Abstract
The existence of tumor-specific T cells, as well as their ability to be primed in cancer patients confirms that the immune response can be deployed to combat cancer. However, there are obstacles that must be overcome to convert the ineffective immune response commonly found in the tumor environment to one that leads to sustained destruction of tumor. Members of the tumor necrosis factor (TNF) superfamily direct diverse immune functions. OX40 and its ligand, OX40L, are key TNF members that augment T-cell expansion, cytokine production, and survival. OX40 signaling also controls regulatory T cell differentiation and suppressive function. Studies over the past decade have demonstrated that OX40 agonists enhance anti-tumor immunity in preclinical models using immunogenic tumors; however, treatment of poorly immunogenic tumors has been less successful. Combining strategies that prime tumor-specific T cells together with OX40 signaling could generate and maintain a therapeutic anti-tumor immune response.
Introduction
Advances in the field of tumor immunotherapy have been borne out of our increased understanding of the mechanisms of the immune system. The ability to elicit immune responses against tumor antigens demonstrates that harnessing the immune system to combat tumors is feasible : however, strategies must be devised that augment and maintain tumor-directed immune responses. Activation of the T cell arm of the immune system requires recognition of cognate antigen by the T- cell receptor (TCR) in conjunction with costimulation provided by antigen-presenting cells (APC). These initial interactions are important for the successful priming of T- cells; however, the tumor microenvironment in which these primed T cells reside will ultimately determine their ability to produce a clinically relevant anti-tumor immune response and establish immunological memory (1). Soon after T-cell priming negative regulatory molecules, such as CTLA-4, are induced on T cells leading to downregulation of the T-cell response (1, 2). Successful tumor immunotherapy will require adequate numbers of tumor-specific T cells that avoid downregulation and survive over an extended period of time. Members of the tumor necrosis factor (TNF) superfamily contribute to this post-activation environment that influences the fate of recently primed T cells. These interactions, exemplified by OX40, 4-1BB, CD27, GITR, and DR3, provide proliferative and survival signals as well as facilitate T-cell differentiation. The goal of this review is to describe our current understanding of OX40/OX40L interactions as they relate to their role in tumor immunotherapy.
OX40 and OX40L Expression
An antibody (MRC OX-40) that bound to activated rat CD4 T cells and augmented the proliferation of those T cells led to the discovery of OX40 (CD134, TNFRSF4) in 1987 (3). OX40 is a 50 kDa type 1 transmembrane receptor containing 277 amino acids (a 49 amino acid cytoplasmic tail and a 186 amino acid extracellular region) (4). Early studies of OX40 expression on T cells demonstrated that T-cell activation was required for its expression (3). Neither naïve T cells nor resting memory T cells express OX40 (3, 5). Potent signaling through the TCR can induce OX40; however optimal expression of OX40 on the surface of T cells requires additional costimulation through CD28 and/or other cytokine receptors (6). Initial work suggested that OX40 expression was limited to CD4+ T cells, but subsequent studies demonstrated that OX40 was also expressed on activated CD8+ T cells (7). Expression on activated CD8+ T cells was more transient. The induction of OX40 occurs within 24 hours and peaks 48–72 hours following initial TCR stimulation. The duration of OX40 expression depends on the potency of TCR signaling and costimulation, but typically lasts 3–4 days. The induction of OX40 on memory T cells following antigen rechallenge occurs much more rapidly than naïve T cells (8). Other cells types e.g.(natural killer T cells, neutrophils, and natural killer cells) also express OX40 (9–11).
OX40 is constitutively expressed on murine FoxP3+CD4+ naturally-occurring Treg cells (nTreg), and is inducible on human Treg cells (12). Thymic development of nTreg cells does not require OX40 as OX40−/− have nTreg cells, which are present at a reduced frequency suggesting a role for OX40 in nTreg cell homeostasis (12). In addition to nTreg cells, naïve T cells can become induced Treg cells (iTreg) when activated in the presence of TGF-β (13). The induction of iTreg cells is affected by OX40, although the results have been controversial. The addition of an agonist anti-OX40 antibody to in vitro conditions that induce iTreg cells inhibited the generation of iTreg cells (14, 15). However, Ruby et al., demonstrated that anti-OX40 agonist treatment increased the polarizing cytokines IFN-γ and IL-4 and diverted naïve T cell differentiation away from the iTreg pathway toward Th1 and Th2 phenotypes. If IL-4 and IFN-γ were blocked during the Treg induction cultures then the agonist anti-OX40 antibody enhanced iTreg generation (16).
