Abstract
There is considerable inter-study and inter-individual variation in the scalp location of parietal sites where transcranial magnetic stimulation (TMS) may modulate visuospatial behaviours (see Ryan, Bonilha, & Jackson 2006); and no clear consensus on methods for identifying such sites. Here we introduce a novel TMS “hunting paradigm” that allows rapid, reliable identification of a site over right anterior intraparietal sulcus (IPS), where short trains (at 10 Hz for 0.5s) of TMS disrupt performance of a task in which subjects judge the presence or absence of a small peripheral gap (at 14 degrees eccentricity), on one or other (known) side of an extended (29 degrees) horizontal line centred on fixation. Signal detection analysis confirmed that TMS at this site reduced sensitivity (d’) for gap targets in the left visual hemifield. A further experiment showed that the same right-parietal TMS increased sensitivity instead for gaps in the right hemifield. Comparing TMS across a grid of scalp locations around the identified ‘hotspot’ confirmed the spatial specificity. Assessment of the TMS intensity required to produce the phenomena found this was linearly related to individuals’ resting motor TMS threshold over hand M1. Our approach provides a systematic new way to identify an effective site and intensity in individuals, at which TMS over right parietal cortex reliably changes visuospatial sensitivity.
Introduction
Previous work has shown that transcranial magnetic stimulation (TMS) can alter performance in some visuospatial tasks when delivered over posterior parietal (PPC) sites; for instance, producing a rightward bias in line bisection or landmark-based tasks (e.g. Fierro et al. 2000, Pourtois et al. 2001, Fierro et al. 2006, Valero-cabre et al. 2006, Fecteau et al. 2006, Muggleton et al. 2008). The effects may be lateralised (with right parietal TMS typically more effective) and may also interact with the visual field tested. For example, numerous studies using right parietal TMS in healthy subjects reveal disruption of visual performance in the contralateral left visual hemifield (e.g. Pascual Leone et al. 1994, Koch et al. 2005, Meister et al. 2006, Dambeck et al. 2006, Muggleton et al 2006, Jin et al. 2008,) and/or enhancement for the right hemifield (see Fecteau et al. 2006 for a detailed review). In one prominent example, Hilgetag, Theoret & Pascual-Leone (2001) reported that extended 1Hz repetitive TMS over right parietal PPC led not only to subsequent contralateral impairment, but also to ipsilateral enhancement of visual target detection. Chambers, Stokes, Janko & Mattingley (2006) reported that short (0.5s) bursts of right PPC TMS at 10Hz may selectively enhance localisation of ipsilateral targets in bilateral arrays.
Clinically, TMS has been used to explore possible therapeutic effects of TMS or repetitive TMS in patients with spatial neglect after unilateral brain injury, when applied over the undamaged hemisphere. The notion of ‘interhemispheric rivalry’ (Kinsbourne, 1977) had led to the possibility that the undamaged hemisphere may become hyperexcitable in neglect, and hence that applying TMS to that hemisphere might potentially rebalance or normalise this (see Koch, Oliveri, Cheeran, Ruge, Lo Gerfo, Salerno, Torriero, Marconi, Mori, Driver, Rothwell & Caltagirone, 2008, for a more extended overview). Single or short trains (up to 5 TMS pulses at 20Hz) of left parietal or frontal TMS have been reported to reduce contralateral extinction for tactile stimuli in unilateral stroke patients(Oliveri, Rossini, Traversa, Cicinelli, Filippi, Pasqualetti et al., 1999). Moreover, 1 Hz stimulation over the unaffected hemisphere may ameliorate a rightward bias in pre-transected line judgements for up to 15 days (Brighina, Bisiach, Oliveri, Piazza, La Bua et al., 2003), and causes some improvement in the perception of chimeric figures (Koch et al, 2008) in neglect patients.
In all of the PPC studies above, TMS was applied over a parietal target defined either with MRI-based frameless stereotaxy (which is not always practical, as in some clinical patient studies); or by simply targeting a point (P3, P4, P5, or P6) defined by the 10/20 EEG electrode placement system. However, neuroimaging studies indicate that the anatomical network underlying visuospatial attention in normals may be rather widely distributed (e.g. see Corbetta & Shulman, 2002; Mort, Malhotra, Mannan, Rorden, et al., 2003). Moreover, at the level of each individual subject or patient, it can be unclear exactly which site of potential parietal TMS stimulation should produce the greatest impact on visuospatial function (Ryan, Bonilha, Jackson, 2006). Recent TMS work in normals has shown that merely using the scalp coordinates of conventional EEG electrode-sites can be rather ineffective (Sack, Kadosh, Schumann, Moerel, Walsh, & Goebel, 2008). Moreover, for electrode sites such as P3 and P4, the anatomical structures underlying them have been shown to vary rather substantially between individuals, e.g. the two structures most likely to underlie P4 are not only the right angular gyrus (~63% of the time), but also the right superior occipital gyrus (~22% of the time); see Okamoto M, Dan H, Sakamoto K et al. (2003).
Using a target site that is defined functionally within each subject, rather than anatomically, might enhance systematic impacts on visuospatial processing, thereby speeding progress both in understanding these effects and in seeking to exploit them clinically. One solution is a ‘hunting procedure’, whereby the effect of TMS on a visuospatial task is assessed briefly over a number of different sites, and the optimal site as defined functionally (in terms of behavioural impact) after such hunting is then selected as the TMS target for more detailed testing, with the same and/or other visuospatial tasks. For example, according to one influential proposal (Ashbridge, Walsh & Cowey, 1997), a 3 × 3 grid can be drawn around P3 or P4 and the best TMS site to disrupt visuospatial search may then be found by comparing the effects for 16 trials at each site. The ‘hotspot’ in this particular protocol has been defined as the point where TMS increases subjects’ reaction time by 100ms or more (Ashbridge, Walsh, and Cowey, 1997). Subsequent TMS over such a pre-defined point was shown to lead to a contralateral deficit in line-judgement tasks but no lateralised deficit in visual search tasks, hinting at some possible mismatch between the hunting procedure and subsequent experimental findings (Ashbridge, Walsh, and Cowey, 1997; see also Ellison, Schindler, Pattison & Milner, 2004).
Although influential, the particular hunting procedure of Ashbridge et al. (1997) is time-consuming, and moreover it relies on reaction-time effects that might not necessarily reflect genuine changes in visuospatial sensitivity (e.g. d’), as can be measured instead via formal signal detection procedures, in tasks that are well below ceiling on accuracy. The aim of the present study was to develop a modified hunting procedure for right parietal TMS effects upon visuospatial performance, in a task which is well suited for application of signal detection theory. We describe a rapid and simple method of localising an effective TMS site over right parietal cortex, which provides an alternative or supplement to the established techniques mentioned above. We go on to validate this new protocol, by showing that it is reliable and specific.
In the first set of studies below we describe the new procedure, test its reproducibility, and identify the most effective right parietal site. In the second and third sets of studies, we use signal detection analysis to examine our findings in detail, to confirm a genuine effect on perceptual sensitivity, and to verify that the induced visuospatial effects differ between contralateral and ipsilateral visual hemifields (in fact inducing opposite effects for the two hemifields during right parietal TMS). Finally, we explore the TMS intensity needed to disrupt visuospatial sensitivity and whether this can be predicted from an individual subject’s resting motor threshold. If so, this would then allow the TMS intensity for visuospatial experiments to be readily adjusted to match each individual subject, along with indivisual parietal TMS-site location as identified via our new hunting procedure.
