Abstract
Decellularization of tissues and organs is a successful platform technology for creating scaffolding materials for tissue engineering and regenerative medicine. It has been suggested that the success of these materials upon implantation is due to the molecular signals provided by the remaining scaffold extracellular matrix (ECM) components presented to probing cells in vivo as they repopulate the surface. For this study, decellularized matrices were created from esophagus, bladder, and small intestine harvested from adult male Fischer 344 rats. The three decellularized matrices (each originating from source tissues which included an epithelial lining on their luminal surfaces) were immunostained for collagen IV and laminin to determine basement membrane retention. Scanning electron micrographs of the surfaces were used to provide insight into the surface topography of each of the decellularized tissues. Time-of-flight secondary ion mass spectrometry (ToF-SIMS) was used to generate high-resolution mass spectra for the surfaces of each scaffold. This surface sensitive technique allows for detailed molecular analysis of the outermost 1–2 nm of a material and has been applied previously to thin protein films and secreted ECM proteins on poly(N-isopropyl acrylamide) (polyNIPAAM) surfaces. To extract trends from within the complex ToF-SIMS dataset, a multivariate analysis technique, principal component analysis (PCA), was employed. Using this method, a molecular fingerprint of each surface was created and separation was seen in the PCA scores between the decellularized esophagus and the decellularized small intestine samples. The PCA scores for the decellularized bladder sample fell between the previous two decellularized samples. Protein films of common extracellular matrix constituents (collagen IV, collagen I, laminin, and Matrigel ) were also investigated. The PCA results from these protein films were used to develop qualitative hypotheses for the relationship of the key fragments identified from the PCA of the decellularized ECMs.
Introduction
Decellularized tissue matrices are widely used for tissue engineering and regenerative medicine[1–4]. It is suggested that their success is due to embedded biospecific signals found within their protein structures. These materials have been created from a variety of source tissues both allogeneic and xenogeneic[5]. The extracellular matrix (ECM) proteins which comprise the bulk of these materials, are highly evolutionarily conserved[6–8]. This sequence similarity helps to explain their favorable immune response upon implantation. The cellular components of tissue are primarily responsible for the antigenicity and adverse response when implanted in non-autologous hosts[5].
It is probable that the body may take embedded signaling cues from the biomolecular structures that comprise these decellularized extracellular matrices and uses these cues to direct the in vivo remodeling process. Acellular tissues have been explored in numerous applications including full heart constructs[8], cardiovascular grafts[9], heart valve[10, 11], nerves[12], skeletal muscle[13], liver[14], bladder[15], esophagus[1, 2], and skin[16] among others.
Decellularized tissue scaffolds present a particularly challenging characterization problem due to their complex arrangement of interacting ECM components and the probable alterations of these structures during the decellularization process. Many different chemical and mechanical decellularization techniques have been employed and each method has the potential to alter the native three-dimensional ultrastructure of the ECM uniquely[5, 17]. However, largely, these ECM scaffolds induce a constructive remodeling response and favorable clinical outcome. To date, characterization methods for decellularized matrices have included mechanical property testing along with imaging techniques such as scanning electron microscopy and immunohistochemistry[5, 17].
Time-of-flight secondary ion mass spectrometry (ToF-SIMS) represents a powerful surface-sensitive analytical method for the characterization of implantable decellularized materials. Using an energetic primary ion beam to eject surface species, the resulting secondary ions are collected and detected with a time-of-flight mass analyzer to yield an information-rich spectrum. This technique can detect all elements with masses ranging from hydrogen to molecules up to several thousands of Daltons without the need for specific markers or the addition of an analysis matrix. However, due to bombardment with highly energetic ions, the analyte is subject to fragmentation, which can complicate data interpretation. Each ToF-SIMS spectrum thus represents a complete molecular fingerprint of the outermost 1–2 nm of the surface under analysis. For every spot analyzed, hundreds of individual spectral features can be identified. Multiple spots (spectra) are taken per sample and the spectra are overlaid so that comparisons of individual peak variance relationships of within-group and between-group changes can be assessed. To understand such complex data sets, multivariate analysis (MVA) techniques have been applied to statistically reduce the complexity to manageable patterns of captured variance[18–21]. For this study, principal component analysis (PCA) was employed. In PCA, a set of new variables called principal components (PCs) are calculated which represent new axes within the data space[22, 23]. These new axes, or PCs, now bisect areas of variance within the original dataset. The first PC will capture the largest percentage of the variance in the dataset while each subsequent PC is orthogonal to its predecessor and captures sequentially less variance until there are no longer identifiable trends. Specifically, PCA has been used previously to determine a set of protein-related peaks within ToF-SIMS data sets which can be used to compare amino acid related structures[18, 19, 24]. Variations in the amino acid fragment intensities can be related to the identity, conformation and orientation of surface bound proteins[25].