The ligand for OX40 (OX40L, CD252, TNFSF4) was initially discovered on HTLV-1 transformed T cells, and termed gp34 (17, 18). Subsequently, gp34 was identified as the binding partner of OX40 (19, 20). OX40L is a type II transmembrane receptor containing 183 amino acids (a 23 amino acid cytoplasmic tail and a 133 amino acid extracellular domain). It is expressed on the surface as a trimer allowing it to bind to three OX40 molecules (21). OX40L is expressed on activated APCs, including dendritic cells, B cells and macrophages (22, 23). Signaling through CD40 as well as inflammatory stimuli that signal through Toll-like receptors (TLR2, TLR4, TLR9) induce OX40L expression on APCs (23). Inducible OX40L expression on APCs ensures that activated OX40+ T cells only receive signaling through OX40 locally when they are in an inflammatory environment. OX40L is also found on NK cells, mast cells, activated T cells, human airway smooth muscle cells, and vascular endothelial cells (24–26).
OX40 and OX40L Signaling
Antigenic stimulation of T cells proceeds through four phases: activation, expansion, contraction, and development of long-lived memory (27). Naïve Ag-specific T cells proliferate upon antigen stimulation and form a pool of effector T cells (Figure 1A). As the antigenic challenge is cleared, the pool of antigen-specific T cells contracts leaving a smaller pool of memory T cells. The temporal expression of OX40 after the priming event suggests its importance in late proliferation and survival of effector T cells. In the absence of OX40, antigen-primed T cells proliferate normally for 2–3 days after antigen stimulation and differentiate into effector T cells, however, there is a significant reduction in their survival by day 12 (Figure 1B)(28, 29). The interaction of OX40 with OX40L results in the recruitment of TNFR-associated (TRAFs) molecules to the intracellular domain of OX40. TRAF2 and 3 activate both the canonical NF-κB1 pathway as well as the noncanonical NF-κB2 pathway, which are known to be important for cell survival. The activation of the anti-apoptotic molecules BCL-2, BCL-xL, and survivin (29, 30) in OX40-stimulated T cells may be responsible for the increased clonal expansion and a larger pool of memory T cells (31), although others have not observed up-regulation of these genes (32, 33).
Figure 1. OX40/OX40L interactions impact the four phases of the T cell lifespan.
A) Activation of T cells begins with the T cell interaction with the antigen-presenting cell. Cognate antigen presented in MHC molecules by the antigen-presenting cell are recognized by the T cell receptor (TCR) on the T cell. Costimulation through CD28 in conjunction with TCR signaling activates the T cell and leads to upregulation of OX40. Similarly, stimulation of antigen-presenting cells through CD40/CD40L (not shown) or TLR agonists induces expression of OX40L. Binding of OX40 to the OX40L enhances proliferation and survival of the T cells leading to a larger expansion of effector T cells and a larger pool of memory T cells. B) In the absence of OX40 on the T cell or OX40L on the antigen-presenting cell, T cell expansion is reduced and the pool of memory T cells is fewer. C) The administration of OX40 agonists bypasses the need for OX40L supplied by mature antigen-presenting cells.
OX40 signaling also augments cytokine secretion (IL-2, IL-4, IL-5, IFN-γ) by activated CD4 T cells (8, 34, 35). Although early reports suggested that OX40 signaling supported Th2 differentiation (22, 34), it has become clear that OX40 signaling can enhance the development of both Th1 and Th2. However, T-cell differentiation is more directly determined by the strength of TCR stimulation, antigen dose, and local cytokine milieu rather than OX40 signaling (36, 37). OX40-stimulated T cells also increase expression of IL-2Rα (CD25), IL-7Rα (CD127), and IL-12Rβ2 (35, 38–40), receptors associated with T-cell survival. For example IL-2Rα signaling activates PKB/AKT which induces the anti-apototic molecules, BCL-2 and BCL-xL (41). Enhanced IL-12Rβ2 expression is required for OX40-mediated survival through a STAT4-dependent mechanism on OX40-treated CD4 T cells (35). Since the initial OX40/OX40L work focused on CD4+ T cells, the understanding of OX40 on CD8+ T cells has lagged behind that of CD4+ T cells. Several studies using agonist anti-OX40 antibody have shown enhanced expansion and survival of CD8+ T cells (7, 42). Clearly though, there is both a direct and indirect effect of OX40 on CD8+ T cells since OX40−/− CD8+ T cells can expand after OX40 treatment although expansion was less than OX40+/+ CD8+ T cells (39, 43, 44).