General Methods
The study was approved by the local ethics committee. Subjects gave written informed consent and were all healthy volunteers with normal or corrected vision by self-report (see individual experimental procedures for detailed information on handedness, age and gender).
In all experiments, subjects sat with head and chin restrained at 50cm from a PC laptop screen (refresh rate 50hz). We used a laptop because the ultimate aim of our study was to introduce a new protocol that may be suitable for clinical TMS studies in a hospital setting.The visual stimuli used are shown in Figure 1, and each comprised a long horizontal line (extending 29 degrees of visual angle), with a small vertical mark at its centrer to indicate the central fixation point. The task was to detect the presence (as in Fig 1B or 1C) or absence (as in Fig 1A) of a small gap, which could appear near the far left (Fig 1B) or far right (Fig 1C) of each line when present, at 14 degrees of eccentricity. Unlike the well-known line-bisection task, our gap-detection task is unambiguous regarding which visual hemifield is most relevant for a particular detection judgement. This is because the gap (when present) was either at the far-left (Experiments 1, 2 and 4) or far-right (Experiment 3), but was never present on both sides concurrently. This contrasts with the horizontal extents that are compared between sides during line-bisection tasks and standard variants upon that, such as judgements of prebisected lines. Moreover, in all our experiments the subjects were instructed regarding which side (far-left or far-right) the gap could appear on, which remained constant throughout each experiment. They nevertheless had to maintain central fixation, as we confirmed with eye-tracking (see below). The foreknown nature of the task-relevant location where the gap might appear constrasts with other paradigms involving potential search of either or both sides, and should minimize any strategies that tradeoff differerent locations, as here a single location was task-relevant and this was always known in advance.
Figure 1.
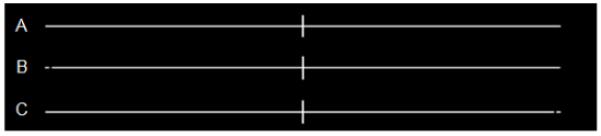
Illustrations of the 3 different stimulus types used: A. ‘No Gap’, B. ‘Left Gap’ C. ‘Right Gap’ (Stimulus C was used in Experiment 3 only, and that experiment did not include Stimulus B; hence, within any one experiment, subjects always knew in advance where the gap might appear, if present). Stimuli were presented using the ‘E-prime’ software package (Psychology Software Tools Inc., Pittsburgh). In all experiments the stimuli comprised white lines on a black background, bisected with a vertical marker that corresponded to the middle of the preceding fixation cross. The lines (Fig. 1) occupied 29.36 degrees of visual angle (26.2cm long at a distance of 50cm) with any gap if present being 1.5 mm (0.17 degrees) wide, situated 2mm from the left or right end of the line (eccentricity of 14.31 to 14.47 degrees from the midline).
Visual stimulus duration was tailored for each subject to achieve a % correct rate of ~95%, using a ‘staircase’ procedure as described in detail below. TMS was delivered over right parietal cortex using a Magstim Super Rapid stimulator (Magstim, Whitland UK). A figure-of-eight coil with diameter 70mm delivered 5 biphasic pulses at 10Hz, starting 100ms before visual display onset and ending with the final pulse being delivered 400 ms after initial visual presentation onset. These TMS bursts were chosen on the basis of previous studies where 5 pulses at 10Hz led to reported ‘neglect-like’ (visuospatial) deficits in line bisection tasks, when given over the right PPC (Bjoertomt et al. 2001, Ellison et al. 2004). Unlike those studies, the train of pulses here started 100ms before visual stimulus onset; we chose this in part because the location of the possible gap ‘target’ was always foreknown in our experiments (see above), so that in principle subjects could covertly monitor that location before visual onset (see Discussion). The initial intensity used was 100% of the subject’s Resting Motor Threshold (RMT), apart from in our final experiment for which TMS intensity was varied. As in other studies in which visual or tactile perception has been affected with TMS over parietal cortex, the coil was held with the handle pointing backwards so as to induce a current with initial phase flowing in the posterior-anterior direction in the underlying brain (Oliveri, Caltagirone et al., 2000; Oliveri, Rossini et al., 2000; Koch, Oliveri et al., 2005). RMT was determined to the nearest 1% of maximum stimulator output, and defined as the minimal stimulus intensity required to produce a Motor Evoked Potential (MEP) of more than 100 microV in at least five of ten consecutive trials (see Rossini et al. 1994).
Experiment 1: Procedure
Following a fixation cross, 9 subjects (8 male and 1 female aged 25-36, Edinburgh Handedness inventory score Mean = 84, SE = 10) were shown on each trial either an unbroken horizontal line (Fig 1A) or a line with a ‘gap’ at the far left (Fig.1B), equiprobably. They were instructed to keep their eyes fixed on the centre of the screen (as confirmed later with eye-tracking, see below) and to indicate their perception (‘gap’ or ‘no gap’) with a key press. Note that the gap, when present, could only appear on the far left in this particular experiment, as was known to the subjects. For each subject, a suitable presentation duration (PD) was determined in the absence of TMS with a staircase procedure, aiming for 95% of the stimuli being correctly identified as containing a gap or no-gap. Using single blocks of 20 trials, the PD was adjusted in 20 ms steps starting at 80 ms. If performance for one block was lower or higher than the desired 1/20 error rate, the PD was adjusted, respectively, one step up or down. The staircase ended if the desired error rate was attained, with the last PD then being deemed suitable. Alternatively, if a reversal in performance occurred around the desired error rate, a retest was administered using the shorter PD of the preceding two blocks. The shorter or longer PD of these two blocks was deemed suitable if the retest error rate was above or below (respectively) the desired rate. For all subjects, the selected PD was typically 20-40 ms (mode of 20 ms, mean of 29 ms).
During the TMS hunting procedure itself, the left ‘gap’ was in fact presented more often (now 90% of trials, unknown to the naïve subjects), but as explained below was often missed nevertheless due to the TMS. We decided to keep the hunting procedure for identifying a hotspot as simple as possible initially, basing it only on ‘misses’ and ‘hits’ (though full signal detection measures that incorporate ‘false alarm’ and ‘correct rejection’ rates were used in subsequent cross-validation experiments, see below). For this reason the proportion of ‘no gap’ trials (which yield neither ‘misses’ nor ‘hits’ and thus did not contribute to initial localisation of the ‘hotspot’) was kept low at 10% (but see also Discussion, and note that in our subsequent cross-validation experiments, gap presence/absence became equiprobable instead). Once the subject was able to correctly identify 4 consecutive ‘gap’ stimuli (as a final confirmation of good performance), TMS was delivered during stimulus presentation as described above (i.e. 5 TMS pulses at 10Hz and 100% RMT, beginning 100 ms prior to display onset).
The coil position at the start of the experiment was EEG 10-20 position P4 in all subjects. This location was selected on the basis of previous TMS studies (Pascual-Leone et al. 1994; Heilgetag et al. 2001, Oliveri, Rossini et al. 2000; Oliveri, Caltagirone et al. 2000; Pourtois et al. 2001; Koch et al. 2005, Dambeck et al. 2006, Jin et al. 2008) in which reliable effects on spatial judgments were found using P4 as the target site. Those past studies suggest that a procedure hunting for a particularly effective parietal-TMS site (as here) may meet with success relatively fast if sites near P4 are sampled initially. Starting at P4, the coil was moved along a spiral-shaped path (see below) using a ‘miss- stay’, ‘hit- shift’ protocol, until a site was reached where the subject missed four consecutive gaps. Hence a TMS site was judged as effective when subjects demonstrated a rise in the ‘miss-rate’ for left gaps as compared to the 4/4 hits scored just before the start of the TMS (see Discussion for the estimated probability of this occurring by chance alone; this probability is rather low).
