined according to Annane et al. as documentation or visually evident focus of infection in the setting of systemic inflammatory response syndrome.7 Patients with CT evidence of POPF but without obvious anastomotic dehiscence and with stable haemodynamics typically underwent a trial of non-operative management.
Figure 1.
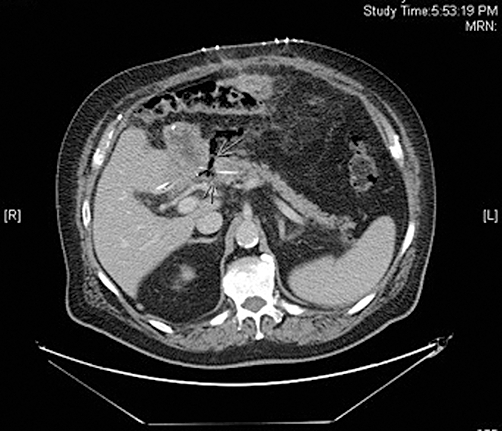
Axial computed tomography demonstrating peripancreatic gas with an associated ‘gap’ (arrows) between the jejunum and the pancreatic neck transaction margin
Figure 2.
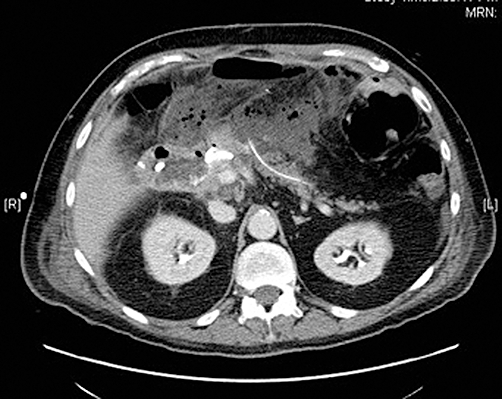
Computed tomography shows that a pancreatic anastomotic stent employed at the construction of the original anastomosis has migrated into the peritoneal cavity
Details of the technique
Initial exploration, via the original bilateral subcostal incision, is undertaken to confirm that the PJ anastomosis is, as suspected, dehisced. The antecolic, isoperistaltic duodenojejunostomy is typically able to be gently retracted laterally to the left, rather than taken down. Care is taken to avoid damage to the transverse colon or mesocolon. The anastomosis is evaluated for: the degree of surrounding inflammation, abscess or necrosis; the extent of anastomotic dehiscence (i.e. anterior vs. complete); the extent of the gap between the pancreas and the jejunal limb, and the quality of the pancreatic parenchyma and the jejunal serosa. The pancreatic remnant is debrided as far as is feasible to healthier, well-vascularized tissue.
When a duct-to-mucosa anastomosis cannot be properly completed as a result of suboptimal conditions, the gap can be ‘bridged’ with a paediatric feeding tube (Fig. 3). This bridge consists of a 5-Fr or 8-Fr silastic feeding tube stent that is placed across the space between the jejunal enterotomy and the pancreatic duct, and secured with an absorbable suture at both ends. The stent size employed is dependent on the receptivity of the pancreatic duct, which is usually very small and can be extremely hard to locate in such conditions. If possible, a larger 8-Fr stent is inserted, thus establishing a larger-calibre channel. The minute (≤2 mm) pancreatic duct may be difficult to access with the feeding tube and care must be taken to avoid a false passage into the pancreatic parenchyma. This difficulty can be addressed by first introducing either a small lacrimal probe or biliary dilator into the duct. Sometimes a small glidewire can be introduced in the duct and the stent employed using the Seldinger technique. The stent can then be anchored in place on the pancreatic side with absorbable sutures placed carefully in the usually friable parenchyma and then applied to the stent.
Figure 3.