ToF-SIMS characterization of decellularized ECM-based scaffolds is analytically challenging because of the scaffolds’ surface chemical and structural complexity. This surface represents the first contact a given cell would encounter once seeded (in vitro) or repopulated (in vivo). With this in mind, characterizing surface chemistry precisely could provide vital insight into the mechanisms behind the in vivo success of decellularized matrices. ToF-SIMS has the power to assess the molecular chemistry and some aspects of the molecular organization associated with the outer layer. The challenge lies in the interpretation of the data. Previously in the characterization of biomaterials, this technique has been used to study adsorbed model protein films[26]. In a recent study, ECM proteins were analyzed after cell liftoff from poly(N-isopropyl acrylamide) (polyNIPAAM)[19, 20] and compared to an adsorbed protein film model. With the detailed molecular characterization of the surfaces investigated in the present study, researchers will have an enhanced understanding of the cellular interactions that occur upon implantation. This knowledge could help to improve functionality of future generations of these naturally-derived materials as well as guide the production of successful synthetic mimetics that incorporate molecular specificity[21].
Materials and Methods
Decellularized ECM Preparation
Fischer 344 adult male retired breeder rats were obtained (Taconic Labs, Inc.) and euthanized with CO2. The esophagus, bladder, and central portion of the small intestine were excised and placed in phosphate buffered saline (PBS, Sigma-Aldrich) plus 1% antibiotic/antimicotic (A/A, Gibco) and shaken gently on an orbital shaker at 25°C for 10 minutes. Tissues were then transferred into a 10mM Tris(Sigma-Aldrich), 5mM ethylene-diaminetetraacetic acid (EDTA, Fisher Scientific), 0.2mM phenylmethanesulfonyl fluoride (PMSF, Sigma-Aldrich) and 1%(v/v) A/A in deionized waster (dH2O) solution at pH 8.0 for 48 hours at 4°C shaken gently on an orbital shaker. For the main decellularization step, tissues were soaked in a Tris/EDTA/PMSF dH2O solution with 0.1%(w/v) sodium dodecyl sulfate (SDS, Fischer) for 24 hours at 25°C and shaken gently on an orbital shaker. After 24 hours, PBS was used to rinse the sections three times each for 10 minutes with gentle shaking. Tissues were then rinsed in a solution of 0.1%(v/v) peracetic acid (Sigma-Aldrich) in a 4%(v/v) ethanol in dH2O solution for 24 hours at 4°C. Sterile PBS was used to rinse the previous solution from the tissue sections (3× each for 10 minutes at 4°C with gentle shaking) and the tissue sections were critical point dried and stored under nitrogen for no longer than three weeks at 4°C before use. The nomenclature scheme used in this work is: decellularized esophagus (dESO), decellularized bladder (dBLAD), decellularized small intestine (dSI) and general decellularized ECM (dECM)..