With our hunting procedure, we aimed to sample a relatively large number of points in a short space of time, rejecting those points unlikely to provide a true ‘hotspot’ as quickly as possible (hence the low proportion of ‘no-gap’ trials), while at the same time maintaining a low risk of declaring a false hotspot (see Discussion). A spiral-shaped path gives a particularly effective spatial coverage of a sampling surface in a time-efficient manner, a property exploited in techniques as diverse as MRI (see fig.6 from Sykora 2006,) or the production of machine tools (Wieczorowski 2001). The coil was moved from P4 in 0.5cm steps along a path which approximated a clockwise spiral drawn through the intersections of a grid (e.g. lateral, posterior, medial, medial, anterior, anterior, lateral, lateral, lateral, posterior and so on). Accuracy was improved by first marking out a grid for the experimenter’s visual reference, centred on the point formed by the coil’s anterior concavity, given that the coil’s initial centre lay over P4 (a grid centered over P4 would hence have been obscured by the coil for most of the hunting procedure). To prevent a sampling bias towards those points postero-lateral (or antero-medial) to P4, the first movement of the coil alternated across subject between medial or lateral (with the overall spiral shifts still clockwise). While even that counterbalancing of first shift still leave some in-principle potential for sampling ‘bias’, in the sense of anterior-lateral and posterior-medial points being sampled somewhat later; but as we show later any such residual sampling bias was in practice very small (see Discussion). The effect of TMS on some aspects of perception (e.g. for visual motion) can be restricted to an area as small as 100mm2 (Beckers & Homberg 1992; Hotson et al. 1994). By moving the coil in steps of 0.5cm here we could be fairly confident that functionally distinct locations (which might include the sought hotspot) should not be missed.
In each individual subject the scalp location of the coil at the end of the hunting procedure, hereafter termed the (parietal) ‘hotspot’, was recorded relative to the EEG ‘10/20’ position P4. In addition, this point was recorded using an infrared positioning system (Northern Digital, Waterloo, Canada), and the Brainsight Frameless software package (Rogue Research, Montreal, Canada). The separate motor TMS hotspot was defined as the optimal site for eliciting MEPs in the left FDI muscle, and was likewise marked on the subject’s structural MRI scan. In a follow-up study the right parietal hotspot was again determined initially by the hunting procedure as before. This time,however, subjects continued with the ‘left gap’/ ‘no gap’ discrimination task for 20 more trials (still with 90% of trials actually containing ‘left gaps’ during TMS), now while wearing an IRIS Skalar Infra-red Eye Tracker. This was to confirm that any reduction in perception of gaps at the far-left of the horizontal line during right parietal TMS over the hotspot could not be due to substantial TMS-induced deviations of the eyes away from such gaps (i.e. towards the right).
Experiment 1 results: Reproducibility of the Hunting Procedure
In all 9 subjects the hunting procedure yielded a point over right parietal cortex where TMS led to increased misses for left gaps, on average taking 62 ±7 trials to find. The average site across all 9 subjects was 2.2 ± 0.3cm (mean ± SE) anterior and 1.3 ±0.3 cm medial to the P4 ‘10/20’ EEG site. In all subjects the site was mapped onto each individual’s structural MRI scan using neuronavigation (see Fig.2). This corresponded to a point along the anterior intraparietal sulcus, just posterior to its junction with primary somatosensory cortex (mean Montreal Neurological Institute coordinates of X = 42.3, Y = −50.3, Z = 64.4) During stimulus presentation (and thus after 2 of the 5 TMS pulses), mean eye position deviated only a very small amount, and to the left rather than right (by 0.46 degrees of visual angle, compared to a total line length of 29 degrees of visual angle, and an eccentricity for the left gap when present of 14 degrees). During TMS, eye blinks occurred during stimulus presentation on less than 2% of trials. Thus, neither changes in eye-position, nor blinks due to TMS, can plausibly explain the substantial impairment of detection for left gaps that we observed in Experiment 2 (see below). Please note also that Experiment 3 subsequently found that the same right-parietal TMS actually enhanced rather than suppressed detection of gaps when present in the right visual field instead. This opposite outcome for the other hemifield is inconsistent with any account in terms merely of TMS-induced blinks obliterating some of the visual displays.
Figure 2.
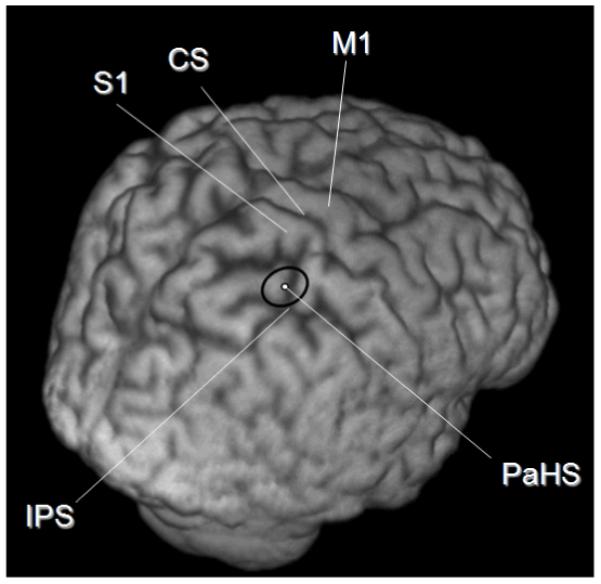
The position of the ‘Parietal Hotspot’ (PaHS) averaged over 9 subjects’ structural MR scans. CS = Central Sulcus, M1 = Primary Motor Cortex, S1= Primary Somatosensory Cortex, IPS = Intra Parietal Sulcus, PaHS = ‘Parietal Hot Spot’. The coordinates of the coil location at the end of the hunting procedure (see main text) as reported in MNI space (ICBM152 template) and using the Talairach stereotaxic convention (Talairach & Tournoux, 1988),were averaged. The coordinates were transformed using the FLIRT programme (FSL 3.2 package, fMRIB, University of Oxford, UK; http://www.fmrib.ox.ac.uk/fsl/) from native space to normalized structural image space. The black ellipse represents the 95% confidence limits. Note that the long-axis of the ellipse lies in the same direction as the TMS coil handle and short axis of the coil, along which the induced magnetic field is more variable. The narrower axis of the ellipse lies along the long axis of the figure of 8 coil where the magnetic field induced is less variable. This may explain the elliptical shape of the 95% confidence limits on site location shown.
Experiment 2: Procedure
For practical reasons only 6 of the original 9 subjects were studied in this time-consuming and demanding follow-up experiment. The six subjects were aged 25-33, all male and with an Edinburgh Handedness Inventory score of 92 ± 4. The parietal hotspot was marked using the scalp coordinates for each subject from the previous experiment. However, in order to confirm that the hotspot does indeed identify the most effective site in its scalp neighbourhood, we now reassigned it as providing the centre of a new 9-point grid (4 × 4 cm, i.e. 2cm between all nearest points in a square grid) which was marked on the scalp. The effect of TMS applied to all of these nine sites was then assessed, to examine how the impact on performance might vary as TMS was shifted away from the putative hotspot. Subjects again had to discriminate between left-gap and no-gap stimuli, as for Experiment 1, but now in blocks of 60 trials (30 no-gap and 30 left-gap stimuli, in randomly intermingled order, with no-gap trials now more common to enable formal signal-detection-theory analyses), first performed without TMS (one block, baseline), and then with TMS disruption (using exactly the same parameters as Experiment 1 and given with each trial, for 10 blocks). TMS was initially delivered over the putative parietal hotspot, and then in randomised order over the 8 other points in the new square grid centred on the hotspot. A final block was then performed with TMS again over the putative hotspot, to provide an average value for this site before and after extended experience, and to assess any impacts of practice (see later). Thus the dual aims of Experiment 2 were to obtain confirmation of the spatial specificity of the identified TMS hotspot TMS, via follow-up testing of a grid of positions on the scalp centred around that site; and to do so while collecting enough psychophysical data to allow full application of signal detection measures (including for sensitivity, d’).