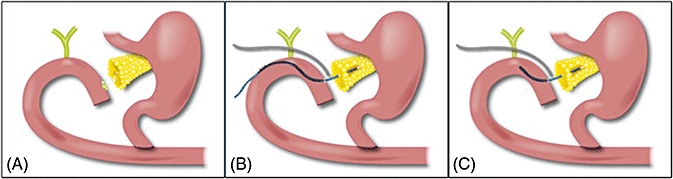
(A) Dehiscence of the pancreatico-jejunal anastomosis is illustrated. Note the gap between the pancreatic remnant and the jejunum. (B) Bridge-Stent Technique with externalized stent, and external drain adjacent to gap. (C) Bridge-Stent Technique with internal stent and external drain adjacent to gap
On the jejunal side, the stent can be placed through the enterotomy employed for the original anastomosis and similarly secured with an absorbable pursestring suture that is again fixed to the stent. Internal stents should be crafted fairly short (6–8 cm at most) in order to allow for ultimate evacuation via bowel peristalsis. Alternatively, if external drainage is desired and is technically feasible, the stent can be externalized several centimetres downstream through the pancreaticobiliary drainage limb in a Witzel fashion and then externalized through the abdominal wall. In some patients, the jejunum has been able to be approximated ‘en masse’ to the pancreatic parenchyma after the bridge stent has been placed, but this is rarely practical. One or two self-suction drains (19-Fr round) are typically placed for external drainage of the surrounding inflammation or abscess and for added control of any residual pancreatic secretion. Ultimately, an internally placed stent will be dislodged and evacuated through the bowels once the absorbable suture dissolves (over weeks or months). Alternatively, an externalized stent can be removed many weeks later once total recovery has been assured. The ultimate objective is to enable a channel (‘neo-duct’) to develop from reactive repair around the periphery of the stent, which will shunt secretions from the pancreatic remnant to the pancreaticobiliary limb.
Results
Over 8 years, 357 PDs were performed with duct-to-mucosa PJ reconstruction. The overall incidence of POPF was 21.8% (n = 78); that of clinically relevant POPF was 12.6% (n = 45) (Fig. 4). Seven ISGPF Grade C fistulae occurred (2.0%). Two of the affected patients ultimately died secondarily to the effects of POPF in the absence of an overt anastomotic dehiscence. One of these patients had been satisfactorily discharged from hospital according to the postoperative care path but was readmitted 12 h later in extremis and died shortly thereafter. Autopsy demonstrated the POPF. The other patient became severely septic as a result of the Grade C POPF, but his family declined re-intervention and he therefore died. The technique described above was used in the other five patients, all of whom had evidence of a grossly disrupted PJ anastomosis (Table 1). In four of these five patients, the manifestation of fistula occurred while they were still in hospital recovering from the index operation.
Figure 4.
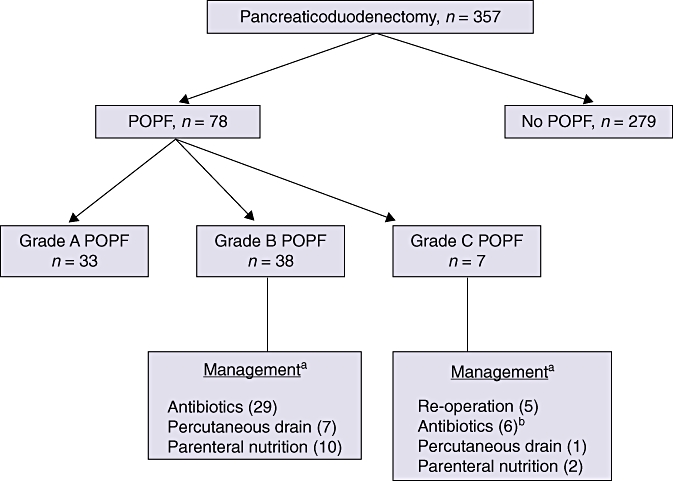
Incidence and management of 78 cases of postoperative pancreatic fistulae (POPF) in 357 cases of pancreaticoduodenectomy. aNot mutually exclusive; bOne patient died prior to initiation of any definitive therapy
Table 1.
Patients managed with the bridge stent technique for Grade C postoperative pancreatic fistulae
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Diagnosis | Ampullary adenoca | Pancreatitis | Ampullary adenoca | Duodenal adenoca | Renal cell metastases |
| Gland texture | Soft | Soft | Soft | Soft | Soft |
| Duct size | 6 mm | 3 mm | 2 mm | 2 mm | 4 mm |
| EBL (index operation) | 400 cc | 600 cc | 550 cc | 500 cc | 200 cc |
| Timing of re-operation | POD 11 | POD 6 | POD 16 | POD 13 | POD 7 |
| Symptoms | Sepsis | Sepsis | Sepsis | Change in drain output character | Abdominal pain after drain removal |
| Gap | 1.5 cm | None | 1 cm | 2 cm | 0.5 cm |
| Type of stent for bridge | 8-Fr Internal | 5-Fr External | 5-Fr Internal | 8-Fr External | 5-Fr External |
| Length of stay, days | 41 | 51 | 77 | 8 | 21 |
| Intensive care unit stay | Yes | Yes | Yes | No | Yes |
| Organ failure | Yes | Yes | Yes | No | Yes |
| Overall cost, US$ | $152 957 | $256 933 | $377 156 | $48 401 | $85 208 |
EBL, estimated blood loss; POD, postoperative day
All five patients originally had at least two or three recognized risk factors for POPF development (pathology other than pancreatic adenocarcinoma or pancreatitis, soft gland texture, PD diameter ≤3 mm, estimated blood loss [EBL]≥1000 ml8). Median EBL during the second operation was 200 cc (range: 50–800 cc). Median operative time was 180.5 min (range: 126–191 min). After re-operation and repair, all patients survived the complication and were discharged from hospital after a median duration of stay of 41 days (range: 8–77 days). Over a median follow-up of 10.7 months (range: 5–68 months), there have been no longterm external pancreatico-cutaneous fistulae, nor any recognized PJ strictures or remnant atrophy. One patient, without a prior diagnosis of diabetes, remains on oral hypoglycaemic agents postoperatively, but no patients have evidence of exocrine insufficiency. Four of the patients are still alive.