Immunohistochemistry
To assess degree of decellularization and protein retention after decellularization, immunohistochemistry was performed. Samples were fixed in Methyl Carnoy’s fixative (60% methanol, 30% acetic acid, 10% chloroform) overnight at 4°C and embedded in paraffin. Serial 5μm thick sections were cut. Deparaffinization of slides was performed as follows: xylenes (3×5min), 100% ethanol (2×3min), 95% ethanol (1×3min), 70% ethanol (1×3min), 50% ethanol (1×3min), phosphate buffered saline (PBS) (2×2min). One set of slides was stained by a standard hematoxylin and eosin protocol (Sigma-Aldrich). For the immunohistochemistry, sections were rinsed in a 3% hydrogen peroxide in methanol solution for 30min and subsequently rinsed in PBS (3×5min). For antigen retrieval, a 0.01M citrate buffer pH 6 was made and heated to a boil in a microwave oven. Slides were placed in the hot buffer for 10–15 minutes and subsequently rinsed in PBS (3×5min). Sections were circled on the slides with a hydrophobic pen and blocked overnight at 4°C in 4% normal goat serum in 0.25% BSA. The following day, sections were incubated with rabbit-anti mouse polyclonal collagen IV antibody (1:500 dilution, Abcam) and rabbit-anti mouse polyclonal laminin antibody (1:25 dilution, Abcam) for 1 hour at 25°C in the 4% blocking solution. Sections were subsequently rinsed in PBS (3×5min). The secondary horse anti-mouse (Vector Labs) was diluted (1:200) in 2% blocking serum and used for incubation of each section for 30 minutes at 25°C and subsequently rinsed in PBS (3×5min). An avidin-biotin kit (ABC, Vector Labs) was used as per manufacturer instructions for 30 minutes at 25°C and sections were subsequently rinsed in PBS (3×5min). The peroxidase substrate, 3, 3′-diaminobenzadine (DAB, Sigma-Aldrich) was prepared as per manufacturer instructions and sections were incubated while being visualized under a microscope to time the color change for subsequent section staining intensities. Tissues were rinsed in PBS (3×5min). Sections were dipped in hematoxylin (Sigma-Aldrich) for 10 minutes for a nuclear counterstain and subsequently rinsed in PBS (3×5min). Sections were dehydrated as follows: 70% ethanol (quick rinse), 95% ethanol (1 min), 100% ethanol (2×1min), Xylene (1 min), Xylene (3 min), Xylene (5 min). Sections were coverslipped with Permount (Sigma-Aldrich). For the additional location of collagen IV and laminin in the alternate fixative solutions, intact rat organs (esophagus, bladder, small intestine) were explanted and fixed overnight at 4°C in both 10% neutral buffered formalin and zinc fixative solution (both from Sigma-Aldrich). Then, the immunohistochemistry procedure from above was repeated with a primary incubation step overnight at 4°C (as opposed to 1 hour at 25°C) with no other changes.
Scanning Electron Microscopy
Dried samples were sputtered with gold and viewed with an FEI Sirion SEM at the UW NanoTech User Facility. SEM images were taken at 70× magnification for gross identification of structures and 1000× magnification for studying the fine structures.
Preparation of Extracellular Matrix Protein Films on Mica
The extracellular matrix proteins collagen I, collagen IV, laminin, and a basement membrane extract (Matrigel) were adsorbed onto mica at 37°C for 2 hours in their respective buffers (all proteins were purchased from. Briefly, mica was cut into 1 centimeter squares and cleaved with tape. Laminin and Matrigel were diluted in 50 nM tris buffered saline (TBS, Invitrogen Corp.) pH 7.5 and collagen I and collagen IV were diluted in 0.05N HCl. All proteins were reconstituted at a concentration of 100 μg/ml. Once protein adsorption was complete, each of the samples were washed in their appropriate buffers from their original reconstitution for 15 minutes (2×). Finally, samples were washed in deionized water (dH2O) for 15 minutes (3×). Samples were dried immediately with a flowing stream of nitrogen gas and stored under nitrogen atmosphere until analysis with ToF-SIMS.
Time of Flight Secondary Ion Mass Spectrometry
ToF-SIMS data were acquired on an ION-TOF ToF.SIMS 5–100 spectrometer using an 25 keV Bi+ ion source in the high current bunched mode, at a pulse width of approximately 2 ns based on the hydrogen peak width, to enhance mass resolution of the spectra. Spectra were acquired in both positive and negative polarities over a mass range of m/z = 0 to 860. Spectra were acquired by rastering the primary ion beam over an area of 0.005 to 0.01 mm2. The primary ion current was 0.8 pA and the total fluence was kept below the static limit to avoid sample damage (< 1013 ions.cm−2). Secondary ions of a given polarity were extracted and detected using a reflectron time-of-flight mass analyzer. Positive ion spectra were mass calibrated using the CH3+, C2H3+, C3H5+ and C7H7+ peaks. Negative ion spectra were not considered in this study as they lacked protein characteristic peaks6. Mass calibration errors were kept below 10 ppm. Mass resolution (m/Δm) for a typical spectrum was 3000–7000 at m/z = 27.