Preclinical Tumor Models
The potent immunological effects mediated by the OX40/OX40L interaction make it an attractive target for improving tumor immunotherapy, especially to increase the frequency and survival of tumor-specific T cells. As stated earlier, TLR agonists and CD40/CD40L are key for the upregulation of OX40L on APCs. However, administration OX40 agonist most likely bypasses the need for DC maturation, at least at the level of OX40 signaling (Figure 1B). Studies have demonstrated therapeutic efficacy when OX40 agonists were delivered three days after subcutaneous tumor challenge with melanoma, glioma, breast and colon carcinoma, sarcoma, renal carcinoma, and prostate cancer (45–49). In each tumor model a percentage of mice (20–55%) remained tumor free. Mice that survived from tumor challenge through OX40 treatment had tumor-specific memory T cells that could protect naïve mice against a tumor challenge after adoptive transfer (45). The ability of OX40-agonists to protect appears to correlate with the immunogenicity of the tumor. Tumor protection was achieved against immunogenic tumors, however poorly immunogenic tumors did not respond to OX40 agonist treatment (50). This is not surprising since OX40 expression on tumor-specific T cells would require sufficient priming likely not provided by poorly immunogenic tumors.
Strategies employing OX40 agonist treatment in conjunction with other agonist antibodies or factors that stimulate T-cell function have been examined. As stated earlier, OX40 engagement upregulates IL-12Rβ2 and the addition of IL-12 to OX40-treated CD4+ T cells resulted in greater IFN-γ secretion (38). The addition of IL-12 and an anti-4-1BB antibody to OX40 treatment resulted in a significantly higher cytotoxic T cell activity against tumor cells, and better survival rates in treated mice compared to controls (51). Combining an agonist OX40 antibody, a blocking CTLA-4 antibody and intratumoral injections of CpG oligonucleotides induced regression of systemic lymphoma tumors and provided long lasting protection against tumor rechallenge (52).
Treg cells and OX40-based tumor immunotherapies
It has become increasingly clear that tumors establish immunosuppressive environments that are an obstacle for successful tumor immunotherapy. One such obstacle is the presence of Treg cells that suppress anti-tumor immune responses (53). As discussed above, recent studies have demonstrated a role for OX40 in the homeostasis of Treg cells (12, 14–16). Specifically, an agonist anti-OX40 antibody reduced the frequency of TGF-β-induced iTregs in vitro (14, 15). Our lab has investigated a model where tumor recurrence correlates with increased frequencies of Treg cells. Briefly, lymphopenic mice with large (150–200 mm2) B16BL6-D5 tumors receive an adoptive transfer of tumor-specific CD4+ (Trp1 TCR transgenic) and CD8+ (pmel TCR transgenic) T cells. Following tumor regression approximately 30% of mice experience tumor recurrence. In an initial experiment mice treated with agonist anti-OX40 antibody had a delay in tumor recurrence (Figure 2A) which correlated with lower frequencies of FoxP3+ CD4+ Treg cells in the peripheral blood (Figure 2B). However, in three subsequent experiments anti-OX40-treated mice had frequencies of FoxP3+ CD4+ Treg cells comparable to rat-Ig treated controls and there was no reduction in tumor recurrence. While there is good correlation with low frequencies of FoxP3+ Treg cells and prevention of tumor growth, we are puzzled by the failure of anti-OX40 treatment to keep Treg low in subsequent experiments. One possible explanation for these conflicting data builds on the observation that OX40 signaling accentuates T-cell differentiation (Th1, Th2, Treg) driven by the local cytokine milieu (16, 54). Possibly, the cytokine milieu was optimal for reducing Treg when anti-OX40 was administered in the first experiment, but not in subsequent studies. These findings underscore the complexity of the anti-tumor immune response and emphasize the importance of further studying the conditions that favor anti-tumor immune responses in recipients of anti-OX40.
