Abstract
Purpose
The aim of this study is to monitor endostatin gene expression and therapy using transferrin receptor (TfR) as reporter gene and transferrin conjugate of ultrasmall supramagnetic iron oxide nanoparticle (Tf–USPIO) as magnetic resonance (MR) reporter probe.
Procedure
A retroviral plasmid (pLP-LNCX) encoding mouse endostatin and TfR was constructed, and packaged with a titer of 4×107colony-forming units per millimeter. MDA-MB-231 breast tumors were established in BALB/c mice by subcutaneous injection of 2×106 MDA-MB-231 cells. Mice were intratumorally injected with recombinant retrovirus and imaged with MR using Tf–USPIO. Western blot, Prussian blue, and immunohistochemical staining were performed to validate the magnetic resonance imaging results. The antitumor effect of retro-endostatin (ES)-TfR was also evaluated by intratumoral injection of the viral vector.
Results
The expression of both endostatin and TfR genes in MDA-MB-231 cells after retroviral transfection was confirmed by Western blot and flow cytometry. Tf–USPIO conjugate binds specifically to cells stably transfected with retro-ES-TfR. After intravenous injection of the Tf–USPIO conjugate, there was a more pronounced decrease in T2 relaxation time in tumors treated with retro-ES-TfR than in tumors treated with empty retrovirus retro-LNCX. The expression of ES gene significantly delayed the growth of MDA-MB-231 tumor and reduction of microvessel density and VEGF level as compared to those without viral transfection or transfected with empty retro-LNCX vector.
Conclusions
Endostatin therapeutic gene expression was visualized successfully using TfR reporter gene and Tf–USPIO MR reporter probe, which indicates that MR reporter gene imaging may be valuable in gene therapy to evaluate therapeutic gene expression and treatment efficacy.
Keywords: Magnetic resonance imaging (MRI), Transferrin receptor (TfR), Ultrasmall super-paramagnetic iron oxide nanoparticle (USPIO), Endostatin (ES), Reporter gene
Introduction
Angiogenesis, the formation of new blood vessels, is one of the most important steps in tumor growth and metastasis [1]. It is controlled by a balance of angiogenic stimulators and inhibitors. Antiangiogenic therapy has recently attracted intense interest because of its broad-spectrum action, low toxicity, and absence of drug resistance [2, 3]. Systemic administration of angiogenic inhibitors has been shown to reduce the growth of established tumors and metastases. Endostatin, a 20-kDa C-terminal fragment of type XVIII collagen, is one of the most potent naturally occurring angiogenesis inhibitors [4]. Although detailed mechanisms of its action are still unclear, it is known to inhibit endothelial cell proliferation and migration, promote apoptosis, and induce cell cycle arrest in endothelial cells [5].
There are, however, some major concerns in translating endostatin therapy to the clinic. One is the difficulty of producing the protein in sufficiently large quantities for chronic treatment, although recently, stable and soluble forms of endostatin produced by Escherichia coli or yeast cells have been reported [6-8]. Additionally, continuous administration of antiangiogenic agent is required over a long period of time. It has been shown that continuous administration of endostatin was much more effective than intraperitoneal or subcutaneous administration [9]. Therefore, gene transfection is one of the most promising methods of administration [10]. How to monitor the efficiency of endostatin transgene expression in target tissues noninvasively, in real time, and at high spatial resolution poses a great challenge [11-13].
In this study, we would like to evaluate the endostatin gene expression and its therapeutic efficacy in a MDA-MB-231 breast cancer model using transferrin receptor (TfR) as magnetic resonance (MR) reporter gene and the transferrin conjugate of ultrasmall supramagnetic iron oxide (Tf–USPIO) as the reporter probe.
Materials and Methods
The Expression of Endostatin and TfR in MDA-MB-231 In Vitro
Cloning of the Murine Endostatin and TfR Genes
Total RNAs were isolated with Trizol reagent from the liver of athymic nude mice (Shanghai Laboratory Animal Center, Shanghai, China) for murine endostatin (ES) gene cloning and from the P3/NSI/1-Ag4-1 cells (China Science Institute Cell Bank, Shanghai, China) for TfR gene cloning, respectively and transcribed reversely into complementary DNAs (cDNAs) with Moloney murine leukemia virus reverse transcriptase (Takara Biotech, Japan). ES and TfR genes were amplified by polymerase chain reaction (PCR) using the following primers: ESFor (5′-GAA GTT ATC AGT CGA CAT GCA TAC TCA TCA GGA CTT TCA-3′), ESRev (5′-GAT CCT GCA GGA ATT CCT ATT TGG AGA AAG AGG TCA T-3′), TfRFor (5′-GAA GTT ATC AGT CGA CAT GAT GGA TCA AGC CAG AT-3′), and TfRRev (5′-ATG GTC TAG AAA GCT TAA AAC TCA TTG TCA ATA TTC C-3′). The two genes were constructed into pIRES plasmid by the connection of the internal ribosomal entry site (IRES), and then, the ES-IRES-TfR fragment was digested and inserted into the multiple cloning sites (MCS) of retrovirus vector pLP-LNCX (BD Clontech, Mountain View, CA), in which endostatin and TfR cDNAs were under the control of the cytomegalovirus (CMV) promoter [14].
Generation of Recombinant Retrovirus Stocks
To obtain retro-ES-TfR retroviral vector, the recombinant plasmid was introduced into the transient packaging cell line retro-PT67 (BD Clontech, Mountain View, CA) by a Lipofectamine2000™ (Invitrogen, Carlsbad, CA). The supernatant was collected 48 h later, filtered through 0.45-μm filters, and used either immediately or frozen at −80°C. Viral titers were determined by exposing 1×105 NIH3T3 cells per well to serial dilutions of filtered virus preparations in the presence of 8 μg/ml polybrene (hexadimethrine bromide; Sigma, St. Louis, MO). After 400 mg/L G418 selection, the survived clone number of the maximum dilution was counted at the tenth day, and the figure of the virus titer was calculated as follows: virus titer= clone number×3×diluted times [11, 15].