Principal Component Analysis (PCA)
For PCA, peaks from the amino acid peak table in Canavan et al. were selected and used for comparison[19]. The selected peaks were then normalized to the total ion intensity of all peaks selected to account for fluctuations in secondary ion yield between different spectra. All spectra were mean-centered before PCA. PCA was then employed to analyze the positive ToF-SIMS data using a set of in house scripts written for Matlab (The MathWorks, Inc., Natick, MA). PCA was used to determine the linear combination of peaks that capture the highest degree of variation in a dataset[22, 23].
Results
In Figures 1 and 2, two known basement membrane proteins (laminin and collagen IV) were identified within the structure and on the surface of the dECMs and the native tissues using immunohistochemistry. Figure 1(A–F) shows a series of images stained for laminin and Figure 1(G–L) shows the same tissue sections stained for collagen IV. Laminin is clearly found lining the luminal structure of dESO (Figure 1A). This was also found to be the case for collagen IV staining of dESO (Figure 1G). In Figure 2A, a clear band of positive staining exists under and throughout the basal epithelium when the esophagus control had been fixed in 10% neutral buffered formalin (NBF). In Figure 2B, a positive line of staining can be seen below the basal epithelium depicting the location of collagen IV in the esophagus control tissue when fixed with a zinc fixative solution.
Figure 1.
Immunohistochemical identification of residual basement membrane proteins in the decellularized rat ECMs and analogous controls. For laminin staining, the following tissues were stained: (A) decellularized rat esophagus (dESO); (B) decellularized rat bladder (dBLAD); (C) decellularized rat small intestine (dSI); (D) native rat esophagus; (E) native rat bladder; (F) native rat small intestine. For collagen IV staining, the following tissues were stained: (G) decellularized rat esophagus (dESO); (H) decellularized rat bladder (dBLAD); (I) decellularized rat small intestine (dSI); (J) native rat esophagus; (K) native rat bladder; (L) native rat small intestine. Images A–F are counterstained with methyl green while images G–L are counterstained with hematoxylin. The scale bars represent 50μm.
Figure 2.
Additional immunohistochemistry for the native esophagus control tissue using alternative fixatives for: (A) laminin fixed in 10% neutral buffered formalin and (B) collagen IV fixed in zinc solution. The images were counterstained with hematoxylin and the scale bars = 50μm.
For the staining and comparison to controls of dBLAD and dSI, the control tissues stained positive for both laminin and collagen IV using methyl Carnoy’s fixative. In Figure 1E and Figure 1K, positive staining for laminin and collagen IV, respectively, can be seen as a broken layer directly beneath the urothelium in the bladder controls. This same broken layer is preserved in dBLAD as shown in Figure 1B and Figure 1H. Finally, for the staining of the small intestine control, laminin and collagen IV can be seen lining the underside of the epithelium in the intestinal villi in Figure 1F and Figure 1L. The dSI images in Figure 1C and Figure 1I, show a preservation of these basement membrane proteins along the luminal surface with a three-dimensional structure of the remains of the villi whose cells have been removed after processing.
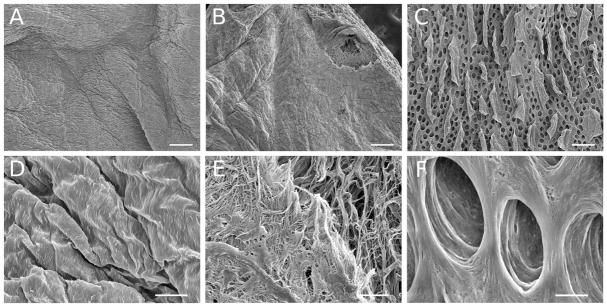
Figure 3 shows scanning electron micrographs of the surfaces of the dECMs at 70× and 1000× respectively. In Figure 3, the surface topography of dESO and dBLAD appears similar at 70× (A–C), but the image for dSI (Figure 3C) shows a series of pores and “flap-like” structures. We speculate that these pores represent the vascular and lymphatic structures of the intestinal villi. In Figure 3D through Figure 3F, scanning electron micrograph images of the same samples are shown at 1000×. In Figure 3E, a section where the outer surface had been damaged during processing is shown and the loose underlying ECM can be seen below what is likely the residual basement membrane structure (based on the positive staining of laminin and collagen IV in Figure 1). The dense basement membrane can also be seen covering the surface of the dESO sample shown in Figure 3D. In Figure 3F, the porous structure of the dSI sample appears to have a tightly connected surface like the dESO and dBLAD samples and this presumably consists mainly of basement membrane proteins as well.