Discussion
Although PJ dehiscence with a Grade C POPF is a rare complication of PD, it has significant clinical impact and implies excessive cost.2,9 The operative management of a disrupted anastomosis is among the most technically challenging procedures a pancreatic specialist will encounter. The operative terrain is generally hindered by significant inflammation from local sepsis and tissue degradation. Often, the presentation is well into the recovery course, and the integrity of the tissues is fragile. In addition, the patient's overall physiology is markedly compromised and his or her nutrition is suboptimal. To salvage this difficult predicament, many options are available, ranging from wide external drainage to anastomotic revision to completion pancreatectomy. In this setting, less may be better.
Smith et al.6 reported their experience with completion pancreatectomy following pancreaticoduodenectomy for pancreatic leak/fistula or haemorrhage. In most cases, completion pancreatectomy was selected as the means of salvage because the severe inflammation in the setting of PJ anastomotic dehiscence precluded safe revision. Of their 11 patients, seven (64%) died in the postoperative period from sepsis and multisystem organ failure. Completion pancreatectomy has also been reported as a salvage procedure in combination with other techniques in the management of associated peritonitis. Guéroult et al.3 described completion pancreatectomy, with a jejunal stump stoma and use of capillary Mikulicz packing in the peripancreatic or pelvic space. Of their eight patients, three died in the postoperative period from multisystem organ failure, jejunal necrosis and leak, and splenic vein erosion with hepaticojejunostomy and gastrojejunostomy leak, respectively. Other results with respect to mortality vary widely, ranging from 0% to 38%.4,5,9 Compared with the now low mortality rates in elective resections, the results following surgery for this problem remain perhaps unreasonably high. This has led some, like Büchler et al., to suggest that there is no longer a role for completion pancreatectomy for Grade C pancreatic fistulae.10
Most groups, even those advocating completion pancreatectomy, make note of its technical difficulty, particularly in terms of operative time and blood loss.5,6 Some have purported that resecting the remnant pancreas in this situation, although morbid, is more expeditious and ultimately more definitive. For instance, a comparison of 21 patients undergoing re-exploration and drainage for PJ leak/dehiscence after pancreaticoduodenectomy vs. eight patients undergoing completion pancreatectomy found that patients in the drainage group were more likely to require additional re-explorations and had a significantly longer length of stay and higher mortality (38% vs. 0%, respectively).4 An alternative modification of completion pancreatectomy, in which a small remnant of the distal pancreas is left in situ, may limit the development of diabetes, but results in higher morbidity and mortality.11 Pancreatogastrostomy has also recently been proposed as an alternative salvage operation in this setting and has been utilized successfully in a small group of patients;12 however, the feasibility of this approach depends on the adequacy of the pancreatic remnant and is likely to require additional dissection in the severely inflamed field. Often, this is technically impossible.
The rationale for the use of this approach developed in response to the perils of re-operating on the pancreas in this unfavourable setting of aggressive local sepsis. The stakes are high for both patient and surgeon, particularly now that limited mortality is expected as a quality measure of surgical performance. In our patients, it was readily evident that revision and re-anastomosis were risky if not impossible, given the extent of separation and/or the tissue quality. However, the procedure may represent an improvement over the more conservative, and perhaps more common, option of wide local drainage or the more drastic option of completion pancreatectomy. The goal of this ‘bridge stent’ technique is to effectively divert most, if not all, of the pancreatic exocrine secretion internally rather than externally. This scenario differs greatly from that in which stent placement across a duct-to-mucosa PJ anastomosis may be considered at the index operation to either protect or facilitate the precise construction of a delicate anastomosis. In fact, several prospective studies of PJ anastomotic stenting at the time of PD have yielded confusing results, showing, for example: a decreased rate of POPF with externalized stents but no change in morbidity, mortality or need for re-operation;13 a fistula rate in soft glands with stents double that in soft glands without stents,14 and no difference in fistula rate between (internally) stented and non-stented patients.15 Given this context, stenting was selectively used at the time of PD. However, the bridge stent is distinct; in effect, a ‘neo-anastomosis’ is developed over a short distance (<1 cm), relying on a fibrotic wall developing around the channel created by the silastic stent.