Gene Transfer Mediated by Retrovirus In Vitro
For retroviral transduction, MDA-MB-231 cells (China Science Institute Cell Bank, Shanghai, China) were cultured and allowed to grow to subconfluence. The medium was then replaced with a 1:1 precipitated mixture of retroviral supernatant and fresh complete media. Polybrene was added to the culture medium at a final concentration of 4 μg/ml in order to enhance the binding of the virus to host cells. The transduction was repeated the following day. Twenty-four hours after the final transduction, the cells were harvested and subcultured into selective medium containing 0.8 g/L of G418 for up to 10 days. G418-resistant cells were pooled, expanded, and tested for ES and TfR gene expression [16, 17].
Analysis of TfR and ES
Western blot was performed to correlate TfR and ES expression in the transfected MDA-MB-231 cells. In brief, the cultured cells were homogenized in protein lysate buffer. Debris was removed by centrifugation at 10,000×g for 10 min at 4°C. The lysates were resolved on 12% polyacrylamide sodium dodecyl sulfate gels and electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were blocked with 3% bovine serum albumin overnight, incubated with primary antibodies (rabbit antimouse endostatin, dilution 1:800, Abcam, Cambridge, MA; rat antimouse TfR, dilution 1:1,000, Santa Cruz, CA), and subsequently with alkaline phosphatase-conjugated secondary antibody. They were developed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (Zhongshan Golden-bridge Biotech Co. Ltd., Beijing, China). Blots were stained with a β-actin antibody to confirm that each lane contained similar amount of tumor homogenate [12, 18]. The expression and binding of Tf to TfR was also assessed by flow cytometry. Briefly, 1×106 retro-ES-TfR or retro-LNCX treated MDA-MB-231 cells were fixed for 5 min in 1 ml of 0.5% paraformaldehyde (Sigma, St. Louis, MO) in phosphate-buffered saline (PBS; pH 7.4) at 4°C and then washed with 1.7 ml of PBS. The cells were stained with Tf–polylysine–fluorescein isothiocyanate (FITC; Sigma, St Louis, MO), a transferrin conjugate that binds specifically to transferrin receptors, and analyzed by flow cytometry. We used a FACSort flow cytometer (Becton Dickinson, Mountain View, CA) utilizing an argon laser with an excitation wavelength of 488 nm. The fluorescence was measured after passage through a 530-nm band-pass filter. A total of 5×103 events were analyzed per sample. Three replicate experiments were performed, and each sample was assayed in triplicate. The data were analyzed using CellQuest software (Becton Dickinson).
Chick Chorioallantoic Membrane Assay
The chick chorioallantoic membrane (CAM) assay was used to measure the activity of endostatin. Briefly, fertilized eggs from white Leghorn chickens were incubated for 7 days at 37°C and 60% humidity. A square window was opened in the shell, and the membrane of the gas chamber was carefully removed to expose the chorioallantoic membrane. Ten eggs were randomly divided into two groups and were doped with 50 μl cell lysate from MDA-MB-231 cells that had been transfected with either retro-ES-TfR or retro-LNCX. The windows were then sealed with sterile parafilm, and the eggs were incubated for 72 h, as described above. The CAM was digitally photographed, and the number and extent of vessel branch points formed by the blood vessels was counted.
Analysis of Tf–USPIO Uptake in MDA-MB-231 Cells
Coupling of Transferrin to USPIO
The USPIO nanoparticles with core size of 10.9 nm and hydrodynamic size of 20.1 nm were synthesized by one-pot reaction, through the thermal decomposition of Fe(acac)3 in 2-pyrrolidone using α,ω-dicarboxyl-terminated poly (ethylene glycol) as surface capping molecule [19]. Transferrin protein was covalently bonded to the USPIOs via an ethyl-3-(dimethylaminopropyl) carbodiimide (EDC)-mediated amidation reaction. Typically, 0.32 mg EDC·HCl (Sigma) and 0.9 mg N-hydroxysulfosuccinimide (Sulfo-NHS, Sigma) were added into 0.32 ml aqueous solution of USPIO (2.5 mg/ml Fe). The solution was stirred with a mechanical stirrer to prevent the nanoparticles from aggregation. After approximately 10 min, 0.24 ml transferrin solution (33 mg/ml) was introduced by adjusting the pH of the reaction to 7.5 using 1 M NaOH. The reaction was allowed for 4 h at 4°C and then purified by gel chromatography using a Sephacryl S-300 column. Native polyacrylamide gel electrophoresis was adopted to check the completion of the conjugation reaction.
Prussian Blue Staining
MDA-MB-231 cells treated with retro-ES-TfR and retro-LNCX were cultivated for 24 h in six-well plates on glass coverslips and incubated with 5-ml culture medium containing Tf–USPIO or plain USPIO at an iron concentration of 0.03 mM for 30 min. The cells were washed thrice with PBS and subsequently fixed with methanol and acetone (−20°C). For Prussian blue staining, the fixed cells were incubated with 10% potassium ferrocyanide for 5 min and 10% potassium ferrocyanide in 20% hydrochloric acid for 30 min, and counterstained with nuclear fast red [20, 21].
Time-Dependent Cell Uptake of Tf–USPIO
The iron concentration of retro-ES-TfR-treated cells incubated with Tf–USPIO (0.03 mM) for different times (5, 10, 30, and 120 min) was assessed using inductively coupled plasma optical emission spectroscopy (ICP-OES). A predetermined number of MDA-MB-231 cells (1×107 cells) were pelleted, and 1 ml of 65% (v/v) nitric acid was added for 1 h at 70°C. The obtained cell extracts were then diluted (1:10) in water. The total iron content was measured at 238.2 nm by means of inductively coupled plasma optical emission spectrometry (ICP-OES; Perkin-Elmer Optima 5300 DV, USA) [22]. The experiments were repeated with three samples.