Experiment 2 Results: Perceptual Effects of Parietal Stimulation on Senstivity for Left Sided Targets, with Spatial Specificity of the TMS effect confirmed via the scalp-grid comparisons
Sensitivity (d’) for left-gaps was indeed found to be impaired during TMS over the right-parietal putative ‘hotspot’ (identified by the hunting procedure), and the spatial-specificity of the hotspot TMS site was then confirmed by comparing 9 TMS positions in a grid centered on the putative hotspot.
Signal detection analysis was used to yield the sensitivity measure ‘d-prime’ (d’) from the 6 subjects’ responses. This was derived from the z-transformed ‘Hit’ (H) and ‘False Alarm’ (FA) rates (d’= z(H)-z(FA)) to provides a measure of accuracy that is independent of bias. Being derived in this way from two z-scores results in d’ being a unit-free value (Green and Swets 1966). The subjects’ intrinsic bias towards giving ‘yes’ or ‘no’ responses for gap-presence was derived as the ‘criterion’ measure (c), where c = −1/2[z(H) + z(F)]. Criterion is independent of sensitivity in signal-detection-theory terms, and is also unit-free. The effect of TMS at the right parietal putative ‘hotspot’ was compared with the averaged effect of TMS applied over the 8 equally spaced surrounding sites, to determine whether or not the hunting procedure had in fact located the optimal site in its neighborhood. To relate the changes caused by TMS to subjects’ pre-existing level of performance, the data were expressed as a percentage of baseline (no TMS) values (the no-TMS) value for d’ at the start of the study was 1.84 ± 0.23). The data showed significantly lower sensitivity (d’) to presence/absence of the gap in the left visual hemifield during TMS over the right parietal hotspot, than during TMS over the surrounding sites in the grid (mean d’ during hotspot TMS fell to 89 ± 0.14% of baseline, while for the surrounding TMS sites in the grid it yielded 112 ± 9%, leading to a significant difference with t(5) = −2.59, p = 0.048, two-tailed; see Fig.3 and Table 1. Finally, there were no significant TMS impact on criterion (c) for left gaps; see Table 2 (all p>0.10).
Figure 3.
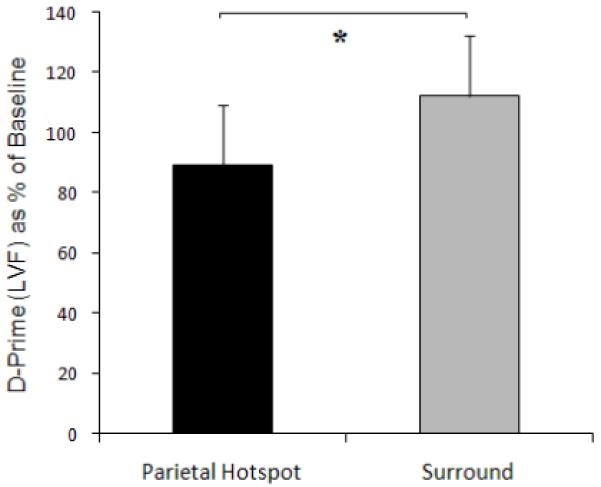
Visual sensitivity (d-prime, as % of the no-TMS baseline) in the left visual field (LVF) for Experiment 2, which found it to be significantly lower with TMS over the right-parietal hotspot as compared to the 8 surrounding sites in the 9-point grid. The asterisk represents a significant difference between the 2 conditions; see main text.
Table 1.
Subjects’ d-prime values, shown as a % of baseline performance (no TMS) for each of the 9 points, during TMS. The standard error of the mean is shown in brackets
| Position Relative to Parietal Hotspot |
2cm Medial | Level | 2cm Lateral |
|---|---|---|---|
| 2cm Anterior | 112 (12) | 120 (13) | 106 (11) |
| Level | 109 (17) | 89 (13) | 114 (11) |
| 2cm Posterior | 119 (17) | 114 (17) | 103 (19) |
Table 2.
Subjects’ criterion values shown as the numerical deviation from baseline (no TMS), for each of the 9 points, during TMS. The standard error of the mean is shown in brackets
| Position Relative to Parietal Hotspot |
2cm Medial | Level | 2cm Lateral |
|---|---|---|---|
| 2cm Anterior | −0.05 (0.23) | 0.10 (0.27) | −0.06(0.16) |
| Level | −0.16 (0.16) | 0.14(0.11) | 0.24 (0.21) |
| 2cm Posterior | −0.16 (0.19) | 0.10(0.14) | 0.16 (0.16) |
Experiment 3: Procedure
In this experiment, the 6 subjects (including five who had participated in both Experiments 1 and 2, and one who had participated in just Experiment 1 before) were 5 males and 1 female, aged 25-33, with a handedness score of 92±4. They were now tested in their ability to detect right sided gaps instead (Fig.1C), when these were intermingled with no-gap stimuli (Fig 1A) in a random order. Subjects now knew in advance that any gap could appear only on the far right. Their ability to detect such gaps was first measured without TMS (one baseline block, 60 trials as in Experiment 2, with gap presence or absence equiprobable) and then with both real, and sham TMS disruption (using the parameters stated above), during two further blocks of 60 trials. TMS was given over the same right parietal hotspot determined by the preceding hunting procedure, with the order of real- and sham TMS blocks counterbalanced across the 6 subjects. Sham stimulation was given at the same intensity, but with the coil first rotated 90° around its (figure-of-eight) long axis before placement on the scalp. With this coil orientation no MEP is produced when held over motor cortex (even at 100% of maximum stimulator output), and substantially less intracerebral TMS-induced voltage is recorded when held over monkey parietal cortex (Lisanby et al. 2001); yet comparable acoustic noise, and non-zero scalp stimulation, still occur.
Based on Hilgetag et al (2001) and the hemispheric-competition notion of Kinsbourne (1997), as briefly reviewed in our introduction, we might expect that TMS over the right parietal hotspot site, selected to impair detection of left gaps, might conversely enhance detection of right gaps instead. But if the TMS disruption for left gaps was somehow nonspecific (e.g. merely reflecting, say, induced blinks), then the same TMS should presumably impair sensitivity to right gaps in the same or similar manner to the impact on performance for left gaps, rather than having an opposite effect.
Experiment 3 Results: Perceptual Effects of Parietal Stimulation on Senstivity for Right Sided Targets
As in Experiment 2, the data were first normalised by being expressed as % of baseline (I,e, no TMS) performance, separately for real and sham TMS conditions. d-prime for gaps in the right visual field (RVF) was found to increase during real TMS over the right parietal hotspot (to 130 ±16% of baseline), less so than during sham TMS (114±15%), leading to a significant difference between real and sham TMS conditions, t(4) = −4.25 p = 0.010, two-tailed; see Fig 4. Note that the enhancement by right-parietal TMS over the hotspot is the opposite outcome to the reduced sensitivity found (in Experiment 2) for gaps in the left visual hemifield instead (see also Experiment 4 below, for a further confirmation of that left hemifield result, when comparing real to sham TMS just as in Experiment 3). As in Experiment 2, there were no significant TMS effects on the criterion (c) measure in Expeirment 3 (p = 0.53 n.s.).