In terms of intensive care unit requirements and organ system failure, the five patients in this series were as physiologically impaired as most patients with a PJ dehiscence previously reported in the literature, and their overall length of hospitalization and associated costs were greatly increased, as would be expected. However, each was ultimately able to be discharged from the hospital. After nearly 2 years of follow-up, four of the five are alive and none has required a subsequent re-operation in either the short- or longterm. The PJ dehiscence was safely and definitively addressed with the bridge stent procedure, which avoided the multiple trips to the operating room that have been reported with wide drainage alone.4 Furthermore, there has been no evidence of PJ stricture development on a chronic basis by follow-up imaging (dilated duct, glandular atrophy) or on clinical grounds (recurrent pancreatitis, diabetes) in these patients.
Conclusions
Pancreaticojejunostomy anastomotic dehiscence following pancreaticoduodenectomy is a rare and difficult problem to manage. Traditionally, re-exploration with completion pancreatectomy and/or wide drainage has been employed, but this process carries with it significant morbidity and mortality. In a compromised patient, the goal remains the conducting of a safe, efficient re-operation that allows meaningful recovery. Here, we have described an alternative approach to the management of this formidable problem that has allowed a small group of patients to recover sufficiently for hospital discharge with limited longterm sequelae.
Acknowledgments
The authors would like to acknowledge the critical contribution of illustrator Michael Cichanowski to this manuscript.
Conflicts of interest
None declared.
References
- 1.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM. Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245:443–451. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guéroult S, Parc Y, Duron F, Paye F, Parc R. Completion pancreatectomy for postoperative peritonitis after pancreaticoduodenectomy. Arch Surg. 2004;139:16–19. doi: 10.1001/archsurg.139.1.16. [DOI] [PubMed] [Google Scholar]
- 4.van Berge Henegouwen MI, De Wit LT, van Gulik TM, Obertop H, Gouma DJ. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg. 1997;185:18–24. doi: 10.1016/s1072-7515(97)00007-0. [DOI] [PubMed] [Google Scholar]
- 5.Farley DR, Schwall G, Trede M. Completion pancreatectomy for surgical complications after pancreaticoduodenectomy. Br J Surg. 1996;83:176–179. [PubMed] [Google Scholar]
- 6.Smith CD, Sarr MG, van Heerden JA. Completion pancreatectomy following pancreaticoduodenectomy: clinical experience. World J Surg. 1992;16:521–524. doi: 10.1007/BF02104459. [DOI] [PubMed] [Google Scholar]
- 7.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 8.Pratt WB, Callery MP, Vollmer CM. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419–428. doi: 10.1007/s00268-007-9388-5. [DOI] [PubMed] [Google Scholar]
- 9.Fuks D, Piessen G, Huet E, Tavernier M, Zerbib P, Michot F, et al. Life-threatening postoperative pancreatic fistula (Grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg. 2009;197:702–709. doi: 10.1016/j.amjsurg.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z'graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310–1314. doi: 10.1001/archsurg.138.12.1310. [DOI] [PubMed] [Google Scholar]
- 11.de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Incidence and management of pancreatic leakage after pancreaticoduodenectomy. Br J Surg. 2005;92:1117–1123. doi: 10.1002/bjs.5047. [DOI] [PubMed] [Google Scholar]
- 12.Bachellier P, Oussoultzoglou E, Rosso E, Scurtu R, Lucescu I, Oshita A, et al. Pancreatogastrostomy as a salvage procedure to treat severe postoperative pancreatic fistula after pancreatoduodenectomy. Arch Surg. 2008;143:966–970. doi: 10.1001/archsurg.143.10.966. [DOI] [PubMed] [Google Scholar]
- 13.Poon RTP, Fan ST, Lo CM, Ng KK, Yuen WK, Yeung C, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 2007;246:425–435. doi: 10.1097/SLA.0b013e3181492c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter JM, Cameron JL, Campbell KA, Chang DC, Riall TS, Schulick RD, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2006;10:1280–1290. doi: 10.1016/j.gassur.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Smyrniotis V, Arkadopoulos N, Kyriazi MA, Derpapas M, Theodosopoulos T, Gennatas C, et al. Does internal stenting of the pancreaticojejunostomy improve outcomes after pancreaticoduodenectomy? A prospective study. Langenbecks Arch Surg. 2010;395:195–200. doi: 10.1007/s00423-009-0585-6. [DOI] [PubMed] [Google Scholar]