Transmission Electron Microscopy Imaging of Intracellular Localization of Tf–USPIO
To assess the uptake and localization of the USPIO particles, transmission electron microscopy (TEM) was performed. Cells grown on glass coverslips were fixed with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate (pH 7.2) at 4°C for overnight. Slides were successively stained with 2% osmium tetroxide and 0.5% uranyl acetate and processed for ultrathin sectioning. Micrographs were taken with a Zeiss EM-10 A electron microscope at 80 kV. The magnification indicator was routinely controlled by the use of a grating replica [20, 21].
Magnetic Resonance Imaging of MDA-MB-231 Cells in Gelatin
MDA-MB-231 cells treated with retro-ES-TfR or retro-LNCX were incubated with 5-ml culture medium containing Tf–USPIO or plain USPIO at an iron concentration of 0.03 mM for 30 min. Competition experiments were done by adding the free ligand Tf to the Tf–USPIO in a molar ratio of 10,000:1. After incubation, the culture medium was removed. The adherent cells were washed thrice with PBS (0.1 mol/L, pH 7.4), trypsinized, and centrifuged for 5 min at 2,000×g. The number of cells was determined using a Neubauer counting chamber. A total of 7×105 cells were embedded in gelatin (200 μl, 10%) at 37°C for MR (Signa HDe 1.5 T, GE Medical Systems, Milwaukee, WI) measurement according to T2w-sequence [FR-FSE (fast-recovery fast spin echo): TR (repetition time), 1,070 ms; TE (echo time): 50, 75,100, and 125 ms; FOV (Field of View), 10×10 cm; matrix, 256×192; slice thickness, 2 mm] [20, 21].
MR Imaging TfR Reporter Gene Expression and Endostatin Gene Therapy In Vivo
Animal Model and Treatments
Tumor xenografts were induced on the back or the flank of 5–6-week-old female athymic BALB/c nude mice (nu/nu; Shanghai Laboratory Animal Center, Shanghai, China) by subcutaneous inoculation of MDA-MB-231 cells (2×106 in 200 μl PBS). Five days after tumor inoculation, mice were randomly divided into three different treatment groups (n=11 per group). The tumor volumes were estimated according to the formula: V = π/6 × a2 × b, where a is the short axis, and b is the long axis [5]. When the tumors reached a volume of 100 mm3 in about 15 days, the mice were daily treated with intratumoral injection of 100 μl retro-ES-TfR or retro-LNCX virus suspension (4×107 colony-forming units per millimeter) for three consecutive days. The control group was daily dosed with 100 μl PBS. The tumor growth was monitored at 5-day intervals [23, 24].
Imaging Gene Expression by MR In Vivo
MRI experiments were performed 4 days after the third intratumoral injection of retro-ES-TfR, retro-LNCX, or PBS. Mice were administrated with Tf–USPIO or plain USPIO at the same dose of 35 mg Fe per kilogram, respectively. Competition experiments in vivo were done by injecting the soluble Tf protein (20 mg/kg) 1 day before MRI. After tail vein injection of USPIO or Tf–USPIO, the tumor-bearing animals were imaged using a 3-in. surface coil according to the following protocol: T1w-sequence (FSE: TR, 160 ms; TE, minimum; FOV, 10×10 cm; matrix, 256×192; slice thickness, 1.5 mm); T2w-sequence (FR-FSE: TR, 1,070 ms; TE, 90 ms; FOV, 10×10 cm; matrix, 256×192; slice thickness, 2 mm); T2*w-sequence (fast spoiled gradient-echo fSPGR): TR, 160 ms; TE, 15 ms; FOV, 10×10 cm; matrix, 160×160; slice thickness, 2 mm; flip angle, 30°). T2 map relaxation times in the tumor before and after particle injection were determined using a multiecho spin echo pulse sequence (TR, 2,000 ms; TE ranged from 15 to 65 ms, eight echoes; number of average, 5; FOV, 8×8 cm; matrix, 128×128; slice thickness, 2.5 mm; voxel size, 1.3×0.9×2 mm3). Three slices were positioned at the tumor at maximum extensions [20, 21].
Ex Vivo Validation of Tumoricidal Effect
The inhibition of tumor angiogenesis by intratumoral injection of retro-ES-TfR was tested by immunohistochemistry and scoring of tumor vessels. Formalin-fixed, paraffin-embedded tumors were collected at 20 days after retroviral treatment and cut in 3-μm sections, deparaffinized, and subjected to endostatin immunohistochemistry (rabbit antimouse endostatin at 1:600 dilution, Abcam, Cambridge, MA) and counterstained with Mayer’s hematoxylin. In a blinded manner, ten high-power fields (200×) were examined per section of tumors in each group. To determine tumor vascularity, the sections were immunostained with a rabbit antimouse CD31 Ab (dilution 1:800; Abcam, Cambridge, MA), as described above. The stained vessels were counted in ten blindly chosen random fields at ×200 magnification, and the mean microvessel density was recorded. To further confirm the in vivo accumulation of Tf–USPIO, tumor tissue slices were prepared and examined by Prussian blue staining. The expression of ES and TfR in the tumors treated with retro-ES-TfR was also confirmed by Western blot.
Statistical Analysis
The data were expressed as mean values ± standard deviation, and paired t test and Wilcoxon rank sum test were used for evaluating statistical significance. A value of less than 0.05 (P<0.05) was considered statistically significant.