Figure 2. OX40 treatment delays tumor recurrence.
A) Lymphopenic (Rag1−/−) mice were challenged with the B16BL6-D5 melanoma cell line. On day 15, Trp1-specific TCR transgenic CD4 T cells together with gp100-specific TCR transgenic CD8 T cells were adoptively transferred into the tumor-bearing mice followed by a vaccination with an irradiated tumor cell vaccine that secretes GM-CSF. Mice received anti-OX40 antibody or Rat IgG on days 15, 20, 24, and 37. B) The percent FoxP3+ cells of the CD4+ T cell population is determined from peripheral blood samples of mice on the indicated days.
It has also been shown that agonistic anti-OX40 antibody can inhibit the suppressive activity of Treg cells. Treg–mediated suppression of GVHD was abrogated by intraperitoneal injection of anti-OX40 antibody, or by in vitro pretreatment of Treg cells with anti-OX40 antibody before transfer (55). Piconese et al., demonstrated in a colon carcinoma model that OX40 stimulation enhanced effector T-cell function and inhibited suppression mediated by Treg cells. Using OX40−/− Treg cells and effector T cells it was shown that OX40 signaling in both Treg cells and effector T cells must occur for the tumor to be rejected (56). Thus, OX40 treatment can affect both Treg-mediated suppression as well as render effector T cells resistant to the inhibition mediated by Treg cells.
Age and OX40-based tumor immunotherapies
It is well established that age-related changes occur in both innate and adaptive immune cells, which may impact tumor immunity and immunotherapy efficacy. Indeed, the systemic administration of anti-OX40 that greatly improved the tumor-free survival of young (2–6 months old) tumor-bearing mice, failed to improve the tumor-free survival in older tumor-bearing mice (12–20 months old) (57). Surprisingly upon further investigation, it was found that older T cells and younger T cells respond comparably following OX40 stimulation, and that the age of the cells of the local innate environment, which promote and support ongoing T cell responses, appear to be responsible for the age-related deficiency in OX40-mediated tumor immunity (57). Further studies are needed to identify and understand the primary age-related defect in order to design optimal OX40-based strategies for the treatment of elderly cancer patients. A potential candidate is IL-12, as administration of this cytokine partially restored OX40-mediated tumor regression in older tumor-bearing mice (57).
Cancer Vaccines
Single agent treatment with OX40 agonist has provided protection against a variety of immunogenic tumors. However, these studies relied on the presence of sufficient endogenous antigen priming to induce the OX40 on tumor-specific T cells, by which the OX40 agonists could exert their effects (Figure 1C). Cancer vaccines prime tumor-specific immune responses, and can be enhanced with the addition of granulocyte macrophage colony-stimulating factor (GM-CSF) (58). Murata et al., showed that the combination of a GM-CSF secreting tumor cell vaccine with anti-OX40 antibody induced a potent CD8+ T-cell response that eliminated established breast cancer in mice (42). Importantly, these authors showed that treatment of OX40 costimulation combined with a GM-CSF-secreting tumor vaccine enhanced the expansion and prolonged the survival of tumor-specific T cells compared to vaccine alone (42). In other studies Hu and colleagues identified that anti-OX40 given in combination with a vaccine generated from tumor autophagosomes was significantly more therapeutic than either agent administered alone (figure 3). These data further support the concept that coordinating immunotherapeutic interventions can improve therapeutic efficacy. Based on these studies our group is preparing to initiate a combination immunotherapy trial of breast cancer autophagosomes from an allogeneic breast cancer cell line together with anti-OX40 antibody.