Figure 3.
Scanning electron micrograph images of the surfaces of critical point dried decellularized rat extracellular matrix scaffolds for: (A) and (D) decellularized esophagus; (B) and (E) decellularized bladder; (C) and (F) decellularized small intestine. Images (A) through (C) are magnified 70X magnified and images (D) through (F) are magnified 1000×. The scale bar = 200 μm in images A–C and 20 μm in images D–F.
In an effort to create comparison surfaces for the ToF-SIMS analysis, we adsorbed a series of ECM protein films onto mica and collected ToF-SIMS spectra. These controls were laminin, collagen IV, Matrigel, and collagen I, each cast onto mica. Figure 4 shows the scores from the ToF-SIMS/PCA for these protein films. PC1 and PC2 combined to capture 89% of the total variance using the peak list published in the paper by Canavan et al.[19] The PC1 vs. PC2 scores plot in Figure 4A shows the distinct separation between the individual protein films. Figure 4A shows that PC1 separates the two collagen isoforms, while PC2 separates the two collagens from laminin and Matrigel. It is interesting to note that the scores for the Matrigel samples fall between the laminin and collagen IV samples. This is reasonable due to the fact that Matrigel consists mainly of these two proteins, as it is a basement membrane extract from Engelbreth-Holm-Swarm mouse sarcoma cells. The placement of the Matrigel samples close to zero for both PC1 and PC2 could be indicative of scores resulting from the combined positive and negative loadings from the collagen IV and laminin fragments. Figure 4B and Figure 4C represent the loadings obtained from PCA of the ToF-SIMS data from the protein films. It should be noted that the PCA loadings did not necessarily directly correlate to the concentrations of components, but represent the relative abundance of one fragment versus another. So a high positive loading for one fragment indicates that fragment is present in higher concentrations in samples with high positive scores. Likewise, fragments with high negative loadings indicate that fragment is present in higher concenctrations in samples with high negative scores. For example, in the Matrigel samples, it is possible that the overall PC scores (computed in conjunction with the loadings) include competing fragments associated with the various components of the protein mixture.
Figure 4.
Principal component analysis (PCA) scores and loadings for ToF-SIMS data from protein films on mica. The protein film samples were: collagen I – blue circles, collagen IV – red circles, laminin – green circles, and Matrigel – black circles. (A) is the PC1 vs. PC2 scores plot. (B) shows the PC1 loadings. (C) shows the PC2 loadings.
In Figure 5, the PC1 scores and loadings from PCA of the dECMs ToF-SIMS data are shown. Clear patterns were not seen in any of the PCs above PC1. In Figure 5A, the dESO samples and the dSI samples are separated at the 95% confidence level. The dBLAD sample scores overlap the dESO and dSI sample scores. In Figure 5B, the loadings associated with PC1 are presented. Of the five major fragments with negative PC1 loadings in Figure 5A, the fragments at m/z 59 (CH5N3+) and m/z 73 (C2H7N3+) were associated with the PC2 loadings from laminin for the protein film studies shown in Figure 4. In addition to these laminin loading fragments, the fragments at m/z 44 (C2H6N+) and m/z 84 (C5H10N+) were associated with PC2 collagen loadings in Figure 4B and Figure 4C. Due to the fact that dESO has a clearly retained basement membrane, we conclude that the fragments that load with dESO in in Figure 5 might be associated with laminin or collagen IV due to its intact basement membrane.
Figure 5.