Result
The Expression of ES and TfR in MDA-MB-231 Cells In Vitro
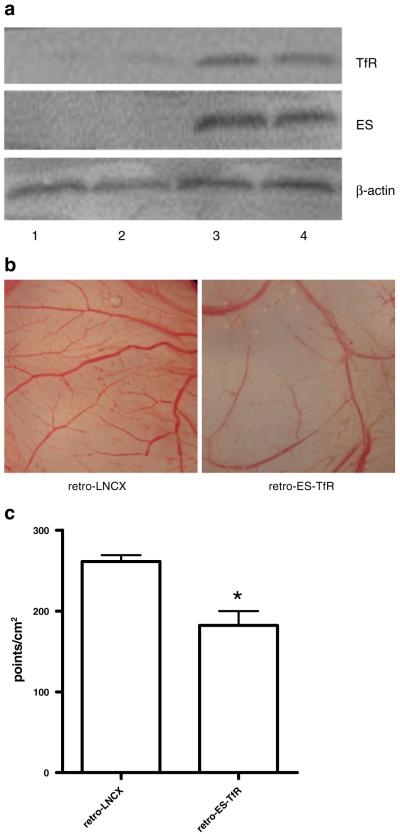
The PCR products amplified from the DNAs of retro-ES-TfR- and retro-LNCX treated-MDA-MB-231 cells were analyzed under ultraviolet light after 10 g/L agarose gel electrophoresis. A 550-bp specific fragment of endostatin gene and a 2,200-bp specific fragment of TfR gene were seen in the PCR product from the DNA of retro-ES-TfR-treated MDA-MB-231 cells, but not from the retro-LNCX control. The presence of recombinant endostatin and TfR in the cell lysate of MDA-MB-231 cells was confirmed by Western blot analysis. An ES protein band of 20 kDa was detected in both the cultured cell and tissue lysate of retro-ES-TfR transfectants, whereas no such band was present in the cells and tumor transfected with empty retrovirus (Fig. 1a). Meanwhile, a significant increase in TfR protein expression was also detected in both cell and tumor tissue lysate after retro-ES-TfR transfection, compared with those treated with retro-LNCX. To further examine whether MDA-MB-231 breast cancer cells express transferrin receptors, cells were stained with Tf–polylysine–FITC, a transferrin conjugate that binds specifically to transferrin receptors, and analyzed by flow cytometry. More than 60% of retro-ES-TfR-transfected MDA-MB-231 cells were positively stained with Tf–polylysine–FITC. The specificity of Tf–polylysine–FITC binding was confirmed by only 5% staining of Tf–polylysine–FITC in MDA-MB-231 cells transfected with retro-LNCX.
Fig. 1.
a Western blot analysis of endostatin (ES) and transferrin receptor (TfR) gene expression in MDA-MB-231 cell lysate (lanes 1 and 3) and tumor tissue lysate (lanes 2 and 4) transfected with retro-LNCX (lanes 1 and 2) and retro-ES-TfR (lanes 3 and 4). b Representative photographs of CAM treated with lysates of MDA-MB-231 cells transfected with retro-LNCX (left) and retro-ES-TfR (right). c The comparison of relative branch points of blood vessels after the treatment with the lysate of MDA-MB-231 cells transfected with retro-ES-TfR and retro-LNCX. *P<0.05.
As shown in Fig. 1b, the lysate of MDA-MB-231 cells transfected with retro-ES-TfR significantly inhibited angiogenesis in the CAM as compared to the lysate of MDA-MB-231 cells treated with retro-LNCX. The number of branch points of the blood vessels was counted and shown in Fig. 1c.
Analysis of Tf–USPIO Uptake in MDA-MB-231 Cells
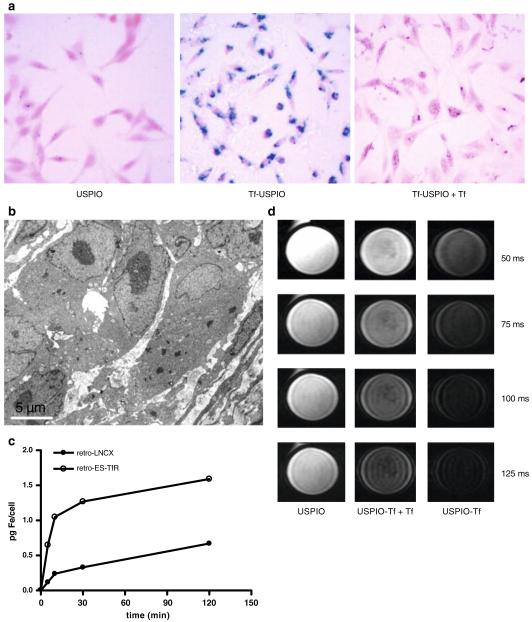
Successful coupling of transferrin protein to USPIOs was confirmed by electrophoresis (data not shown). The accumulation of Tf–USPIO conjugate was assessed histologically using Prussian blue staining (Fig. 2a). After 30 min incubation, a strong uptake of Tf–USPIO was observed in cells treated with retro-ES-TfR, whereas there was no significant uptake in cells treated with retro-LNCX. After blocking TfR with free Tf, the amount of blue granules in the cytoplasm of MDA-MB-231 was reduced, indicating that the fast accumulation of these particles was mediated by TfR. The subcellular localization of the particles was observed by TEM after incubation with Tf–USPIO for 30 min (Fig. 2b). Large amount of USPIO particles were internalized and accumulated in the cytoplasm of MDA-MB-231 cells transfected with retro-ES-TfR. On the contrary, very little USPIO were seen in MDA-MB-231 cells transfected with retro-LNCX (data not shown).
Fig. 2.
a Prussian blue-stained and nucleus fast red-counterstained retro-ES-TfR-transfected MDA-MB-231 cells incubated with USPIO, Tf–USPIO, and Tf–USPIO + Tf at an iron concentration of 0.03 mM for 30 min. b TEM image of retro-ES-TfR-transfected MDA-MB-231 cells incubated with Tf–USPIO (bar 0.5 μm). c The measurement of cellular iron contents using ICP-OES after incubation of retro-LNCX- or retro-ES-TfR-treated MDA-MB-231 with Tf–USPIO for different time intervals (iron concentration, 0.03 mM of growth medium). d T2-weighted MR images of retro-ES-TfR-transfected MDA-MB-231 cells treated with USPIO, Tf–USPIO + Tf, and Tf–USPIO, respectively. The echo times used to acquire the images were 50, 75, 100, and 125 ms, respectively.