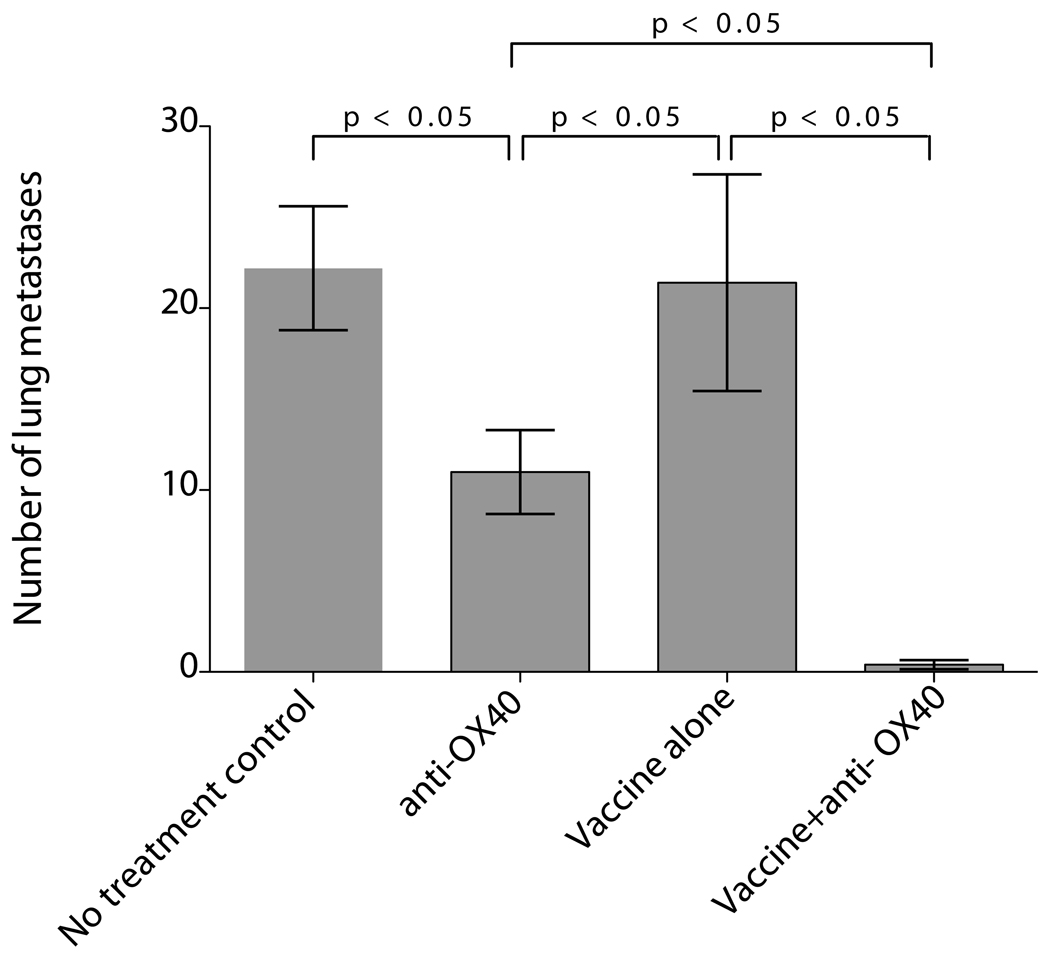
Figure 3. OX40 treatment significantly improves therapeutic response to vaccination with tumor-derived antophagosomes.
Mice received 2×105 3LL tumor cells i.v. and on day 3 were treated with 3LL DRibble vaccine alone (antophagosomes), 3LL DRibble vaccine combined with anti-OX40, or anti-OX40 alone. Untreated mice were included as a control. 3LL DRibble vaccine (20µg) was injected into both inguinal lymph nodes. Anti-OX40 (75 µg/mouse) was administered ip. All mice were sacrificed on day 28, the lungs were collected and metastases were enumerated. Typically five mice were included in each group. Results are representative of three independent experiments.
Radiation and Chemotherapy
While it is likely tumors that shed small quantities of antigen as cancer cells turn over in the process of tumor growth and invasion (59), engineering large-scale death of cancer cells in vivo is a potential source of antigen to prime new immune responses. Thus, over the course of radiation therapy, patients can develop new tumor antigen-specific immune responses (60). In immunocompetent animal models, CD8 T cells significantly contribute to the efficacy of radiation therapy (61, 62). The combination of agonistic anti-OX40 antibodies and radiation therapy has been shown to be significantly more effective than either therapy alone (62, 63). Importantly, OX40 therapy was shown to increase tumor antigen-specific cytotoxicity (63) and result in increased infiltration of activated CD8 T cells into the tumor (62).