Figure 4.
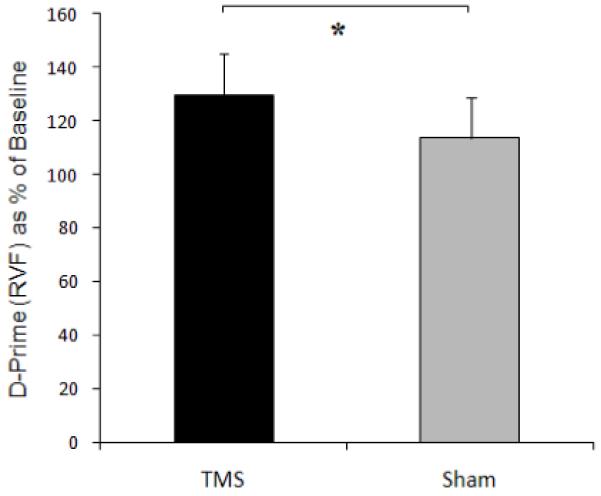
The effect of TMS over the right-parietal hotspot on d-prime (as % of no-TMS baseline) for targets in the right visual field (RVF), showing a significant rise compared to the baseline (no-TMS) condition, that also differs significantly from the intermingled Sham-TMS condition; see main text.
Experiment 4: Procedure
The aim of this final study was to examine how effective TMS intensity over the right parietal hotspot, for disrupting sensitivity to left gaps, might relate to individual motor thresholds when stimulating over M1 instead. 8 subjects (from the original 9 in Experiment 1, 7 male, 1 female, handedness score 82±10) were asked to discriminate between ‘left gap’ or ‘no gap’ stimuli in blocks of 60 trials, just as in Experiment 2. But blocks were now performed in successive pairs: one with TMS delivered over the right parietal hotspot and one using sham TMS (coil still held over the same hotspot, but now at 90 degrees to the scalp), with the order of these within each successive block-pair being counterbalanced (see below). Each block-pair was randomly assigned 1 of 10 different TMS intensities (10% RMT, or 20% RMT, or 30% RMT, and so on up to 100% RMT). Thus each of the 10 TMS intensity levels was performed in a different, pseudorandomized order for each subject. Within each block-pair, the sequence (TMS first or Sham first) alternated with each 10% increase in TMS intensity. For half the subjects (chosen at random) the sequence was: TMS first for 10% RMT, Sham first for 20% RMT, TMS first for 30% RMT and so on. For the other half, the sequence also alternated in similar fashionm but starting with Sham first for 10% RMT. This was done to avoid weighting the higher TMS intensity blocks with more TMS-first block pairs (and thus to avoid potential misleadingly poor performances for TMS relative to Sham at high intensities, due to potential intra-block-pair practice effects). In this way we could determine how the impact of TMS at different intensities over the right parietal hotspot, on visuospatial sensitivity to left gaps, might relate to the intensity of TMS required to reach resting motor threshold in individual subjects.
Experiment 4 Results: Relationship between perceptual effect at parietal hotspot for different intensities, and motor threshold for each subject.
For the 8 subjects the RMT range was 40-69% of maximum stimulator output, with a mean of 53±9.7%. For each subject, sensitivity for gaps in the left visual hemifield fell as right-parietal TMS intensity over the hotspot was increased. The rate at which this occurred was studied by comparing the change in d’ after real TMS (expressed as a % of sham TMS d’), against TMS intensity (% of maximum stimulator output) in each subject, and then fitting a linear trend line to each resulting function. Further analysis tested whether the disruptive effect of right-parietal TMS (over the hotspot) at different intensities, upon visuospatial sensitivity to left gaps, was linked to subjects’ RMT. If so, we would expect a given level of TMS intensity to produce more disruption in subjects with a lower RMT (and less in those with a higher RMT). We can expect accordingly that the best spread of d’ values (for comparison with individual subject RMT values) should be found at a TMS intensity corresponding to the average RMT across subjects. For each subject, we therefore read off along their linear trend-line the % drop in d’ at the average RMT (53% of maximum stimulator output). A significant correlation (Spearmans’s rho, rs(6) = 0.794, p 0.019, two- tailed) was found between, on the one axis, the d’ drop due to TMS over the hotspot (as a % of Sham) for each subject (at 53% of maximum stimulator output) with, on the other axis, their RMT; see Fig 5.. Hence a key finding from Experiment 4 is that for TMS over the right parietal hotspot, the amount by which left-gap-senstivity declines (relative to sham) at a given level of TMS intensity relates systematically to each individuals’ resting motor threshold. Since the latter can be readily assessed for any healthy subject or patient, it can now provide a natural way to scale TMS intensity when targeting a right parietal site with the aim of changing visuospatial sensitivity for peripheral targets, as for the gaps used here. Note that this may not always apply for other TMS effects, for which scaling by motor threshold may be inappropriate (see Stokes et al, 2005, 2007, plus Boroojerdi et al., 2002; Stewart, Walsh, & Rothwell, 2001, Antal et al. 2004). But here we have been able to shown that the approach is clearly viable for the present right-parietal TMS effects upon visuospatial sensitivity for left gaps.
Fig.5.
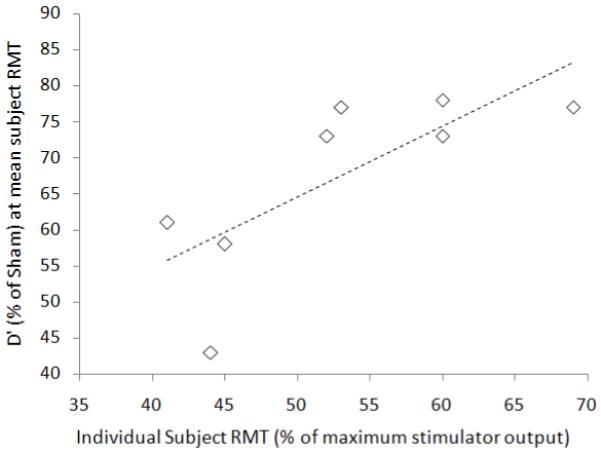
Scatterplot illustrating the positive correlation (dotted line: rs(6) = 0.79, p = .019) between the d’ drop (as a % of d’ scores during Sham rather than real TMS, along the y-axis) for each subject (at the average RMT across subjects i.e. 53% of maximum stimulator output), with each subject’s individual RMT shown along the x-axis, for the 8 subjects of Experiment 4.
We next examined the 8 structural MRI scans of the subjects participating in Experiment 4, to investigate possible underlying causes of the correlation described above (as illustrated in Figure 5). We found that the reconstructed scalp-to-cortex depths at each individual parietal or motor hotspot (for parietal, mean 16.2mm, SD 2.5mm; for motor,14.0 mm, SD 2.5mm) correlated tightly with the other across subjects (rs(6) = 0.94, p<0.05, two-tailed). To assess the influence of scalp-cortex distance on the relationship we had found between individuals’ RMT and their parietal-hotspot TMS impact on left-gap-sensitivity (figure 5), we next performed partial correlations, now entering the individual scalp-cortex distances at parietal or motor hotspots as further controlling factors. Either of these each rendered the original correlation less significant. Specifically, entering RMT as factor W, % real-versus-sham d’ at mean RMT as factor X, scalp-to-cortex distance for the parietal hotspot as factor Y, and this distance for the motor hotspot as factor Z, yielded: rs(5)WX.Y = 0.72, t = 2.10, p = 0.10, and rs(5)WX.Z = 0.70 t = 1.92, p = 0.13). This implies that individual differences in scalp-to-cortex distance contribute to the observed relationship between individual RMTs and the impact of parietal-hotspot TMS on left-gap sensitivity (cf. Fig 5). But this does not undermine the usefulness of scaling TMS intensity relative to RMT, when seeking an individually-effective TMS intensity for the parietal hotspot.