Additionally, quantification of the cellular iron content by ICP-OES of retro-ES-TfR treated MDA-MB-231 cells proved the higher time-dependent uptake of Tf–USPIO compared with the retro-LNCX-treated cells. After 5-min incubation with Tf–USPIO, the mean cellular iron content in retro-LNCX-treated MDA-MB-231 cells incubated with Tf–USPIO was 0.1±0.07 pg Fe per cell. In cells treated with retro-ES-TfR, it was increased to 0.7±0.08 pg Fe per cell, and with time, the cellular iron content increased obviously, reaching 1.2±0.09 at 10 min, 1.4±0.08 at 30 min and 1.7±0.10 pg Fe per cell at 2 h. The internalization of Tf–USPIO by retro-LNCX-treated MDA-MB-231 was less prominent after incubation (0.2±0.08 at 10 min, 0.4±0.10 at 30 min, and 0.7±0.11 pg Fe per cell at 2 h; Fig. 2c).
MRI of MDA-MB-231 cells treated with retro-ES-TfR in gelatin (Fig. 2d) showed the similar result to the histological staining in vitro. T2-weighted images of the cells incubated with Tf–USPIO exhibited rapid signal decay. In contrast, no obvious difference can be seen in the cells incubated with plain USPIO. After Tf blocking, the signal was less reduced compared with the unblocking group, which suggests that Tf–USPIO can effectively target the retro-ES-Tf- transfected MDA-MB-231 cells and be detected by MR.
Imaging Gene Expression via TfR/Tf–USPIO Using MR In Vivo
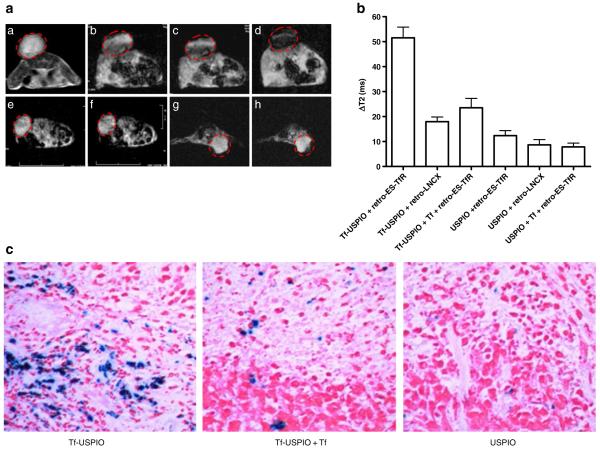
Following gene transfer, the TfR reporter gene expression was detected by MRI in vivo. The Tf–USPIO was injected via tail vein into MDA-MB-231 tumor-bearing mice. The injected dose in all cases was 35 mg Fe per kilogram body weight. T2*-weighted MR images acquired before and at different times after injection are shown in Fig. 3a, and the change of T2 relaxation time under different conditions are shown in Fig. 3b. In MDA-MB 231 tumors treated with retro-ES-TfR, T2 relaxation time was decreased by 51.5±10.5 ms or 63.2±12.1% at 24 h after Tf–USPIO injection. As a comparison, retro-ES-TfR-transfected tumor only showed the decrease of T2 relaxation time by 12.4±4.9 ms or 13.7±4.4% at 24 h after USPIO injection. Tf blocking (1 day before Tf–USPIO injection) showed limited tumor signal reduction from T2 weighted images and T2 relaxation time decrease of 23.5±9.2 ms or 26.7±7.9%. In MDA-MB-231 tumors transfected with retro-LNCX, Tf–USPIO injection led to T2 signal decrease by 17.9±4.7 ms or 22.5±7.0% at 24 h after Tf–USPIO injection. The signal decrease and T2 relaxation time change in tumors treated with retro-LNCX was likely due to the endogenous expression of TfR, which was also confirmed by fluorescence-activated cell sorting (FACS) and western blot. The appearance of blue granules in retro-ES-TfR treated the tumors (Fig. 3c), which is not present in the control group, indicates the existence of USPIO nanocrystals in the tumor and causes the change of MRI in vivo.
Fig. 3.
a T2*-weighted MR images of nude mice bearing MDA-MB-231 tumors before and after intravenous (i.v.) injection of Tf–USPIO, Tf–USPIO + Tf, or USPIO. a–d Tumors transfected with retro-ES-TfR pre (a), 1 (b), 6 (c), and 24 h (d) postinjection of Tf–USPIO. e, f Tumors transfected with retro-LNCX pre (e) and 24 h (f) postinjection of Tf–USPIO. g, h Tumors transfected with retro-ES-TfR and injected with Tf followed by baseline (g) and 24 h postinjection of Tf–USPIO (h). b Change of T2 relaxation times in retro-ES-TfR- and retro-LNCX-transfected tumors after injection of Tf–USPIO, Tf–USPIO + Tf, and USPIO, respectively. c Prussian blue-stained and nucleus fast red-counterstained retro-ES-TfR-transfected MDA-MB-231 tumor tissue 24 h after intravenous administration of Tf–USPIO, Tf–USPIO + Tf, and USPIO.
Inhibitions of Tumor Growth Following ES Gene Expression In Vivo
The MDA-MB-231 tumors were subjected to intratumoral injection of retroviral vectors for three consecutive days for gene therapy when the tumor volume reached about 100 mm3 (n=11 per group). The growth delay of experimental groups was monitored via serial caliper measurements. On day25, retro-ES-TfR-treated tumors showed a final fractional tumor volume (vfinal/vinitial) of 7.38±0.55, significantly less than the retro-LNCX treated (vfinal/vinitial = 13.2±1.98) and PBS control (vfinal/vinitial =14.9±2.52; P<0.01; Fig. 4a). CD31 staining of paraffin sections of retro-ES-TfR-treated tumors revealed marked degradation and weakening of tumor neovasculature. In contrast, control mice received retro-LNCX showed healthy, mature tumor endothelium (Fig. 4b). Fewer microvessels in tumors treated with retro-ES-TfR (24.6±3.8/mm2) were also found than those treated with retro-LNCX (41.9±2.7/mm2; Fig. 4c, P<0.05).
Fig. 4.
a MDA-MB-231 tumor mice treatment with retro-ES-TfR, retro-LNCX, and PBS. Three doses of intratumor injection of retro-ES-TfR (n=11 per group) led to significant growth delay compared to the retro-LNCX (n=11 per group) and PBS groups (n=6 per group). b CD31 staining of the paraffin slices of tumors injected with retro-LNCX (left) and retro-ES-TfR (right). c Tumor microvessel density (MVD) measurement.