While many chemotherapy drugs are toxic to lymphocytes, many chemotherapeutic agents have also been shown to engender an immune response that is responsible for some portion of their efficacy (64). With careful timing to take advantage of lymphocyte repopulation following lymphodepleting chemotherapy, agonistic anti-OX40 antibodies have been shown to enhance control of tumors in animal models (54). In this system the cytotoxic therapy is posited to provide tumor antigen to tumor-draining lymph nodes, priming T cells and inducing OX40 expression. Based on these data our group has just received funding to initiate a clinical investigation to evaluate anti-OX40 in combination with chemotherapy and radiation therapy in patients with metastatic prostate cancer.
Surgery
During cancer surgery, particular care is to avoid disruption of the tumor and minimize release of cancer cells; therefore, tumor antigen release during the operation may be minimal. However, it has been proposed that surgical removal of the primary tumor has a net positive effect on anti-tumor immunity by removal of a source of immune suppression (65). In animal models, CD8 T cells have been shown to play a significant role in controlling residual disease following surgical removal of the primary tumor (62). In an animal model of surgical therapy, treatment with agonistic anti-OX40 antibodies resulted in increased tumor antigen specific T cell cytotoxicity and significantly enhanced control of residual disease (62).
Adoptive Immunotherapy
Vaccination strategies rely on the ability to prime the endogenous immune system sufficiently to eradicate tumor. The ability to expand tumor-specific cells ex vivo enables adoptive immunotherapy to achieve potent antitumor effects above what is often achievable by vaccination alone. The role of OX40 in augmenting proliferation and survival makes it a logical candidate for combination with adoptive immunotherapy. Intracranial tumors that had been established for 10 days could be cured with the adoptive transfer of 3-fold fewer T cells if the adoptive transfer was combined with anti-OX40 treatment (66). Using OX40−/− CD8 T cells it was shown that OX40 signaling was important for the survival and accumulation of CD8 T cells at the tumor site (67). These data show that agonistic anti-OX40 antibodies may combine well with adoptive immunotherapy to increase the activity and survival of tumor antigenspecific T cells.
Our group has also investigated whether adding soluble anti-OX40 to cultures of tumor-infiltrating lymphocytes (TIL) augmented the production of tumor-specific T cells. Analysis of 128 independent and parallel cultures of TIL cloids (64 with anti-OX40 and 64 without) from melanoma surgical specimens from 10 different patients failed to show any striking difference in either the frequency or number of tumor-specific T cells recovered. It is still an open question whether immobilized anti-OX40, which typically provides T cells with a stronger proliferative signal, may increase TIL recovery. Another option is to employ anti-OX40 treatment during the TIL rapid expansion cycle or REP. The REP is the massive expansion phase where TIL are cultured with excess feeders and anti-CD3 alone or together with anti-CD28. Given the importance of OX40 signaling in providing a survival signal to T cells we anticipate that applying anti-OX40 during the REP may increase recovery of TIL.
Clinical Studies
A mouse monoclonal agonistic antibody to OX40 was developed by our group under the direction of Dr. Andrew Weinberg and produced under GMP conditions for administration to patients with cancer. A first-in-man phase I trial was performed to establish a safe dose with biologic activity. Three doses of anti-OX40 were administered on days 1, 3 and 5 of a treatment cycle at 0.1, 0.4 and 2 mg/kg. The dose levels were chosen based on pre-clinical primate models showing immunological effect and good tolerability (68). The schedule was chosen because multiple doses of anti-OX40 induced tumor regression in animal models. Furthermore, it is known that OX-40 cycles over a 24–48 hour period in activated T cells as reviewed above. Finally, the murine anti-OX40 that we are using induces HAMA, although not during the first week of anti-OX40 exposure. Thus, repetitive weekly dosing was not feasible with this particular antibody. KLH and tetanus were injected at the same time as the OX40 agonist and used as reporter antigens. Thirty patients received anti-OX40. Ten patients were treated at each dose level to learn more about the immunological effects of the OX40 pathway engagement in humans. Toxicities were mild with fatigue and transient lymphopenia as the most commonly observed side effects (69). The clinical results and assessment of immunological effects are currently being prepared for publication.
As noted above future plans include clinical trials combining anti-OX40 with chemotherapy, radiation or vaccines. Other studies are planned to examine tumor-specific T-cell immune responses induced by OX40 in patients with metastatic melanoma. A human OX40L-Ig fusion protein (70) and a humanized antibody to OX40 are in preparation and will be used to study the effects of repetitive dosing of OX40 agonists in patients with cancer.