Principal component analysis (PCA) scores and loadings for decellularized esophagus, bladder and small intestine. The ToF-SIMS/PCA were obtained from the surfaces of the following decellularized tissue samples: (B) decellularized bladder – blue circles, (E) decellularized esophagus – red diamonds, and (S) decellularized small intestine – green cross. (A) shows the PC1 scores, which captured 38% of the total variance in the dataset. (B) shows the PC1 loadings.
Discussion
In tissue engineering and regenerative medicine, the end goal is to reconstruct functional tissues and organs that can replace or repair damaged or lost tissues or organs. Some of the first efforts towards this end goal involved the implantation of cell-seeded synthetic scaffolds[27–29]. Scaffolds of biological origin also showed promise and it was proposed that natural scaffolding materials, often consisting of extracellular matrix components, held signaling cues embedded within the biomacromolecules present in these materials[30].
One major approach to regenerative medicine has focused on decellularized tissues and whole organs[5]. These decellularized extracellular matrix scaffolds have the advantage of being comprised of combinations of the natural biomacromolecules associated with the source tissue of the material. The three-dimensional structure and molecular composition have been engineered by nature to present the “correct” set of signaling moieties and ligands to maintain a tissue in homeostasis and dynamic equilibrium. Decellularized extracellular matrix scaffolds have characteristics of both synthetically derived materials and natural, intact donor tissues. The decellularization of tissues creates a unique set of molecular surface characteristics not found in nature but derived from nature and with proven regenerative potential. The materials elicit functional regenerated structures[1, 8, 31] and we assume these surface characteristics are at least in part responsible for this response.
To study the molecular surface characteristics and investigate the mechanisms behind the functionality of decellularized ECM scaffolds, ToF-SIMS has been used here to shed light on molecular distinctions between decellularized ECMs derived from different organs in a rat model. The three-dimensional structure and molecular composition of a decellularized tissue-based scaffold is affected by the processing steps involved. The SEM images and immunohistochemistry (Figures 1–3) revealed that the processing used in this study successfully created three decellularized ECMs that retained a high degree of the original fine structure of the tissues. This preservation of the fine structure is probably more readily attainable using thin rat tissues in contrast to thicker porcine or human tissue. On the other hand, this fine structure preservation is important for the ToF-SIMS analysis so that the studies are performed with a more specific knowledge of the surface proteins and topography.
Protein films of known basement membrane components were cast onto mica to create standard spectra for the subsequent analysis of the decellularized ECMs. These protein films (Figures 4 and 5) provide insights into which fragments could be associated with each of the basement membrane components in the decellualrized ECMs. However, there are limitations in this approach. First, the available basement membrane proteins are derived from mice, but this is not likely to be a significant issue because of the high level of sequence homology between mouse and rat proteins. A second limitation is associated with the orientation of the proteins on the mica surface. Using laminin as an example, the protein has a cruciform structure in its native conformation[32]. In the deposition of a film of laminin, there might be a preferential orientation or conformation due to geometric factors and intermolecular interactions, or the deposited proteins may be randomly oriented. It is unlikely that this will be the same conformation as is found in the native basement membrane. The sampling depth of static SIMS (1–2nm) is shallower than the average thickness of the basement membrane (~100nm)[33], so the differences in orientation or conformation of the laminin proteins in the decellularized ECMs may affect the observed ToF-SIMS fragment intensity pattern[34, 35]. Differences in protein size and arrangement in multi-component protein films can also affect the observed ToF-SIMS fragment intensities[36]. Thus, the structure of deposited protein films may impact the ability to use the deposited protein film as a standard material to understand the ECM composition. It can be assumed that the protein films are relatively uniform due to small 95% confidence levels observed in the PCA results. An effort was made to correlate the two models (ECM and deposited protein standards) using a partial-least squares regression and other multivariate classification tools (data not shown), but the two data sets had too many differences to be statistically comparable. However, it may still be possible to qualitatively compare the two, as was attempted in this study.
This study shows that despite some limitations in the experiments, much complexity can be described with the ToF-SIMS and PCA combinatorial approach. However, caution is appropriate when trying to identify the structure of large complex molecules such as proteins from the pattern of mass fragments in a PC model, so the conclusions drawn here can be used to form testable hypotheses.