Discussion
Recent advances in molecular and cell biology techniques have fueled a rapid increase in our understanding of human diseases. Molecular therapeutic approaches to correct “defects” are also attempted by expressing exogenous genes, imparting additional functions to the cells. Simultaneously, increasing effort has been directed toward identifying ways of monitoring gene delivery and expression noninvasively in vivo [13, 25-27]. Here, we demonstrated that TfR, as an imageable marker gene, could be used to evaluate endostatin therapeutic gene expression in vivo.
First of all, we designed and produced the coexpression retroviral vector with two different transgenes ES and TfR under the control of CMV promoter. Using the recombinant retrovirus, the exogenous genes were integrated into the genome of tumor cells, and continuous expression was obtained. But we reasoned whether the expression of ES was reflected by the expression of TfR. The expression of ES and TfR gene were tested both in vitro and in vivo. As evidenced by Western blot, the ES expression increased in cells treated with retro-ES-TfR, meanwhile, the expression of TfR also was significantly upregulated. After sorting by FACS, the similar result in TfR expression was observed. Western blot also confirmed the simultaneous expression of ES and TfR in vivo after injection of retro-ES-TfR intratumorally. Taken together, these data indicate that: (1) the ES and TfR genes could be successfully transferred into tumor cells, (2) the two different genes were simultaneously expressed after gene transfer, and (3) the expression of TfR could reflect the expression of ES. As the expression of TfR followed with the expression of ES gene on the downstream of the vector, we would be able to use the imageable marker gene to provide readout on the expression of the therapeutic transgenes expressed next [28].
The expression of endostatin gene produced a 20-kD protein fragment, which had been shown to be an inhibitor of angiogenesis in primary tumors. Antiangiogenic therapy is relatively less toxic and less drug resistance, and antiangiogenic gene therapy appears to be more reasonable, as it has the potential to produce the therapeutic agents in a local area for a sustained period, avoiding continuous administrations of antiangiogenic agents. The expression of endostatin may decrease the neovascularization; lead to the loss of an adequate vasculature, which would deprive the tumor of oxygen and nutrients; inhibit the growth of tumor. It has recently been reported that endostatin gene transfer inhibits breast cancer and metastases in mice [29] and inhibits the growth of HT29 human colon cancer cells [5, 11]. In our work, endostatin gene mediated by retrovirus was administrated intratumorally, resulting in the expression of endostatin protein in situ. Tumor volumes within the treated group were shown to be significantly smaller than those in the PBS control group and retro-LNCX group. The delayed tumor growth might be explained by the expression of therapeutic gene followed by decreased microvascular density and downregulation of VEGF protein (data not shown).
TfR, a cell surface receptor which mediated iron internalization, has been used as a marker gene to visualize gene expression probed by targeted USPIO. Using this imaging system, the internalization and the accumulations of USPIO nanoparticles in cells can be measured and correlated to receptor expression level [13, 22, 25, 30-34]. In this study, we imaged TfR expression with Tf–USPIO conjugate to detect the antiangiogenic therapeutic gene endostatin. The incorporation of Tf–USPIO conjugate in cells transfected with retro-ES-TfR led to significantly more cell uptake and T2 signal decrease as compared to those transfected with retro-LNCX. Following intravenous injection of Tf–USPIO in MDA-MB-231 tumor-bearing mice, the mice with intratumoral injection of retro-ES-TfR showed T2*-weighted signal drop as early as 1 h after the Tf–USPIO injection, mainly in the peripheral areas of the tumor. The hypointensity of the whole tumor became more pronounced with time. The effective TfR receptor-mediated internalization of surface bound Tf–USPIO is likely the reason for the sustained MR signal and signal amplification with time. It is of note that MDA-MB-231 tumors with PBS and retro-LNCX both showed much less but detectable decrease in MR signal intensity, possibly due to the endogenous expression of TfR in the wild-type MDA-MB-231 cells that have been confirmed in our FACS analysis and earlier reports in the literature [35].
Despite the success of demonstrating the feasibility of MR reporter gene imaging to quantify therapeutic gene expression and the ability of endostatin gene therapy in a subcutaneous breast cancer model, there are several limitations exist in this study. Firstly, a retroviral vector was used for gene transfer, which has potential limitations and risks, including limited packaging capacity and unfavorable immunological features. Future studies should test nonviral vectors to achieve high transfection efficiency, excellent safety profile with low immunogenicity, and minimal risk for insertional mutagenesis [17, 36, 37]. Secondly, although intratumoral delivery route has been frequently used to evaluate the efficacy of virotherapy, targeted gene transduction to specific tissues and organs through intravenous injection would be the preferred method of gene delivery. Targeting vectors that circulate and home to specific cells could allow early therapeutic intervention. It can also enhance the safety of gene therapeutic applications by reducing inadvertent infection of irrelevant cells or tissues and decrease the side effect of gene therapy [38]. Thirdly, the delay of tumor growth by retro-ES-TfR was significant compared to the control groups, however, the antitumor effect was considered satisfactory probably due to the suboptimal single-agent therapy strategy. Combining gene therapy with conventional therapy might be advantageous, as gene therapy could be employed to reduce the dose of chemotherapeutic agents to spare the side-effects of cytotoxic drugs, without impairing antitumor efficacy. It was reported that the combination of gene therapy and conventional chemotherapy showed a stronger effect than monotherapies, so further investigation of this possibility is warranted [5, 12]. Lastly, MR reporter genes typically have low sensitivity. To increase the sensitivity of imaging, optical and radionuclide approaches should be the alternatives. Using luciferase gene, optical imaging could provide an accurate and sensitive imaging in rodents to detect low levels of gene expression. Positron emission tomography and single photon emission computed tomography are sufficiently sensitive and quantitative to measure the expression of reporter gene in both preclinical models and human patients. Many of the shortcomings of each modality alone also can be overcome by the use of combinational imaging of multiple reporter genes [39-41].