General,Discussion
This study introduces, explores and validates a novel hunting procedure for identifying a distinct point over right posterior parietal cortex at which TMS disrupts visuospatial sensitivity in the contralateral visual hemifield (while leading to enhanced visuospatial sensitivity instead for the ipsilateral hemifield). The right-parietal site identified lies along the antero-superior edge of parietal regions commonly implicated in ‘neglect-related’ lesions (temporo-parietal junction, angular and supramarginal gyri (see Parton & Husain, 2004; Golay, Schnider & Ptak 2008), though it should be noted that extensive lesions after stroke versus TMS disruptions in normals may be very different in their physiological consequences. The location of the identified effective TMS site along the anterior intraparietal sulcus (IPS) appears consistent with several other TMS studies in normals that disrupted visuospatial processing (e.g. Fierro, Brighina et al., 2000; Oliveri, Rossini et al., 1999; Hilgetag, Theoret, & Pascual-Leone, 2001); Sack, Kohler et al., 2007; Schenkluhn et al. 2008). Here we introduce a systematic and cross-validated way to identify the optimal site functionally, and an effective TMS intensity, for individual subjects.
It has been suggested that when judging non-foveal targets (as when discriminating whether or not there was an eccentric ‘gap’ in the extended horizontal line here), several processes may be involved that draw on specific circuits involving parital cortex, such as decoupling attention from fixation and shifting the attentional focus covertly to the target location (e.g., Posner et al. 1984; Giesbrecht and Mangun 2005). In the present experiments, the location of the possible gap target on one or other side was always known in advance, but the burst of TMS pulses began shortly before display onset. This may have disrupted the intended covert attentional focus. We found significantly reduced sensitivity (d’) for target gaps in the left hemifield during bursts of TMS over the identified right-parietal hotspot. But the same TMS actually led to enhanced rather than impaired sensitivity for right hemifield gaps (Experiment 3), illustrating that the TMS did not simply disrupt any visual judgements. Future studies using variations on the new paradigm introduced here could study the timing of these TMS effects in more detail, either by using single TMS pulses at different points in time relative to the visual displays, and/or by jittering the 10Hz bursts relative to those stimuli. Our aim here was not to specify the timing of our effects, but rather to introduce a speedy yet formal ‘hunting’ procedure, for identifying an effective right-parietal site, and intensity, in individuals.
A recent brain imaging study compared activation during holding or shifting of covert attention for both central and peripheral locations (Kelley et al., 2008). Their analysis revealed activity during maintenance of covert attention at peripheral locations (during central eye fixation, as here) in anterior PPC, with a peak approximately 1 cm medial to the location found in the present study. This activation for maintained peripheral attention fell closer to our stimulation site than those for shifting attention from central fixation to periphery. This may accord with our present use of a paradigm in which peripheral target location was known, and constant, throughout each experiment, hence requiring covert attention to be held there rather than frequently shifted.
The ‘hunting procedure’ introduced here (see Experiment 1) is intended to provide a quick and practical heuristic for locating a right parietal area that influences visuospatial sensitivity (impairing this for the contralateral hemifield, as further confirmed in Experiment 2, while enhancing it for the ipsilateral hemifled, see Experiment 3). But despite offering a quick heuristic technique, the risk of falsely identifying the wrong area as the ‘hot spot’ appears relatively low. If subjects were to respond at random during the TMS part of Experiment 1, it can be argued that there should only be a 0.0625 (p(4 consecutive misses) = 0.54) probability of prematurely ending the procedure (i.e. finding a false ‘hot spot’ defined by four consecutive misses) at any location tested. If we consider the hunting procedure as a whole (which includes titration of display durations to produce ~95% correct baseline performance prior to TMS), ‘random’ responses actually generate a substantially lower (conditional) probability of producing a false hotspot. The probability for 4 initial consecutive hits followed by 4 consecutive misses (as the ‘hotspot’ was defined) would be 0.0039 for each location tested (i.e. p(correct)4 × p(incorrect)4 = 0.54 × 0.54 = 3.9 × 10−3). Moreover, if the apparent hotspot was a complete false positive, because subjects can in principle still maintain the 95 % correct performance level achieved during the ‘staircase’ thresholding trials during TMS at the particular site, the risk of a false positive during the hunting procedure arguably falls still further, to just 5.1 × 10−6 per stimulation site (p(correct)4 × p(incorrect)4 = 0.954 × 0.054 = 5.1 × 10−6). Finally, a less hypothetical estimate of the subjects’ % correct score during the hunting procedure can be derived using actual data from Experiment 4. Recall that in this study one of the blocks was performed with sham TMS given over the hotspot with stimulator intensity set to 100% of the subjects RMT. This provides a suitable ‘test model’ in which TMS effects should be absent, yet the click-sound due to TMS (and to a lesser extent scalp artifact) are still included. The average % correct score from this block for all subjects in Experiment 4 was 85±7% (understandably less than the 95% scored without any distraction from the coil, but far greater than chance levels of accuracy). When we insert this value into our previous hypothetcical, calculation the probability of finding a false hotspot, by chance, in the absence of any TMS effect, still remains very low: 0.854 × 0.154 = 2.6 × 10−4 per location tested. Thus we suggest that despite its simplicity and speed, the hunting procedure introduced in Experiment 1 should not be particularly susceptible to false-positive apparent ‘hotspots’. In any case, we note also that our further experiments confirmed, validated and refined the impact of TMS at the identified parietal hotspot on visuospatial performance, using the formal methods of signal detection theory.
As touched on in the Procedure section for Experiment 1, some slightly potential spatial ‘sampling-bias’ might still arise during the hunting procedure, despite the alternating start direction of the spiral path. Because the spiral search pattern ran clockwise for all subjects during the hunting procedure, anterior-lateral and posterior-medial points would be sampled somewhat later. However by analysing the actual sampling paths of all subjects we found that the anterior-lateral or posterior-medial locations (when grouped into quadrants) were tested only 0.8 (on average) sites later than the other two quadrants. As implemented, our hunting procedure thus seems sufficiently robust in practice not to be substantially affected by spatial samploing bias, though for future work any such bias could be reduced still further by adding anterior and posterior starting directions (to the existing medial and lateral ones).
The change in gap-present proportions from 50 % to 90% when moving from the initial ‘staircase’ thresholding stage to the hunting procedure of Experiment 1 might pose a potential problem if subjects were informed about this change, and correspondingly shifted their response criteria. The 9 subjects we tested were naïve, and this may be important for future studies with a similar procedure. But we note that our TMS findings remained robust (and spatially specific to the identified right-parietal hotspot) in Experiment 2, where gap presence/absence remained equiprobable throughout (see also Experiments 3 and 4).
Further refinement and cross-validation of the hotspot’s properties in the subsequent experiments included: a) our confirmation of genuine effects upon visuospatial sensitivity (d’), with signal-detection measures (Experiments 2-4); b) confirmation that the site yielded by our hunting procedure was indeed significantly the most effective within a 9-point grid subsequently tested around it, via collection of further data independent of the original hunting procedure (Experiment 2); c) demonstration that sensitivity to targets in the right visual hemifield actually showed the opposite pattern to left hemifield targets, for the same right-parietal TMS site, with enhanced sensitivity for right targets but impaired for left targets; and finally (d) demonstration that the effect of right-parietal TMS as a function of intensity related systematically to each individual’s motor threshold for TMS over M1 (in a manner that may in turn relate to scalp-to-cortex distances, as implied by partial correlations with those for both parietal and M1 sites).