A complete basement membrane structure appears to be intact on the luminal surface of the dESO samples and this structure dominates the results in the PCA results. The partially intact basement membrane in the dBLAD and dSI samples could explain the separation from the dESO samples in the PCA results shown in Figure 5 although surface topographical aspects of the dSI sample could also be responsible for some of the differences.
Conclusions
This study successfully determined the parameters for ToF-SIMS (time-of-flight secondary ion mass spectrometry) spectral acquisition from the surfaces of decellularized ECMs and established the groundwork for PCA (principal component analysis) of that data. The surface molecular functionality of the decellularized ECMs as seen in the PCA scores identified distinct differences between the dSI and dESO samples (decellularized small intestine and decellularized esophagus respectively). The PCA of the protein films led to a hypothesis describing the specifics of the PCA scores differences. We have attempted to use patterns of amino acid fragments for intact decellularized ECMs to identify ECM proteins without success. Instead, the fragments can be used empirically as descriptors of molecular surface functionality. Thus, this description of the surface molecular functionality coupled with future studies to elucidate the effects of these differences on biological processes could provide valuable design parameters for future materials or for the creation of synthetic mimetics designed to simulate the pro-regenerative surfaces associated with extracellular matrix scaffolds.
Acknowledgments
The authors would like to acknowledge funding and facilities provided by the University of Washington Engineered Biomaterials 21st Century (UWEB) center which is a National Science Foundation Engineering Research Center. Additional funding and facilities were also provided by the National ESCA and Surface Analysis Center for Biomedical Problems (NESAC/BIO) through grant EB-002027 from the National Institutes of Health. The SEM portion of this research was conducted at the University of Washington NanoTech User Facility, a member of the NSF National Nanotechnology Infrastructure Network (NNIN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, et al. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Bhrany AD, Beckstead BL, Lang TC, Farwell DG, Giachelli CM, Ratner BD. Development of an esophagus acellular matrix tissue scaffold. Tissue Eng. 2006;12:319–30. doi: 10.1089/ten.2006.12.319. [DOI] [PubMed] [Google Scholar]
- 3.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519–26. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 4.Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, et al. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15:S29–S40. doi: 10.3727/000000006783982368. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Bernard MP, Chu ML, Myers JC, Ramirez F, Eikenberry EF, Prockop DJ. Nucleotide-sequences of complementary deoxyribonucleic acids for the pro-alpha-1 chain of human type-I procollagen - statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983;22:5213–23. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- 7.Exposito JY, Dalessio M, Solursh M, Ramirez F. Sea-urchin collagen evolutionarily homologous to vertebrate pro-alpha-2(I) collagen. J Biol Chem. 1992;267:15559–62. [PubMed] [Google Scholar]
- 8.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 9.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 10.Rieder E, Kasimir MT, Silberhumer G, Seebacher G, Wolner E, Simon P, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127:399–405. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Zeltinger J, Landeen LK, Alexander HG, Kidd ID, Sibanda B. Development and characterization of tissue-engineered aortic valves. Tissue Eng. 2001;7:9–22. doi: 10.1089/107632701300003250. [DOI] [PubMed] [Google Scholar]
- 12.Kim BS, Yoo JJ, Atala A. Peripheral nerve regeneration using acellular nerve grafts. J Biomed Mater Res A. 2004;68A:201–9. doi: 10.1002/jbm.a.10045. [DOI] [PubMed] [Google Scholar]
- 13.Borschel GH, Dow DE, Dennis RG, Brown DL. Tissue-engineered axially vascularized contractile skeletal muscle. Plast Reconstr Surg. 2006;117:2235–42. doi: 10.1097/01.prs.0000224295.54073.49. [DOI] [PubMed] [Google Scholar]