Conclusion
Our findings indicate that it is possible to noninvasively characterize gene expression in tumors with the presence of transferring receptor reporter gene and Tf–USPIO conjugate reporter probe using MR scanner in vivo. Thus, MR reporter gene imaging may provide insights into gene delivery in vivo and may have the potential to optimize tumor gene therapy.
Acknowledgments
This work was supported, in part, by the Foundation of Heilongjiang Educational Committee (10551123), Heilongjiang Technical Plan Project (WC30307), the Key Program of Heilongjiang Scientific Problem Tackling Project (GB04C30201), and the Ministry of Personnel Foundation for Returnees (2007275).
Footnotes
Kai Wang and Kezheng Wang contributed equally to the work.
References
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Shojaei F, Ferrara N. Role of the microenvironment in tumor growth and in refractoriness/resistance to anti-angiogenic therapies. Drug Resist Updat. 2008;11:219–230. doi: 10.1016/j.drup.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Tozer GM, Kanthou C, Lewis G, Prise VE, Vojnovic B, Hill SA. Tumour vascular disrupting agents: combating treatment resistance. Br J Radiol. 2008;81:S12–S20. doi: 10.1259/bjr/36205483. Spec No 1. [DOI] [PubMed] [Google Scholar]
- 4.Wickstrom SA, Alitalo K, Keski-Oja J. Endostatin signaling and regulation of endothelial cell-matrix interactions. Adv Cancer Res. 2005;94:197–229. doi: 10.1016/S0065-230X(05)94005-0. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Dong X, Xu Z, Jiang X, Jiang H, Krissansen GW, et al. Endostatin gene therapy enhances the efficacy of paclitaxel to suppress breast cancers and metastases in mice. J Biomed Sci. 2008;15:99–109. doi: 10.1007/s11373-007-9201-3. [DOI] [PubMed] [Google Scholar]
- 6.Xu HM, Zhang GY, Ji XD, Cao L, Shu L, Hua ZC. Expression of soluble, biologically active recombinant human endostatin in Escherichia coli. Protein Expr Purif. 2005;41:252–258. doi: 10.1016/j.pep.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Dhanabal M, Ramchandran R, Volk R, Stillman IE, Lombardo M, Iruela-Arispe ML, et al. Endostatin: yeast production, mutants, and antitumor effect in renal cell carcinoma. Cancer Res. 1999;59:189–197. [PubMed] [Google Scholar]
- 8.Huang X, Wong MK, Zhao Q, Zhu Z, Wang KZ, Huang N, et al. Soluble recombinant endostatin purified from Escherichia coli: anti-angiogenic activity and antitumor effect. Cancer Res. 2001;61:478–481. [PubMed] [Google Scholar]
- 9.Hansma AH, Broxterman HJ, van der Horst I, Yuana Y, Boven E, Giaccone G, et al. Recombinant human endostatin administered as a 28-day continuous intravenous infusion, followed by daily subcutaneous injections: a phase I and pharmacokinetic study in patients with advanced cancer. Ann Oncol. 2005;16:1695–1701. doi: 10.1093/annonc/mdi318. [DOI] [PubMed] [Google Scholar]
- 10.Ning T, Yan X, Lu ZJ, Wang GP, Zhang N, Yang J, et al. Gene therapy in orthotopic lung cancer murine model with angiogenesis inhibitor, endostatin. Hum Gene Ther. 2009 doi: 10.1089/hum.2008.098. in press. [DOI] [PubMed] [Google Scholar]
- 11.Coutinho EL, Andrade LN, Chammas R, Morganti L, Schor N, Bellini MH. Anti-tumor effect of endostatin mediated by retroviral gene transfer in mice bearing renal cell carcinoma. FASEB J. 2007:3153–3161. doi: 10.1096/fj.07-8412com. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Tan G, Li J, Dong X, Krissansen GW, Sun X. Gene transfer of endostatin enhances the efficacy of doxorubicin to suppress human hepatocellular carcinomas in mice. Cancer Sci. 2007;98:1381–1387. doi: 10.1111/j.1349-7006.2007.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waerzeggers Y, Monfared P, Viel T, Winkeler A, Voges J, Jacobs AH. Methods to monitor gene therapy with molecular imaging. Methods. 2009 doi: 10.1016/j.ymeth.2009.03.007. 2009. (in press) [DOI] [PubMed] [Google Scholar]
- 14.Wei X, Lin L, Hou Y, Fu X, Zhang J, Mao Z, et al. Construction of recombinant adenovirus co-expression vector carrying the human transforming growth factor-β1 and vascular endothelial growth factor genes and its effect on anterior cruciate ligament fibroblasts. Chin Med J. 2008;121:1426–1432. [PubMed] [Google Scholar]
- 15.Jin X, Bookstein R, Wills K, Avanzini J, Tsai V, LaFace D, et al. Evaluation of endostatin antiangiogenesis gene therapy in vitro and in vivo. Cancer Gene Ther. 2001:982–989. doi: 10.1038/sj.cgt.7700396. [DOI] [PubMed] [Google Scholar]
- 16.Huszthy PC, Brekken C, Pedersen TB, Thorsen F, Sakariassen PO, Skaftnesmo KO, et al. Antitumor efficacy improved by local delivery of species-specific endostatin. J Neurosurg. 2006;104:118–128. doi: 10.3171/jns.2006.104.1.118. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z, Teng GJ, Chen BA, Xu ZF, Hu J, Shao ZY. Local effects of retrovirally transduced endostatin-expressing human umbilical cord blood CD34+ cells on transplanted malignancy in a mouse model of hepatic cancer. Cell Transplant. 2008;17:969–975. doi: 10.3727/096368908786576525. [DOI] [PubMed] [Google Scholar]
- 18.Pawliuk R, Bachelot T, Zurkiya O, Eriksson A, Cao Y, Leboulch P. Continuous intravascular secretion of endostatin in mice from transduced hematopoietic stem cells. Mol Ther. 2002;5:345–351. doi: 10.1006/mthe.2002.0572. [DOI] [PubMed] [Google Scholar]