Conclusions
Combining OX40 treatment with other anticancer approaches, including immunotherapy, that are consistent with the functional role of OX40 in augmenting expansion and survival of tumor-specific T cells makes OX40 a promising partner in combinatorial therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
ADW is a founder of Agonox, a company that has licensed OX40 agonists for cancer treatment. H-MH and BAF are founders of UbiVac, a company developing autophagosome vaccines for treatment of cancer.
References
- 1.Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev. 2010;235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 3.Paterson DJ, Jefferies WA, Green JR, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 4.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. EMBO J. 1990;9:1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soroosh P, Ine S, Sugamura K, Ishii N. Differential requirements for OX40 signals on generation of effector and central memory CD4+ T cells. J Immunol. 2007;179:5014–5023. doi: 10.4049/jimmunol.179.8.5014. [DOI] [PubMed] [Google Scholar]
- 6.Redmond WL, Ruby CE, Weinberg AD. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit Rev Immunol. 2009;29:187–201. doi: 10.1615/critrevimmunol.v29.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 8.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 9.Baumann R, Yousefi S, Simon D, Russmann S, Mueller C, Simon HU. Functional expression of CD134 by neutrophils. Eur J Immunol. 2004;34:2268–2275. doi: 10.1002/eji.200424863. [DOI] [PubMed] [Google Scholar]
- 10.Zaini J, Andarini S, Tahara M, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Invest. 2007;117:3330–3338. doi: 10.1172/JCI32693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C, Lou Y, Lizee G, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda I, Ine S, Killeen N, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 13.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 14.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 16.Ruby CE, Yates MA, Hirschhorn-Cymerman D, et al. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka Y, Inoi T, Tozawa H, Yamamoto N, Hinuma Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-I (HTLV-I) Int J Cancer. 1985;36:549–555. doi: 10.1002/ijc.2910360506. [DOI] [PubMed] [Google Scholar]
- 18.Miura S, Ohtani K, Numata N, et al. Molecular cloning and characterization of a novel glycoprotein, gp34, that is specifically induced by the human T-cell leukemia virus type I transactivator p40tax. Mol Cell Biol. 1991;11:1313–1325. doi: 10.1128/mcb.11.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godfrey WR, Fagnoni FF, Harara MA, Buck D, Engleman EG. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med. 1994;180:757–762. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baum PR, Gayle RB, 3rd, Ramsdell F, et al. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Compaan DM, Hymowitz SG. The crystal structure of the costimulatory OX40-OX40L complex. Structure. 2006;14:1321–1330. doi: 10.1016/j.str.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- 24.Burgess JK, Carlin S, Pack RA, et al. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J Allergy Clin Immunol. 2004;113:683–689. doi: 10.1016/j.jaci.2003.12.311. [DOI] [PubMed] [Google Scholar]
- 25.Imura A, Hori T, Imada K, et al. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–2195. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendel I, Shevach EM. Activated T cells express the OX40 ligand: requirements for induction and costimulatory function. Immunology. 2006;117:196–204. doi: 10.1111/j.1365-2567.2005.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masopust D, Ahmed R. Reflections on CD8 T-cell activation and memory. Immunol Res. 2004;29:151–160. doi: 10.1385/IR:29:1-3:151. [DOI] [PubMed] [Google Scholar]
- 28.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 29.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 30.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prell RA, Evans DE, Thalhofer C, Shi T, Funatake C, Weinberg AD. OX40-mediated memory T cell generation is TNF receptor-associated factor 2 dependent. J Immunol. 2003;171:5997–6005. doi: 10.4049/jimmunol.171.11.5997. [DOI] [PubMed] [Google Scholar]
- 33.Huddleston CA, Weinberg AD, Parker DC. OX40 (CD134) engagement drives differentiation of CD4+ T cells to effector cells. Eur J Immunol. 2006;36:1093–1103. doi: 10.1002/eji.200535637. [DOI] [PubMed] [Google Scholar]
- 34.Ohshima Y, Yang LP, Uchiyama T, et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- 35.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol. 2008;180:2140–2148. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 36.Rogers PR, Croft M. CD28, Ox-40, LFA-1, and CD4 modulation of Th1/Th2 differentiation is directly dependent on the dose of antigen. J Immunol. 2000;164:2955–2963. doi: 10.4049/jimmunol.164.6.2955. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins SJ, Perona-Wright G, Worsley AG, Ishii N, MacDonald AS. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol. 2007;179:3515–3523. doi: 10.4049/jimmunol.179.6.3515. [DOI] [PubMed] [Google Scholar]