The opposite pattern of effects for left versus right hemifield visuospatial sensitivity (i.e., impaired sensitivity for the left hemifield, but enhanced sensitivity for the right during our right-parietal TMS) rules out a nonspecific disruption of all visual processing (as might have arisen if, say, our TMS had induced actual blinks, or some internal ‘attentional blink’ regardless of target location). The opposing pattern for the two hemifields accord with classic notions of hemispheric rivalry (Kinsbourne, 1977) and with other TMS work (Hilgetag, Theoret, & Pascual-Leone, 2001), though now confirming this opposing pattern for actual perceptual sensitivity, d’, in signal-detection terms for the first time.
The significant correlation between the effectiveness of parietal TMS (here on visuospatial sensitivity) at different intensities, with motor threshold in individual subjects here, builds on a previous observation (Oliveri, Caltagirone et al., 2000) that the average level of TMS intensity (given over P4 in that study) required to disrupt tactile perception can relate to RMT. But it contrasts with other work reporting little or no correlation of RMT with phosphene thresholds over occipital cortex (Boroojerdi et al., 2002; Stewart, Walsh, & Rothwell, 2001, Antal et al. 2004). The latter outcome might reflect idiosyncrasies in the depth of early visual cortical structures from the scalp in individuals (cf. Stokes et al, 2005). By contrast the MR-reconstructed depth from the scalp, to parietal or motor cortex under either of our hotspot sites, did show parietal-motor correlations within our subjects. Subsequent partial correlations implicated this underlying anatomical relationship one contributor to the initial correlation demonstrated in Experiment 4 (and Figure 5), between individual RMT, and the impact of real versus sham parietal-hotspot TMS on left-visuospatial sensitivity. Nevertheless, our data still show that by using TMS over the individually-hunted, functional definied parietal hotspot, at an intensity equal to the individually-determined RMT over M1, one can expect to obtain a reliable effect on visuospatial sensitivity.
Given the greater scalp-to-cortex distance for the parietal than the motor site, over-stimulation of underlying parietal cortex seems unlikely. Thus right-parietal TMS at the spot identified via our “hunting” procedure, at an intensity equal to RMT, should have the dual virtues of inducing a robust effect on visuospatial sensitivity (as shown in Experiments 2 and 4, figs. 3 and 5) yet with a low risk of any adverse effect. Experiment 4 may also illustrate the potential importance of tailoring the intensity of stimulation used in each subject (e.g. in relation to RMT) rather than using a single constant intensity across subjects (which may result in over-or under stimulation of the underlying cortex for some). Contrary to studies on earlier visual areas (Boroojerdi et al., 2002; Stewart, Walsh, & Rothwell, 2001, Antal et al. 2004), which may differ for the reasons noted earlier (e.g. highly variable distance from the scalp), RMT may thus still provide a useful and easily measured physiological surrogate for some other sites, in this case for the intensity of stimulation over right-parietal cortex needed to disrupt visuospatial sensitivity.
Conclusion
In this study we identified a right-parietal ‘hotspot’ or node that may form a pivotal part of the network that subserves visuospatial awareness. The use of signal detection theory revealed significant impacts on true visual sensitivity (i.e. d’), with right-parietal TMS at the identified site and at an appropriate intensity disrupting visuospatial sensitivity for left targets but enhancing this for right targets. We cross-validated the derived parietal site in several ways, to confirm the efficacy of our new hunting procedure. It provides a systematic new way to identify an effective right-parietal site for inducing specific effects on visuospatial sensitivity, with the effective intensity now also being guided in a principled manner by that ‘anchor’ for TMS researchers, the RMT.
Supplementary Material
Footnotes
This work was carried out at the Sobell Department of Motor Neuroscience, UCL Institute of Neurology, University College London, 33 Queen Square London WC1N 3BG, UK
References
- 1.Antal A, Nitsche M, Kincses T, Lampe C, Paulus W. No correlation between moving phosphene and motor thresholds: a transcranial magnetic stimulation study. Neuroreport. 2004;15(2):297–302. doi: 10.1097/00001756-200402090-00017. [DOI] [PubMed] [Google Scholar]
- 2.Ashbridge E, Walsh V, Cowey A. Temporal aspects of visual search studied by transcranial magnetic stimulation. Neuropsychologia. 1997;35:1121–1131. doi: 10.1016/s0028-3932(97)00003-1. [DOI] [PubMed] [Google Scholar]
- 3.Boroojerdi B, Meister I, Foltys H, Sparing R, Cohen L, Topper R. Visual and motor cortex excitability: a transcranial magnetic stimulation study. Clinical Neurophysiology. 2002;113:1501–1504. doi: 10.1016/s1388-2457(02)00198-0. [DOI] [PubMed] [Google Scholar]
- 4.Brighina F, Bisiach E, Oliveri M, Piazza A, La Bua V, Daniele O, Fierro B. 1Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neuroscience Letters. 2003;336:131–133. doi: 10.1016/s0304-3940(02)01283-1. [DOI] [PubMed] [Google Scholar]
- 5.Chambers CD, Stokes MG, Janko NE, Mattingley JB. Enhancement of visual selection during transient disruption of parietal cortex. Brain Research. 2006;1097(1):149–155. doi: 10.1016/j.brainres.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 6.Corbetta M, Schulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 7.Corthout E, Hallett M, Cowey A. Interference with vision by TMS over the occipital pole: a fourth period. Cognitive Neuroscience and Neuropsychology. 2003;14(4):651–655. doi: 10.1097/00001756-200303240-00026. [DOI] [PubMed] [Google Scholar]
- 8.Dambeck N, Sparing R, Meister IG, Wienemann M, Weidermann J, Topper R, Boroojerdi A. Interhemispheric imbalance during visuospatial attention investigated by unilateral and bilateral TMS over human parietal cortices. Brain Res. 2006;1072(1):194–9. doi: 10.1016/j.brainres.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 9.Drew AS, van Donkelaar P. The contribution of the human PPC to the orienting of visuospatial attention during smooth pursuit. Experimental Brain Research. 2007;179(1):65–73. doi: 10.1007/s00221-006-0769-z. [DOI] [PubMed] [Google Scholar]
- 10.Ellison A, Schindler I, Pattison L, Milner D. An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain. 2004;127:2307–2315. doi: 10.1093/brain/awh244. [DOI] [PubMed] [Google Scholar]
- 11.Fecteau S, Pascual-Leone A, Theoret H. Paradoxical facilitation of attention in healthy humans. Behavioural Neurology. 2006;(17):159–162. doi: 10.1155/2006/632141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fierro B, Brighina F, Giglia G, Palermo A, Francolini M, Scalia S. Paired pulse TMS over the right posterior parietal cortex modulates visuospatial perception. Journal of the Neurological Sciences. 2006;247:144–148. doi: 10.1016/j.jns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Fierro B, Brighina F, Oliveri M, Piazza A, La Bua V, Buffa D, Bisiach E. Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport. 2000;11(7):1519–1521. [PubMed] [Google Scholar]
- 14.Giesbrecht B, Mangun GR. Identifying the neural systems of topdown attentional control: a meta-analytic approach. In: Itti L, Rees G, Tsotsos J, editors. Neurobiology of attention. Academic Press/Elsevier; New York: 2005. pp. 53–56. [Google Scholar]