- 19.Hu F, Wei L, Zhou Z, Ran Y, Li Z, Gao M. Preparation of Biocompatible Magnetite Nanocrystals for In Vivo Magnetic Resonance Detection of Cancer. Adv Mater Deerfield. 2006;18:2553–2556. [Google Scholar]
- 20.Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Chen K, Lee HY, Xu C, Hsu AR, Peng S, et al. Ultrasmall c(RGDyK)-coated Fe3O4 nanoparticles and their specific targeting to integrin alpha(v)beta3-rich tumor cells. J Am Chem Soc. 2008;130:7542–7543. doi: 10.1021/ja802003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mowat P, Franconi F, Chapon C, Lemaire L, Dorat J, Hindré F, et al. Evaluating SPIO-labelled cell MR efficiency by three-dimensional quantitative T2* MRI. NMR Biomed. 2007;20:21–27. doi: 10.1002/nbm.1084. [DOI] [PubMed] [Google Scholar]
- 23.Yamanaka R, Zullo SA, Ramsey J, Onodera M, Tanaka R, Blaese M, et al. Induction of therapeutic antitumor antiangiogenesis by intratumoral injection of genetically engineered endostatin producing Semliki Forest virus. Cancer Gene Ther. 2001;8:796–802. doi: 10.1038/sj.cgt.7700367. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Liu FK, Li X, Li JS, Xu GX. Retrovirus-mediated gene transfer of human endostatin inhibits growth of human liver carcinoma cells SMMC7721 in nude mice. World J Gastroenterol. 2002:1045–1049. doi: 10.3748/wjg.v8.i6.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogemann D, Basilion JP. “Seeing inside the body”: MR imaging of gene expression. Eur J Nucl Med Mol Imaging. 2002;29:400–408. doi: 10.1007/s00259-002-0765-x. [DOI] [PubMed] [Google Scholar]
- 26.Brack SS, Dinkelborg LM, Neri D. Molecular targeting of angiogenesis for imaging and therapy. Eur J Nucl Med Mol Imaging. 2004;31:1327–1341. doi: 10.1007/s00259-004-1648-0. [DOI] [PubMed] [Google Scholar]
- 27.Schäfer R, Kehlbach R, Wiskirchen J, Bantleon R, Pintaske J, Brehm BR, et al. Transferrin receptor upregulation: in vitro labeling of rat mesenchymal stem cells with superparamagnetic iron oxide. Radiology. 2007;244:514–523. doi: 10.1148/radiol.2442060599. [DOI] [PubMed] [Google Scholar]
- 28.Ichikawa T, Högemann-Savellano D, Saeki Y, Tyminsk E, Terada K, Weissleder R, et al. MRI of transgene expression: correlation to therapeutic gene expression. Neoplasia. 2002;4:523–530. doi: 10.1038/sj.neo.7900266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauter BV, Martinet O, Zhang WJ, Mandeli J, Woo SL. Adenovirus-mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases. Proc Natl Acad Sci USA. 2000;97:4802–4807. doi: 10.1073/pnas.090065597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore A, Josephson L, Bhorade RM, Basilion JP, Weissleder R. Human transferrin receptor gene as a marker gene for MR imaging. Radiology. 2001;221:244–250. doi: 10.1148/radiol.2211001784. [DOI] [PubMed] [Google Scholar]
- 31.Hogemann D, Josephson L, Weissleder R, Basilion JP. Improvement of MRI Probes To Allow Efficient Detection of Gene Expression. Bioconjug Chem. 2000;11:941–946. doi: 10.1021/bc000079x. [DOI] [PubMed] [Google Scholar]
- 32.Moore A, Basilion JP, Chiocca EA, Weissleder R. Measuring transferrin receptor gene expression by NMR imaging. Biochim Biophys Acta. 1998;1402:239–249. doi: 10.1016/s0167-4889(98)00002-0. [DOI] [PubMed] [Google Scholar]
- 33.Thorek DL, Tsourkas A. Size, charge and concentration dependent uptake of iron oxide particles by non-phagocytic cells. Biomaterials. 2008;29:3583–3590. doi: 10.1016/j.biomaterials.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakase I, Gallis B, Takatani-Nakase T, Oh S, Lacoste E, Singh NP, et al. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009;274:290–298. doi: 10.1016/j.canlet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Inoue T, Cavanaugh PG, Steck PA, Brunner N, Nicolson GL. Differences in transferrin response and numbers of transferrin receptors in rat and human mammary carcinoma lines of different metastatic potentials. J Cell Physiol. 1993;156:212–217. doi: 10.1002/jcp.1041560128. [DOI] [PubMed] [Google Scholar]
- 36.Kealy BLA, McMahon JM, Ritter T, O’Doherty A, Hoare M, Greiser U, Vaughan EE, Maenz M, O’Shea C, Barry F, O’Brien T. Comparison of viral and nonviral vectors for gene transfer to human endothelial progenitor cells. Tissue Eng Part C Methods. 2009 doi: 10.1089/ten.tec.2008.0323. in press. [DOI] [PubMed] [Google Scholar]
- 37.Evans V, Foster H, Graham I, Foster K, Athanasopoulos T, Simons J, et al. Human apolipoprotein E expression from mouse skeletal muscle by electrotransfer of nonviral DNA (plasmid) and pseudotyped recombinant adeno-associated virus (AAV2/7) Hum Gene Ther. 2008;19:569–578. doi: 10.1089/hum.2007.169. [DOI] [PubMed] [Google Scholar]
- 38.Morizono K, Xie Y, Ringpis G, Johnson M, Nassanian H, Lee B, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 39.Blasberg R. In vivo molecular-genetic imaging: multi-modality nuclear and optical combinations. Nucl Med Biol. 2003;30:879–888. doi: 10.1016/s0969-8051(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 40.Bhaumik S, Gambhir S. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci USA. 2002;99:377–382. doi: 10.1073/pnas.012611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacLaren D, Toyokuni T, Cherry S, Barrio J, Phelps M, Herschman H, et al. PET imaging of transgene expression. Biol Psychiatry. 2000;1:337–348. doi: 10.1016/s0006-3223(00)00970-7. [DOI] [PubMed] [Google Scholar]