- 38.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–3743. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 39.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J Immunol. 2007;179:7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 40.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37:157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 41.Gaffen SL. Signaling domains of the interleukin 2 receptor. Cytokine. 2001;14:63–77. doi: 10.1006/cyto.2001.0862. [DOI] [PubMed] [Google Scholar]
- 42.Murata S, Ladle BH, Kim PS, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974–983. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 43.Yu Q, Yue FY, Gu XX, Schwartz H, Kovacs CM, Ostrowski MA. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J Immunol. 2006;176:2486–2495. doi: 10.4049/jimmunol.176.4.2486. [DOI] [PubMed] [Google Scholar]
- 44.Serghides L, Bukczynski J, Wen T, et al. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J Immunol. 2005;175:6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 45.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 46.Morris A, Vetto JT, Ramstad T, et al. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res Treat. 2001;67:71–80. doi: 10.1023/a:1010649303056. [DOI] [PubMed] [Google Scholar]
- 47.Ali SA, Ahmad M, Lynam J, et al. Anti-tumour therapeutic efficacy of OX40L in murine tumour model. Vaccine. 2004;22:3585–3594. doi: 10.1016/j.vaccine.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 48.Sadun RE, Hsu WE, Zhang N, et al. Fc-mOX40L fusion protein produces complete remission and enhanced survival in 2 murine tumor models. J Immunother. 2008;31:235–245. doi: 10.1097/CJI.0b013e31816a88e0. [DOI] [PubMed] [Google Scholar]
- 49.Redmond WL, Gough MJ, Weinberg AD. Ligation of the OX40 co-stimulatory receptor reverses self-Ag and tumor-induced CD8 T-cell anergy in vivo. Eur J Immunol. 2009;39:2184–2194. doi: 10.1002/eji.200939348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 51.Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6:528–536. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 52.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113:3546–3552. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 54.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 56.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruby CE, Weinberg AD. OX40-enhanced tumor rejection and effector T cell differentiation decreases with age. J Immunol. 2009;182:1481–1489. doi: 10.4049/jimmunol.182.3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marzo AL, Lake RA, Lo D, et al. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162:5838–5845. [PubMed] [Google Scholar]
- 60.Nesslinger NJ, Sahota RA, Stone B, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 61.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gough MJ, Crittenden M, Sarff M, et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control following surgical or radiation therapy of cancer in mice. J Immunother. 2010;33:798–809. doi: 10.1097/CJI.0b013e3181ee7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yokouchi H, Yamazaki K, Chamoto K, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci. 2008;99:361–367. doi: 10.1111/j.1349-7006.2007.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 65.Morton DL. Changing concepts of cancer surgery: surgery as immunotherapy. Am J Surg. 1978;135:367–371. doi: 10.1016/0002-9610(78)90067-3. [DOI] [PubMed] [Google Scholar]
- 66.Kjaergaard J, Peng L, Cohen PA, Drazba JA, Weinberg AD, Shu S. Augmentation versus inhibition: effects of conjunctional OX-40 receptor monoclonal antibody and IL-2 treatment on adoptive immunotherapy of advanced tumor. J Immunol. 2001;167:6669–6677. doi: 10.4049/jimmunol.167.11.6669. [DOI] [PubMed] [Google Scholar]
- 67.Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J Immunol. 2005;175:3534–3541. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- 68.Weinberg AD, Thalhofer C, Morris N, et al. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother. 2006;29:575–585. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 69.Kovacsovics-Bankowski M, Walker E, Floyd K, Urba W, Curti B, Weinberg AD. Increased CD4 and CD8 Memory T-cell Proliferation After Anti-OX40 Administration to Cancer Patients: Immunologic Assessment of a Phase I Clinical Trial [abstract] J Immunother. 2009;32:952. [Google Scholar]
- 70.Morris NP, Peters C, Montler R, et al. Development and characterization of recombinant human Fc:OX40L fusion protein linked via a coiled-coil trimerization domain. Mol Immunol. 2007;44:3112–3121. doi: 10.1016/j.molimm.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]