- 14.Lin P, Chan WCW, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046–53. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 15.Freytes DO, Badylak SF, Webster TJ, Geddes LA, Rundell AE. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials. 2004;25:2353–61. doi: 10.1016/j.biomaterials.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Wainwright DJ. Use of an acellular allograft dermal matrix (alloderm) in the management of full-thickness burns. Burns. 1995;21:243–8. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 17.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Wagner MS, Castner DG. Characterization of adsorbed protein films by time-of-flight secondary ion mass spectrometry with principal component analysis. Langmuir. 2001;17:4649–60. doi: 10.1002/1097-4636(20011205)57:3<432::aid-jbm1186>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 19.Canavan HE, Graham DJ, Cheng XH, Ratner BD, Castner DG. Comparison of native extracellular matrix with adsorbed protein films using secondary ion mass spectrometry. Langmuir. 2007;23:50–6. doi: 10.1021/la062330o. [DOI] [PubMed] [Google Scholar]
- 20.Canavan HE, Cheng XH, Graham DJ, Ratner BD, Castner DG. Surface characterization of the extracellular matrix remaining after cell detachment from a thermoresponsive polymer. Langmuir. 2005;21:1949–55. doi: 10.1021/la048546c. [DOI] [PubMed] [Google Scholar]
- 21.Brown BN, Barnes CA, Kasick RT, Michel R, Gilbert TW, Beer-Stolz D, et al. Surface characterization of extracellular matrix scaffolds. Biomaterials. 2010;31:428–37. doi: 10.1016/j.biomaterials.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson JE. A user’s guide to principal components. New York: Wiley; 1991. [Google Scholar]
- 23.Graham DJ, Wagner MS, Castner DG. Information from complexity: challenges of ToF-SIMS data interpretation. Appl Surf Sci. 2006;252:6860–8. [Google Scholar]
- 24.Lhoest JB, Wagner MS, Tidwell CD, Castner DG. Characterization of adsorbed protein films by time of flight secondary ion mass spectrometry. J Biomed Mater Res. 2001;57:432–40. doi: 10.1002/1097-4636(20011205)57:3<432::aid-jbm1186>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Tidwell CD, Castner DG, Golledge SL, Ratner BD, Meyer K, Hagenhoff B, et al. Static time-of-flight secondary ion mass spectrometry and x-ray photoelectron spectroscopy characterization of adsorbed albumin and fibronectin films. Surf Interface Anal. 2001;31:724–33. [Google Scholar]
- 26.Michel R, Castner DG. Advances in time-of-flight secondary ion mass spectrometry analysis of protein films. Surf Interface Anal. 2006;38:1386–92. [Google Scholar]
- 27.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 28.Ochoa ER, Vacanti JP. An overview of the pathology and approaches to tissue engineering. Ann N Y Acad Sci. 2002 Dec;979:10–26. doi: 10.1111/j.1749-6632.2002.tb04863.x. discussion 35–8. [DOI] [PubMed] [Google Scholar]
- 29.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 30.Bissell MJ, Hall HG, Parry G. How does the extracellular-matrix direct gene expression. J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 31.Atala A, Vacanti JP, Peters CA, Mandell J, Retik AB, Freeman MR. Formation of urothelial structures in vivo from dissociated cells attached to biodegradable polymer scaffolds in vitro. J Urol. 1992;148:658–62. doi: 10.1016/s0022-5347(17)36685-5. [DOI] [PubMed] [Google Scholar]
- 32.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin - glycoprotein from basement-membranes. J Biol Chem. 1979;254:9933–7. [PubMed] [Google Scholar]
- 33.Stanley JR, Woodley DT, Katz SI, Martin GR. Structure and function of basement-membrane. J Invest Dermatol. 1982;79:S69–S72. doi: 10.1111/1523-1747.ep12545830. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Castner DG, Ratner BD, Jiang SY. Probing the orientation of surface-immobilized immunoglobulin G by time-of-flight secondary ion mass spectrometry. Langmuir. 2004;20:1877–87. doi: 10.1021/la035376f. [DOI] [PubMed] [Google Scholar]
- 35.Xia N, May CJ, McArthur SL, Castner DG. Time-of-flight secondary ion mass spectrometry analysis of conformational changes in adsorbed protein films. Langmuir. 2002;18:4090–7. [Google Scholar]
- 36.Wagner MS, Horbett TA, Castner DG. Characterization of the structure of binary and ternary adsorbed protein films using electron spectroscopy for chemical analysis, time-of-flight secondary ion mass spectrometry, and radiolabeling. Langmuir. 2003;19:1708–15. doi: 10.1016/s0142-9612(02)00612-9. [DOI] [PubMed] [Google Scholar]