- 15.Golay L, Schnide r A., Ptak R. Cortical and subcortical anatomy of chronic spatial neglect following vascular damage. Behavioural and Brain Functions. 2008;4:43. doi: 10.1186/1744-9081-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green M, Swets J. Signal Detection and Psychophysics. John Wiley and Sons; New York: 1966. [Google Scholar]
- 17.Hilgetag C, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nature Neuroscience. 2001;4(9):953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- 18.Jin Y, Hilgetag C. Perturbation of visuospatial attention by high-frequency offline rTMS. Experimental Brain Research. 2008;189:121–128. doi: 10.1007/s00221-008-1449-y. [DOI] [PubMed] [Google Scholar]
- 19.Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cerebral Cortex. 2008;18(1):114–125. doi: 10.1093/cercor/bhm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsbourne M. Hemi-neglect and hemisphere rivalry. In: Weinstein EA, Friedland RP, editors. Hemi-inattention and hemisphere specialisation. Advances in neurology. Vol. 18. Raven Press; New York: 1977. pp. 41–9. [PubMed] [Google Scholar]
- 21.Koch G, Oliveri M, Cheran B, Ruge D, Lo Gerfo E, Salerno S, Torriero S, Marconi B, Mori F, Driver J, Rothwell J, Caltagirone C. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008 doi: 10.1093/brain/awn273. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch G, Oliveri M, Torriero S, Caltagirone C. Modulation of excitatory and inhibitory circuits for visual awareness in the human right parietal cortex. Experimental Brain Research. 2005;160:510–516. doi: 10.1007/s00221-004-2039-2. [DOI] [PubMed] [Google Scholar]
- 23.Lisanby S, Gutman D, Luber B, Schroeder C, Sackeim H. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biological Psychiatry. 2001;49:460–463. doi: 10.1016/s0006-3223(00)01110-0. [DOI] [PubMed] [Google Scholar]
- 24.Meister IG, Wienemann M, Buelte C, Grunewald R, Sparing N, Dambeck N, Boroojerdi B. Hemiextinction induced by transcranial magnetic stimulation over the right temporo-parietal junction. Neuroscience. 2006;142:119–123. doi: 10.1016/j.neuroscience.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Mort D, Malhotra P, Mannan S, Rorden C, Pambakian A, Kennard C, Husain M. The Anatomy of Visual Neglect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- 26.Muggleton N, Postma P, Moutsopoulou K, Nimmo-Smith I, Marce IA, Walsh V. TMS over right posterior parietal cortex induces neglect in a scene-based frame of reference. Neuropsychologia. 2006;44(7):1222–9. doi: 10.1016/j.neuropsychologia.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Muggleton N, Cowey A, Walsh V. The role of the angular gyrus in visual conjunction search investigated using signal detection analysis and transcranial magnetic stimulation. Neuropsychologia. 2008;46:2198–2202. doi: 10.1016/j.neuropsychologia.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Oda I, Isobe S, Suzuki T, Kohyama K, Dan I. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10-20 system oriented for transcranial functional brain mapping. Neuroimage. 2003;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Oliveri M, Rossini P, Pasqualetti P, Traversa R, Cicinelli P, Palmieri M, Tomaioulo F, Caltagirone C. Interhemispheric asymmetries in the perception of unimanual and bimanual cutaneous stimuli. Brain. 1999;122:1721–1729. doi: 10.1093/brain/122.9.1721. [DOI] [PubMed] [Google Scholar]
- 30.Oliveri M, Caltagirone M, Filippi M, Traversa P, Cicinelli P, Pasqualetti P, Rossini M. Paired transcranial magnetic stimulation protocols reveal a pattern of inhibition and facilitation in the human parietal cortex. The Journal of Physiology. 2000;529:461–468. doi: 10.1111/j.1469-7793.2000.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveri M, Rossini P, Filippi M, Traversa R, Cicinelli P, Palmieri M, Pasqualetti P, Caltagirone C. Time-dependent activation of parieto-frontal networks for directing attention to tactile space. Brain. 2000;123:1939–1947. doi: 10.1093/brain/123.9.1939. [DOI] [PubMed] [Google Scholar]
- 32.Parton A, Husain M. Spatial Neglect. Advances in Clinical Neuroscience and Rehabilitation. 2004;4(4):17–18. [Google Scholar]
- 33.Pascual-Leone A, Gomez-Tortosa E, Grafman J, Alway D, Nichelli P, Hallett M. Induction of visual extinction by rapid-rate transcranial magnetic stimulation of parietal lobe. Neurology. 1994;44:494–498. doi: 10.1212/wnl.44.3_part_1.494. [DOI] [PubMed] [Google Scholar]
- 34.Paus T, Grosbras M-H. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. European Journal of Neuroscience. 2003;18:3121–3126. doi: 10.1111/j.1460-9568.2003.03055.x. [DOI] [PubMed] [Google Scholar]
- 35.Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pourtois G, Vandermeeren Y, Olivier E, de Gelder B. Event related TMS over the right posterior parietal cortex induces ipsilateral visuo-spatial interference. Neuroreport. 2001;12(11):2369–2374. doi: 10.1097/00001756-200108080-00017. [DOI] [PubMed] [Google Scholar]
- 37.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, Maertens de Noordhout A, Marsden C, Murray N, Rothwell JC, Swash M, Thomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 38.Ryan S, Bonilha L, Jackson S. Individual variation in the location of the parietal eye fields: a TMS study. Experimental Brain Research. 2006;173:398–394. doi: 10.1007/s00221-006-0379-9. [DOI] [PubMed] [Google Scholar]
- 39.Sack A, Kadosh R, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. The Journal of Cognitive Neuroscience. 2008 doi: 10.1162/jocn.2009.21126. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Sack A, Kohler A, Bestmann S, Linden D, Dechent P, Goebel R, Baudewig J. Imaging the brain activity changes underlying impaired visuospatial judgements: Simultaneous fMRi TMS, and behavioral studies. Cerebral Cortex. 2007;17(12):2841–52. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- 41.Schenkluhn B, Ruff C, Heinen K, Chambers C. Parietal Stimulation Decouples Spatial and Feature-Based Attention. The Journal of Neuroscience. 2008;28(44):11106–11110. doi: 10.1523/JNEUROSCI.3591-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart L, Walsh V, Rothwell J. Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia. 2001;39:415–419. doi: 10.1016/s0028-3932(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 43.Stokes MG, Chambers CD, Gould IC, English T, McNaught E, McDonald O, Mattingley JB. Distance adjusted motor threshold for transcranial magnetic stimulation. Clinical Neurophysiology. 2007;118:1617–1625. doi: 10.1016/j.clinph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, Mattingley JB. Simple metric for scaling motor threshold based on scalp-cortex distance: application to studies using transcranial magnetic stimulation. The Journal of Neurophysiology. 2005;94:4520–4527. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- 45.Sykora S. K-space formulation of MRI. In: Sykora S, editor. Stan’s Library. Vol. 1. 2005. www.ebyte.it/library. [Google Scholar]
- 46.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart; Thieme: 1988. [Google Scholar]
- 47.Valero-Cabre A, Rushmore R, Payne B. Low frequency transcranial magnetic stimulation on the posterior parietal cortex induces visuotopically specific neglect-like syndrome. Experimental Brain Research. 2006;172:14–21. doi: 10.1007/s00221-005-0307-4. [DOI] [PubMed] [Google Scholar]
- 48.Wieczorowski M. Spiral sampling as a fast way of data acquisition in surface topography. International Journal of Machine Tools & Manufacture. 2001;41:2017–2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


